Abstract
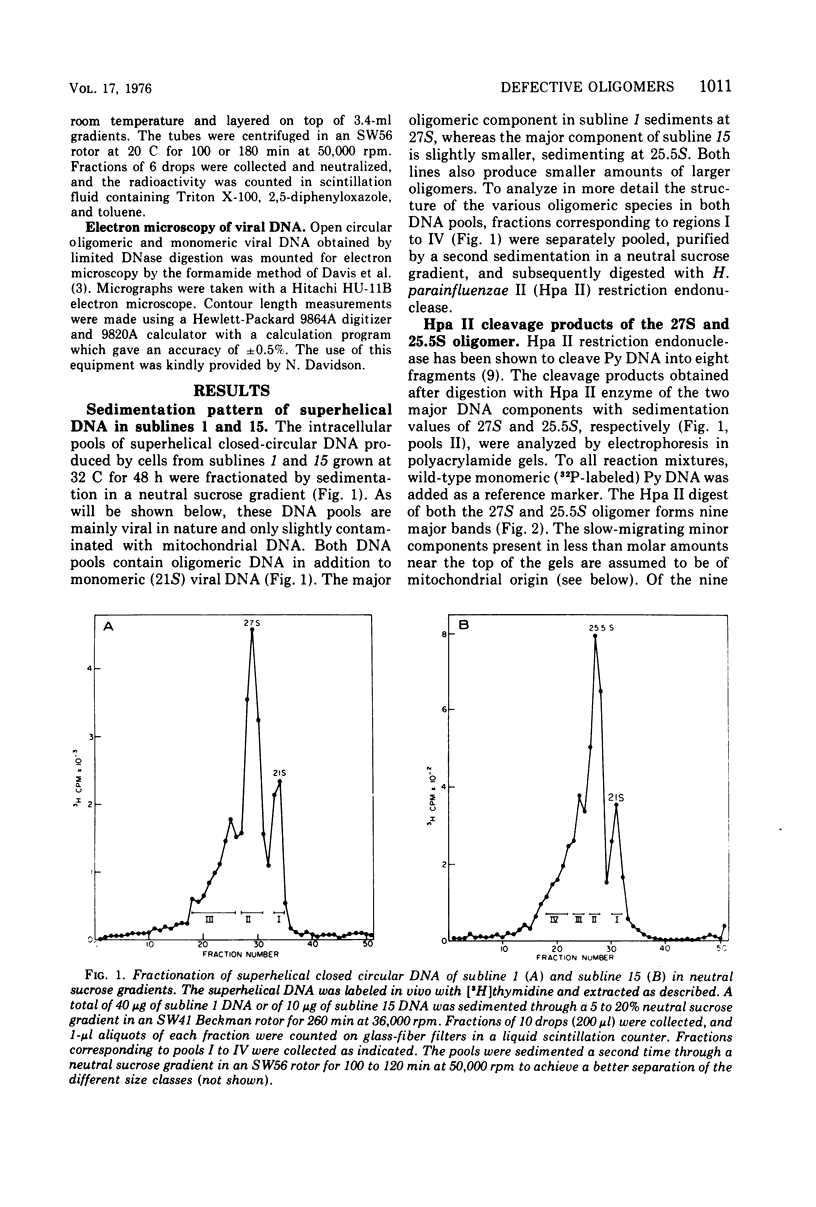
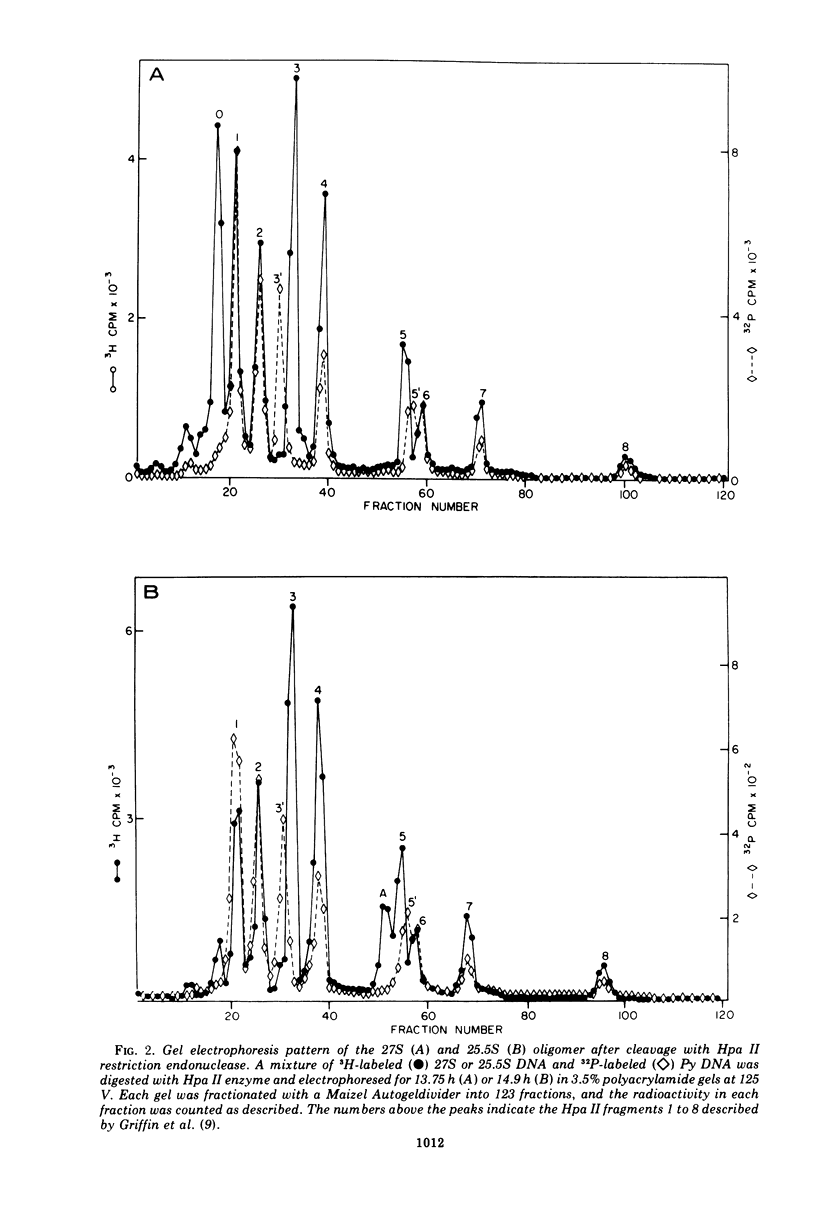
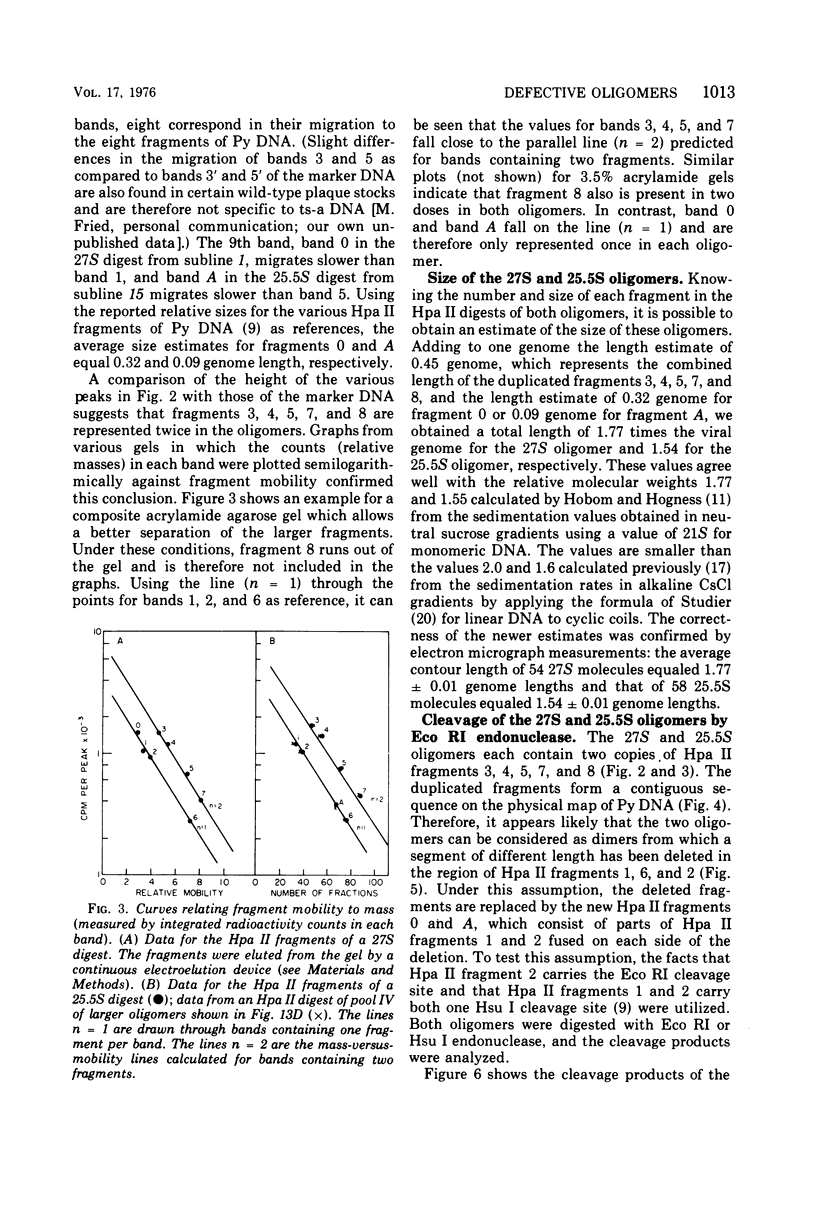
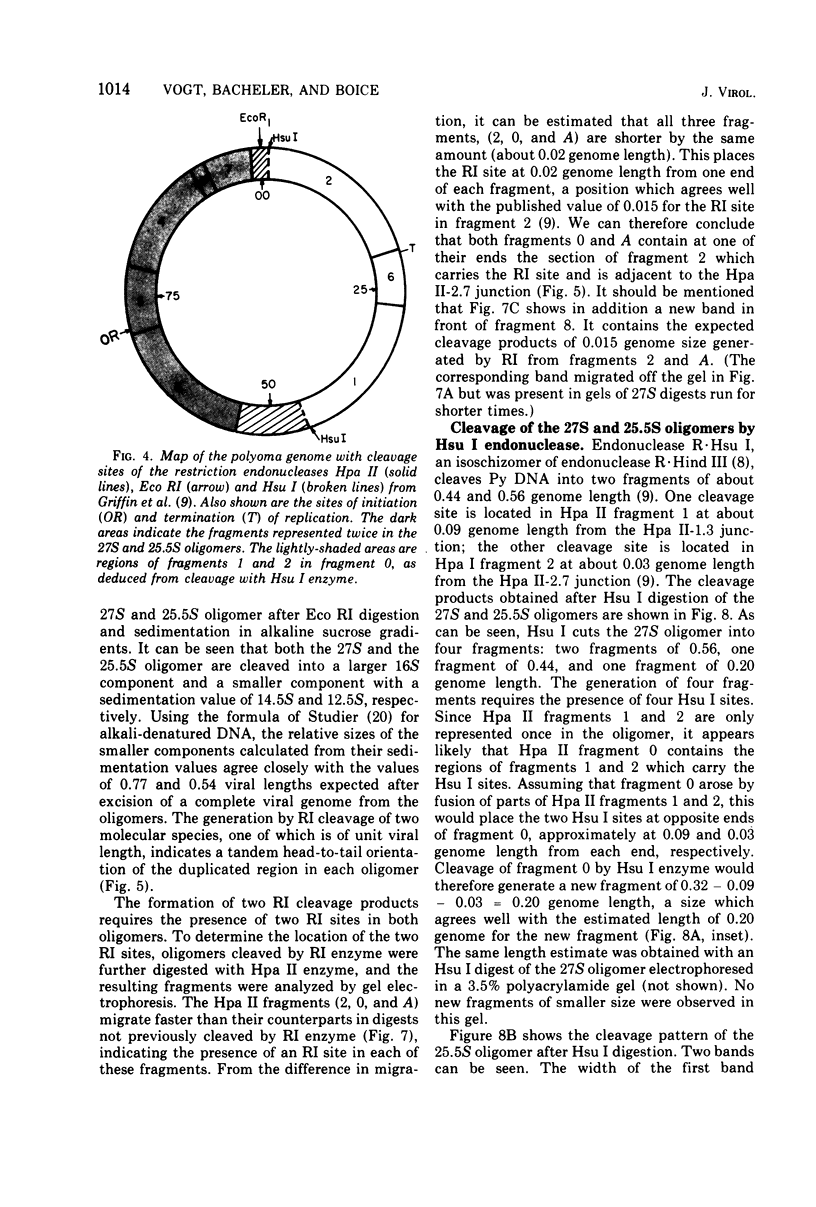
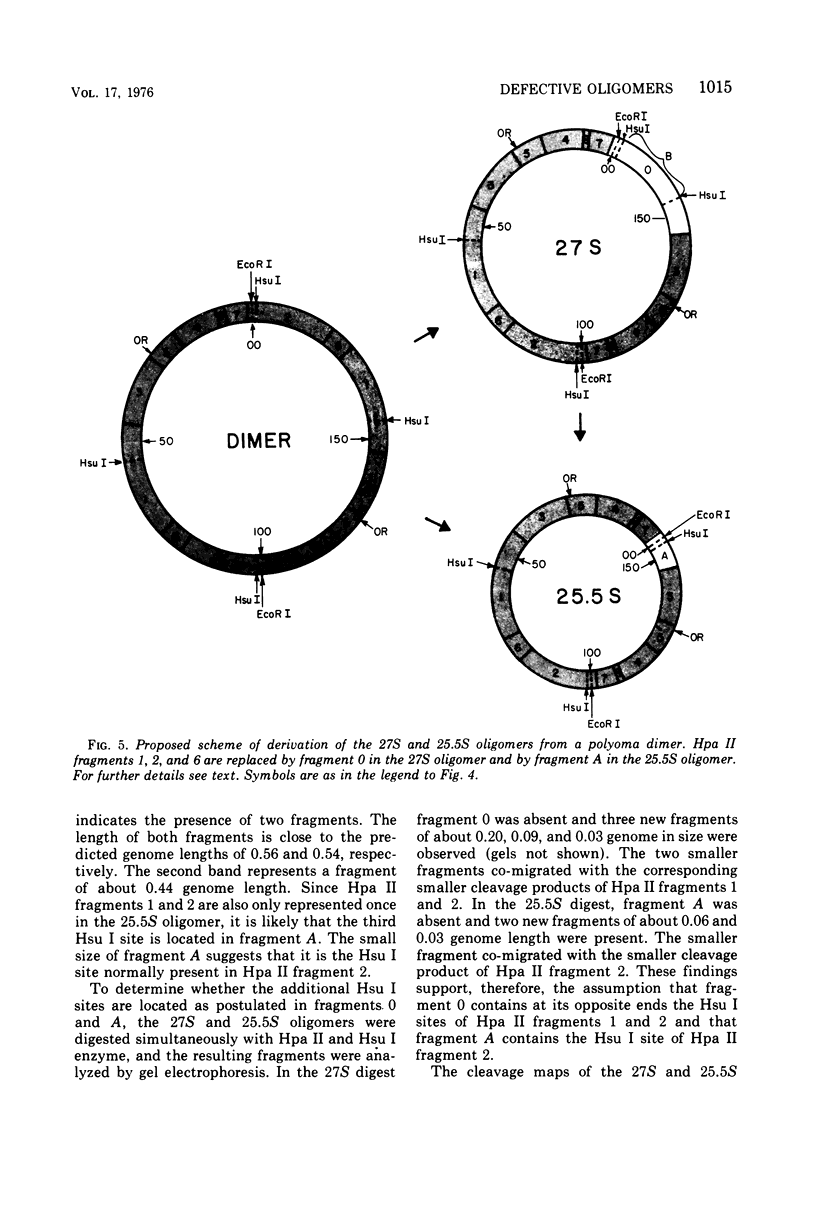
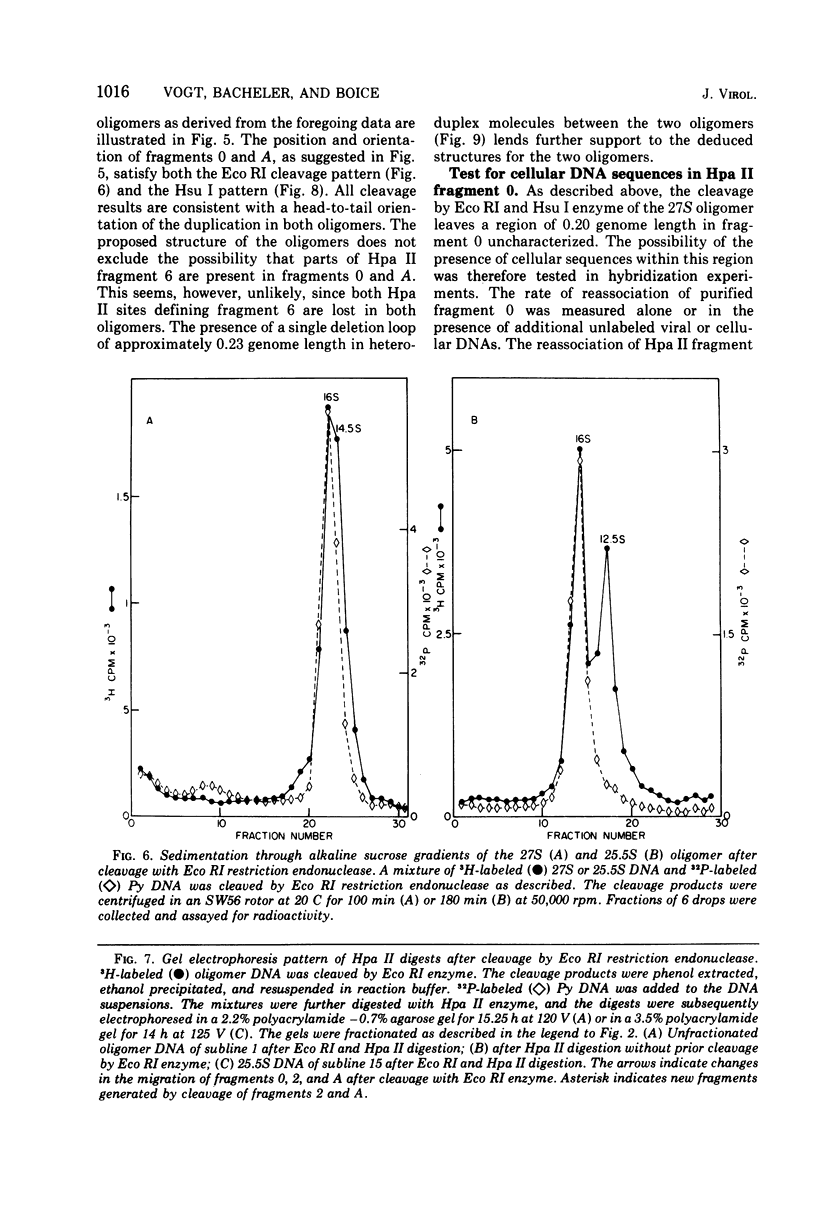
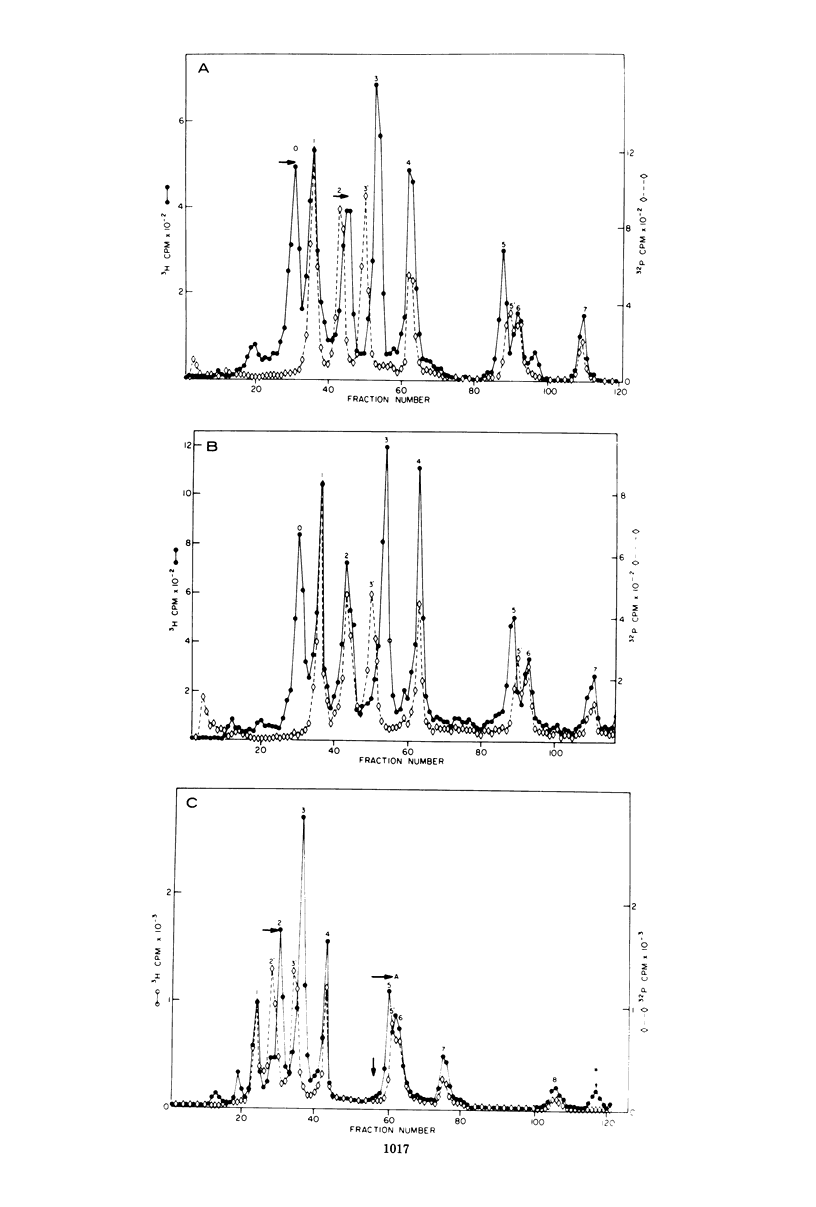
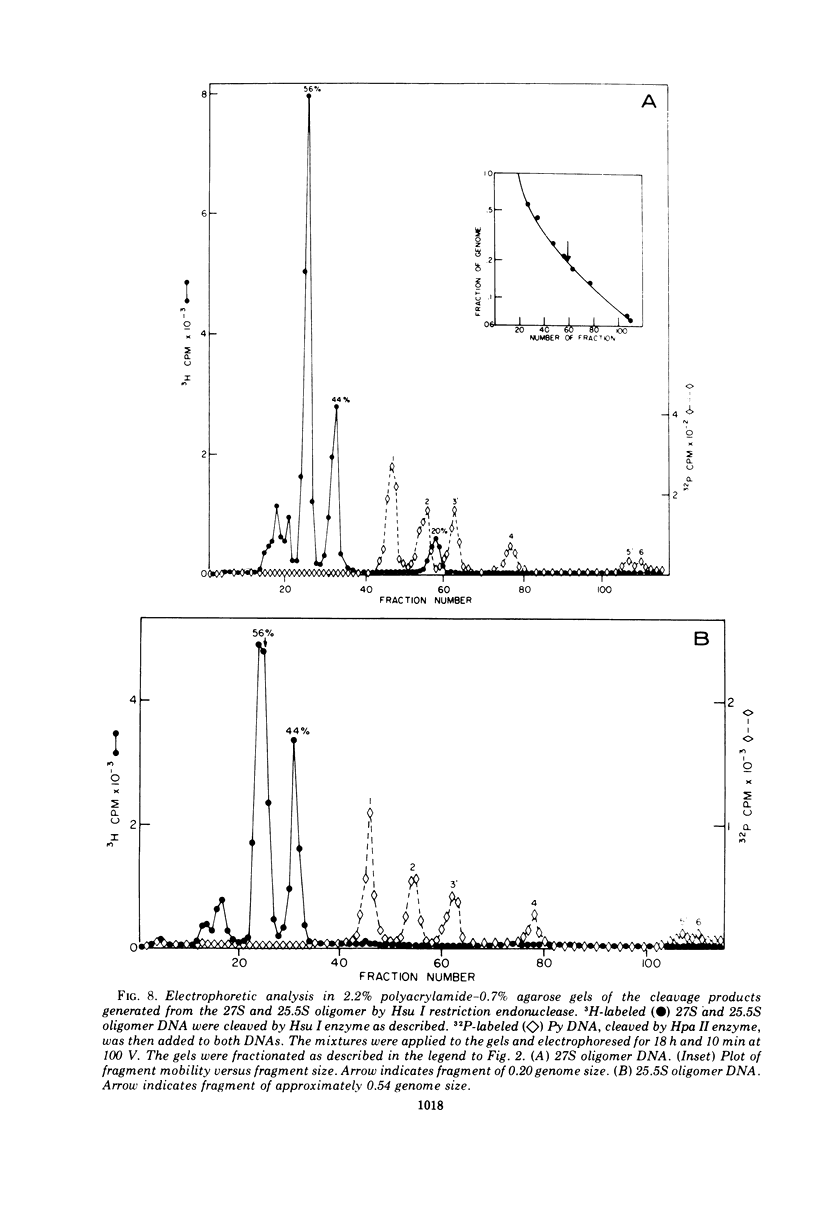
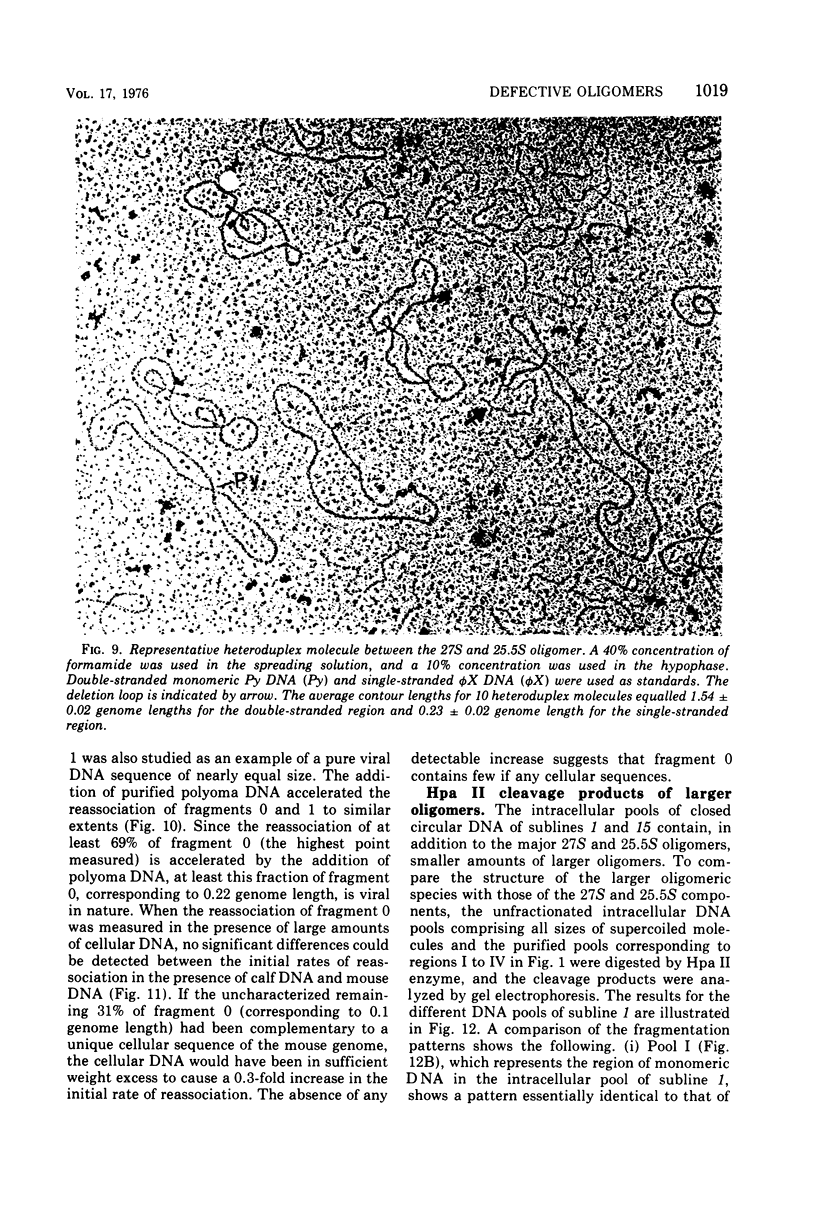
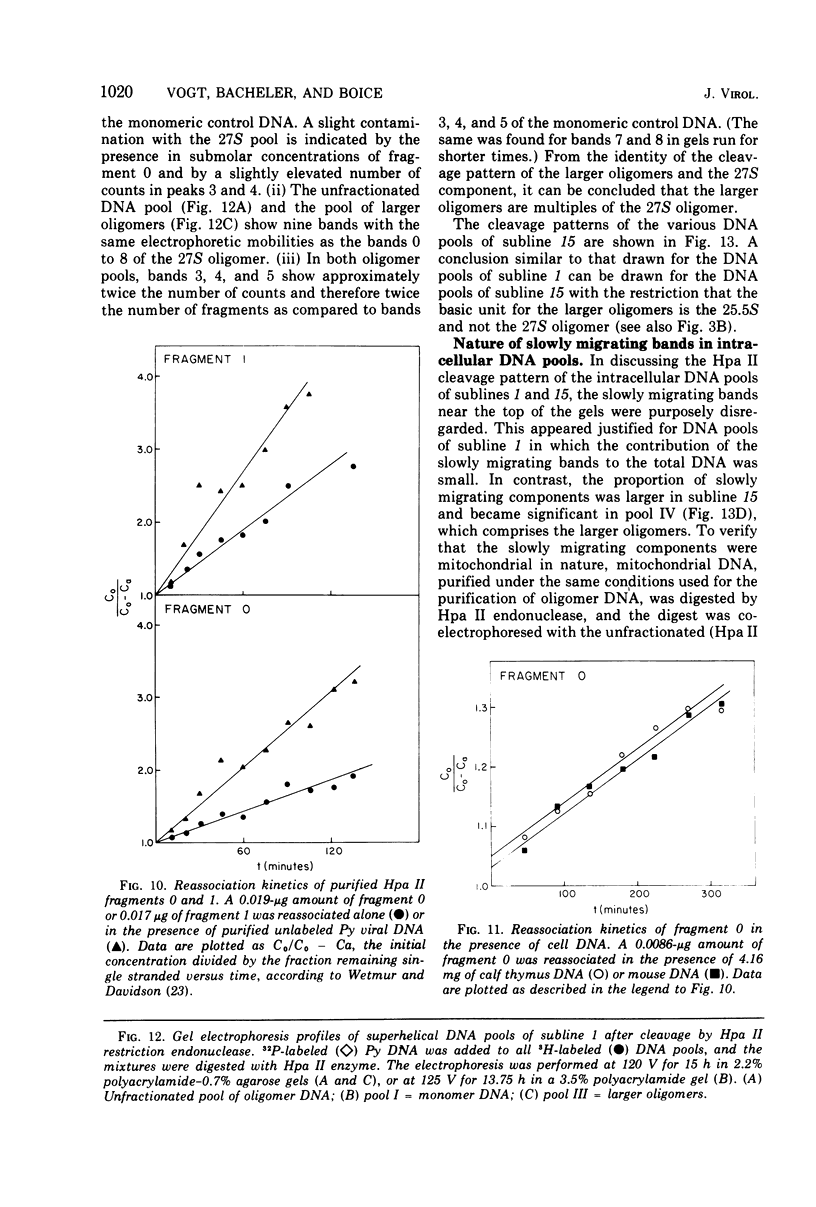
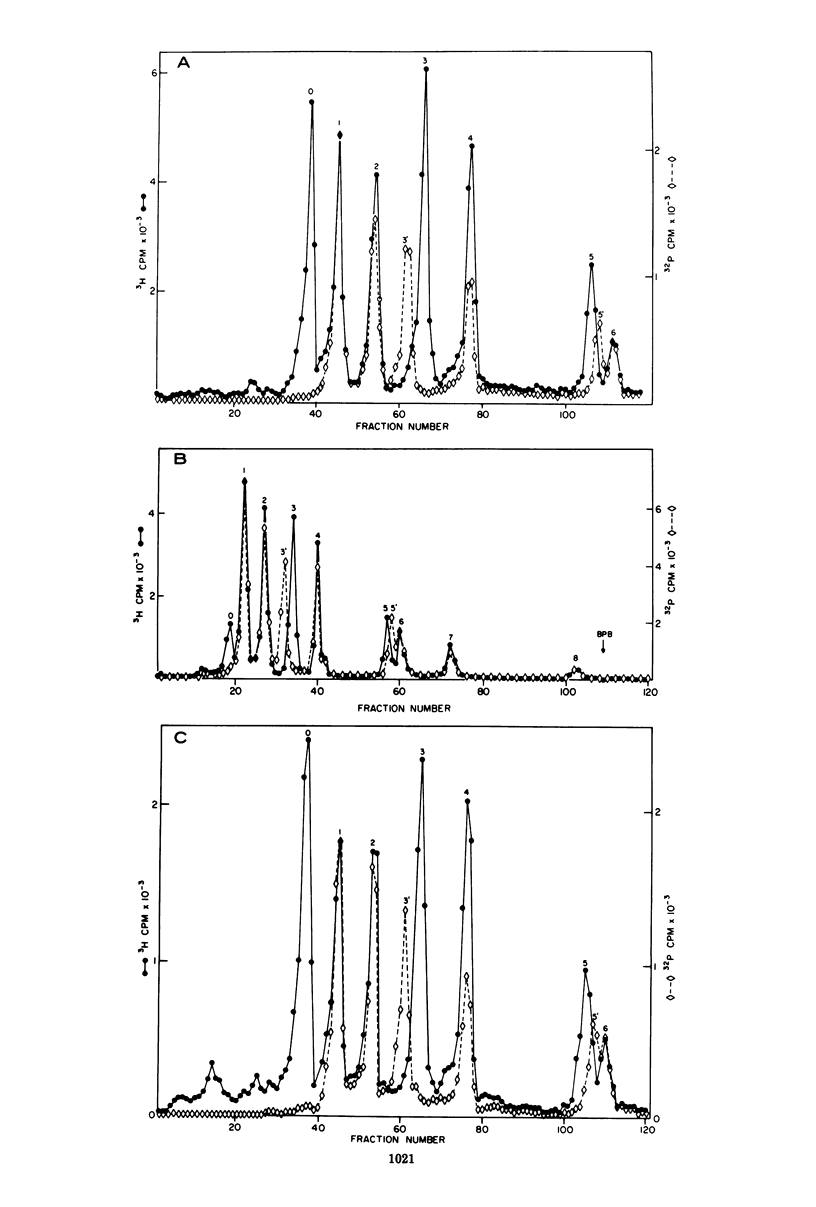
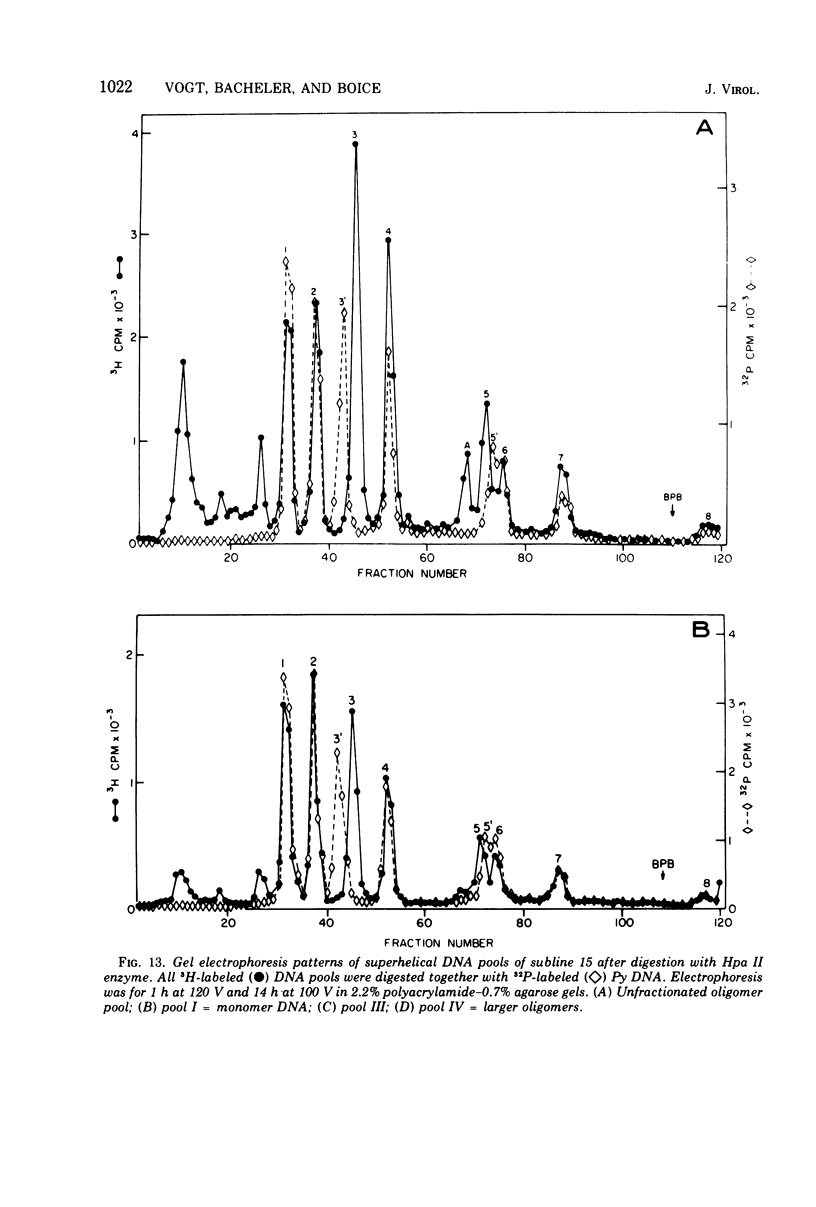
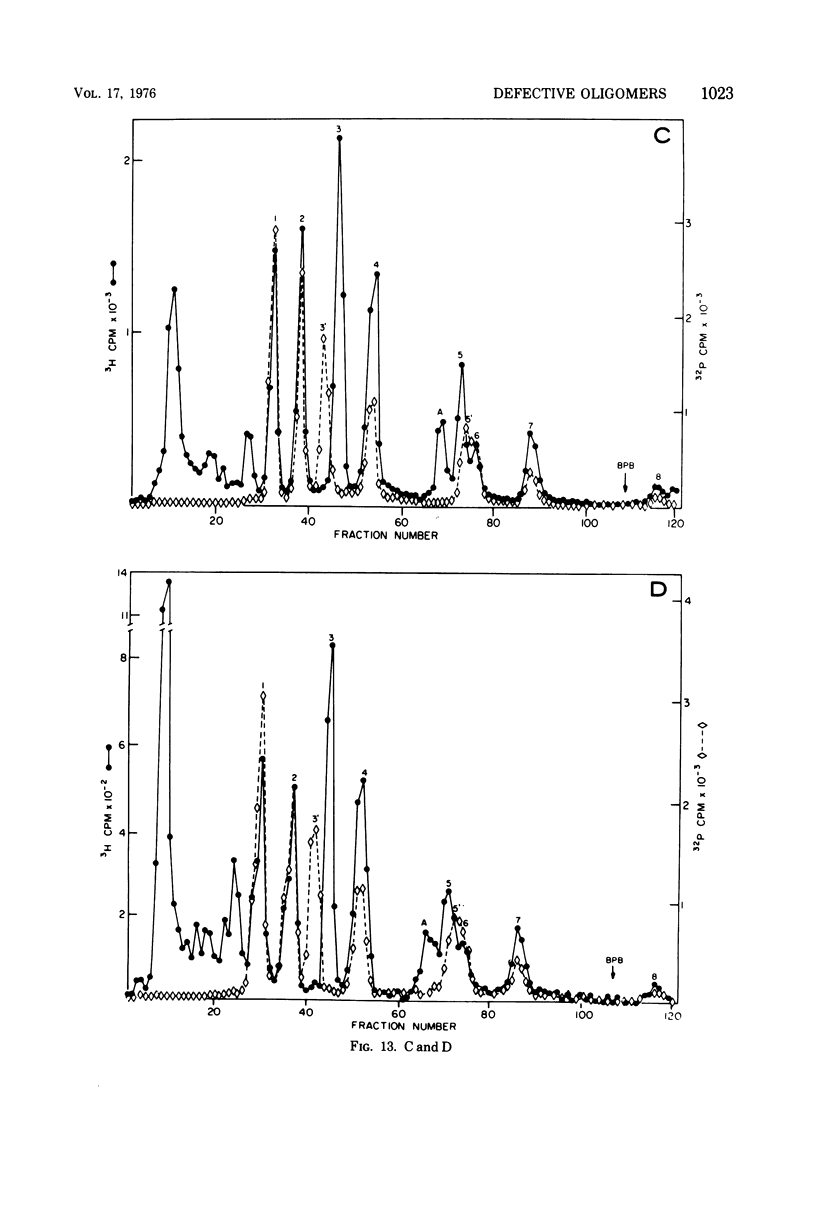
