Abstract
Despite improvements made in stroke rehabilitation, motor impairment and gait deficits persist at discharge. New interventions are needed. Mirror therapy has promise as one element of a rehabilitation program. The primary objectives were to 1) describe a bilateral, lower extremity mirror therapy (LE-MT) device and training protocol and 2) investigate the feasibility of LE-MT. A LE-MT device was constructed to train bilateral LE movements for 30 min, 3 times/week for 4 weeks, as an adjunct to physiotherapy in three individuals post-stroke. Sessions were digitally recorded and reviewed to extract feasibility measures; repetitions, rests and session duration. Pre and post measures of gait and motor impairment were taken. Two participants completed 100% of the sessions and a third completed 83% due to a recurrence of pre-existing back pain. Repetitions increased and session duration was maintained. Number of rests decreased for two participants and increased for one participant. Participants reported fatigue and mild muscle soreness but also that the intervention was tolerable. Positive gait changes included increased velocity and decreased variability. LE motor impairment also improved. A bilateral LE-MT adjunct intervention for stroke is feasible and may have positive effects. A history of low back pain should be a precaution.
Keywords: Health sciences, Medicine, Rehabilitation
1. Introduction
Impairments in strength, coordination, and balance lead to gait complications post-stroke [1] and improving gait is the number one rehabilitation goal stated by individuals with stroke [2]. Despite improvements made with rehabilitation, post-stroke gait remains slower, more variable, and asymmetric compared to healthy adults [3, 4, 5]. Therefore, new approaches to gait rehabilitation are required to improve outcomes. It is likely that the most effective approach is comprised of exercises and interventions that specifically address stroke-related deficits contributing to gait dysfunction [6]. Mirror therapy (MT) has promise as one element of such a multidimensional gait rehabilitation program. First used in the mid-1990s to treat phantom limb pain [7], it has since been adopted for the treatment of a variety of conditions including stroke [8, 9, 10].
The main goal of MT is to provide visual feedback about the affected limb movement which is generated from the mirror reflection of unaffected limb movement [11]. Visual information is critical to the performance and monitoring of skilled movements including postural control [12] and gait [13]. Furthermore, based on evidence of neuroplastic reorganization in the absence of sensory input, it has been suggested that sensory input is integral to the preservation of cortical representations and therefore motor function following stroke [14]. It is proposed that MT acts to restore the correspondence between motor output (commands sent to the affected limb) and sensory input (visual feedback of the affected limb moving as commanded) [11], and thus enhances recovery of motor control in the affected limb. Motor outcomes associated with upper limb MT include motor recovery as measured by the Brunnstrom stages and Fugl-Meyer [15, 16, 17], and improved speed and accuracy of movement [10] (see Thieme et al. [18], Rothgangel et al. [19] and Ezendam et al. [20] for reviews).
Despite success with MT in the upper limb post-stroke, there is little work on the benefits of MT for the lower extremities (LE-MT). One randomized controlled trial (RCT) of a MT intervention that used ankle dorsiflexion movement for 5 days a week, 2 to 5 h a day, for 4 weeks in individuals with chronic stroke reported improved motor recovery and functional independence, but no difference in functional ambulation category (FAC) [21]. A second RCT included six LE-MT exercises for individuals with acute stroke for 30 min a day, six days a week, for two weeks [22]. Findings included improved ambulation as measured by FAC but no differences between groups in Brunnel Balance Assessment scores or motor recovery [22].
Both of these studies demonstrate the potential for LE-MT to improve motor recovery, balance and gait outcomes post-stroke. But there is an opportunity for improvement. Both previous MT studies employed unilateral movements of the non-paretic LE only. The paretic LE remained inactive throughout the MT intervention. We questioned whether bilateral LE movements could be combined with MT to improve outcomes. Bilateral movement training of the upper extremities post-stroke is associated with increased activation of the non-affected motor cortex during paretic UE movements [23] and during MT [24] compared to unilateral training. This is because bilateral movements involve a facilitory drive from the intact hemisphere to increase excitability in motor pathways of the paretic limb [25]. Therefore, we proposed that coupling bilateral LE movements with the visual feedback about the affected LE movement provided by the mirror would improve outcomes achieved with MT. Since the emphasis in MT is on the visual feedback created by the mirror, the paretic LE movements occurring behind the mirror (and obscured from the patient’s view) need not be exact. Rather, it is the best attempt by the individual to move the LE [10] and the associated descending motor command that are required [11].
