Abstract
AIM
To investigate the morphological changes of meibomian glands in patients with type 2 diabetes mellitus (DM).
METHODS
Of 118 eyes (118 patients) with type 2 DM (DM group) and 100 eyes of 100 control subjects (control group) were enrolled. After completing an ocular surface disease index (OSDI) questionnaire, the non-invasive tear film break-up time (NI-BUT) and the structure of the meibomian glands (MGs, meibography) were assessed by the Keratograph 5M system. Partial or complete loss of MG was scored for each eyelid from grade 0 (no loss) to grade 3 (lost area was >2/3 of the total MG area), which were also examined by laser scanning confocal microscopy (LSCM). The primary outcomes were meibomian gland acinar unit density (MGAUD), meibomian gland acinar longest diameter (MGALD) and meibomian gland acinar shortest diameter (MGASD).
RESULTS
Compared with control group, the OSDI was significantly higher in DM group (Z=-5.916; P<0.001), while the NI-BUT was significantly lower (Z=-7.765; P<0.001). Keratograph showed that there were more MGs dropout in DM group than that in control group. The meiboscore was significantly higher in DM group compared with control group (Z=-3.937; P<0.001). LSCM revealed that there were cytological alterations of MGs in DM group compared with control group, which included enlargement of MG acinar units and decreased in density of MG acinar units. Specifically, there were lower MGAUD, larger MGALD and MGASD in DM group than control group (Z=-10.120, -9.4442, -7.771; P<0.001).
CONCLUSION
Compared with the normal control participants, the patients with type 2 DM had more unstable tear films and severe symptoms of dry eye. Using Keratograph 5M system and LSCM, we found that the patients with type 2 DM had more significant morphological and cytological changes and dysfunction in MGs.
Keywords: meibomian glands, diabetes, tear films, keratograph, laser scanning confocal microscopy
INTRODUCTION
With the incidence of diabetes increased year by year, diabetes-related eye complications have drawn the attention of ophthalmologists increasingly, and the ocular surface changes caused by diabetes become a new focus in ophthalmology. Relevant research has shown that patients with type 2 diabetes mellitus (DM) are more likely to have abnormalities on stability of tear film[1].
The constituents of the tear film are mucins, water and lipids. Lipids are secreted by the meibomian glands (MGs) that are squeezed out when blinking[2]. As the largest sebaceous gland in the body, MG secretes lipid which is the main component of the lipid layer of tear film and plays a significant role in the building of the surface tension, the stability of the tear film and the prevention of evaporation[3]. The abnormalities and dysfunction of MGs may lead to instability of tear film, rapid evaporation of tear, increasing of tear osmolarity and ultimately result in ocular surface disorders[4].
Several studies have been conducted to investigate the ocular surface disorder in patients with type 2 DM, however, we know little about morphological and cytological changes of MGs in these subjects. By observing the alterations of MGs in patients with type 2 DM and analyzing its correlation with ocular surface changes, this study was aimed to better understand the pathogenesis and influencing factors of MGs changes in diabetic patients, thus to avoid further damages to ocular surface and to provide guidance for clinical diagnosis and treatment.
SUBJECTS AND METHODS
Subjects
This was a comparative study which evaluated 118 eyes of 118 patients with type 2 DM and 100 eyes of 100 control participants seen in the Department of Ophthalmology of Shandong Provincial Qianfoshan Hospital during the period from October 2014 to November 2015. The right eye was examined in each subject. Exclusion criteria included contact lens wear, a history of eye surgery, ocular inflammation, blepharitis, trauma, continuous eye drop, Sjögren syndrome, and systemic diseases or treatments that would affect the quality and stability of tear film. This study was conducted in accordance with the ethical standards stated in the Declaration of Helsinki and approved by the Ethics Committee of Shandong Provincial Qianfoshan Hospital, with informed consent obtained from all participants.
Methods
Symptom assessment
For assessment of ocular surface symptoms and the severity of dry eye, all subjects were required to complete a symptom questionnaire [ocular surface disease index (OSDI)][5]. The questionnaire gives a range of 0 (no symptoms) till 100 (severe symptoms), with higher scores representing greater levels of symptoms. A score ≥13 was considered dry eye[6].
