Abstract
AIM
To evaluate the advantage of canaloplasty compared to trabeculectomy for patients with open angle glaucoma.
METHODS
Potentially relevant studies were systematically searched using various databases from inception until December 2015. The outcome analyses performed automatically using Revman 5.3 included intraocular pressure reduction (IOPR), postoperative success rate, anti-glaucoma medications reduction and the incidence of adverse events.
RESULTS
We included four qualified studies incorporating a total of 215 eyes for quantitative synthesis. The weighted mean difference (WMD) of IOPR between canaloplasty and trabeculectomy from baseline to 12mo was -2.33 (95%CI: -4.00, -0.66). There was not significant improvement in the complete or qualified success rate (OR: 0.58, 95%CI: 0.26, 1.31; OR: 0.50, 95%CI: 0.10, 2.44, respectively). Similarly, no statistically significance was observed in anti-glaucoma mediations reduction (WMD: -0.54, 95%CI: -1.18, 0.09). Sensitivity analysis of the primary outcome estimate confirmed the stability of the Meta-analysis result.
CONCLUSION
Trabeculectomy seems to be more effective in lowering IOP up to 12mo when comparing with canaloplasty. Canaloplasty does not seem to be inferior to trabeculectomy considering the postoperative success rate or the number of postoperative anti-glaucoma medications. Meanwhile, it has an advantage of less bleb related complications.
Keywords: canaloplasty, trabeculectomy, open angle glaucoma, Meta-analysis
INTRODUCTION
Glaucoma is a chronic neurodegenerative disorder of the optic nerve, if untreated, resulting in irreversible loss of sight. It is estimated that the number of people with glaucoma worldwide will increase to 111.8 million by the year 2040[1]. Among different subtypes of glaucoma, open angle glaucoma (OAG) is the most popular form of the disease. Glaucoma should not only be regarded as a medical problem but also as a socioeconomic burden.
Trabeculectomy is the most popular and effective treatment for patients with medically uncontrolled glaucoma, and therefore plays an important role in the management of this blinding disease[2]. Filtering blebs most often fail due to the process of wound healing and subsequent scarring in the conjunctiva and episclera. A variety of anti-fibrotic agents have been widely used and significantly improved the outcome. Unfortunately, due to their nonspecific mechanism of action, they are far from ideal and can be associated with an increased incidence of sight-threatening complications including wound leak, hypotony, and endophthalmitis[3]. The shortcomings have spurred interest in new alternative surgical methods to increase the success rate of glaucoma surgery.
Canaloplasty, a new interventional nonpenetrating surgical technique, has become an appealing alternative to traditional incisional glaucoma therapies. The procedure intends to enhance the outflow of aqueous humor by using a flexible microcatheter to enlarge the Schlemm's canal and stretch out the trabecular meshwork (TM), thus relieving intraocular pressure (IOP)[4]. It is less invasive, independent of bleb formation and intraoperative mitomycin C. Patients with OAG might be good candidates for this procedure. Canaloplasty combined with phacoemulsification may also be a useful surgical option in patients with coexistent cataract and OAG[5]–[6].
Given the evidence available to date, several studies have compared canaloplasty with standard trabeculectomy from different aspects in the management of OAG and have reported conflicting results[7]–[10]. To our knowledge, a quantitative synthesis of published data has not yet been reported. Therefore, we believe it is necessary to perform a Meta-analysis of all currently available data to assess the advantage of canaloplasty over trabeculectomy.
MATERIALS AND METHODS
This Meta-analysis was underwent according to the Preferred Reporting Items for Systematic Review and Meta-Analyses (PRISMA) statement (Checklist S1)[11].
Literature Search
The initial systematic search was performed using the following computer databases: PubMed, EMBASE, ISI Web of Knowledge and the Cochrane Library. Following the systematic search, we also went through the reference lists of all relevant articles manually to avoid missing additional clinical trials. We included potentially relevant articles published from inception until December 2015. There was no language or time restriction through the whole searching process. Systematic searches were conducted using the key words “canaloplasty” or “tensioning of Schlemm canal”, “trabeculectomy”, “open angle glaucoma”.