Abnormal muscle activation timing, increased passive tone and spasticity act to resist joint movement and interfere with voluntary motor control of the affected LE following a stroke [26]. These factors may explain why the previous studies of MT for the LE [21], [22] chose to train unilateral movement of the unaffected LE only. Therefore, the challenge of our proposed combination of bilateral LE movement training with MT was to design a device that facilitated movement of the affected LE behind the mirror. Furthermore, it was important to determine whether such an intervention involving bilateral LE movement could be tolerated by individuals with stroke. To our knowledge, the combination of bilateral training with MT post-stroke has not been investigated for the LE before. Therefore, the primary objective of this study was to investigate the feasibility of a bilateral LE-MT intervention. A secondary objective was to explore pre- to post- intervention changes in spatiotemporal gait parameters and motor impairment.
2. Materials and methods
Participants were individuals with stroke receiving outpatient physiotherapy from a private clinic. They were selected for inclusion in the study by their physiotherapist, who was privy to the study objectives and intervention protocol. To be included in the study participants had to: 1) be greater than 3 months post unilateral stroke, 2) be able to walk independently for 10 m, and 3) have a Chedoke McMaster Stroke Assessment (CMSA) leg and foot score of 2 or greater. The study was approved by the local Research Ethics Board and all participants provided written informed consent to participate in the LE-MT intervention and pre-post assessments. After participants completed the study, separate informed consent was obtained to review their physiotherapy documentation. These clinical notes were reviewed retrospectively to determine the number of physiotherapy visits and types of exercises and training activities the participant received while enrolled in the MT intervention.
2.1. Bilateral lower extremity mirror therapy device
Bilateral LE-MT is MT that involves volitional movement of the paretic LE behind the mirror performed simultaneously with unaffected LE movement and has not been studied previously to the best of our knowledge. The LE-MT device (Fig. 1a) designed for the investigational purposes of this study cost approximately $500 in materials and took 4 h to construct. The device includes a base constructed of wood that securely houses the mirror in a vertical position and two slider boards on either side which facilitate the bilateral LE movement. The base also houses a vertical wall made of styrofoam. Attached to the mirror and draped over the styrofoam wall, is a black curtain which serves to obscure the affected LE movement from the participant’s view.
Fig. 1.
The device used for the LE-MT intervention (a). The recording view of the intervention (b).
2.2. Bilateral lower extremity mirror therapy intervention
The LE-MT intervention involved 12 sessions completed over a 4 week period. The intervention sessions were conducted as an adjunct to each participant’s conventional physiotherapy which continued throughout the study. Given the eventual intended application of the current MT intervention (as one component of a multidimensional gait rehabilitation program), administering MT as an adjunct to participants’ usual therapy was appropriate.
The device was positioned so that the mirror was between the LEs with the affected extremity obstructed from view by a black curtain. The participant’s feet were secured in heel blocks of slider boards. In long-sitting, the participants performed simultaneous bilateral LE flexion-extension movements at the hip and knee while viewing the reflection of unaffected limb on the mirror’s surface. Participants were instructed to perform the movements with the affected LE behind the mirror to the best of their ability [10]. Participants were also instructed to perform as many repetitions as they could, at their own pace and to take rests as needed. The goal for session duration was 30 min but ultimately depended upon participant tolerance. Since greater intensity of training (defined here by number of repetitions) enhances functional recovery after stroke [27] participants were allowed to complete as many repetitions as they could until fatigue or the 30 min was complete.
A study investigator (LC) observed all sessions for every participant. At the beginning of each session, LC questioned participants about their tolerance of the previous session and then monitored for fatigue throughout the session by soliciting self-reported level of fatigue from the participant. MT requires that the affected LE is obscured from the patient’s view so an illusion of intact movement is created by the mirror. However, we required the ability to monitor the movement of the affected LE behind the mirror in order to assess the number of repetitions. Therefore, sessions were also recorded with a digital video camera positioned at the individual’s midline in such a way that both LEs were in view (Fig. 1b). This recording ensured an accurate count of repetitions performed and rest periods taken.
2.3. Intervention feasibility
We were interested in determining the potential use of the bilateral LE-MT intervention post-stroke. To verify the intervention’s potential use, we looked to ensure that the desired bilateral LE movements could be completed by the participants and that the intervention was well tolerated. Therefore, we defined feasibility as: a) adherence to the training protocol, b) an increase or maintenance of the number of bilateral movement repetitions performed over the intervention period, c) a maintenance or decrease in the number of rest periods accumulated per session over the intervention period, and d) positive subjective report of tolerance from the participants with stroke.
