Abstract
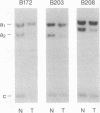
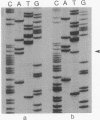
Loss of heterozygosity (LOH) on the short arm of chromosome 17 (17p) was found in 27 of 52 (52%) previously untreated primary breast cancers. There was a significant correlation between this 17p allelic loss and two parameters associated with aggressive tumor behavior: high cellular proliferative fraction and DNA aneuploidy. These correlations with high cellular proliferative fraction and DNA aneuploidy were not found in tumors with LOH at nine other chromosome locations. The p53 gene, a putative tumor suppressor gene located at 17p13, was examined for aberrations to determine whether it is the target for the 17p LOH in breast cancer. Unlike other types of human cancer, there were no homozygous deletions or rearrangements of the p53 gene, and only 2 of 13 (15%) were mutated in the conserved region where mutational "hot spots" have been previously located. Therefore, we hypothesize that, in breast cancer, either loss or inactivation of gene(s) on chromosome 17p other than the p53 gene or a different mechanism of p53 gene inactivation may be responsible for the observed high labeling index and DNA aneuploidy associated with LOH at 17p.
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Baker S. J., Fearon E. R., Nigro J. M., Hamilton S. R., Preisinger A. C., Jessup J. M., vanTuinen P., Ledbetter D. H., Barker D. F., Nakamura Y. Chromosome 17 deletions and p53 gene mutations in colorectal carcinomas. Science. 1989 Apr 14;244(4901):217–221. doi: 10.1126/science.2649981. [DOI] [PubMed] [Google Scholar]
- Bartek J., Iggo R., Gannon J., Lane D. P. Genetic and immunochemical analysis of mutant p53 in human breast cancer cell lines. Oncogene. 1990 Jun;5(6):893–899. [PubMed] [Google Scholar]
- Bos J. L., Fearon E. R., Hamilton S. R., Verlaan-de Vries M., van Boom J. H., van der Eb A. J., Vogelstein B. Prevalence of ras gene mutations in human colorectal cancers. 1987 May 28-Jun 3Nature. 327(6120):293–297. doi: 10.1038/327293a0. [DOI] [PubMed] [Google Scholar]
- Buchman V. L., Chumakov P. M., Ninkina N. N., Samarina O. P., Georgiev G. P. A variation in the structure of the protein-coding region of the human p53 gene. Gene. 1988 Oct 30;70(2):245–252. doi: 10.1016/0378-1119(88)90196-5. [DOI] [PubMed] [Google Scholar]
- Cogswell P. C., Morgan R., Dunn M., Neubauer A., Nelson P., Poland-Johnston N. K., Sandberg A. A., Liu E. Mutations of the ras protooncogenes in chronic myelogenous leukemia: a high frequency of ras mutations in bcr/abl rearrangement-negative chronic myelogenous leukemia. Blood. 1989 Dec;74(8):2629–2633. [PubMed] [Google Scholar]
- Coles C., Thompson A. M., Elder P. A., Cohen B. B., Mackenzie I. M., Cranston G., Chetty U., Mackay J., Macdonald M., Nakamura Y. Evidence implicating at least two genes on chromosome 17p in breast carcinogenesis. Lancet. 1990 Sep 29;336(8718):761–763. doi: 10.1016/0140-6736(90)93236-i. [DOI] [PubMed] [Google Scholar]
- Cropp C. S., Lidereau R., Campbell G., Champene M. H., Callahan R. Loss of heterozygosity on chromosomes 17 and 18 in breast carcinoma: two additional regions identified. Proc Natl Acad Sci U S A. 1990 Oct;87(19):7737–7741. doi: 10.1073/pnas.87.19.7737. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Devilee P., Cornelisse C. J., Kuipers-Dijkshoorn N., Jonker C., Pearson P. L. Loss of heterozygosity on 17p in human breast carcinomas: defining the smallest common region of deletion. Cytogenet Cell Genet. 1990;53(1):52–54. doi: 10.1159/000132893. [DOI] [PubMed] [Google Scholar]