Inclusion and Exclusion Criteria
Relevant controlled clinical trials with participates undergoing canaloplasty compared to trabeculectomy for OAG were included in our Meta-analysis. Apart from this, at least one of the main outcome measures should be expressed in the studies: intraocular pressure reduction (IOPR), postoperative success rate, adverse events or relevant data. Abstracts from conferences, case reports, duplicate publications, letters and reviews were excluded.
Data Extraction
Two review authors (Lin ZJ and Xu S) extracted the following required data independently from eligible articles: author name, publication year, country, study design, patient characteristics, follow-up period, sample size, mean age, IOP measurements, the postoperative success rate, the number of glaucoma medications as well as the proportion of adverse events. We resolved any differences by discussion.
Quality Assessment
Two reviewers (Lin ZJ and Xu S) subjectively scored each selected article using a system previously reported by Downs and Black[12], which could evaluate randomized controlled trials as well as non-randomized studies. This system evaluated the methodology quality from the following five aspects: reporting, external validity, bias, confounding and power. Also, we resolved any differences by discussion. If the quality score was over 50%, we believed there was adequate quality of the study.
Outcome Measures
We used IOPR from preoperative to a certain time point after the surgery for the primary efficacy estimate. When mean±standard deviation (SD) of the IOPR were available from the trial, we used these directly. Otherwise, we calculated them as Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0[13] describes:
Similarly, we used this method to evaluate the number of glaucoma medication reduction. Another efficacy estimate was the rate of complete success (maximum postoperative IOP of 18 mm Hg without medications) and qualified success (maximum IOP of 18 mm Hg with or without medications)[14]. Pooled estimates were also performed for the rates of postoperative complications.
Statistical Analysis
All statistical analyses were completed using Revman software (version 5.3; Cochrane Collaboration, Oxford, UK). We examined heterogeneity with the Chi2 and I2 tests. P<0.05 for Chi2 or the I2 measure >50% was considered as obvious heterogeneity. If heterogeneity was observed, a random effects model was used, or we chose a fixed effects model. We presented dichotomous outcomes with pooled odds ratio (OR) and a 95% confidence interval (CI). For continuous outcomes, the weighted mean difference (WMD) with 95%CI was measured. P<0.05 represented a statistically significant difference for overall effect.
Sensitivity Analysis and Publication Bias
Sensitivity analysis was investigated by omitting each individual study to identify the reliability and credibility of the results. The possibility of publication bias was performed by funnel plot. In addition, if available, the Begg's and Egger's measures were also calculated to assess the result.
RESULTS
Literature Search
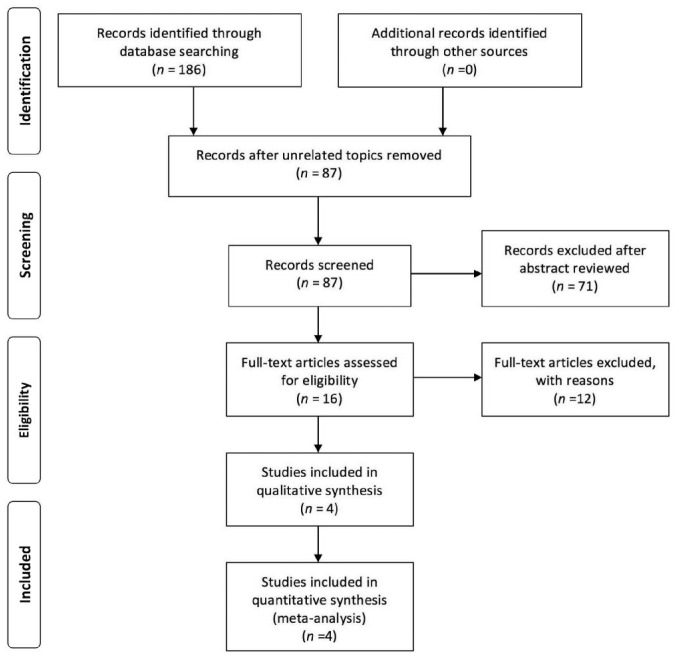
The flow diagram of study selection process is displayed clearly in Figure 1. The initial literature search identified 186 articles, and no additional studies were identified from other sources. After reviewing the abstracts, 99 papers were unrelated topics and 71 papers were not clinical controlled trials, thus these were excluded. Subsequently, the remaining 16 articles with potentially relevant trials were assessed. Twelve studies were excluded for different reasons: 2 surgical technique combined phacoemulsification[15]–[16], 6 unqualified control groups (2 included viscocanalostomy as control groups[17]–[18], 1 included non-penetrating deep sclerectomy as control group[19], 3 included phacoemulsification as control groups[5]–[6],[20]) and 4 without outcomes related to IOP[21]–[24]. Finally, only four studies were included in this Meta-analysis.