Feasibility measures were extracted from the session recordings. These were viewed by a study investigator (LC) who recorded the number of repetitions completed and the amount of rest periods taken. A repetition was defined as one simultaneous and synchronous flexion and extension movement of both LEs. Out of sync movements were not counted as this was believed to indicate the illusion of MT was lost and thus the goal of MT was not being achieved. Comparable range of motion in the affected limb to the unaffected limb was not expected but some activation in the affected limb was required. A rest period was defined as a stop from movement for any amount of time in excess of 10 s. A second study investigator (SM) viewed 3 randomly selected recordings (one from each participant) and recorded repetitions and rest periods using the same criteria. The average percent difference in recorded repetitions and rest periods between investigators was 1.1% and 5.7% respectively.
2.4. Measuring change in gait and motor impairment
To address the secondary study objective, gait and motor impairment were measured before (BASE) and after (POST) completion of the LE-MT intervention. Over-ground spatiotemporal gait parameters were measured with a pressure-sensitive mat (Protokinetics, Havertown, PA, USA). Participants performed 4 walking trials each at their preferred and fastest possible pace with their customary gait aid (if applicable). The parameters were averaged over the 4 trials and included gait velocity, step length and swing time variability (standard deviation, SD) [28], and step length and swing time symmetry [29]. Symmetry is calculated as the ratio of the affected and unaffected limb values for step length in centimeters and swing time in seconds, with the larger value in the numerator as per published recommendations [29]. Stroke severity was characterized with the National Institutes of Stroke Scale (NIHSS) [30] and motor impairment of the leg was measured with the CMSA leg and foot [31].
Change scores were calculated for each of the measures over the LE-MT intervention period as follows: CHANGE = POST − BASE. Changes in velocity and variability were reported in terms of multiples of the meaningful clinical importance difference (MCID) values; 6 cm/s for velocity, 0.25 cm for step length variability and 0.01 s for swing time variability [32], [33]. Since MCIDs were not available for gait symmetry, changes were analyzed in reference to the reported upper confidence interval thresholds for symmetry ratios in step length (1.08) and swing time (1.06) [29]. A ratio greater than the threshold indicates asymmetric gait.
2.5. Participants
Participant 1 was a 56 year old female 43 months post-stroke with left hemiparesis and walked with a cane. Baseline NIHSS and CMSA scores are outlined in Table 1. Her physiotherapy goals included improved balance and left LE function. The intervention period lasted 29 days. During this period she received 9 visits of physiotherapy which included treadmill, bike and elliptical training, balance training, and strengthening of the LEs.
Table 1.
Summary of clinical measure scores.
| NIHSS |
CMSA (leg/foot) |
|||
|---|---|---|---|---|
| BASE | POST | BASE | POST | |
| Participant 1 | 4 | 3 | 5/4 | 6/4 |
| Participant 2 | 4 | 3 | 5/4 | 5/4 |
| Participant 3 | 5 | 4 | 5/5 | 5/5 |
NIHSS = National Institutes of Health Stroke Scale, CMSA = Chedoke McMaster Stroke Assessment.
Participant 2 was a 48 year old female 54 months post-stroke with left hemiparesis and walked with a cane. NIHSS and CMSA scores at baseline are summarized in Table 1. Her therapy goals were to improve gait and walk without her cane. The intervention period lasted 29 days. During this period she received 8 visits of physiotherapy which included LE strengthening and stretching exercises, treadmill and bike training, mobilization with activator pole, and balance training including yoga.
Participant 3 was a 69 year old male 58 months post-stroke with left hemiparesis and walked without an aid. Baseline NIHSS and CMSA values are outlined in Table 1. The intervention period lasted 27 days. He received 2 visits of physiotherapy during this period. After he completed the MT intervention and POST assessment he discontinued his conventional physiotherapy for personal reasons. As a result, he could not be contacted and therefore we were unable to obtain retrospective consent specifically for the chart review.
3. Results
3.1. Intervention feasibility
The LE-MT intervention was completed with 100% adherence by Participants 1 and 2 with no adverse events observed during the sessions or reported by the participants. Participant 3 completed 83% of sessions. He missed the final two sessions due to an acute recurrence of pre-existing back pain. However, Participant 3 did return to complete the POST assessment session. All participants reported fatigue following each session and mild muscle soreness the day after a session, but also reported that the intervention was tolerable.
