Abstract
Paracoccidioidomycosis is a systemic fungal infection caused by Paracoccidioides brasiliensis and endemic in certain areas of Central and South America. We report a case of a 62-year-old-man with a complex history of tuberculosis and imaging findings of a cerebral lesion and bilateral adrenal enlargement. Biopsy of adrenal gland revealed Paracoccidioides brasiliensis. This case highlights the importance of travel history for diagnosis of paracoccidioidomycosis in non-endemic areas and emphasizes the clinical and histopathological similarities with tuberculosis.
Keywords: Paracoccidioidomycosis, Paracoccidioides brasiliensis, Endemic systemic mycosis, Bilateral adrenal enlargement, Tuberculosis
1. Introduction
Paracoccidioidomycosis is a systemic fungal infection caused by the thermally dimorphic fungus Paracoccidioides brasiliensis [1]. This disease is endemic in certain South and Central American countries with the highest prevalence observed in Brazil (approximately 80%) [2]. People who work in agriculture and live in rural areas are at a particularly high risk for infection [1]. Due to its specific geographical distribution, paracoccidioidomycosis is only observed in patients who have lived or travelled in endemic regions [3]. An increase in migration and travel activities might contribute to a higher prevalence of paracoccidioidomycosis and other systemic mycoses also in Europe.
The thermally dimorphic fungus Paracoccidioides brasiliensis grows as a filamentous fungus at 20–26 °C and as yeast at 37 °C [2]. Diagnosis is based on the direct identification of the fungus with microscopy, culture or histology from clinical specimen [2]. In addition, molecular biological techniques such as polymerase chain reaction (PCR) demonstrate high sensitivity suitable for diagnosis and monitoring of treatment [4], [5]. Serological tests are used for evaluation of treatment response and disease recurrence [6].
Paracoccidioidomycosis clinically appears in two different forms: an acute/subacute juvenile form and a chronic adult form [2]. More than 90% of cases are chronic forms predominantly affecting men 30–60 years old and are characterized by slow progression over months or even years. This form can occur either as unifocal if only the lungs are affected (approximately 25%) or multifocal with extrapulmonary dissemination to oral or nasal mucosa, skin, lymph nodes or adrenal glands. Less frequently, involvement of the eyes, central nervous system (CNS), bones, vascular system and genital lesions may occur [2].
The fungus has been detected in soil, but the natural habitat is unknown. Human to human transmission has not been described, yet [7]. Similar to other systemic mycoses, Paracoccidioides brasiliensis enters the host via the respiratory tract and is usually inhaled during agriculture-related activities. Depending on the patient's immune status, it may stay inactive or spread by lymphatic and haematogenous dissemination to various secondary sites [1], [2]. The choice of treatment with azole derivatives, amphotericin B and sulfonamides depends on the severity of the disease [8].
Diagnosis of imported paracoccidioidomycosis in non-endemic countries is challenging [9]. First, the latency period between inoculation of the pathogen and manifestation of overt symptoms may be very long [2]. Second, physicians in non-endemic countries have limited clinical experience with endemic systemic mycosis. Third, clinical and histopathological presentation of this fungal infection resembles several other infectious and non-infectious diseases.
We report on a rare case of paracoccidioidomycosis in Europe that was initially misdiagnosed and treated as tuberculosis.
Aim of this case report is to strengthen awareness for imported endemic systemic mycosis - in particular paracoccidioidomycosis - and emphasize the similarities to tuberculosis and importance of travel history.
2. Case
A 62-year-old man was transferred from a community hospital to our department due to suspected diagnosis of reactivated tuberculosis of the lungs and adrenal glands in February 2015 (day 0). He reported left-sided chest and abdominal pain, weight loss of 12 kg during the last 3 weeks as well as night sweats and cough. The punctum maximum of the abdominal pain was located at the left costal margin and aggravated at inspiration.