Figure 1. Flow diagram of literature search and study selection in this Meta-analysis.
Characteristics and Quality Assessment
Table 1 showed the detailed characteristics of the eligible studies. Overall, a total of 215 eyes from OAG patients were included in our Meta-analysis. Of 100 eyes had canaloplasty, while 115 eyes underwent trabeculectomy. Three of the four studies were conducted in Germany, and one was performed in America. Only one of the included studies was randomized trial while the other three were retrospective studies. Table 2 displayed the results of quality assessment (Checklist S2). For Downs and Black score, it was suggested that all of the four included studies possessed adequate quality.
Table 1. Characteristics of eligible clinical studies.
| First author (a) | Country | Design | Type of glaucoma | Follow-up (mo) | Treatment | No. of eyes | Mean age (a) | Baseline IOP (mm Hg) |
| Ayyala[7] (2011) | America | Retrospective | OAG | 12 | Canaloplasty | 33 | 68.3±10.0 | 21.2±6.6 |
| Trabeculectomy | 46 | 64.5±11.7 | 23.4±10.4 | |||||
| Brüggemann[8] (2013) | Germany | Retrospective | POAG, PEXG, NTG | 12 | Canaloplasty | 15 | 69.1 (33-86) | 26.73±6.4 |
| Trabeculectomy | 15 | 66.0 (33-84) | 26.3±10.9 | |||||
| Matlach[10] (2015) | Germany | RCT | POAG, PEXG, PG | 24 | Canaloplasty | 30 | 66.5±11.3 | 23.7±5.1 |
| Trabeculectomy | 32 | 67.9±9.3 | 22.2±5.3 | |||||
| Thederan[9] (2014) | Germany | Retrospective | POAG, PEXG, NTG, PG | 12 | Canaloplasty | 22 | 72.9±5.2 | 23.7±7.6 |
| Trabeculectomy | 22 | 71.9±11.4 | 23.9±10.7 |
RCT: Randomized controlled trial; OAG: Open angle glaucoma; POAG: Primary open angle glaucoma; PEXG: Pseudoexfoliate glaucoma; PG: Pigmentary glaucoma; NTG: Normal tension glaucoma.
Table 2. Quality scoring system for the included studies.
| First author (a) | Quality score component |
Score |
|||||
| I | II | III | IV | V | Over all | Percentage | |
| Ayyala[7] (2011) | 9 | 2 | 5 | 3 | 3 | 22 | 68.75 |
| Brüggemann[8] (2013) | 9 | 2 | 5 | 3 | 2 | 21 | 65.63 |
| Matlach[10] (2015) | 10 | 3 | 7 | 4 | 3 | 27 | 84.38 |
| Thederan[9] (2014) | 9 | 2 | 5 | 3 | 3 | 22 | 68.75 |
I: Reporting; II: External validity; III: Bias; IV: Confounding; V: Power.
Intraocular Pressure Reduction
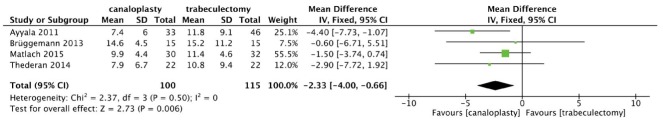
Our primary analysis enrolling 215 eyes compared canaloplasty with trabeculectomy in terms of IOPR up to 12mo (Figure 2). No significant heterogeneity was found between the two groups (χ2=2.37, P=0.50, I2=0.0), and the combined results showed both the surgical techniques significantly reduced postoperative IOP. Trabeculectomy was found to achieve a numerically greater IOPR from baseline and the differences in IOPR were statistically significant (WMD=-2.33, 95%CI: -4.00, -0.66).
