Abstract
Accumulating evidence indicates that glaucoma is a multifactorial neurodegenerative disease characterized by the loss of retinal ganglion cells (RGC), resulting in gradual and progressive permanent loss of vision. Reducing intraocular pressure (IOP) remains the only proven method for preventing and delaying the progression of glaucomatous visual impairment. However, the specific role of IOP in optic nerve injury remains controversial, and little is known about the biomechanical mechanism by which elevated IOP leads to the loss of RGC. Published studies suggest that the biomechanical properties of the sclera and scleral lamina cribrosa determine the biomechanical changes of optic nerve head, and play an important role in the pathologic process of loss of RGC and optic nerve damage. This review focuses on the current understanding of biomechanics of sclera in glaucoma and provides an overview of the possible interactions between the sclera and IOP. Treatments and interventions aimed at the sclera are also discussed.
Keywords: biomechanics, glaucoma, sclera, intraocular pressure, finite element models
INTRODUCTION
Glaucoma is the foremost cause of irreversible blindness, affecting more than 70 million people around the globe[1]. Accumulating evidence reveals that glaucoma is a multifactorial neurodegenerative disease resulting from the loss of retinal ganglion cells (RGC) and from damage to the optic nerve. Both defects ultimately result in progressive permanent vision loss.
The main pathological factor of most glaucoma is elevated intraocular pressure (IOP), and reducing IOP continuously and effectively remains the only proven method for preventing and delaying the progression of glaucomatous visual impairment[2]. However, normotensive glaucoma also exists and there are significant numbers patients with classical glaucoma who have statistically normal levels of IOPs. In these patients, irreversible sustained injury of the optic nerve, gradual narrowing of the visual field and progressive loss of visual function persist even though the IOP is normal or below normal levels. These findings suggest that biomechanical factor involved in the pathogenesis of glaucoma cannot be neglected [3]–[6]. In depth knowledge to the biomechanics mechanism contributing to glaucomatous damage may ultimately lead to early detection, early diagnosis and better treatment. There has been ongoing research investigating the mechanical properties of pupillary blocking force, the involvement of the iris, and mechanisms of the aqueous humor flow, as well as studies evaluating morphological changes in the anterior chamber[7]–[10]. In recent years, research has suggested that the biomechanical properties of the sclera and scleral lamina cribrosa (LC) determine biomechanical changes of the optic nerve head (ONH)[11]–[12], thus playing an important role in the pathologic process of the loss of RGC and contributing to optic nerve damage[13]–[16].
SCLERA AS A BIOMECHANICAL STRUCTURE
Biomechanics is a newly developed interdisciplinary subject which applies mechanical principles and technology to biological systems. Biologic soft tissues such as the sclera are viscoelastic materials, and so exhibit the features of relaxation, creep and hysteresis[17]. The structure of sclera means that its geometry, material properties, and structural stiffness responds dynamically to the mechanical behavior of the surrounding tissues.
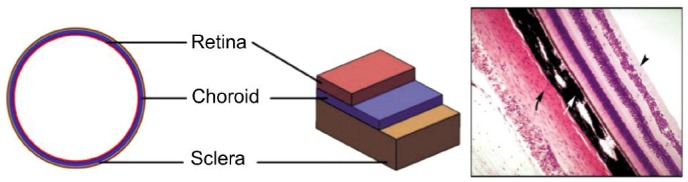
The eyeball is nearly spherical, the anterior part of the wall of the eyeball wall forms the cornea, and the posterior part consists of three layers: the sclera, choroid and retina[18]. Among these three tissues, the sclera is the toughest, and the retina is the softest. The thicknesses of these three layers are not constant throughout the whole surface[19]. The most inner layer, the retina, is only present in 72% of the orbit, more precisely in the distal part of the eyeball (Figure 1). Thus when subjected to the same stress, the tangent modulus of the retina, choroid and sclera are an order of magnitude higher than its each other. Thus the sclera plays a crucial role in maintaining the shape of eyeball.
Figure 1. Three layers of the eyeball, schematic diagram and tissue slices figure.