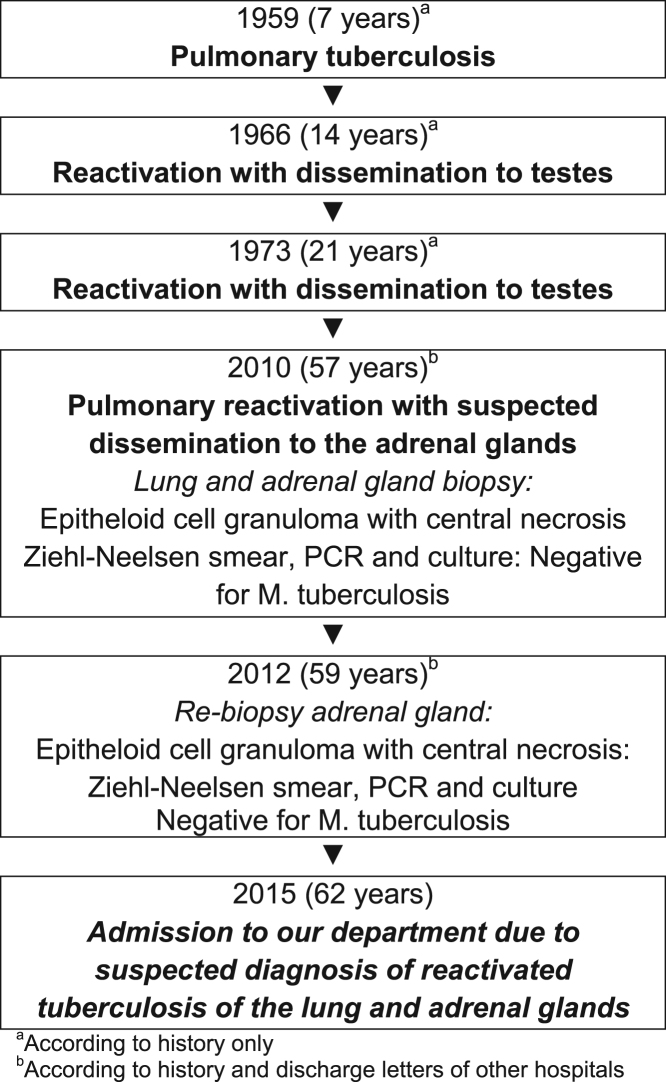
Past medical history revealed several episodes of tuberculosis with involvement of the lungs, testes and adrenal glands (Fig. 1). The patient reported that tuberculosis was diagnosed for the first time at the age of 7 years, followed by reactivations in the lungs with dissemination to the testes at the age of 14 and 21. Therefore, the patient had received tuberculostatic therapy, repeatedly. In 2010, he underwent CT-guided biopsy of a suspicious pulmonary nodule in the right lower lobe and left adrenal gland due to considerable enlargement of adrenal glands. Based on the histopathological finding of epithelioid cell granuloma with central necrosis, the diagnosis of tuberculosis of lungs and adrenal glands was made. At that time, sputum smear, PCR and culture for acid fast bacilli were negative. Due to increasing size of the adrenal glands the patient underwent a second biopsy in the same hospital in 2012. Histopathological finding were similar to previous biopsy and again considered to be tuberculosis.
Fig. 1.
Timeline of patient's tuberculosis.
From the year 1997 until 2001 he made yearly visits – each for several weeks - to a village in Regio de Pasco (Peru), a rural area located next to the rainforest of the Amazonas. He lived there in a house with a garden for approximately three years (2002–2004) and helped to construct a hospital. He did no agricultural or field work. During his stay in Peru he had no injury or disease worth mentioning. Except for two days in Arica (Chile) he did not visit any other South or Central American regions or countries. In addition, he reported that one day he recognized bats in the ceiling of his house and inhaled their excrements during cleaning.
In addition to tuberculosis our patient had a past medical history of chronic bronchitis, Billroth II surgery due to peptic ulcer disease, cholecystectomy and prostate cancer treated with radiation therapy approximately 10 years ago. The patient was a retired patient transport service driver in an Austrian hospital. Family history revealed a tuberculosis infection of his father. He was a current smoker with a history of 45 pack years, reported occasional alcohol consumption and no known allergies. He was on no chronic medication.
Upon admission, the patient appeared in a good general condition with normal body temperature and a blood pressure of 120/70 mmHg. Auscultation and percussion of lungs and heart were unremarkable. The abdomen was soft and showed periumbilical tenderness without resistance. Peripheral edema was not observed. Basic neurological examination and skin were unremarkable. No enlarged lymph nodes were found.