Figure 2. Forest plot for IOPR comparing canaloplasty with trabeculectomy at 12mo.
Complete and Qualified Success
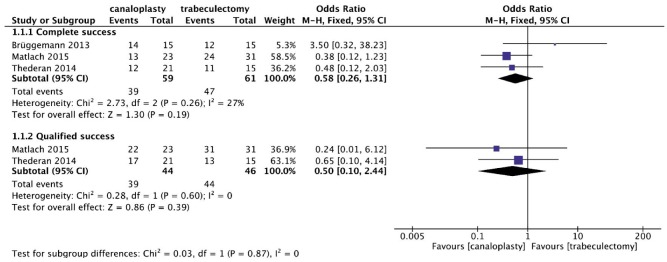
With respect to the postoperative success, non-significant proportions of canaloplasty patients were found to achieve better complete success or qualified success than trabeculectomy patients at 12mo (OR: 0.58, 95%CI: 0.26, 1.31; OR: 0.50, 95%CI: 0.10, 2.44, respectively) (Figure 3). No apparent heterogeneities across these studies were observed (both P>0.05; I2<50%).
Figure 3. Forest plot for the complete and qualified success comparing canaloplasty with trabeculectomy at 12mo.
Anti-glaucoma Medications Reduction
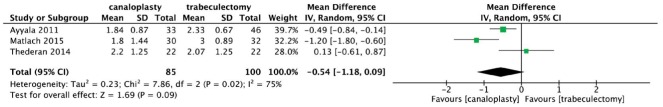
Figure 4 provided the postoperative change in anti-glaucoma medications use. Results showed significant heterogeneity among studies (χ2=7.86, P=0.02, I2=75%), and the difference in postoperative anti-glaucoma medications reduction was not statistically significant at 12mo (WMD: -0.54, 95%CI: -1.18, 0.09).
Figure 4. Forest plot for anti-glaucoma medications reduction comparing canaloplasty with trabeculectomy at 12mo.
Safety Analysis
All eligible studies reported more than one postoperative adverse event. The most common adverse events in both groups were listed in Table 3. Canaloplasty presented a lower incidence of choroidal detachment than trabeculectomy with a pooled OR of 0.12 (0.03 to 0.48), while with a significantly higher incidence of hyphema with OR of 8.80 (2.25 to 34.51). Statistically fewer patients in the canaloplasty group compared to the trabeculectomy group had some other adverse events, such as hypotony (IOP<5 mm Hg) and shallow anterior chamber, with the pooled ORs being 0.36 (0.13 to 0.99) and 0.32 (0.05 to 2.14), respectively.
Table 3. Postoperative complications comparing canaloplasty with trabeculectomy at 12 months.
| Complication | No. of studies | OR (95%CI) | Test for heterogeneity |
Overall effect |
||||
| χ2 | df | P | I2 | Z | P | |||
| Hyphema | 3 | 8.80 (2.25, 34.51) | 0.47 | 2 | 0.79 | 0% | 3.12 | 0.002 |
| Descemet detachment | 3 | 4.49 (0.72, 27.82) | 0.18 | 2 | 0.92 | 0% | 1.61 | 0.11 |
| Choroidal effusions | 4 | 0.12 (0.03, 0.48) | 1.04 | 3 | 0.79 | 0% | 3.04 | 0.002 |
| Hypotony (IOP<5 mm Hg) | 2 | 0.36 (0.13, 0.99) | 0.26 | 1 | 0.61 | 0% | 1.98 | 0.05 |
| Shallow anterior chamber | 2 | 0.32 (0.05, 2.14) | 0.17 | 1 | 0.68 | 0% | 1.18 | 0.24 |
OR: Odds ratio, which was calculated using a fixed effects model.
Sensitivity Analysis and Publication Bias
The sensitivity analyses suggested that each study did not significantly influence the estimation of IOPR (Table 4). Publication bias was not assessed due to the limited number of included studies in our Meta-analysis.