The number of movement repetitions, rests and session duration per LE-MT session are displayed for each participant in Fig. 2. The number of repetitions (mean (standard deviation)) was greater in the final session (937 (141)) compared to the first session (531 (44)) across all three participants. The number of rests for the first and last session was 6 and 31 for Participant 1, 10 and 1 for Participant 2 and 12 and 11 for Participant 3, respectively. Session duration increased from the first session to the last (Participants 1 and 3), or was maintained at 30 min throughout the intervention period (Participant 2).
Fig. 2.
Performance during LE-MT intervention. a. duration of each LE-MT session; b. number of repetitions completed per LE-MT session; and c. number of rest periods taken per LE-MT session for Participant 1 (solid line), Participant 2 (dotted line), and Participant 3 (dashed line).
3.2. Observed performance during LE-MT
For Sessions 1 through 7 Participant 1 completed movement repetitions and took rests as needed until she ultimately terminated the session due to fatigue. For Sessions 8 through 12 Participant 1 performed repetitions in blocks of 25 which was always followed by a rest. This pattern would carry on until she terminated the session due to fatigue. Participant 2 completed movement repetitions and took rests as needed until the session duration goal of 30 min was reached. She performed the repetitions in this manner for all MT sessions. Participant 3 completed movement repetitions and took rests as needed until he either terminated the session due to fatigue (sessions 1 and 3) or until the session duration goal of 30 min was reached.
3.3. Changes in gait and motor impairment
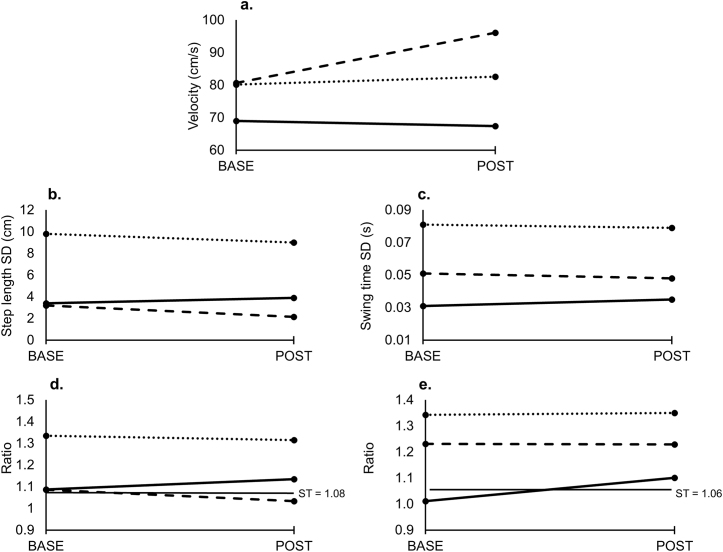
Gait parameters (velocity, variability and symmetry) measured at BASE and POST are summarized in Fig. 3 (preferred pace condition) and Fig. 4 (fast pace condition) for all three participants. Change in gait parameters as measured by multiples of MCID values for each participant are summarized in Table 2. Motor impairment scores (NIHSS and CMSA) at BASE and POST are summarized for each participant in Table 1.
Fig. 3.
Spatiotemporal gait parameters (a. gait velocity; b. step length variability (SD); c. swing time variability (SD); d. step length symmetry; e. swing time symmetry measured at each assessment time point (BASE and POST) for the preferred pace with usual walking aid condition for Participant 1 (solid line), Participant 2 (dotted line) and Participant 3 (dashed line). SD = standard deviation; ST = symmetry threshold.
Fig. 4.
Spatiotemporal gait parameters (a. gait velocity; b. step length variability (SD); c. swing time variability (SD); d. step length symmetry; e. swing time symmetry measured at each assessment time point (BASE and POST) for the fast pace with usual walking aid condition for Participant 1 (solid line), Participant 2 (dotted line) and Participant 3 (dashed line). SD = standard deviation; ST = symmetry threshold.
Table 2.