Electrocardiogram showed normal sinus rhythm with 78 beats per minute and no pathological findings. There was a mild eosinophilia (6%) and monocytosis (16%), but total white cell count (8.1 G/l), hemoglobin (13.9 g/dl), and platelet count (366 G/l) were within normal limits. Levels of serum creatinine, and blood urea nitrogen (BUN) were normal. The concentration of serum gamma glutamyltransferase (GGT, 193 U/l), alkaline phosphatase (224 U/l), and C-reactive protein (9.0 mg/dl), plasma fibrinogen (560 mg/dl), and erythrocyte sedimentation rate (ESR, 54/81 mm) were elevated. Serum albumin (3.4 g/dl) and sodium (135 mmol/l) were slightly decreased while other electrolytes were normal. Myocardial biomarkers such as high sensitive Troponin-T (hs-TnT), creatine kinase (CK) and muscle-brain type creatine kinase (CK-MB) were within normal range.
Computed tomography (CT) showed postspecific pulmonary lesions. Compared to previous examinations, abdominal CT (day +0) revealed an increasing inhomogeneous enlargement of the adrenal glands (4.0×6.3 cm) (Fig. 2). Additionally, several enlarged retroperitoneal and mesenterial lymph nodes were found. Subsequently performed positron emission tomography (PET) (day +18) revealed an increased metabolic activity in bilaterally enlarged adrenal glands. Furthermore, hypermetabolic activity of cervical lymph nodes and the laryngeal area was found.
Fig. 2.
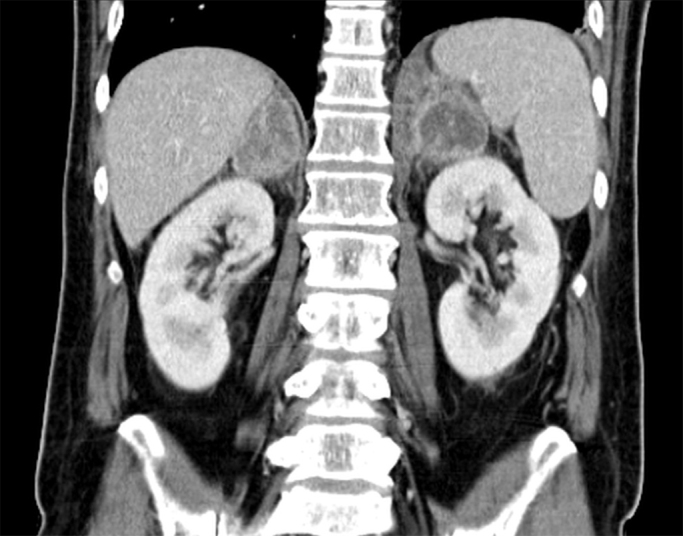
Abdominal CT scan showing considerable enlargement of adrenal glands.
Clinical and radiographic findings were suspicious for reactivation of tuberculosis. Interferon-gamma release assay (Quantiferon) (day +7) was positive but Ziehl-Neelsen smear, polymerase chain reaction (PCR) and culture of the sputum were negative for Mycobacterium tuberculosis. Serological tests for human immunodeficiency virus (HIV), lues and adrenal autoantibodies were negative. Serum levels of adrenocorticotropic hormone (ACTH) and cortisol confirmed primary adrenal insufficiency.
Symptoms such as tremor, disturbance of sensitivity and weakness of the extremities, triggered a neurological work-up. Brain magnetic resonance imaging (MRI) (day +31) revealed a round temporo-parietal located lesion with perifocal edema, suspicious for infection or metastasis (Fig. 3). Cerebrospinal fluid (CSF) obtained by lumbar puncture (day +34) was unremarkable without any sign for infection, inflammation or neoplasm. Neurosurgeons did not consider surgical intervention.
Fig. 3.