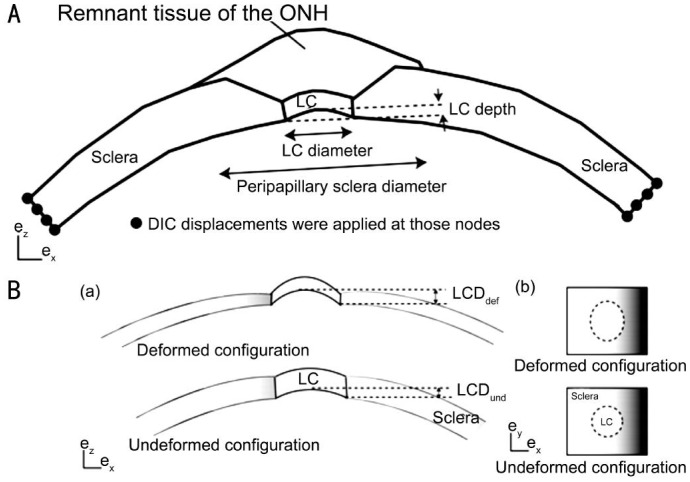
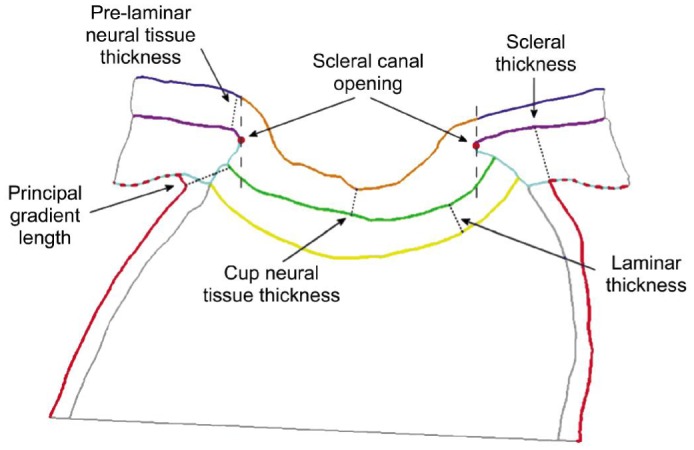
The sclera is the fibrous outer protective coating of the eye, consisting almost entirely of dense bands of parallel and interlacing collagen. It is a tough, white tissue that is continuous with the cornea anteriorly and with the dural sheath of the optic nerve posteriorly. The posterior sclera is divided into two layers where it meets the optic nerve[20]–[21]. The outer two-thirds forms the scleral canal which transitions to the optic sheath, and the inner third forms the LC which is an irregular mesh-like structure through which the optic nerves traverse the eyeball (Figure 2). The mechanical load to the sclera is mainly derived from IOP-related stress and strain.
Figure 2. Definitions of the thicknesses and distances measured from the specimen-specific models.
The normal human sclera is composed from the outside to the inside of episclera, scleral stroma and lamina fascia. The episclera contains an irregular arrangement of interwoven small collagen fibers. The scleral stroma is composed of dense collagen fiber bundles containing many collagenous fibrils which lie parallel to each other on the outer surface, and which interlace and fuse together on the inner surface. The lamina fascia is composed of smaller collagen fiber bundles. Thus collagen plays a major role in maintaining the structure, function and the biomechanical properties of the sclera[22].
BIOMECHANICAL PROPERTIES OF SCLERA
Ophthalmologists and biomechanics researchers have adopted a series of computer modeling and experimental studies to research the biomechanical properties of sclera. As far back as 1969, it was demonstrated that under the same stress, the anterior sclera underwent the least deformation and the posterior sclera the maximum deformation with the peripheral sclera being intermediate[23]. The following year a preliminary exploration of the biomechanical properties of sclera was conducted using a static uniaxial test.
Later research used finite element models (FEMs) to study the biomechanical characteristics of human sclera[24]. This enabled the stress-strain relationships within the sclera to be calculated by applying an axial force and measuring the change in length, cross-sectional area and the load. These experiments indicated that the sclera was anisotropic and viscoelastic. Other researchers showed that the stress borne by ocular tissues was determined by the 3D geometrical shape of the tissue and it was found that IOP-generated stress and the eye's corresponding response took effect in different regions of the sclera[25].
Experiments conducted in rabbits and monkeys reported no significant differences in the stress-strain curves for 0-10% strain between the four quadrants surrounding the optic nerve for monkey sclera, however, differences were detected in the quadrants of rabbit sclera at strains below 4%. Analogical other research suggested that sclera can be stored up to 3d without risking mechanical deterioration[26]. This series of experiments and earlier experiments both had an analogous defect and inaccuracy resulting from the slow strain rate (1%/s) that was used and also from the use of uniaxial loading mode.
In 2009, the defects of traditional experimental design were overcome by the use of a high-rated pressurization system (hydraulic system). A study of the dynamic changes in the sclera of 12 excised human eyes determined the relationship between true stress and true strain in the sclera tissue in both equatorial and meridional directions, by measuring the internal pressure, the diameter of the globe, the thickness of the sclera, and the changing coordinates of optical markers[27]. Based on these measurements it was concluded that the human sclera has a higher degree of stiffness in the equatorial direction than in the meridional direction. This implies that it has stronger resistance to deformation in equatorial direction.
An additional study measured the age-related and glaucoma-related changes in isolated posterior sclera from normal and glaucomatous human eyes subjected to time-dependent and rate-dependent inflation tests[28]. The normal and glaucoma specimens both exhibited a similar regional variation in thickness, but there was a 15% decrease in thickness in normal eyes from subjects aged between 40 and 90y in normal eyes, and there was a threefold increase in stiffness between the ages of 40 and 80y. Similar results were obtained from a study of biomechanical properties in monkey eyes[15]. However the study in isolated human eyes[28], showed that at the same stress level, circumferential and meridional strain was larger in the peripapillary sclera than in other regions. The largest deformation occurred in the nasal-inferior quadrant. The scleral tissues showed a nonlinear, rate-dependent, and time-dependent biomechanical response to the inflation testing, showing creep at constant pressure and hysteresis upon unloading. The presence of heterogeneous collagen fiber organization in the peripapillary sclera appears effective in limiting LC strain and it may reduce strain levels at the scleral canal boundary, which a transition zone prone to LC disinsertion, focal LC defects in glaucoma[29].
Based on results of correlated computer modeling and laboratory research, academia has drawn the conclusion that sclera strain originating from IOP-related stress is not only dependent on the different geometry and material properties of scleral collagens in different regions, but is also influenced by the objective conditions of IOP.