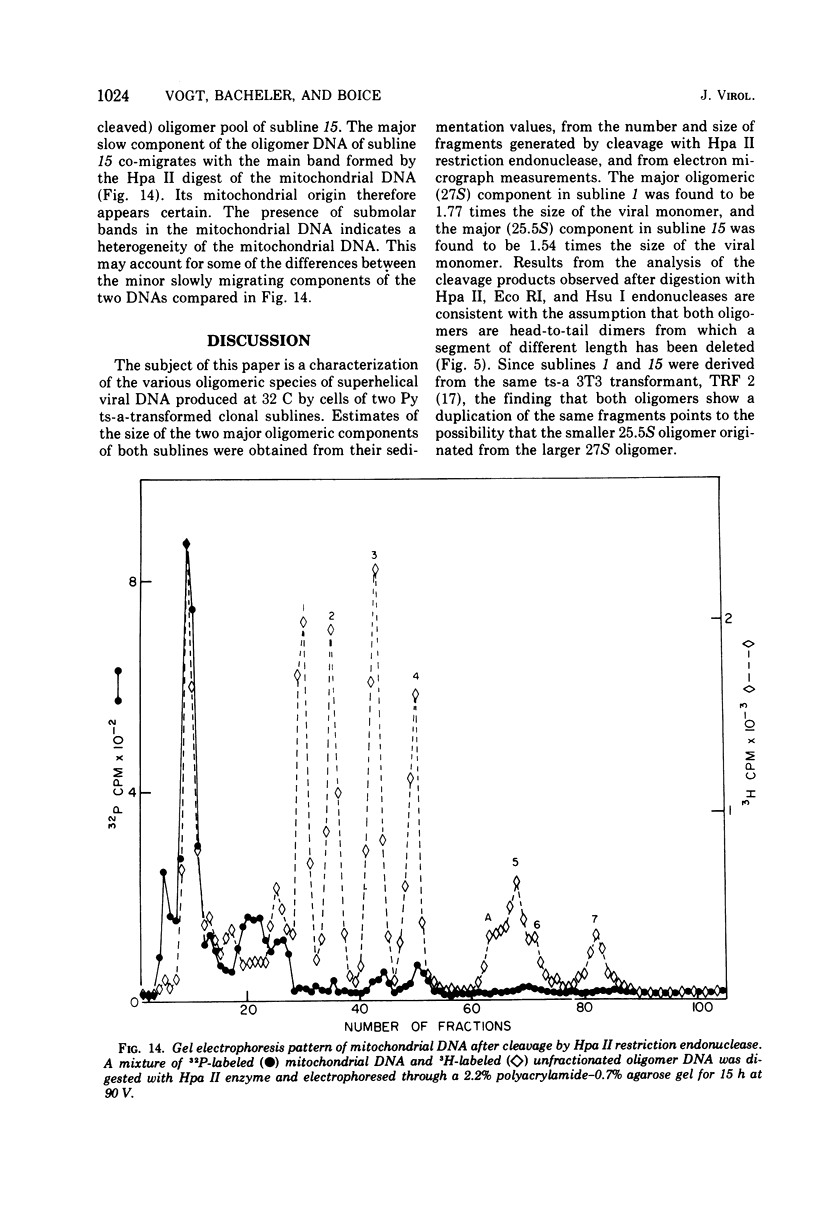
The various oligomeric viral DNA species produced at 32 C by two related syblines of ts-a-transformed mouse 3T3 cells were characterized. Results from the analysis of the cleavage products observed after digestion with restriction endonucleases from Haemophilus parainfluenzae, Escherichia coli RI, and Haemophilus suis are consistent with the assumption that in both sublines, the major oligomeric component is a dimer from which a segment of different length is deleted. The major oligomeric (27S) component