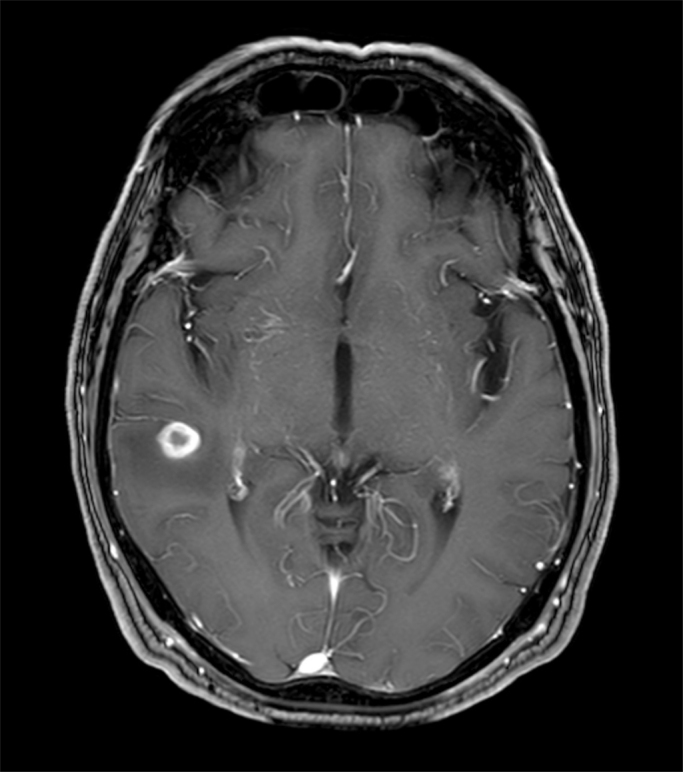
Cerebral MRI showing round temporo-parietal located lesion.
The cause of hypermetabolic bilateral adrenal enlargement remained unclear, initially. Tuberculosis was suspected but could not be confirmed by microbiology. Hence, CT guided biopsy of the left adrenal gland and extirpation of a right cervical lymph node (day +42) were performed for histopathological and microbiological work-up.
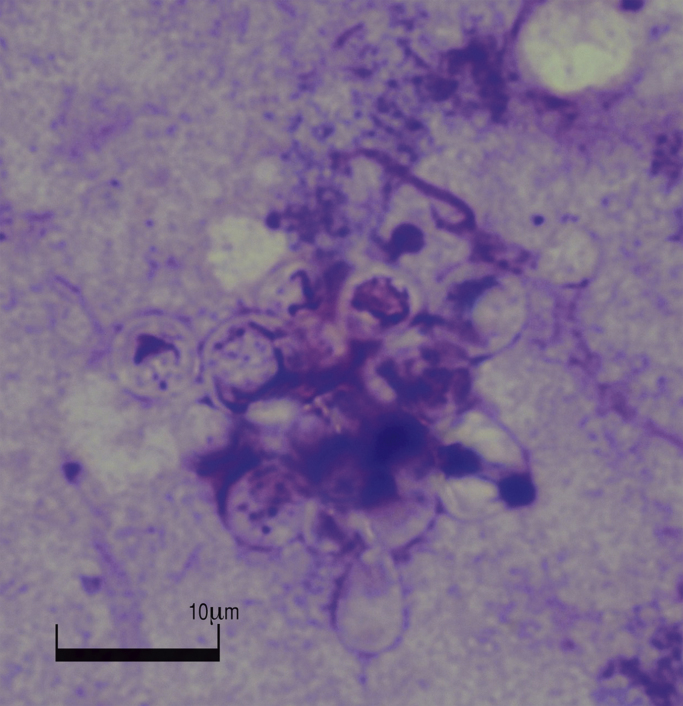
Neither in the biopsy specimens of left adrenal gland nor the right cervical lymph node, Ziehl-Neelsen and PCR revealed any evidence for mycobacterium tuberculosis infection (day +45). Serology for various systemic fungal infections and broad spectrum PCR from blood and aspirate fluids from biopsy (day +49) were negative as well. Histology of lymph node and left adrenal gland biopsy showed granulomas with central necrosis (day +48). In addition, in the left adrenal gland periodic acid–schiff (PAS), McManus, and methenamine silver stain (Grocott) revealed evidence of fungal pathogens. Giemsa stain of the left adrenal gland biopsy specimen (day +49) showed characteristic yeast elements with multipolar budding of variable size thus indicating the presence of Paracoccidioides brasiliensis (Fig. 4). Cultures of the left adrenal gland biopsy specimen, were positive after incubation for 5 days (day +54) at 37 °C and 28 °C on Columbia agar (with 5% sheep blood; bioMérieux, Austria), 2% Sabouraud dextrose agar (Oxoid, Austria) and brain-heart infusion agar (BHI agar, Merck, Austria). Sequence analysis of the isolate confirmed the presence of Paracoccidioides brasiliensis. Sequence similarity was assessed by a search of homology not only with GenBank sequences, but also with the ISHAM ITS database. 100% identity was achieved with both databases (ISHAM identification number: MITS2102).