Summary of change in gait parameters from BASE to POST measured in multiples of MCID and symmetry upper thresholds.
| Change in velocity in multiples of MCID (6 cm/s) |
Change in step length variability in multiples of MCID (0.25 cm) |
Change in swing time variability in multiples of MCID (0.01 s) |
Change in step length symmetry with respect to normal threshold (1.08) |
Change in swing time symmetry with respect to normal threshold (1.06) |
||||||
|---|---|---|---|---|---|---|---|---|---|---|
| preferred | fast | preferred | fast | preferred | fast | preferred | fast | preferred | fast | |
| Participant 1 | 0.3 (-) | 0.7 (+) | 2.0 (-) | 0.1 (+) | 0.4 (-) | 1.0 (-) | n/c | n/c | worsened | n/c |
| Participant 2 | 0.4 (+) | 0.5 (+) | 3.2 (+) | 5.2 (-) | 0.2 (+) | 0.2 (+) | n/c | n/c | n/c | n/c |
| Participant 3 | 2.6 (+) | 1.1 (+) | 4.2 (+) | 7.1 (+) | 0.3 (+) | 0.5 (-) | improved | n/c | n/c | n/c |
Change scores of 1.0 MCIDs or greater are in bold for emphasis. MCID = meaningful clinical important difference, (+) = improvement of gait parameter, (-) = worsening of gait parameter, n/c = no change to symmetry.
4. Discussion
This study demonstrated that a 4 week bilateral LE-MT adjunct intervention can be administered to individuals with chronic stroke. By the last LE-MT session, all participants performed a greater number of repetitions compared to their initial session. It is important to emphasize that this intervention combined MT with bilateral training of LE movements which to our knowledge is the first post-stroke MT intervention for the LE to do so. While previous MT interventions for the UE incorporated bilateral movements [10], those for the LE have employed unilateral movement of the unaffected limb only [21], [22]. In the present study, participants demonstrated improved tolerance and endurance for bilateral MT training over the intervention period. The number of movement repetitions performed by each participant at their final MT session ranged from 820–1093, which is in line with the lower end of repetitions associated with neuroplastic change and/or recovery in animal models neurologic injury (i.e. 600 repetitions of UE movement and 600–1800 steps on a treadmill) [34].
Minor fatigue but no pain was reported during LE-MT sessions. Unfortunately, one participant experienced an acute recurrence of pre-existing back pain outside of the LE-MT intervention. Although the exact cause that precipitated this acute episode was not identified, it is possible that it was related to the long-sitting position and repeated bilateral hip flexion movements required by the intervention. Future work will consider pre-existing back pain as a possible precaution for the LE-MT intervention. Additionally, the next iteration of the MT device design and intervention protocol will incorporate LE abduction/adduction movements that can be alternated with the flexion/extension movement pattern. This will alleviate the repetitive nature of current training protocol which may decrease the risk of exacerbating a pre-existing chronic condition such as low back pain.
There was variation in the way participants performed the LE movements during the sessions. During each session, Participants 2 and 3 would perform LE movement repetitions until fatigue, take a rest and then recommence the LE movement. This approach was associated with a steady increase in movement repetitions and session duration over the 4 weeks. In contrast, Participant 1 started out with this performance strategy, but switched to completing LE movements in set blocks of 25 repetitions which were consistently followed by a rest. This explains the sharp increase in the number of rest periods from Session 7 to 8. It should be noted that while participants received standardized instructions to focus on the mirror image of their leg and perform the bilateral movements to the best of their ability, they were not instructed as to the number of repetitions to perform or the manner in which to perform the movements (i.e. in set blocks repetitions or repetitions to the point of fatigue). In keeping with the underlying purpose of mirror therapy (i.e. to provide appropriate visual feedback of successful movement), future work will include instructions to participants that prioritize and reinforce the focus of attention on the mirror reflection of the leg rather than on the number of movements performed in a session.
This study also demonstrated some changes in gait and motor impairment of the leg. For instance, Participant 2 exhibited a decrease in step length variability at the preferred pace. Participant 3 also showed decreased step length variability at preferred and fast pace as well as increased velocity and improved step length symmetry. Conversely, Participant 1 and 2 exhibited some negative gait changes such as increased gait asymmetry and variability. Despite the negative changes in gait, all 3 participants exhibited an improvement in LE motor control as measured by the CMSA (Participant 1) and the NIHSS; Item 6–Motor Leg (Participant 3) and Item 7 − Limb Ataxia (Participants 1 and 2). This variation in responsiveness to MT across individuals with stroke has been noted in a previous case series [35]. Ultimately, the effects of this bilateral LE MT intervention will need to be evaluated in a randomized controlled trial.