Table 4. Results of leave-one-out sensitivity analysis of IOPR.
| Excluded study | WMD (95%CI) | Test for heterogeneity |
Overall effect |
||||
| χ2 | df | P | I2 | Z | P | ||
| Ayyala[7] (2011) | -1.63 (-3.56, 0.29) | 0.39 | 2 | 0.82 | 0 | 1.66 | 0.10 |
| Brüggemann[8] (2013) | -2.47 (-4.20, -0.73) | 2.04 | 2 | 0.36 | 2% | 2.79 | 0.005 |
| Matlach[10] (2015) | -3.36 (-5.86, -0.86) | 1.19 | 2 | 0.55 | 0 | 2.63 | 0.008 |
| Thederan[9] (2014) | -2.25 (-4.03, -0.47) | 2.31 | 2 | 0.32 | 13% | 2.48 | 0.01 |
WMD: Weighted mean difference, which was calculated using a fixed effects model.
DISCUSSION
Glaucoma can cause visual impairment irreversibly in different age groups, which is characterized by progressive optic neuropathy and loss of visual field. With the growing emphasis on this devastating disease, ophthalmologists have attempted to seek for new surgical techniques that combine high effectiveness with fewer complications. Over the years, various minimally invasive surgical techniques offer an alternative choice for our glaucoma patients[25]. Trabeculectomy has been recognized as the classic surgical procedure in the management of glaucoma[26]. Canaloplasty is designed to increase the outflow by the natural drainage system and prevent many postoperative complications. The issue of IOP control and the success rate of canaloplasty compared to trabeculectomy remained controversial.
In this study, we found there was a trend towards better IOP outcomes with trabeculectomy up to 12mo which was statistically significant when comparing with canaloplasty. The IOP results after canaloplasty were similar to previous publications[27]–[29]. The most comprehensive evaluation performed by Lewis et al[30] demonstrated 34.7% IOP decrease from baseline at 12mo. According to our definition of success, no significant difference was detected between the two groups. One possible explanation was the small sample size, making it difficult to find positive results. There were similar results with regard to the number of anti-glaucoma medications of the two surgical techniques.
When it came to the postoperative complications, no significant difference was found except hyphema and choroidal effusions. Hyphema appeared to be more common in canaloplasty groups, which was in accordance with previous studies[19],[31]–[32]. However, the bleb-related complications were more commonly reported in the trabeculectomy groups. Theoretically, due to bleb-free procedures with proper tension of Schlemm's canal, canaloplasty has the significant advantage of lowering IOP from early stage and reducing the risk of the bleb-related complications compared to trabeculectomy. Several experts shared the same opinion that the occurrence of hyphema was associated with intraoperative or early postoperative decrease of IOP which leads to blood reflux into the anterior chamber from distal outflow pathways[19],[33]. Grieshaber et al[34] considered hyphema as a positive prognostic factor rather a complication.
Several limitations in this Meta-analysis should be demonstrated when considering the results. Firstly, we conducted a broad and exhaustive search of relevant studies, only to find very few randomized controlled clinical trials comparing the efficacy, safety and tolerability of canaloplasty with trabeculectomy for patients with OAG. Thus, the major limitation is lack of randomized controlled trials as well as the small sample size, which could affect the outcome measurements and introduce bias to our findings. Secondly, the forest plot for publication bias was not performed due to insufficient number of included studies. Likewise, publication bias estimated by Begg's and Egger's tests was not carried out. To avoid publication bias, the reference lists of all relevant articles were reviewed after electronic search. In addition, our analysis of IOPR was based on the data pooled from the same phases of follow-up. Considering another fact that researches may not go publish if they produce negative data, we believe that publication bias is unavoidable. A third limitation of this Meta-analysis was the short term follow-up. Most studies had a 12mo follow-up. Extending follow-up period may reveal different incidence of late adverse events of this new surgical technique.
What is more, as canaloplasty is newly developed, the level of surgical expertise is related with outcomes, which could have influenced the direction of the conclusion. None of the included studies evaluated the surgical expertise of the surgeons who performed the procedure. This should be taken into consideration when evaluating the results. Also, the staging of glaucoma was not specified. Taken together, the positive associations observed between the two groups need to be considered with caution and further studies are needed to verify the results.