Fig. 4.
Microscopy (Giemsa stain) appearance of Paracoccidioides brasiliens showing characteristic yeast form with multipolar budding.
Thus, we diagnosed paracoccidioidomycosis with dissemination to adrenal glands and highly suspicious involvement of cervical lymph nodes and the brain.
When paracoccidioidomycosis was suspected, the specimen and all cultures were processed in a special laboratory for biosafety level 3 (S3-laboratory). This laboratory has all the equipment and safety features required for biosafety level 3 with controlled air flow and filtered ventilation systems. All personnel wore personal protective equipment (specialized clothing, gloves whenever handling the specimens or cultures). In accordance to this, personnel had the utmost protection against accidental infection.
After identification of Paracoccidioides brasiliensis antifungal treatment was initiated (Table 1). In addition, replacement therapy with fludrocortisone and hydrocortisone was prescribed. Due to the evidence of cerebral involvement the patient was initially treated with intravenous liposomal amphotericin B. The patient's general clinical condition improved and the antifungal therapy was switched to oral itraconazole. However, itraconazole never reached therapeutic level - neither with capsule nor liquid formulation, most probably due to the previous Billroth II resection. After deterioration of the patient and an increase in inflammatory parameters, liposomal amphotericin B was restarted. Associated with worsening of the systemic inflammation, hypotension, prerenal kidney failure and subsequent Addison crises occurred, so that hydrocortisone and fludrocortisone doses had to be adapted and high volume intravenous (IV) fluids substituted.
Table 1.
Prescribed antifungal treatment.
| Nr | Drug | Route | Duration | Days after admission |
|---|---|---|---|---|
| 1. | Liposomal amphotericin B | IV | 16 days | day+57 until day+72 |
| 2. | Itraconazole | Oral | 32 days | day+73 until day+104 |
| 3. | Liposomal amphotericin B | IV | 44 days | day+104 until day+147 |
| 1 day | day+150a | |||
| 3 days | day+152a, day+154a, day+157a | |||
| 1 day | day+159a | |||
| 4. | Posaconazole | IV | 12 days | day+161 until day+172 |
| Oral | 8 days | day+173 until day+180 | ||
| Oral | since day+181a |
IV, intravenous.
Outpatient treatment.
Several ultrasound imaging studies showed a continuous decrease in size of the bilateral adrenal gland enlargement and a follow-up MRI of the brain revealed a shrinking size of the cerebral lesion. Overall clinical improvement was observed. After consultation of an infectious disease specialist, our patient was switched to oral posaconazole. Finally, the patient was discharged in a good general condition on oral posaconazole.
3. Discussion
Our case report describes the complex journey from presentation to diagnosis and treatment of a patient with paracoccidioidomycosis in a non-endemic area and a history of tuberculosis.
Evidence of paracoccidioidomycosis in non-endemic regions such as Europe is based on case reports, case series and reviews. Numerous cases were observed in Spain and other European countries [9], [10]. To the best of our knowledge, we diagnosed the second case of chronic paracoccidioidomycosis in Austria. The first case of chronic paracoccidioidomycosis in a Cuban female living in Austria was published in the year 2004 [11]. Initially, this case was misdiagnosed and treated as tuberculosis.
Since Paracoccidioides brasiliensis is endemic in several countries of South- and Central America, the patient was most likely infected by inhalation during one of his stays in Peru. The first manifestation in the lung obviously remained undiagnosed and therefore - in agreement with the literature - it seems that there has been a latency period of several years from infection to first clinical presentation already with dissemination to lymph nodes, brain and adrenal glands [2]. Various conditions such as smoking, previous tuberculosis with tuberculostatic treatment and prostate cancer with radiation therapy may have facilitated infection by altering the patient's immune status.