Biomechanical Response of Sclera to Elevated Intraocular Pressure
Studies based on optical tracking of pressurized shells[12],[27],[30] and uniaxial testing of scleral strips have made important contributions to our understanding of scleral biomechanics[31]–[32]. It has been shown that the sclera undergoes IOP-related deformation and corresponding mechanical responses in early experimental glaucoma and that these responses are transmitted to the ONH, ultimately resulting in the development or deterioration of glaucoma.
In one set of experiments, monkey scleral shells were mounted on a custom-built pressurization apparatus, and electronic speckle pattern interferometry was introduced to measure the 3-D displacements, the geometry and the thickness of the posterior sclera exposed to acute IOP elevations of 5 to 45 mm Hg[33]. An inverse finite element method was used to determine the tangent modulus and structural stiffness of the sclera. The results showed that the displacement magnitude in all directions was lowest as IOP increased from 30 to 45 mm Hg and greatest as IOP increased from 5 to 10 mm Hg. Compliance was also best in this interval of IOP. There was a high degree of nonlinearity in the tangent modulus and structural stiffness as IOP increased from 5 to 45 mm Hg and the mean tangent modulus was 3- to 6-times higher at 45 mm Hg than at 10 mm Hg in all regions. However, there was an inverse relationship between scleral tangent modulus and scleral thickness in all regions as IOP increased. Structural stiffness and thickness was found to be higher in the peripapillary sclera. From these results it was concluded that the posterior sclera adopts heterogeneous, anisotropic, and nonlinear behavior when exposed to acute elevations of IOP, causing it to stiffen dramatically when IOP increases beyond 30 mm Hg. IT was postulated that the increased structural stiffness in the peripapillary sclera might be a protective mechanism that minimizes deformations in the vicinity of the ONH.
These results were confirmed by a later study[15], showing that the posterior sclera stiffened as a result of extracellular matrix (ECM) remodeling at high levels of cumulative IOP insult. It was also demonstrated that the increase in tangent modulus at elevated IOPs was a consequence of collagen and elastin remodeling. It was reported that a stiffer scleral shell is biomechanically beneficial as it is less prone to deformation and remodeling in response to increased IOP[15].
This observation coincided with the results from several studies showing that[34]–[35], mechanical strain applied to scleral fibroblasts activates the release of matrix metalloproteinases (MMPs) and tissue inhibitors of metalloproteinase, resulting on reconstruction of the scleral ECM. The results show that IOP-generated scleral shell expansion increases scleral strain in all eyes, but in eyes with the stiffest sclera, these increases may be too small to initiate a fibroblast biomechanical response. Surprisingly, this hypothesis seems to contradict the fact that the sclera stiffens with age and the elderly are more vulnerable to glaucoma[20],[31]. To reconcile these findings, it should be noted that collagen fibers become fragile with age and the mechanism scleral stiffening with age is different to that of scleral stiffening induced by experimental remodeling.
A study of isolated posterior sclera reported the occurrence of pressure-controlled inflation[28]. The results showed that the mean thickness of peripapillary sclera in normal and glaucoma specimens was on average 100 µm thicker than that of mid-posterior scleral, and the damaged glaucoma specimens displayed an appreciably thicker sclera. It was also demonstrated that there was no difference in the mid-posterior region, in terms of stress-strain response and fiber stiffness of the sclera, but in the peripapillary region, glaucoma specimens showed a smaller strain ratio than normal specimens, and the meridional strain was lower still.
Based on previous findings in animal experiments, it was reasoned that biomechanical response of sclera to elevated IOP is predominantly centered in the tissue immediately surrounding the ONH[28]. IOP-generated stress in the sclera is inversely related to the thickness of the sclera, and scleral thickening in a damaged glaucomatous eye might be a protective mechanism to decrease the IOP-generated stresses. It is also possible that different creep mechanisms are activated at different stress levels resulting from IOP[36]. When IOP initially increases, the displacement caused by the increased stress is thought to be regulated by the uncrimping of collagen fibers. However, as IOP continues to increase, further displacement may be caused by stretching the collagen fibrils. In undamaged glaucoma specimens, the slower creep rates that are observed in the peripapillary sclera are consistent with the speculated deposition of fibrillar components, which in turn generate a denser network and prevent fibril mobility.
Influence of Sclera to Optic Nerve Head Biomechanics
From a biomechanical perspective, the eyeball is a vessel with inflow and outflow mechanisms that control its internal pressure. IOP applies the normal pressure load to the inner surface of the ocular wall, engendering an in-wall circumferential stress defined as the hoop stress. Most of this IOP-induced stress is borne by the stiff, collagenous sclera. The more compliant retina and nerve fiber tissues can only bear little of this load and are therefore the first to collapse when IOP increased abnormally[37]. Evidence from previous studies showed that strains of 5%-8% induce a wide range of biological effects in neural cells[38]–[39]. In the ONH, IOP is borne primarily by the fenestrated connective tissues of the LC, and the response is influenced by the geometry and degree of scleral canal expansion[40]. Studies have shown that scleral biomechanics are closely related to ONH biomechanics. The material properties of the sclera, especially its structural stiffness, directly affects the biomechanical stimulus and the severity of injury to RGC and optic nerve astrocytes that results from elevated IOP[41].