Keratograph 5M evaluation
All the subjects underwent imaging with the Keratograph 5M (Oculus GmbH, Wetzlar, Germany) equipped with a modified tear film scanning function intended for the evaluation of the ocular surface. Non-invasive tear film break-up time (NI-BUT) was automatically detected with the Keratograph 5M, as described previously[7]. For further analysis, the time of the first tear film break-up was used and the value below 5 mm was considered abnormal[8].
Using the infrared meibography model of the Keratograph 5M, the upper eyelids and lower eyelids were performed and evaluated after eyelids eversion[9]–[11]. Partial or complete loss of the MGs was scored according to the following grades for each eyelid as meiboscore: grade 0 (no loss of MGs); grade 1 (less than one-third loss of MGs); grade 2 (less than two-thirds loss of MGs); grade 3 (more than two-thirds loss of MGs). The meiboscore of each eye was calculated as the total meiboscore from both upper and lower eyelids, which made the total meiboscore per eye to a range of 0-6[12]–[13]. The score greater than 1 was defined as abnormal[12].
In vivo laser confocal microscopy
In vivo laser confocal microscopy (CM) was performed on all subjects with Heidelberg Retina Tomograph III-Rostock Cornea Module (Heidelberg Engineering GmbH, Dossenheim, Germany), as described previously[13]–[15]. Briefly, after the eyelid was everted, the central of the Tomo-Cap was applanated onto the palpebral conjunctiva, and the MGs were scanned while moving the applanating lens. Three randomized, nonoverlapping, high-quality digital images of the nasal, middle, and temporal lower eyelid (total of 9 images per eyelid) were used for the CM-based assessments. We measured the meibomian gland acinar unit density (MGAUD), the meibomian gland acinar longest diameter (MGALD) and the meibomian gland acinar shortest diameter (MGASD) to evaluate the morphologic changes in the MGs as described previously[16]. The MGAUD were manually marked for each 400×400 µm frame and automatically calculated with the internal Cell Count software, while the MGALD and MGASD were calculated by using the Image J software (Java software program developed by the National Institutes of Health; available: http://rsb.info.nih.gov/ij/).
Statistical Analysis
Statistical analysis was performed by using the Statistical Package for the Social Sciences version 17.0 (SPSS Inc, Chicago, USA, III). The χ2 test was used to compare gender differences between DM patients and normal control patients. The Mann-Whitney U test was used to determine age and the examinations (OSDI, NI-BUT, meibography score, MGAUD, MGALD, MGASD) differences among DM patients and normal control subjects. A P value less than 0.05 was considered to be statistically significant difference.
RESULTS
A total of 118 patients with type 2 DM (58 males, 60 females; mean age 59.68±7.81y) and 100 normal control subjects (52 males, 48 females; mean age 60.27±7.59y) were recruited for the study. Table 1 showed that there was no significant difference in gender and age distribution (P=0.675; Z=-0.474, P=0.636) between the 2 groups.
Table 1. Characteristics of the study subjects.
| Parameters | DM group (n=118) | Control group (n=100) | 1P | Z |
| Gender (M:F) | 58:60 | 52:48 | 0.675 | |
| Age (a) | 59.68±7.81 | 60.27±7.59 | 0.636 | -0.474 |
| OSDI | 23.02±13.13 | 12.11±6.48 | 0.000 | -5.916 |
| NI-BUT | 4.44±2.40 | 8.42±3.79 | 0.000 | -7.765 |
| MGAUD | 77.55±25.66 | 115.38±19.05 | 0.000 | -10.120 |
| MGALD | 89.30±21.99 | 58.01±14.99 | 0.000 | -9.444 |
| MGASD | 47.00±12.33 | 31.34±34.00 | 0.000 | -7.771 |
1The χ2 test (gender) and the Mann-Whitney test were performed to compare these parameters between the 2 groups.
The OSDI results showed that 68.64% of people in DM group had symptom of dry eye compared to 30% in the control group. The OSDI was significantly higher in DM group than that in control group (Z=-5.916; P<0.001) (Table 1).