in subline 1 was estimated to be 1.77 times the size of the viral monomer, and the major (25.5S) component in subline 15 was estimated to be 1.54 times the size of the viral monomer. These size estimates were confirmed by electron micrograph measurements. The larger oligomers produced by both sublines were found to be multiples of the major oligomeric component of each subline.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bishop D. H., Claybrook J. R., Spiegelman S. Electrophoretic separation of viral nucleic acids on polyacrylamide gels. J Mol Biol. 1967 Jun 28;26(3):373–387. doi: 10.1016/0022-2836(67)90310-5. [DOI] [PubMed] [Google Scholar]
- Edgell M. H., Hutchison C. A., 3rd, Sclair M. Specific endonuclease R fragments of bacteriophage phiX174 deoxyribonucleic acid. J Virol. 1972 Apr;9(4):574–582. doi: 10.1128/jvi.9.4.574-582.1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- FRIED M. ISOLATION OF TEMPERATURE-SENSITIVE MUTANTS OF POLYOMA VIRUS. Virology. 1965 Apr;25:669–671. doi: 10.1016/0042-6822(65)90098-x. [DOI] [PubMed] [Google Scholar]
- Fried M. Characterization of a temperature-sensitive mutant of polyoma virus. Virology. 1970 Mar;40(3):605–617. doi: 10.1016/0042-6822(70)90205-9. [DOI] [PubMed] [Google Scholar]
- Griffin B. E., Fried M., Cowie A. Polyoma DNA: a physical map. Proc Natl Acad Sci U S A. 1974 May;71(5):2077–2081. doi: 10.1073/pnas.71.5.2077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hirt B. Selective extraction of polyoma DNA from infected mouse cell cultures. J Mol Biol. 1967 Jun 14;26(2):365–369. doi: 10.1016/0022-2836(67)90307-5. [DOI] [PubMed] [Google Scholar]