It was originally thought that the direct effects of elevated IOP pushed the LC posteriorly, without causing a significant degree of scleral deformation[4],[12]. However, data from the majority of numerical and experimental modeling studies show that as IOP increases, the sclera deforms, and these deformations when transmitted to the ONH often have a significant influence on the displacement of the LC[33],[42]–[43]. In these models, lateral deformation transmitted to the LC by the sclera may be even larger than the posterior deformations induced directly by the elevated IOP on the LC. This has been confirmed by studies using 3D histomorphometry and OCT[25],[32],[44]. Some experts now agree that LC by itself does not undergo biomechanical change in response to elevated IOP, but rather the sclera, LC and ONH behave as a complete biomechanical system which responds to increased IOP.
The process can be visualized by imagining the effect of attaching a solid piece of metal (the sclera) to an elastic sheet (the LC). If the metal is pulled, the elastic sheet has to follow. This means that displacements of the LC and neural tissue depend mainly on how much the sclera deforms[11]. The direct effect of elevated IOP is to cause scleral mechanical displacement, which is transmitted to the scleral canal causing it to expand. This causes the LC to become taut and thin. As the IOP continues to increase, the LC is markedly displaced posteriorly and ultimately becomes cupped. In vitro studies using fluorescein labeling and confocal scanning laser tomography showed that the volume and surface tension of LC of the human eye increases in response to an increase in pressure (Figure 3)[45]. In experimental glaucoma, lasting posterior deformation of LC results in early damage to the load-bearing connective tissue of the ONH. This in turn makes the ONH increasingly sensitive to any level of IOP insult[40].
Figure 3. The finite element model of the posterior scleral and LC.
A: Schematic of the finite element model for the posterior scleral cup with the peripapillary sclera and the optic nerve head. The peripapillary sclera thins over a distance equal to the LC diameter; B: a) Schematic representation of the LC and the sclera in the (ex, ez) plane, showing the LCP; b) Schematic representation of the LC and sclera in the (ex, ey) plane.
Studies measuring LC thickness and position, scleral canal geometry and eccentricity in normal and early glaucoma monkey eyes have been undertaken in an attempt to further understand the biomechanical response of scleral canal and LC to elevated IOP. In this study, when the IOP increased from 0 mm Hg to 10 mm Hg, the peripapillary sclera displaced posteriorly, the scleral canal expanded and causing the LC to become taut and thin. However, when the IOP increased from 10 to 30 mm Hg, the LC became deformed and then remodeled into a deep cupped structure. These finding have been reproduced by other researchers[42]. A later study concluded that the relationship between LC deformation and scleral canal expansion also depended on the structural stiffness and thickness of sclera (Figure 4). The corneoscleral shell of enucleated human donor eyes was investigated by means of FEM[46], the results indicated that the scleral geometry, especially the thickness of the posterior sclera, significantly influenced the biomechanical response of ONH to IOP-related stress. It was shown that a thinner posterior sclera deformed more easily and to a greater extent to a given strain, and that scleral canal expansion and the LC deformation would be larger than seen with a thicker sclera. This would lead to a higher biomechanical load in the ONH. Other workers[47], demonstrated that following an acute elevation in IOP, the stiffness of the sclera scleral samples taken from normal eyes of human donors (defined as selected as “compliant”, “median” and “stiff”) dramatically influenced the biomechanics of ONH[48]. The results suggested that a compliant sclera underwent a much greater strain than a stiff sclera in all regions (including the corneoscleral shell, the peripheral sclera, the peripapillary sclera and the LC), whether at a normal IOP (15 mm Hg) or an acutely elevated IOP (50 mm Hg). The researchers commented that individuals with diseases that weaken connective tissues (e.g. Marfan's syndrome, Ehlers-Danlos syndrome, orthogenesis imperfecta), might have weakened scleral collagen and might predict high risk of IOP-induced deformation at the ONH, exposing them to high risk of glaucomatous optic neuropathy.
Figure 4. Schematic of finite element for peripapillary sclera in different condition.
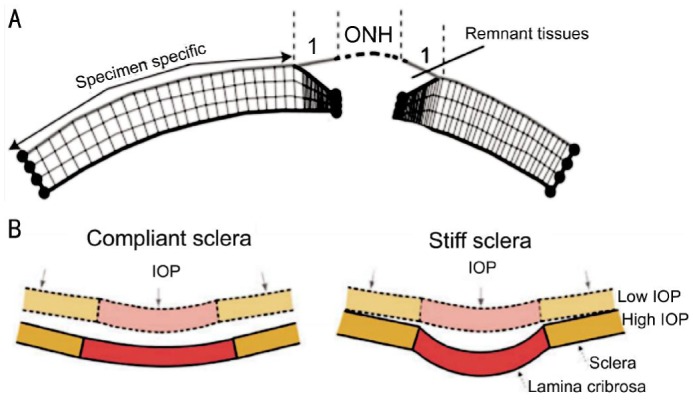
A: Schematic of the transverse view of the finite element mesh showing the thickness profile in the generic model of the peripapillary sclera; B: IOP induces large scleral canal expansions in eyes with compliant sclera (left) that pulls the contained lamina taut despite the direct posterior force of IOP on the laminar surface. Conversely, a stiff sclera allows relatively little canal expansion with IOP elevation (right) and less stretching of the contained lamina, thus allowing the lamina to be displaced posteriorly by the direct action of IOP on its anterior surface.