The NI-BUT measurements of DM group and control group were 4.44±2.40s (range 0.4-10.3s) and 8.42±3.79s (range 1.7-17.5s) respectively (Z=-7.765; P<0.001) (Table 1, Figure 1). Keratograph 5M showed more MGs dropout in DM group than that in control group. And there was 57.63% of people in DM group had MG dropout while it was only 33% in control group. The differences between the two groups were significant (Z=-3.937; P<0.001) (Table 2, Figure 2).
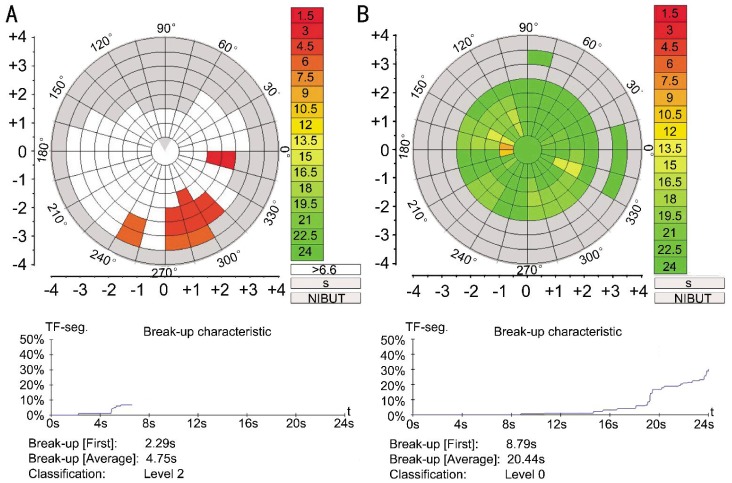
Figure 1. Output of the NI-BUT measurement by Keratograph 5M system.
The NI-BUT is automatically marked with different colors in the video. The bar chart on the right shows the corresponding relation between color and time. The first NI-BUT in a subject in DM group was 2.29s (A), whereas that in control group was 8.79s (B).
Table 2. Characteristics of the study subjects.
| Parameters | Stage | Group |
1P | Z | |
| DM (n=118) | Control (n=100) | ||||
| MD score | 0 | 50 | 67 | 0.000082 | -3.937 |
| 1 | 26 | 18 | |||
| 2 | 21 | 10 | |||
| 3 | 18 | 5 | |||
| 4 | 3 | 0 | |||
1The the Mann-Whitney test were performed to compare this parameter between the 2 groups.
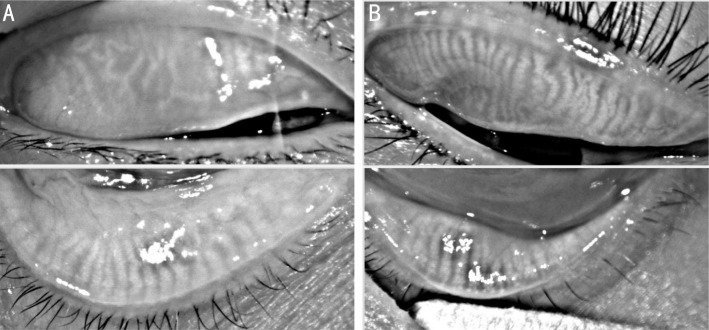
Figure 2. Infrared meibography of the upper eyelids and lower eyelids were performed by Keratograph 5M system.
The MG dropout was scored in a subject in DM group was 4 (A), whereas that in control group was 0 (B).
Laser scanning confocal microscopy (LSCM) revealed cytological alterations of MGs in patients with diabetes, including the enlargement of MG acinar units and the decreases in density of MG acinar units (Figure 3A) compared with control subjects (Figure 3B). Compared with the control group, the MGAUD mean value was significantly lower in DM group (77.55±25.66 U/mm2 vs 115.38±19.05 U/mm2; Z=-10.120; P<0.001), while the MGALD mean value was significantly longer (89.30±21.99 µm vs 89.30±21.99 µm; Z=-9.444; P<0.001) and the MGASD mean value was significantly shorter (47.00±12.33 µm vs 31.34±34 µm; Z=-7.771; P<0.001) (Table 1).