- Hobom G., Hogness D. S. The role of recombination in the formation of circular oligomers of the lambda plasmid. J Mol Biol. 1974 Sep 5;88(1):65–87. doi: 10.1016/0022-2836(74)90295-2. [DOI] [PubMed] [Google Scholar]
- Hudson B., Vinograd J. Sedimentation velocity properties of complex mitochondrial DNA. Nature. 1969 Jan 25;221(5178):332–337. doi: 10.1038/221332a0. [DOI] [PubMed] [Google Scholar]
- Jaenisch R., Levine A. J. DNA replication of SV40-infected cells. VII. Formation of SV40 catenated and circular dimers. J Mol Biol. 1973 Jan 10;73(2):199–212. doi: 10.1016/0022-2836(73)90323-9. [DOI] [PubMed] [Google Scholar]
- Lee A. S., Sinsheimer R. L. A continuous electroelution method for the recovery of DNA restriction enzyme fragments. Anal Biochem. 1974 Aug;60(2):640–644. doi: 10.1016/0003-2697(74)90279-6. [DOI] [PubMed] [Google Scholar]
- Peacock A. C., Dingman C. W. Molecular weight estimation and separation of ribonucleic acid by electrophoresis in agarose-acrylamide composite gels. Biochemistry. 1968 Feb;7(2):668–674. doi: 10.1021/bi00842a023. [DOI] [PubMed] [Google Scholar]
- STUDIER F. W. SEDIMENTATION STUDIES OF THE SIZE AND SHAPE OF DNA. J Mol Biol. 1965 Feb;11:373–390. doi: 10.1016/s0022-2836(65)80064-x. [DOI] [PubMed] [Google Scholar]
- Schmeckpeper B. J., Smith K. D. Use of formamide in nucleic acid reassociation. Biochemistry. 1972 Mar 28;11(7):1319–1326. doi: 10.1021/bi00757a032. [DOI] [PubMed] [Google Scholar]
- Wetmur J. G., Davidson N. Kinetics of renaturation of DNA. J Mol Biol. 1968 Feb 14;31(3):349–370. doi: 10.1016/0022-2836(68)90414-2. [DOI] [PubMed] [Google Scholar]