The finding of bilateral adrenal enlargement with primary adrenal insufficiency and the history of tuberculosis revealed reactivation and dissemination of tuberculosis as the most likely diagnosis upon presentation to our center. However, no available report from the past five years showed clear evidence of infection with Mycobacterium tuberculosis and no detailed medical information was available regarding the first episodes of tuberculosis. Diagnosis and therapy of the last episodes labeled as tuberculosis reactivations three and five years before presentation to our hospital was based solely on histopathological findings of adrenal epithelioid cell granuloma with central necrosis. Despite long-term tuberculostatic therapy, no decrease in adrenal gland enlargement was noted, requiring reconsideration of the diagnosis.
The clinical and histopathological presentation not only of paracoccidioidomycosis, but also of other systemic fungal infections, may resemble tuberculosis [12]. Both tuberculosis and systemic mycosis induce formation of granulomas [13]. Decreased cellular immunity and production of certain cytokines and their receptors facilitate both infections. Paracoccidioidomycosis and tuberculosis may be present concomitantly, occur sequentially or might be misdiagnosed due to similar clinical presentations. Previous studies have reported a rate of coexistence of both diseases ranging from 5.5% to 19% [14].
Another common cause of adrenal enlargement and insufficiency are metastases in cancer patients, ranging from 20% to 45% in autopsy studies of lung cancer patients [15]. In our patient with a history of prostate cancer this was excluded by histopathological findings.
A variety of pathogens (fungi, bacteria, viruses and parasites) may affect adrenal glands. Particularly in the presence of bilateral adrenal enlargement, infections caused by Mycobacterium tuberculosis, Paracoccidioides brasiliensis, Histoplasma capsulatum, Blastomyces dermatitidis or Cryptococcus neoformans must be considered for differential diagnosis [16]. Due to the high affinity of Paracoccidioides for adrenal glands, adrenal gland involvement in paracoccidioidomycosis is common (85–90% in autopsy studies) [16], [17].
An autoimmune process is the most common reason for primary adrenal insufficiency (Addison's disease) in Western countries, whereas tuberculosis and other infectious diseases, metastatic cancer, adrenal hemorrhage, infarction or drugs are less frequent [18].
In conclusion, paracoccidioidomycosis is rare in Europe and can mimic more familiar diseases like tuberculosis. Depending on the patient's clinical presentation, travel history, and treatment success, endemic systemic fungal infections should be considered for differential diagnosis. With an increase in migration and travel activities these infections might also gain more relevance in Europe.
Funding
This research did not receive any specific grant from funding agencies in the public, commercial, or not-for-profit sectors.
Conflict of Interest
Gernot Wagner has received travel support from Gilead Sciences.
Acknowledgements
None.
References
- 1.Ameen M., Talhari C., Talhari S. Advances in paracoccidioidomycosis. Clin. Exp. Dermatol. 2010;35(6):576–580. doi: 10.1111/j.1365-2230.2009.03647.x. [DOI] [PubMed] [Google Scholar]
- 2.Brummer E., Castaneda E., Restrepo A. Paracoccidioidomycosis: an update. Clin. Microbiol Rev. 1993;6(2):89–117. doi: 10.1128/cmr.6.2.89. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Colombo A.L., Tobon A., Restrepo A., Queiroz-Telles F., Nucci M. Epidemiology of endemic systemic fungal infections in Latin America. Med Mycol. 2011;49(8):785–798. doi: 10.3109/13693786.2011.577821. [DOI] [PubMed] [Google Scholar]