AIM OF TREATMENTS AND INTERVENTIONS FOR SCLERA
Some research represent that biomechanical behavior of sclera influences the physiology and pathophysiology of the optic nerve head, and the biomechanical theory of optic nerve may help to explain how certain eyes, suffering high IOP, are predisposed to the development of glaucomatous optic neuropathy[3]. The biomechanical behavior of scleral and LC may be a new way to manipulate optic neuropathy serve as a potential therapeutic target. It is possible to strengthen and stiffen the sclera using collagen cross-linking techniques in vivo in animals, which undoubtedly have the potential to stiffen the sclera. Other research indicated that sulphated-glycosaminoglycan were found to represent on average only 0.6% of the dry weight of the porcine posterior sclera. Buffer-treatment significantly changed the scleral mechanical behavior leading to an increase in low-pressure stiffness, hysteresis, and creep rate, whereas a decrease in high-pressure stiffness[49]. These findings represent a significant effect of sulphated-glycosaminoglycan on both the stiffness and time-dependent behavior of the sclera, and the biomechanical characters of sclera in glaucoma eyes, such as creep or stiffness, may be influenced with the alterations in s-GAG content.
One specific experiment operated by Kimball et al[50] demonstrated that glyceraldehyde-treated eyes had greater RGC axon loss from elevated IOP by alter of scleral composition. Glyceraldehyde treatment increased scleral cross-linking, decreased scleral permeability, and increased scleral stiffness. It can produced a steeper pressure-strain behavior. The results of this research indicated that the characters of sclera such as cross-linking, permeability and stiffness play very important role in development of glaucoma. The dynamic responses of sclera may present therapeutic approaches that could be additive to IOP lowering.
LIMITATIONS OF PRESENT STUDIES
The design of experimental device in present kinds of experiment are various, some of which have their own specialty[51]. But maybe some of designs have some defects. Such as impermeability of some device may not achieve the desired results, some devices cannot recreate real physiological status of eyeball. Biomechanical study, as an implement to predict pathogenesis of glaucoma, is a long-term process. To make the consequence comparably, it is better to unify the equipment design. Similarly, the choice of biomechanical formula should be unified in order to prevent that variously miscellaneous formula may disturb uniformity of result.
There are some shortages in the results from many experimental groups. In order to use those kinds of result in next step of experiment or in clinical guidance, uniform experimental parameters are needed. We should choose the same material models (constitutive model), having similar characters with human sclera, to obtain analogical computer modeling. We also need find out the best appropriate finite element analysis method. By comparison and research of several FEMs, a finite element model should be established for the sclera and ONH structures. It is necessary to uniform the understanding with the concept of material properties about sclera and LC, which influence remarkably the selection of computational formula about stress, strain and elasticity modulus, etc.
Some biomechanical researches are based on extracorporeal experimental study, especially the biomechanical properties of sclera. It is hard to judge the facticity to real condition using in finite element research. So the verification by inverse analysis seems to be crucial. By studying and summarizing a large number of predecessors' researches. In our own research project, the research team improved device design of biomechanics research, which makes it more semblable to the eyeball physiological state by making sure leakproofness, controlling the change of IOP more precisely. We also improve the precision of biomechanical data of scleral tissue through multi-direction measurements. With the inverse analysis, applying effectiveness is close to the real situation and the results could be applied to future clinical work much better.
Implications and Directions
The important factors influencing scleral biomechanics include the density and arrangement of collagen fibers in each region (Figure 5), the sclera geometry, the rate of change in IOP, and the duration of IOP-related stress. Most experts agree that the biomechanical properties of sclera influence the biomechanics of the ONH, and the particular scleral properties in different individuals may be the critical risk factor leading to glaucomatous optic nerve damage[45]. Until now, there no specific scientific technique to predict accurately what level of IOP will result in mechanical scleral deformation and failure, resulting in loss of RGC and the damage to the optic nerve.
Figure 5. Regional differences in laminar microarchitecture in a normal eye, and the predicted relationship between regional laminar density and strain.
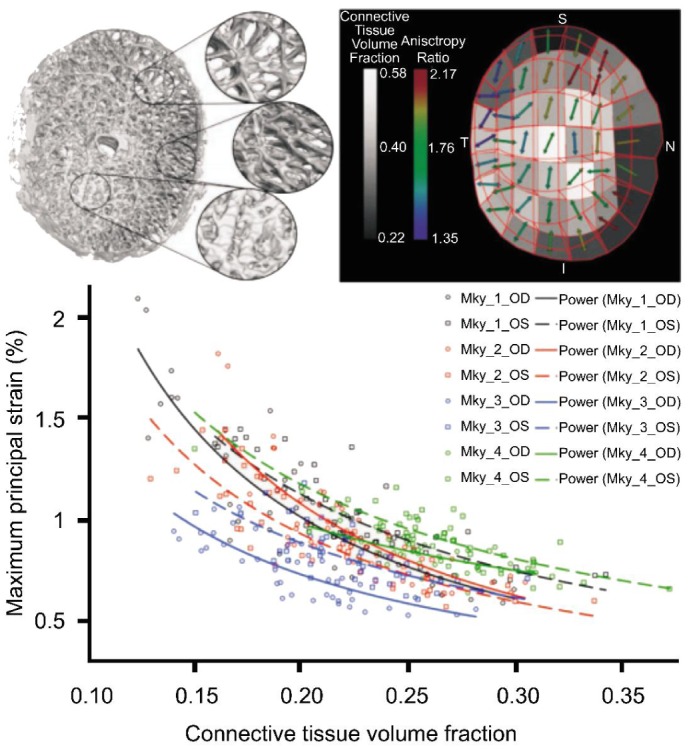
Finite element modeling is a computational tool for predicting how a complex biological tissue will behave under varying levels of load[46]. Finite element modeling in monkey and human cadaver eyes has been used to study the mechanical response of the sclera to different levels of IOP. These experiments have sought to identify the most important factor governing the ability of a given sclera and ONH to maintain structural integrity, nutritional homeostasis and axoplasmic transport at physiologic and pathologic levels of IOP[41].