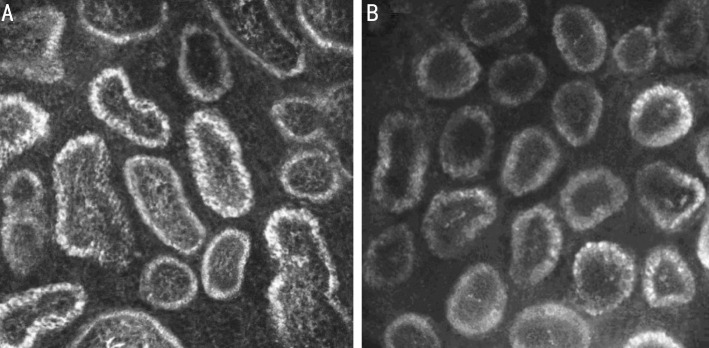
Figure 3. The morphologic changes in the MGs were evaluated by LSCM.
DM group (A) revealed more decrease in density of MG acinar units and more enlargement acinar units compared with control subjects (B).
DISCUSSION
Previous research has proved that the abnormal surface diseases in diabetes were associated with the superficial punctate keratitis, functional abnormalities of corneal innervation and severity of diabetic retinopathy, etc[17]. However, there was scarce report investigating the morphological and structural alterations of MGs and their impact on the ocular surface disease in patients with type 2 DM. The recent technological progress of keratograph and LSCM provide us a noninvasive method to elucidate the structural and functional changes in the ocular surface. In this study, the MGs and its acinus units were observed directly from the eyelid by using the two devices to evaluate the lipid secretions of MGs.
Our result showed that 57.63% of people in DM group had MG dropout, while it was 33% in control group. Compared with the control subjects, patients with type 2 DM had significantly higher meiboscores. LSCM revealed that a large proportion of the people with DM appeared alterations of MGs, including enlargement of acinar units, irregular shape with acinus and decrease in density of acinar units. Thus we speculated that there may be a certain degree of obstruction of the MG ductal in patients with type 2 DM. The obstruction of the MG ductal lead to accumulation of meibum, thus causing the cystic dilatation and the irregular morphology of the acini, and further causing the atrophy of the acini and decrease of the acinar density. In the meantime it was found that there was a lot of high density shadows in the acini and gland bubble wall of DM patients, which was different from normal people and suggested there was abnormality in the acini. Besides that, inflammatory cells infiltration were seen around acini, which suggesting inflammation factors might also play role in the development of pathological changes. Also, the results showed that the DM group suffered more severe ocular discomfort and tear film abnormal than the control group, which was consistent with the previous studies[18]. These findings supported that these alterations in MGs might account for the discomfort and the tear film instability in diabetes patients.
The mechanism underlying the above changes in the MGs was still unclear. It has been demonstrated that the MG was an androgen target organ. There were androgen receptor protein expressed in acinar epithelial cell nuclei[19]. Sullivan et al[20] indicated that androgens could regulate the function of MGs, enhanced the quality and/or quantity of lipids produced by this tissue, and promoted the formation of the tear film's lipid layer. It was found that androgen levels in diabetic patients were significantly lower than those in non-diabetic population[21]. This deficiency of androgen in diabetes patients might lead to the dysfunction of MG.
The alterations of MGs might be adversely affected by a neuropathic mechanism. Studies proved that MGs were innervated by parasympathetic fibres with a smaller contribution from sympathetic and sensory neurons[22]. Disease or any damage of these neurons could result in dry eye in the animal model[23]. The neuropathy, a frequent complication of diabetes, might result in dysfunction of the MGs via their innervation.
In addition, the secretion of meibum mainly relies on blink movements. During the process of blink, the mechanical action of the lid muscles can deliver and disperse lipid onto the ocular surface and subsequently form the tear film lipid layer[24]. Thus, blink movements play an important role in the secretion of meibum. However, the patients with DM previously exhibited reduction of corneal sensitivity and blinks movements[25], which would lead to less secretion of lipid, obstruction of gland duct, secretion stasis of meibum and further dysfunctions of MGs.
However, our study had several limitations. Firstly, MGs changes related with diabetes were complicated, and we think there may be more factors, such as blood sugar and severity of diabetic retinopathy. Future research with larger study populations may be grouped by blood sugar level and severity of diabetic retinopathy. Secondly, we only evaluated morphologic changes in the MGs and had not involved MG expressibility and eyelid evaluation.