However, currently published data show canaloplasty may be a reasonable additional surgical procedure for a selected group of patients diagnosed with OAG, which provides further insight into this important topic. A four-year follow-up study from Brusin [35] also revealed that canaloplasty was promising for glaucoma patients. Though it is far from ideal and the learning curve effect may have played a role in outcomes, further rigorous RCTs with carefully planned protocols are required to strengthen the application of canaloplasty.
In conclusion, our Meta-analysis indicated that trabeculectomy appeared to be more effective in postoperative IOP lowering than canaloplasty. Canaloplasty and trabeculectomy had the similar efficacy in anti-glaucoma medications reduction, the complete and the qualified success rate. Canaloplasty was associated with higher rates of hyphema but lower rates of bleb related complications than trabeculectomy. However, clinicians should be aware that the present results drawn from our analysis should be interpreted cautiously and further investigations are required on this topic.
Acknowledgments
Conflicts of Interest: Lin ZJ, None; Xu S, None; Huang SY, None; Zhang XB, None; Zhong YS, None.
REFERENCES
- 1.Tham YC, Li X, Wong TY, Quigley HA, Aung T, Cheng CY. Global prevalence of glaucoma and projections of glaucoma burden through 2040: a systematic review and meta-analysis. Ophthalmology. 2014;121(11):2081–2090. doi: 10.1016/j.ophtha.2014.05.013. [DOI] [PubMed] [Google Scholar]
- 2.Rulli E, Biagioli E, Riva I, Gambirasio G, De Simone I, Floriani I, Quaranta L. Efficacy and safety of trabeculectomy vs nonpenetrating surgical procedures: a systematic review and meta-analysis. JAMA Ophthalmol. 2013;131(12):1573–1582. doi: 10.1001/jamaophthalmol.2013.5059. [DOI] [PubMed] [Google Scholar]
- 3.Lama PJ, Fechtner RD. Antifibrotics and wound healing in glaucoma surgery. Surv Ophthalmol. 2003;48(3):314–346. doi: 10.1016/s0039-6257(03)00038-9. [DOI] [PubMed] [Google Scholar]
- 4.Harvey BJ, Khaimi MA. A review of canaloplasty. Saudi J Ophthalmol. 2011;25(4):329–336. doi: 10.1016/j.sjopt.2011.08.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Gesser C, Matthaei M, Meyer-Rüsenberg B, Richard G, Klemm M. Effect of combined cataract surgery and canaloplasty on postoperative intraocular pressure (IOP) Ophthalmologe. 2012;109(8):770–776. doi: 10.1007/s00347-012-2613-0. [DOI] [PubMed] [Google Scholar]
- 6.Arthur SN, Cantor LB, WuDunn D, Pattar GR, Catoira-Boyle Y, Morgan LS, Hoop JS. Efficacy, safety, and survival rates of IOP-lowering effect of phacoemulsification alone or combined with canaloplasty in glaucoma patients. J Glaucoma. 2014;23(5):316–320. doi: 10.1097/IJG.0b013e3182741ca9. [DOI] [PubMed] [Google Scholar]
- 7.Ayyala RS, Chaudhry AL, Okogbaa CB, Zurakowski D. Comparison of surgical outcomes between canaloplasty and trabeculectomy at 12 months' follow-up. Ophthalmology. 2011;118(12):2427–2433. doi: 10.1016/j.ophtha.2011.05.021. [DOI] [PubMed] [Google Scholar]
- 8.Brüggemann A, Despouy JT, Wegent A, Müller M. Intraindividual comparison of canaloplasty versus trabeculectomy with mitomycin C in a single-surgeon series. J Glaucoma. 2013;22(7):577–583. doi: 10.1097/IJG.0b013e318255bb30. [DOI] [PubMed] [Google Scholar]
- 9.Thederan L, Grehn F, Klink T. Comparison of canaloplasty with trabeculectomy. Klin Monbl Augenheilkd. 2014;231(3):256–261. doi: 10.1055/s-0033-1360392. [DOI] [PubMed] [Google Scholar]
- 10.Matlach J, Dhillon C, Hain J, Schlunck G, Grehn F, Klink T. Trabeculectomy versus canaloplasty (TVC study) in the treatment of patients with open-angle glaucoma: a prospective randomized clinical trial. Acta Ophthalmol. 2015;93(8):753–761. doi: 10.1111/aos.12722. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Moher D, Liberati A, Tetzlaff J, Altman DG, PRISMA Group Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. J Clin Epidemiol. 2009;62(10):1006–1012. doi: 10.1016/j.jclinepi.2009.06.005. [DOI] [PubMed] [Google Scholar]
- 12.Downs SH, Black N. The feasibility of creating a checklist for the assessment of the methodological quality both of randomised and non-randomised studies of health care interventions. J Epidemiol Community Health. 1998;52(6):377–384. doi: 10.1136/jech.52.6.377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Higgins J, Green S, editors. Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0 [updated March 2011]. The Cochrane Collaboration 2011. Available at: www.cochrane-handbook.org.