In practice the IOP experiences acute, short-term or long-term fluctuations resulting from blinks, eye rubbing and circadian rhythms. The material properties and the geometry of sclera change with age and with various pathological factors that may or may not be related to the IOP. In order to increase the accuracy of research and getting more visual results, some experimental devices or technologies used on other ocular researches may apply to the biomechanical analysis of sclera and computational modeling. The recent use of non-invasive imaging of the sclera, including polarization-sensitive optical coherence tomography[52] and magic angle-enhanced magnetic resonance imaging, can reveal the structural details of the scleral shell and their changes upon IOP elevation[53]. We can also utilize the non-invasive imaging of whole globe[54]–[55] and the brain's visual system[56] for systematic and longitudinal evaluation of the interactions between IOP loading, ocular dynamics, and the resulting RGC loss and visual pathway damages in experimental animal models and possibly humans as parts of future directions[57].
The collagen and elastin fibers of sclera are highly anisotropic[58], and the microstructure of ECM is extremely complex[59]. Even more difficulty in this area of research is related the fact that gene mutations also involve scleral biomechanical properties[60]. It is expected that future biomechanical models of sclera and ONH can be undertaken during ocular examination. This will identify the changes in IOP that are related to the intracellular environment of the optic nerve, the mechanical strain of the sclera and the mechanical deformation of LC and ONH. If successful, the ophthalmologists will be able to accurately predict the physiological and pathological IOP for different individuals, and eventually evaluate a safe target IOP for different glaucoma patients. These advances will aid the development of newer treatments and interventions aimed at the sclera of glaucomatous patients and will open a new chapter for glaucoma research.
Acknowledgments
Foundation: Supported by National Natural Science Foundation of China (No.81370913).
Conflicts of Interest: Jia X, None; Yu J, None; Liao SH, None; Duan XC, None.
REFERENCES
- 1.Quigley HA, Broman AT. The number of people with glaucoma worldwide in 2010 and 2020. Br J Ophthalmol. 2006;90(3):262–267. doi: 10.1136/bjo.2005.081224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Fujino Y, Asaoka R, Murata H, Miki A, Tanito M, Mizoue S, Mori K, Suzuki K, Yamashita T, Kashiwagi K, Shoji N, Japanese Archive of Multicentral Databases in Glaucoma Construction G Evaluation of glaucoma progression in large-scale clinical data: the Japanese archive of multicentral databases in glaucoma (JAMDIG) Invest Ophthalmol Vis Sci. 2016;57(4):2012–2020. doi: 10.1167/iovs.15-19046. [DOI] [PubMed] [Google Scholar]
- 3.Strouthidis NG, Girard MJ. Altering the way the optic nerve head responds to intraocular pressure-a potential approach to glaucoma therapy. Curr Opin Pharmacol. 2013;13(1):83–89. doi: 10.1016/j.coph.2012.09.001. [DOI] [PubMed] [Google Scholar]
- 4.Sigal IA, Ethier CR. Biomechanics of the optic nerve head. Exp Eye Res. 2009;88(4):799–807. doi: 10.1016/j.exer.2009.02.003. [DOI] [PubMed] [Google Scholar]
- 5.Roberts MD, Sigal IA, Liang Y, Burgoyne CF, Downs JC. Changes in the biomechanical response of the optic nerve head in early experimental glaucoma. Invest Ophthalmol Vis Sci. 2010;51(11):5675–5684. doi: 10.1167/iovs.10-5411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Roberts MD, Liang Y, Sigal IA, Grimm J, Reynaud J, Bellezza A, Burgoyne CF, Downs JC. Correlation between local stress and strain and lamina cribrosa connective tissue volume fraction in normal monkey eyes. Invest Ophthalmol Vis Sci. 2010;51(1):295–307. doi: 10.1167/iovs.09-4016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Zheng C, Cheung CY, Aung T, Narayanaswamy A, Ong SH, Friedman DS, Allen JC, Baskaran M, Chew PT, Perera SA. In vivo analysis of vectors involved in pupil constriction in Chinese subjects with angle closure. Invest Ophthalmol Vis Sci. 2012;53(11):6756–6762. doi: 10.1167/iovs.12-10415. [DOI] [PubMed] [Google Scholar]
- 8.Whitcomb JE, Amini R, Simha NK, Barocas VH. Anterior-posterior asymmetry in iris mechanics measured by indentation. Exp Eye Res. 2011;93(4):475–481. doi: 10.1016/j.exer.2011.06.009. [DOI] [PubMed] [Google Scholar]