- Devilee P., van den Broek M., Kuipers-Dijkshoorn N., Kolluri R., Khan P. M., Pearson P. L., Cornelisse C. J. At least four different chromosomal regions are involved in loss of heterozygosity in human breast carcinoma. Genomics. 1989 Oct;5(3):554–560. doi: 10.1016/0888-7543(89)90023-2. [DOI] [PubMed] [Google Scholar]
- Dicker A. P., Volkenandt M., Adamo A., Barreda C., Bertino J. R. Sequence analysis of a human gene responsible for drug resistance: a rapid method for manual and automated direct sequencing of products generated by the polymerase chain reaction. Biotechniques. 1989 Sep;7(8):830–838. [PubMed] [Google Scholar]
- Donis-Keller H., Green P., Helms C., Cartinhour S., Weiffenbach B., Stephens K., Keith T. P., Bowden D. W., Smith D. R., Lander E. S. A genetic linkage map of the human genome. Cell. 1987 Oct 23;51(2):319–337. doi: 10.1016/0092-8674(87)90158-9. [DOI] [PubMed] [Google Scholar]
- Feinberg A. P., Vogelstein B. A technique for radiolabeling DNA restriction endonuclease fragments to high specific activity. Anal Biochem. 1983 Jul 1;132(1):6–13. doi: 10.1016/0003-2697(83)90418-9. [DOI] [PubMed] [Google Scholar]
- Finlay C. A., Hinds P. W., Levine A. J. The p53 proto-oncogene can act as a suppressor of transformation. Cell. 1989 Jun 30;57(7):1083–1093. doi: 10.1016/0092-8674(89)90045-7. [DOI] [PubMed] [Google Scholar]
- Forrester K., Almoguera C., Han K., Grizzle W. E., Perucho M. Detection of high incidence of K-ras oncogenes during human colon tumorigenesis. 1987 May 28-Jun 3Nature. 327(6120):298–303. doi: 10.1038/327298a0. [DOI] [PubMed] [Google Scholar]
- Hinds P., Finlay C., Levine A. J. Mutation is required to activate the p53 gene for cooperation with the ras oncogene and transformation. J Virol. 1989 Feb;63(2):739–746. doi: 10.1128/jvi.63.2.739-746.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Isobe M., Emanuel B. S., Givol D., Oren M., Croce C. M. Localization of gene for human p53 tumour antigen to band 17p13. Nature. 1986 Mar 6;320(6057):84–85. doi: 10.1038/320084a0. [DOI] [PubMed] [Google Scholar]
- James C. D., Carlbom E., Dumanski J. P., Hansen M., Nordenskjold M., Collins V. P., Cavenee W. K. Clonal genomic alterations in glioma malignancy stages. Cancer Res. 1988 Oct 1;48(19):5546–5551. [PubMed] [Google Scholar]
- Lane D. P., Benchimol S. p53: oncogene or anti-oncogene? Genes Dev. 1990 Jan;4(1):1–8. doi: 10.1101/gad.4.1.1. [DOI] [PubMed] [Google Scholar]
- Mackay J., Steel C. M., Elder P. A., Forrest A. P., Evans H. J. Allele loss on short arm of chromosome 17 in breast cancers. Lancet. 1988 Dec 17;2(8625):1384–1385. doi: 10.1016/s0140-6736(88)90584-3. [DOI] [PubMed] [Google Scholar]
- McGuire W. L., Tandon A. K., Allred D. C., Chamness G. C., Clark G. M. How to use prognostic factors in axillary node-negative breast cancer patients. J Natl Cancer Inst. 1990 Jun 20;82(12):1006–1015. doi: 10.1093/jnci/82.12.1006. [DOI] [PubMed] [Google Scholar]
- Menon A. G., Anderson K. M., Riccardi V. M., Chung R. Y., Whaley J. M., Yandell D. W., Farmer G. E., Freiman R. N., Lee J. K., Li F. P. Chromosome 17p deletions and p53 gene mutations associated with the formation of malignant neurofibrosarcomas in von Recklinghausen neurofibromatosis. Proc Natl Acad Sci U S A. 1990 Jul;87(14):5435–5439. doi: 10.1073/pnas.87.14.5435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meyer J. S., McDivitt R. W. Reliability and stability of the thymidine labeling index of breast carcinoma. Lab Invest. 1986 Feb;54(2):160–164. [PubMed] [Google Scholar]