- 4.Buitrago M.J., Merino P., Puente S., Gomez-Lopez A., Arribi A., Zancope-Oliveira R.M. Utility of real-time PCR for the detection of Paracoccidioides brasiliensis DNA in the diagnosis of imported paracoccidioidomycosis. Med Mycol. 2009;47(8):879–882. doi: 10.3109/13693780802713208. [DOI] [PubMed] [Google Scholar]
- 5.Gomes G.M., Cisalpino P.S., Taborda C.P., de Camargo Z.P. PCR for diagnosis of paracoccidioidomycosis. J. Clin. Microbiol. 2000;38(9):3478–3480. doi: 10.1128/jcm.38.9.3478-3480.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Wanke B., Aide M.A. Chapter 6--paracoccidioidomycosis. J. Bras. Pneumol. 2009;35(12):1245–1249. doi: 10.1590/s1806-37132009001200013. [DOI] [PubMed] [Google Scholar]
- 7.Bocca A.L., Amaral A.C., Teixeira M.M., Sato P.K., Shikanai-Yasuda M.A., Soares Felipe M.S. Paracoccidioidomycosis: eco-epidemiology, taxonomy and clinical and therapeutic issues. Future Microbiol. 2013;8(9):1177–1191. doi: 10.2217/fmb.13.68. [DOI] [PubMed] [Google Scholar]
- 8.Shikanai-Yasuda M.A., Telles Filho Fde Q., Mendes R.P., Colombo A.L., Moretti M.L. [Guidelines in paracoccidioidomycosis] Rev. Soc. Bras. Med. Trop. 2006;39(3):297–310. doi: 10.1590/s0037-86822006000300017. [DOI] [PubMed] [Google Scholar]
- 9.Buitrago M.J., Bernal-Martinez L., Castelli M.V., Rodriguez-Tudela J.L., Cuenca-Estrella M. Histoplasmosis and paracoccidioidomycosis in a non-endemic area: a review of cases and diagnosis. J. Travel Med. 2011;18(1):26–33. doi: 10.1111/j.1708-8305.2010.00477.x. [DOI] [PubMed] [Google Scholar]
- 10.Ajello L., Polonelli L. Imported paracoccidioidomycosis: a public health problem in non-endemic areas. Eur. J. Epidemiol. 1985;1(3):160–165. doi: 10.1007/BF00234089. [DOI] [PubMed] [Google Scholar]
- 11.Mayr A., Kirchmair M., Rainer J., Rossi R., Kreczy A., Tintelnot K. Chronic paracoccidioidomycosis in a female patient in Austria. Eur. J. Clin. Microbiol. Infect. Dis. 2004;23(12):916–919. doi: 10.1007/s10096-004-1239-9. [DOI] [PubMed] [Google Scholar]
- 12.Bonifaz A., Vazquez-Gonzalez D., Perusquia-Ortiz A.M. Endemic systemic mycoses: coccidioidomycosis, histoplasmosis, paracoccidioidomycosis and blastomycosis. J. Dtsch Dermatol Ges. 2011;9(9):705–714. doi: 10.1111/j.1610-0387.2011.07731.x. (quiz 15) [DOI] [PubMed] [Google Scholar]
- 13.Zumla A., James D.G. Granulomatous infections: etiology and classification. Clin. Infect. Dis. 1996;23(1):146–158. doi: 10.1093/clinids/23.1.146. [DOI] [PubMed] [Google Scholar]
- 14.Quagliato Junior R., Grangeia Tde A., Massucio R.A., De Capitani E.M., Rezende Sde M., Balthazar A.B. Association between paracoccidioidomycosis and tuberculosis: reality and misdiagnosis. J. Bras. Pneumol. 2007;33(3):295–300. doi: 10.1590/s1806-37132007000300011. [DOI] [PubMed] [Google Scholar]
- 15.Allard P., Yankaskas B.C., Fletcher R.H., Parker L.A., Halvorsen R.A., Jr. Sensitivity and specificity of computed tomography for the detection of adrenal metastatic lesions among 91 autopsied lung cancer patients. Cancer. 1990;66(3):457–462. doi: 10.1002/1097-0142(19900801)66:3<457::aid-cncr2820660310>3.0.co;2-r. [DOI] [PubMed] [Google Scholar]
- 16.Upadhyay J., Sudhindra P., Abraham G., Trivedi N. Tuberculosis of the adrenal gland: a case report and review of the literature of infections of the adrenal gland. Int. J. Endocrinol. 2014;2014:876037. doi: 10.1155/2014/876037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Agudelo C.A., Munoz C., Ramirez A., Gutierrez J., Velez S., Perez J.C. Identification of Paracoccidioides brasiliensis in adrenal glands biopsies of two patients with paracoccidioidomycosis and adrenal insufficiency. Rev. Inst. Med. Trop. Sao Paulo. 2009;51(1):45–48. doi: 10.1590/s0036-46652009000100008. [DOI] [PubMed] [Google Scholar]
- 18.Ten S., New M., Maclaren N. Clinical review 130: addison's disease 2001. J. Clin. Endocrinol. Metab. 2001;86(7):2909–2922. doi: 10.1210/jcem.86.7.7636. [DOI] [PubMed] [Google Scholar]