- 14.Heuer DK, Barton K, Grehn F, Shaarawy T, Sherwood M. Consensus on definitions of success. In: Shaarawy TM, Sherwood MB, Grehn F, editors. World Glaucoma Association Guidelines on design and reporting of glaucoma surgical trial. Amsterdam: Kugler Publications; 2009. pp. 15–24. [Google Scholar]
- 15.Matlach J, Freiberg FJ, Leippi S, Grehn F, Klink T. Comparison of phacotrabeculectomy versus phacocanaloplasty in the treatment of patients with concomitant cataract and glaucoma. BMC Ophthalmol. 2013;13(1):.1. doi: 10.1186/1471-2415-13-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Schoenberg ED, Chaudhry AL, Chod R, Zurakowski D, Ayyala RS. Comparison of surgical outcomes between phacocanaloplasty and phacotrabeculectomy at 12 months' follow-up. J Glaucoma. 2015;24(7):543–549. doi: 10.1097/IJG.0000000000000023. [DOI] [PubMed] [Google Scholar]
- 17.Peckar C, Körber N. Canaloplasty for open angle glaucoma: a three years critical evaluation and comparison with viscocanalostomy. Spektrum der Augenheilkunde. 2008;22(4):240–246. [Google Scholar]
- 18.Koerber NJ. Canaloplasty in one eye compared with viscocanalostomy in the contralateral eye in patients with bilateral open-angle glaucoma. J Glaucoma. 2012;21(2):129–134. doi: 10.1097/IJG.0b013e31820277c0. [DOI] [PubMed] [Google Scholar]
- 19.Rękas M, Byszewska A, Petz K, Wierzbowska J, Jünemann A. Canaloplasty versus non-penetrating deep sclerectomy - a prospective, randomised study of the safety and efficacy of combined cataract and glaucoma surgery: 12-month follow-up. Graefes Arch Clin Exp Ophthalmol. 2015;253(4):591–599. doi: 10.1007/s00417-015-2931-4. [DOI] [PubMed] [Google Scholar]
- 20.Tetz M, Koerber N, Shingleton BJ, von Wolff K, Bull H, Samuelson TW, Lewis RA. Phacoemulsification and intraocular lens implantation before, during, or after canaloplasty in eyes with open-angle glaucoma: 3-year results. J Glaucoma. 2015;24(3):187–194. doi: 10.1097/IJG.0b013e318285ff13. [DOI] [PubMed] [Google Scholar]
- 21.Brüggemann A, Müller M. Trabeculectomy versus canaloplasty-utility and cost-effectiveness analysis. Klin Monbl Augenheilkd. 2012;229(11):1118–1123. doi: 10.1055/s-0032-1315100. [DOI] [PubMed] [Google Scholar]
- 22.Quaranta L, Biagioli E, Riva I, Tosoni C, Brusini P, Centofanti M, Katsanos A, Floriani I, Konstas AG. Effect of trabeculectomy and canaloplasty on intra-ocular pressure modifications after postural changes in open-angle glaucoma. Acta Ophthalmol. 2014;92(6):498–499. doi: 10.1111/aos.12470. [DOI] [PubMed] [Google Scholar]
- 23.Klink T, Sauer J, Körber NJ, Grehn F, Much MM, Thederan L, Matlach J, Salgado JP. Quality of life following glaucoma surgery: canaloplasty versus trabeculectomy. Clin Ophthalmol. 2015;9:7–16. doi: 10.2147/OPTH.S72357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Matlach J, Klink T. Trabeculectomy versus canaloplasty. Ophthalmologe. 2015;112(4):325–331. doi: 10.1007/s00347-014-3160-7. [DOI] [PubMed] [Google Scholar]