- 9.Rasmussen CA, Gabelt BT, Kaufman PL. Aqueous humor dynamics in monkeys in response to the kappa opioid agonist bremazocine. Trans Am Ophthalmol Soc. 2007;105 [PMC free article] [PubMed] [Google Scholar]
- 10.Zhang K, Qian X, Mei X, Liu Z. An inverse method to determine the mechanical properties of the iris in vivo. Biomed Eng Online. 2014;13:66. doi: 10.1186/1475-925X-13-66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Ethier CR. Scleral biomechanics and glaucoma-a connection? Can J Ophthalmol. 2006;41(1):9–12,14. doi: 10.1016/S0008-4182(06)80060-8. [DOI] [PubMed] [Google Scholar]
- 12.Burgoyne CF, Downs JC, Bellezza AJ, Suh JK, Hart RT. The optic nerve head as a biomechanical structure: a new paradigm for understanding the role of IOP-related stress and strain in the pathophysiology of glaucomatous optic nerve head damage. Prog Retin Eye Res. 2005;24(1):39–73. doi: 10.1016/j.preteyeres.2004.06.001. [DOI] [PubMed] [Google Scholar]
- 13.Yang H, Ren R, Lockwood H, Williams G, Libertiaux V, Downs C, Gardiner SK, Burgoyne CF. The connective tissue components of optic nerve head cupping in monkey experimental glaucoma part 1: global change. Invest Ophthalmol Vis Sci. 2015;56(13):7661–7678. doi: 10.1167/iovs.15-17624. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Girard MJ, Suh JK, Bottlang M, Burgoyne CF, Downs JC. Scleral biomechanics in the aging monkey eye. Invest Ophthalmol Vis Sci. 2009;50(11):5226–5237. doi: 10.1167/iovs.08-3363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Girard MJ, Suh JK, Bottlang M, Burgoyne CF, Downs JC. Biomechanical changes in the sclera of monkey eyes exposed to chronic IOP elevations. Invest Ophthalmol Vis Sci. 2011;52(8):5656–5669. doi: 10.1167/iovs.10-6927. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Grytz R, Meschke G, Jonas JB. The collagen fibril architecture in the lamina cribrosa and peripapillary sclera predicted by a computational remodeling approach. Biomech Model Mechanobiol. 2011;10(3):371–382. doi: 10.1007/s10237-010-0240-8. [DOI] [PubMed] [Google Scholar]
- 17.Valero C, Navarro B, Navajas D, Garcia-Aznar JM. Finite element simulation for the mechanical characterization of soft biological materials by atomic force microscopy. J Mech Behav Biomed Mater. 2016;62:222–235. doi: 10.1016/j.jmbbm.2016.05.006. [DOI] [PubMed] [Google Scholar]
- 18.Yoshida M, Yamazaki J, Mizunuma H. A finite element analysis of the retinal hemorrhages accompanied by shaken baby syndrome/abusive head trauma. J Biomech. 2014;47(14):3454–3458. doi: 10.1016/j.jbiomech.2014.09.014. [DOI] [PubMed] [Google Scholar]
- 19.Wu J, Nasseri MA, Eder M, Azqueta Gavaldon M, Lohmann CP, Knoll A. The 3D eyeball fea model with needle rotation. APCBEE Procedia. 2013;7(2013):4–10. [Google Scholar]
- 20.Downs JC. Optic nerve head biomechanics in aging and disease. Exp Eye Res. 2015;133:19–29. doi: 10.1016/j.exer.2015.02.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Sigal IA, Flanagan JG, Tertinegg I, Ethier CR. 3D morphometry of the human optic nerve head. Exp Eye Res. 2010;90(1):70–80. doi: 10.1016/j.exer.2009.09.013. [DOI] [PubMed] [Google Scholar]
- 22.Coudrillier B, Pijanka J, Jefferys J, Sorensen T, Quigley HA, Boote C, Nguyen TD. Collagen structure and mechanical properties of the human sclera: analysis for the effects of age. J Biomech Eng. 2015;137(4):041006. doi: 10.1115/1.4029430. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Curtin BJ. Physiopathologic aspects of scleral stress-strain. Trans Am Ophthalmol Soc. 1969;67:417–461. [PMC free article] [PubMed] [Google Scholar]
- 24.Al-Sukhun J, Lindqvist C, Kontio R. Modelling of orbital deformation using finite-element analysis. J R Soc Interface. 2006;3(7):255–262. doi: 10.1098/rsif.2005.0084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Yang H, Downs JC, Sigal IA, Roberts MD, Thompson H, Burgoyne CF. Deformation of the normal monkey optic nerve head connective tissue after acute IOP elevation within 3-D histomorphometric reconstructions. Invest Ophthalmol Vis Sci. 2009;50(12):5785–5799. doi: 10.1167/iovs.09-3410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Girard M, Suh JK, Hart RT, Burgoyne CF, Downs JC. Effects of storage time on the mechanical properties of rabbit peripapillary sclera after enucleation. Curr Eye Res. 2007;32(5):465–470. doi: 10.1080/02713680701273792. [DOI] [PubMed] [Google Scholar]
- 27.Bisplinghoff JA, McNally C, Manoogian SJ, Duma SM. Dynamic material properties of the human sclera. J Biomech. 2009;42(10):1493–1497. doi: 10.1016/j.jbiomech.2009.03.043. [DOI] [PubMed] [Google Scholar]