In summary, we have completed the first study elucidating the alterations of MGs in the patients with type 2 DM by Keratograph 5M system and LSCM. Our study found that diabetes patients had more significant morphological changes and dysfunction in MGs when compared with normal control participants.
Acknowledgments
Conflicts of Interest: Yu T, None; Shi WY, None; Song AP, None; Gao Y, None; Dang GF, None; Ding G, None.
REFERENCES
- 1.Najafi L, Malek M, Valojerdi AE, Khamseh ME, Aghaei H. Dry eye disease in type 2 diabetes mellitus; comparison of the tear osmolarity test with other common diagnostic tests: a diagnostic accuracy study using STARD standard. J Diabetes Metab Disord. 2015;14:39. doi: 10.1186/s40200-015-0157-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Wang Y, Dong N, Wu H. Meibomian gland morphology study progression. Zhonghua Yan Ke Za Zhi. 2014;50(4):299–302. [PubMed] [Google Scholar]
- 3.Craig JP, Tomlinson A. Importance of the lipid layer in human tear film stability and evaporation. Optom Vis Sci. 1997;74(1):8–13. doi: 10.1097/00006324-199701000-00014. [DOI] [PubMed] [Google Scholar]
- 4.Palamar M, Degirmenci C, Ertam I, Yagci A. Evaluation of dry eye and meibomian gland dysfunction with meibography in patients with rosacea. Cornea. 2015;34(5):497–499. doi: 10.1097/ICO.0000000000000393. [DOI] [PubMed] [Google Scholar]
- 5.Schiffman RM, Christianson MD, Jacobsen G, Hirsch JD, Reis BL. Reliability and validity of the ocular surface disease index. Arch Ophthalmol. 2000;118(5):615–621. doi: 10.1001/archopht.118.5.615. [DOI] [PubMed] [Google Scholar]
- 6.Feng Y, Gao Z, Feng K, Qu H, Hong J. Meibomian gland dropout in patients with dry eye disease in China. Curr Eye Res. 2014;39(10):965–972. doi: 10.3109/02713683.2014.891748. [DOI] [PubMed] [Google Scholar]
- 7.Hong J, Sun X, Wei A, Cui X, Li Y, Qian T, Wang W, Xu J. Assessment of tear film stability in dry eye with a newly developed keratograph. Cornea. 2013;32(5):716–721. doi: 10.1097/ICO.0b013e3182714425. [DOI] [PubMed] [Google Scholar]
- 8.Johnson ME, Murphy PJ. The effect of instilled fluorescein solution volume on the values and repeatability of TBUT measurements. Cornea. 2005;24(7):811–817. doi: 10.1097/01.ico.0000154378.67495.40. [DOI] [PubMed] [Google Scholar]
- 9.Menzies KL, Srinivasan S, Prokopich CL, Jones L. Infrared imaging of meibomian glands and evaluation of the lipid layer in Sjogren's syndrome patients and nondry eye controls. Invest Ophthalmol Vis Sci. 2015;56(2):836–841. doi: 10.1167/iovs.14-13864. [DOI] [PubMed] [Google Scholar]
- 10.Srinivasan S, Menzies K, Sorbara L, Jones L. Infrared imaging of meibomian gland structure using a novel keratograph. Optom Vis Sci. 2012;89(5):788–794. doi: 10.1097/OPX.0b013e318253de93. [DOI] [PubMed] [Google Scholar]
- 11.Abdelfattah NS, Dastiridou A, Sadda SR, Lee OL. Noninvasive imaging of tear film dynamics in eyes with ocular surface disease. Cornea. 2015;34(Suppl. 10):S48–52. doi: 10.1097/ICO.0000000000000570. [DOI] [PubMed] [Google Scholar]
- 12.Arita R, Itoh K, Inoue K, Amano S. Noncontact infrared meibography to document age-related changes of the meibomian glands in a normal population. Ophthalmology. 2008;115(5):911–915. doi: 10.1016/j.ophtha.2007.06.031. [DOI] [PubMed] [Google Scholar]