The rationale for training in-phase LE movement in order to improve gait function which features antiphase LE movement could be questioned. However, it is important to emphasize two points. First we present this bilateral LE-MT intervention as an adjunct to conventional physiotherapy which typically includes ample practice of anti-phase LE movement during gait training. Second, the ultimate goal of MT is to restore the motor command and visual sensory feedback loop that has been disrupted [36]. The illusion of intact movement of the affected LE combined with bilateral LE movement can only be created with in-phase LE movement when employing a mirror. Future work could investigate the use of virtual reality and motion capture equipment such that the movements of the unaffected LE could be superimposed over the affected LE while performing anti-phase movements during walking. However, such an intervention would be far less accessible in the clinical setting compared to the present MT device. Finally, we reported changes in the ataxia item on the NIHSS scale which is typically used during the acute phase of stroke. Future randomized controlled trials will include a specific measure of ataxia more appropriate to the subacute and chronic phase of stroke.
5. Conclusion
Mirror therapy is a simple and inexpensive adjunct intervention. The current study demonstrates that a bilateral LE-MT intervention is easily performed and well tolerated by individuals with chronic stroke. This study differs from two previous studies on LE-MT post-stroke; the present intervention combined mirror therapy with bilateral LE movements whereas previous work employed unaffected LE movements only. Further investigation of this adjunct intervention is warranted while considering pre-existing back pain as a precaution. The results of the present study will be used to guide refinement of the mirror therapy device and training protocol and will also inform the design of a randomized controlled pilot study.
Declarations
Author contribution statement
Lucas D. Crosby: Conceived and designed the experiments; Performed the experiments; Analyzed and interpreted the data; Wrote the paper.
Stephanie Marrocco: Analyzed and interpreted the data.
Janet Brown: Contributed reagents, materials, analysis tools or data.
Kara K. Patterson: Contributed reagents, materials, analysis tools or data; Wrote the paper.
Funding statement
This study was supported by a grant from the Physiotherapy Foundation of Canada and the Neurosciences Division of the Canadian Physiotherapy Association. Kara K. Patterson was supported by a personnel award from the Heart and Stroke Foundation and the Canadian Stroke Network.
Competing interest statement
Some of the authors of this study (LC, JB, and KP) have signed an agreement with a technology transfer and business development company to bring the device to market.
Additional information
No additional information is available for this paper.
Acknowledgements
We are grateful for the support of Sari Shatil and Shelialah Pereira (Neuphysio, London, ON) who provided assistance with recruitment and the facilities for data collection. Finally, we would like to thank the participants without whom this study would not have been possible.
References
- 1.Brouwer B., Parvataneni K., Olney S.J. A comparison of gait biomechanics and metabolic requirements of overground and treadmill walking in people with stroke. Clin. Biomech. Nov 2009;24(9):729–734. doi: 10.1016/j.clinbiomech.2009.07.004. [DOI] [PubMed] [Google Scholar]
- 2.Bohannon R.W., Williams Andrews A., Smith M.B. Rehabilitation goals of patients with hemiplegia. Int. J. Rehabil. Res. 1988;11(2):181–183. [Google Scholar]
- 3.Michael K.M., Allen J.K., Macko R.F. Reduced ambulatory activity after stroke: the role of balance, gait, and cardiovascular fitness. Arch. Phys. Med. Rehabil. 2005;86(8):1552–1556. doi: 10.1016/j.apmr.2004.12.026. [DOI] [PubMed] [Google Scholar]