- 25.Brandão LM, Grieshaber MC. Update on minimally invasive glaucoma surgery (MIGS) and new implants. J Ophthalmol. 2013;2013:705915. doi: 10.1155/2013/705915. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Arora KS, Robin AL, Corcoran KJ, Corcoran SL, Ramulu PY. Use of various glaucoma surgeries and procedures in medicare beneficiaries from 1994 to 2012. Ophthalmology. 2015;122(8):1615–1624. doi: 10.1016/j.ophtha.2015.04.015. [DOI] [PubMed] [Google Scholar]
- 27.Bull H, von Wolff K, Körber N, Tetz M. Three-year canaloplasty outcomes for the treatment of open-angle glaucoma: European study results. Graefes Arch Clin Exp Ophthalmol. 2011;249(10):1537–1545. doi: 10.1007/s00417-011-1728-3. [DOI] [PubMed] [Google Scholar]
- 28.Fujita K, Kitagawa K, Ueta Y, Nakamura T, Miyakoshi A, Hayashi A. Short-term results of canaloplasty surgery for primary open-angle glaucoma in Japanese patients. Case Rep Ophthalmol. 2011;2(1):65–68. doi: 10.1159/000324808. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Lopes-Cardoso I, Esteves F, Amorim M, Calvão-Santos G, Freitas ML, Salgado-Borges J. Circumferential viscocanalostomy with suture tensioning in Schlemm canal (canaloplasty)-one year experience. Arch Soc Esp Oftalmol. 2013;88(6):207–215. doi: 10.1016/j.oftal.2012.06.021. [DOI] [PubMed] [Google Scholar]
- 30.Lewis RA, von Wolff K, Tetz M, Koerber N, Kearney JR, Shingleton BJ, Samuelson TW. Canaloplasty: three-year results of circumferential viscodilation and tensioning of Schlemm's canal using a microcatheter to treat open-angle glaucoma. J Cataract Refract Surg. 2011;37(4):682–690. doi: 10.1016/j.jcrs.2010.10.055. [DOI] [PubMed] [Google Scholar]
- 31.Shingleton B, Tetz M, Korber N. Circumferential viscodilation and tensioning of Schlemm canal (canaloplasty) with temporal clear corneal phacoemulsification cataract surgery for open-angle glaucoma and visually significant cataract: one-year results. J Cataract Refract Surg. 2008;34(3):433–440. doi: 10.1016/j.jcrs.2007.11.029. [DOI] [PubMed] [Google Scholar]
- 32.Brusini P, Caramello G, Benedetti S, Tosoni C. Canaloplasty in open-angle glaucoma: mid-term results from a multicenter study. J Glaucoma. 2016;25(5):403–407. doi: 10.1097/IJG.0000000000000103. [DOI] [PubMed] [Google Scholar]
- 33.Grieshaber MC, Pienaar A, Olivier J, Stegmann R. Canaloplasty for primary open-angle glaucoma: long-term outcome. Br J Ophthalmol. 2010;94(11):1478–1482. doi: 10.1136/bjo.2009.163170. [DOI] [PubMed] [Google Scholar]
- 34.Grieshaber MC, Schoetzau A, Flammer J, Orgül S. Postoperative microhyphema as a positive prognostic indicator in canaloplasty. Acta Ophthalmol. 2013;91(2):151–156. doi: 10.1111/j.1755-3768.2011.02293.x. [DOI] [PubMed] [Google Scholar]
- 35.Brusini P. Canaloplasty in open-angle glaucoma surgery: a four-year follow-up. Scientific World Journal. 2014;2014:469906. doi: 10.1155/2014/469609. [DOI] [PMC free article] [PubMed] [Google Scholar]