- 13.Finis D, Ackermann P, Pischel N, Konig C, Hayajneh J, Borrelli M, Schrader S, Geerling G. Evaluation of meibomian gland dysfunction and local distribution of meibomian gland atrophy by non-contact infrared meibography. Curr Eye Res. 2015;40(10):982–989. doi: 10.3109/02713683.2014.971929. [DOI] [PubMed] [Google Scholar]
- 14.Ibrahim OM, Matsumoto Y, Dogru M, Adan ES, Wakamatsu TH, Goto T, Negishi K, Tsubota K. The efficacy, sensitivity, and specificity of in vivo laser confocal microscopy in the diagnosis of meibomian gland dysfunction. Ophthalmology. 2010;117(4):665–672. doi: 10.1016/j.ophtha.2009.12.029. [DOI] [PubMed] [Google Scholar]
- 15.Matsumoto Y, Sato EA, Ibrahim OM, Dogru M, Tsubota K. The application of in vivo laser confocal microscopy to the diagnosis and evaluation of meibomian gland dysfunction. Mol Vis. 2008;14:1263–1271. [PMC free article] [PubMed] [Google Scholar]
- 16.Matsumoto Y, Shigeno Y, Sato EA, Ibrahim OM, Saiki M, Negishi K, Ogawa Y, Dogru M, Tsubota K. The evaluation of the treatment response in obstructive meibomian gland disease by in vivo laser confocal microscopy. Graefes Arch Clin Exp Ophthalmol. 2009;247(6):821–829. doi: 10.1007/s00417-008-1017-y. [DOI] [PubMed] [Google Scholar]
- 17.Misra SL, Patel DV, McGhee CN, Pradhan M, Kilfoyle D, Braatvedt GD, Craig JP. Peripheral neuropathy and tear film dysfunction in type 1 diabetes mellitus. J Diabetes Res. 2014;2014:848659. doi: 10.1155/2014/848659. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Seifart U, Strempel I. The dry eye and diabetes mellitus. Ophthalmologe. 1994;91(2):235–239. [PubMed] [Google Scholar]
- 19.Rocha EM, Wickham LA, da Silveira LA, Krenzer KL, Yu FS, Toda I, Sullivan BD, Sullivan DA. Identification of androgen receptor protein and 5alpha-reductase mRNA in human ocular tissues. Br J Ophthalmol. 2000;84(1):76–84. doi: 10.1136/bjo.84.1.76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Sullivan DA, Sullivan BD, Ullman MD, Rocha EM, Krenzer KL, Cermak JM, Toda I, Doane MG, Evans JE, Wickham LA. Androgen influence on the meibomian gland. Invest Ophthalmol Vis Sci. 2000;41(12):3732–3742. [PubMed] [Google Scholar]
- 21.Corona G, Mannucci E, Petrone L, Schulman C, Balercia G, Fisher AD, Chiarini V, Forti G, Maggi M. A comparison of NCEP-ATPIII and IDF metabolic syndrome definitions with relation to metabolic syndrome-associated sexual dysfunction. J Sex Med. 2007;4(3):789–796. doi: 10.1111/j.1743-6109.2007.00498.x. [DOI] [PubMed] [Google Scholar]
- 22.Chung CW, Tigges M, Stone RA. Peptidergic innervation of the primate meibomian gland. Invest Ophthalmol Vis Sci. 1996;37(1):238–245. [PubMed] [Google Scholar]
- 23.Song XJ, Li DQ, Farley W, Luo LH, Heuckeroth RO, Milbrandt J, Pflugfelder SC. Neurturin-deficient mice develop dry eye and keratoconjunctivitis sicca. Invest Ophthalmol Vis Sci. 2003;44(10):4223–4229. doi: 10.1167/iovs.02-1319. [DOI] [PubMed] [Google Scholar]
- 24.Knop E, Knop N, Millar T, Obata H, Sullivan DA. The international workshop on meibomian gland dysfunction: report of the subcommittee on anatomy, physiology, and pathophysiology of the meibomian gland. Invest Ophthalmol Vis Sci. 2011;52(4):1938–1978. doi: 10.1167/iovs.10-6997c. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Sagdik HM, Ugurbas SH, Can M, Tetikoglu M, Ugurbas E, Ugurbas SC, Alpay A, Ucar F. Tear film osmolarity in patients with diabetes mellitus. Ophthalmic Res. 2013;50(1):1–5. doi: 10.1159/000345770. [DOI] [PubMed] [Google Scholar]