- 4.Balasubramanian C.K., Neptune R.R., Kautz S.A. Variability in spatiotemporal step characteristics and its relationship to walking performance post-stroke. Gait Posture. 2009;29(3):408–414. doi: 10.1016/j.gaitpost.2008.10.061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Patterson K.K., Nadkarni N.K., Black S.E., McIlroy W.E. Gait symmetry and velocity differ in their relationship to age. Gait Posture. 2012;35(4):590–594. doi: 10.1016/j.gaitpost.2011.11.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Lindsay M., Gubitz G., Bayley M., Phillips S., editors. 4th Edition. Ottawa : Heart and Stroke Foundation of Canada; 2013. Canadian best practice recommendations for stroke care. [Google Scholar]
- 7.Ramachandran V.S., Rogers-Ramachandran D. Synaesthesia in phantom limbs induced with mirrors. Proc. Biol. Sci. 1996;263:377–386. doi: 10.1098/rspb.1996.0058. [DOI] [PubMed] [Google Scholar]
- 8.Rosén B., Lundborg G. Training with a mirror in rehabilitation of the hand. Scand. J. Plast. Reconstr. Surg. Hand Surg. 2005;39(2):104–108. doi: 10.1080/02844310510006187. [DOI] [PubMed] [Google Scholar]
- 9.McCabe C.S., Haigh R.C., Blake D.R. Mirror visual feedback for the treatment of complex regional pain syndrome (type 1) Curr. Pain Headache Rep. 2008;12:103–107. doi: 10.1007/s11916-008-0020-7. [DOI] [PubMed] [Google Scholar]
- 10.Altschuler E.L., Wisdom S.B., Stone L., Foster C., Galasko D., Llewellyn D.M.E., Ramachandran V.S. Rehabilitation of hemiparesis after stroke with a mirror. Lancet. 1999;353:2035–2036. doi: 10.1016/s0140-6736(99)00920-4. [DOI] [PubMed] [Google Scholar]
- 11.Ramachandran V.S., Altschuler E.L. The use of visual feedback, in particular mirror visual feedback, in restoring brain function. Brain. Jul. 2009;132:1693–1710. doi: 10.1093/brain/awp135. [DOI] [PubMed] [Google Scholar]
- 12.Collins J., De Luca C. The effects of visual input on open-loop and closed-loop postural control mechanisms. Exp. Brain Res. 1995;103(1):151–163. doi: 10.1007/BF00241972. [DOI] [PubMed] [Google Scholar]
- 13.Patla A. How is human gait controlled by vision? Ecol. Psychol. 1988;10(3-4):287–302. [Google Scholar]
- 14.Schabrun S.M., Hillier S. Evidence for the retraining of sensation after stroke: a systematic review. Clin. Rehabil. 2009;23(1):27–39. doi: 10.1177/0269215508098897. [DOI] [PubMed] [Google Scholar]
- 15.Michielsen M.E., Selles R.W., van der Geest J.N., Eckhardt M., Yavuzer G., Stam H.J., Smits M., Ribbers G.M., Bussmann J.B.J. Motor recovery and cortical reorganization after mirror therapy in chronic stroke patients: a phase II randomized controlled trial. Neurorehabil. Neural Repair. 2011;25(3):223–233. doi: 10.1177/1545968310385127. [DOI] [PubMed] [Google Scholar]
- 16.Dohle C., Püllen J., Nakaten A., Küst J., Rietz C., Karbe H. Mirror therapy promotes recovery from severe hemiparesis: a randomized controlled trial. Neurorehabil. Neural Repair. 2008;23(3):209–217. doi: 10.1177/1545968308324786. [DOI] [PubMed] [Google Scholar]
- 17.Yavuzer G., Selles R., Sezer N., Sütbeyaz S., Bussmann J.B., Köseoğlu F., Atay M.B., Stam H.J. Mirror therapy improves hand function in subacute stroke: a randomized controlled trial. Arch. Phys. Med. Rehabil. 2008;89(3):393–398. doi: 10.1016/j.apmr.2007.08.162. [DOI] [PubMed] [Google Scholar]
- 18.Thieme H., Mehrholz J., Pohl M., Behrens J., Dohle C. Mirror therapy for improving motor function after stroke. Stroke. 2012;44(1):e1–e2. doi: 10.1161/strokeaha.112.673087. [DOI] [PubMed] [Google Scholar]
- 19.Rothgangel A.S., Braun S.M., Beurskens A.J., Seitz R.J., Wade D.T. The clinical aspects of mirror therapy in rehabilitation: a systematic review of the literature. Int. J. Rehabil. Res. 2011;34(1):1–13. doi: 10.1097/MRR.0b013e3283441e98. [DOI] [PubMed] [Google Scholar]
- 20.Ezendam D., Bongers R., Jannink M. Systematic review of the effectiveness of mirror therapy in upper extremity function. Disabil. Rehabil. 2009:1–15. doi: 10.3109/09638280902887768. [DOI] [PubMed] [Google Scholar]
- 21.Sütbeyaz S., Yavuzer G., Sezer N., Koseoglu B.F. Mirror therapy enhances lower-extremity motor recovery and motor functioning after stroke: a randomized controlled trial. Arch. Phys. Med. Rehabil. 2007;88(5):555–559. doi: 10.1016/j.apmr.2007.02.034. [DOI] [PubMed] [Google Scholar]