- Mulligan L. M., Matlashewski G. J., Scrable H. J., Cavenee W. K. Mechanisms of p53 loss in human sarcomas. Proc Natl Acad Sci U S A. 1990 Aug;87(15):5863–5867. doi: 10.1073/pnas.87.15.5863. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nigro J. M., Baker S. J., Preisinger A. C., Jessup J. M., Hostetter R., Cleary K., Bigner S. H., Davidson N., Baylin S., Devilee P. Mutations in the p53 gene occur in diverse human tumour types. Nature. 1989 Dec 7;342(6250):705–708. doi: 10.1038/342705a0. [DOI] [PubMed] [Google Scholar]
- Ponder B. Cancer. Gene losses in human tumours. Nature. 1988 Sep 29;335(6189):400–402. doi: 10.1038/335400a0. [DOI] [PubMed] [Google Scholar]
- Rochlitz C. F., Scott G. K., Dodson J. M., Liu E., Dollbaum C., Smith H. S., Benz C. C. Incidence of activating ras oncogene mutations associated with primary and metastatic human breast cancer. Cancer Res. 1989 Jan 15;49(2):357–360. [PubMed] [Google Scholar]
- Rodenhuis S., van de Wetering M. L., Mooi W. J., Evers S. G., van Zandwijk N., Bos J. L. Mutational activation of the K-ras oncogene. A possible pathogenetic factor in adenocarcinoma of the lung. N Engl J Med. 1987 Oct 8;317(15):929–935. doi: 10.1056/NEJM198710083171504. [DOI] [PubMed] [Google Scholar]
- Sager R. Tumor suppressor genes: the puzzle and the promise. Science. 1989 Dec 15;246(4936):1406–1412. doi: 10.1126/science.2574499. [DOI] [PubMed] [Google Scholar]
- Saiki R. K., Gelfand D. H., Stoffel S., Scharf S. J., Higuchi R., Horn G. T., Mullis K. B., Erlich H. A. Primer-directed enzymatic amplification of DNA with a thermostable DNA polymerase. Science. 1988 Jan 29;239(4839):487–491. doi: 10.1126/science.2448875. [DOI] [PubMed] [Google Scholar]
- Sanger F., Nicklen S., Coulson A. R. DNA sequencing with chain-terminating inhibitors. Proc Natl Acad Sci U S A. 1977 Dec;74(12):5463–5467. doi: 10.1073/pnas.74.12.5463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith H. S., Wolman S. R., Dairkee S. H., Hancock M. C., Lippman M., Leff A., Hackett A. J. Immortalization in culture: occurrence at a late stage in the progression of breast cancer. J Natl Cancer Inst. 1987 Apr;78(4):611–615. [PubMed] [Google Scholar]
- Takahashi T., Nau M. M., Chiba I., Birrer M. J., Rosenberg R. K., Vinocour M., Levitt M., Pass H., Gazdar A. F., Minna J. D. p53: a frequent target for genetic abnormalities in lung cancer. Science. 1989 Oct 27;246(4929):491–494. doi: 10.1126/science.2554494. [DOI] [PubMed] [Google Scholar]
- Vindeløv L. L., Christensen I. J., Nissen N. I. A detergent-trypsin method for the preparation of nuclei for flow cytometric DNA analysis. Cytometry. 1983 Mar;3(5):323–327. doi: 10.1002/cyto.990030503. [DOI] [PubMed] [Google Scholar]
- Wadey R. B., Pal N., Buckle B., Yeomans E., Pritchard J., Cowell J. K. Loss of heterozygosity in Wilms' tumour involves two distinct regions of chromosome 11. Oncogene. 1990 Jun;5(6):901–907. [PubMed] [Google Scholar]
- Yokota J., Wada M., Shimosato Y., Terada M., Sugimura T. Loss of heterozygosity on chromosomes 3, 13, and 17 in small-cell carcinoma and on chromosome 3 in adenocarcinoma of the lung. Proc Natl Acad Sci U S A. 1987 Dec;84(24):9252–9256. doi: 10.1073/pnas.84.24.9252. [DOI] [PMC free article] [PubMed] [Google Scholar]