- 22.Mohan U., Karthik babu S., Vijay Kumar K., Suresh B., Misri Z., Chakrapani M. Effectiveness of mirror therapy on lower extremity motor recovery, balance and mobility in patients with acute stroke: a randomized sham-controlled pilot trial. Ann. Indian Acad. Neurol. 2013;16(4):634–639. doi: 10.4103/0972-2327.120496. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Summers J.J., Kagerer F.A., Garry M.I., Hiraga C.Y., Loftus A., Cauraugh J.H. Bilateral and unilateral movement training on upper limb function in chronic stroke patients: a TMS study. J. Neurol. Sci. 2007;252(1):76–82. doi: 10.1016/j.jns.2006.10.011. [DOI] [PubMed] [Google Scholar]
- 24.Michielsen M.E., Smits M., Ribbers G.M., Stam H.J., van der Geest J.N., Bussmann J.B.J., Selles R.W. The neuronal correlates of mirror therapy: an fMRI study on mirror induced visual illusions in patients with stroke. J. Neurol. Neurosurg. Psychiatry. 2011;82(4):393–398. doi: 10.1136/jnnp.2009.194134. [DOI] [PubMed] [Google Scholar]
- 25.Cauraugh J.H., Summers J.J. Neural plasticity and bilateral movements: a rehabilitation approach for chronic stroke. Prog. Neurobiol. 2005;75(5):309–320. doi: 10.1016/j.pneurobio.2005.04.001. [DOI] [PubMed] [Google Scholar]
- 26.Arene N., Hidler J. Understanding motor impairment in the paretic lower limb after a stroke: a review of the literature. Top. Stroke Rehabil. 2009;16(5):346–356. doi: 10.1310/tsr1605-346. [DOI] [PubMed] [Google Scholar]
- 27.Kwakkel G. Impact of intensity of practice after stroke: issues for consideration. Disabil. Rehabil. 2006;28(13):823–830. doi: 10.1080/09638280500534861. [DOI] [PubMed] [Google Scholar]
- 28.Chisholm A.E., Makepeace S., Inness E.L., Perry S.D., Mcilroy W.E., Mansfield A. Spatial-temporal gait variability poststroke: variations in measurement and implications for measuring change. Arch. Phys. Med. Rehabil. 2014;95(7):1335–1341. doi: 10.1016/j.apmr.2014.02.014. [DOI] [PubMed] [Google Scholar]
- 29.Patterson K.K., Gage W.H., Brooks D., Black S.E., McIlroy W.E. Evaluation of gait symmetry after stroke: a comparison of current methods and recommendations for standardization. Gait Posture. 2010;31(2):241–246. doi: 10.1016/j.gaitpost.2009.10.014. [DOI] [PubMed] [Google Scholar]
- 30.Brott T., Adams H.P., Olinger C.P., Marler J.R., Barsan W.G., Biller J., Spilker J., Holleran R., Eberle R., Hertzberg V. Measurements of acute cerebral infarction: a clinical examination scale. Stroke. Jul. 1989;20(7):864–870. doi: 10.1161/01.str.20.7.864. [DOI] [PubMed] [Google Scholar]
- 31.Gowland C., Stratford P., Ward M., Moreland J., Torresin W., Van Hullenaar S., Sanford J., Barreca S., Vanspall B., Plews N. Measuring physical impairment and disability with the Chedoke-McMaster Stroke Assessment. Stroke. 1993;24:58–63. doi: 10.1161/01.str.24.1.58. [DOI] [PubMed] [Google Scholar]
- 32.Perera S., Mody S.H., Woodman R.C., Studenski S.A. Meaningful change and responsiveness in common physical performance measures in older adults. J. Am. Geriatr. Soc. 2006;54(5):743–749. doi: 10.1111/j.1532-5415.2006.00701.x. [DOI] [PubMed] [Google Scholar]
- 33.Brach J.S., Perera S., Studenski S., Katz M., Hall C., Verghese J. Meaningful change in measures of gait variability in older adults. Gait Posture. 2010;31(2):175–179. doi: 10.1016/j.gaitpost.2009.10.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Lang C.E., MacDonald J.R., Gnip C. Counting repetitions: an observational study of outpatient therapy for people with hemiparesis post-stroke. J. Neurol. Phys. Ther. 2007;31(1):3–10. doi: 10.1097/01.npt.0000260568.31746.34. [DOI] [PubMed] [Google Scholar]
- 35.Stevens J.A., Stoykov M.E.P. Using motor imagery in the rehabilitation of hemiparesis. Arch. Phys. Med. Rehabil. 2003;84(7):1090–1092. doi: 10.1016/s0003-9993(03)00042-x. [DOI] [PubMed] [Google Scholar]
- 36.Deconinck F.J.A., Smorenburg A.R.P., Benham A., Ledebt A., Feltham M.G., Savelsbergh G.J.P. Reflections on mirror therapy: a systematic review of the effect of mirror visual feedback on the brain. Neurorehabil. Neural Repair. 2014:1–13. doi: 10.1177/1545968314546134. [DOI] [PubMed] [Google Scholar]