- 28.Coudrillier B, Tian J, Alexander S, Myers KM, Quigley HA, Nguyen TD. Biomechanics of the human posterior sclera: age-and glaucoma-related changes measured using inflation testing. Invest Ophthalmol Vis Sci. 2012;53(4):1714–1728. doi: 10.1167/iovs.11-8009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Zhang L, Albon J, Jones H, Gouget CL, Ethier CR, Goh JC, Girard MJ. Collagen microstructural factors influencing optic nerve head biomechanics. Invest Ophthalmol Vis Sci. 2015;56(3):2031–2042. doi: 10.1167/iovs.14-15734. [DOI] [PubMed] [Google Scholar]
- 30.Pierscionek BK, Asejczyk-Widlicka M, Schachar RA. The effect of changing intraocular pressure on the corneal and scleral curvatures in the fresh porcine eye. Br J Ophthalmol. 2007;91(6):801–803. doi: 10.1136/bjo.2006.110221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Schultz DS, Lotz JC, Lee SM, Trinidad ML, Stewart JM. Structural factors that mediate scleral stiffness. Invest Ophthalmol Vis Sci. 2008;49(10):4232–4236. doi: 10.1167/iovs.08-1970. [DOI] [PubMed] [Google Scholar]
- 32.Yang H, Downs JC, Girkin C, Sakata L, Bellezza A, Thompson H, Burgoyne CF. 3-D histomorphometry of the normal and early glaucomatous monkey optic nerve head: lamina cribrosa and peripapillary scleral position and thickness. Invest Ophthalmol Vis Sci. 2007;48(10):4597–4607. doi: 10.1167/iovs.07-0349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Girard MJ, Downs JC, Bottlang M, Burgoyne CF, Suh JK. Peripapillary and posterior scleral mechanics-part II: experimental and inverse finite element characterization. J Biomech Eng. 2009;131(5):051012. doi: 10.1115/1.3113683. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Shelton L, Rada JS. Effects of cyclic mechanical stretch on extracellular matrix synthesis by human scleral fibroblasts. Exp Eye Res. 2007;84(2):314–322. doi: 10.1016/j.exer.2006.10.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Wang G, Chen W. Effects of mechanical stimulation on viscoelasticity of rabbit scleral fibroblasts after posterior scleral reinforcement. Exp Biol Med (Maywood) 2012;237(10):1150–1154. doi: 10.1258/ebm.2012.012196. [DOI] [PubMed] [Google Scholar]
- 36.Nguyen TD, Jones RE, Boyce BL. A nonlinear anisotropic viscoelastic model for the tensile behavior of the corneal stroma. J Biomech Eng. 2008;130(4):041020. doi: 10.1115/1.2947399. [DOI] [PubMed] [Google Scholar]
- 37.Downs JC, Roberts MD, Burgoyne CF. Mechanical environment of the optic nerve head in glaucoma. Optom Vis Sci. 2008;85(6):425–435. doi: 10.1097/OPX.0b013e31817841cb. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Patton DA, McIntosh AS, Kleiven S. The biomechanical determinants of concussion: finite element simulations to investigate tissue-level predictors of injury during sporting impacts to the unprotected head. J Appl Biomech. 2015;31(4):264–268. doi: 10.1123/jab.2014-0223. [DOI] [PubMed] [Google Scholar]
- 39.Patton DA, McIntosh AS, Kleiven S. The biomechanical determinants of concussion: finite element simulations to investigate brain tissue deformations during sporting impacts to the unprotected head. J Appl Biomech. 2013;29(6):721–730. doi: 10.1123/jab.29.6.721. [DOI] [PubMed] [Google Scholar]
- 40.Sigal IA, Yang H, Roberts MD, Grimm JL, Burgoyne CF, Demirel S, Downs JC. IOP-induced lamina cribrosa deformation and scleral canal expansion: independent or related? Invest Ophthalmol Vis Sci. 2011;52(12):9023–9032. doi: 10.1167/iovs.11-8183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Coudrillier B, Pijanka JK, Jefferys JL, Goel A, Quigley HA, Boote C, Nguyen TD. Glaucoma-related changes in the mechanical properties and collagen micro-architecture of the human sclera. PLoS One. 2015;10(7):e0131396. doi: 10.1371/journal.pone.0131396. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Sigal IA. Interactions between geometry and mechanical properties on the optic nerve head. Invest Ophthalmol Vis Sci. 2009;50(6):2785–2795. doi: 10.1167/iovs.08-3095. [DOI] [PubMed] [Google Scholar]
- 43.Sigal IA, Flanagan JG, Tertinegg I, Ethier CR. Modeling individual-specific human optic nerve head biomechanics. Part II: influence of material properties. Biomech Model Mechanobiol. 2009;8(2):99–109. doi: 10.1007/s10237-008-0119-0. [DOI] [PubMed] [Google Scholar]
- 44.Agoumi Y, Sharpe GP, Hutchison DM, Nicolela MT, Artes PH, Chauhan BC. Laminar and prelaminar tissue displacement during intraocular pressure elevation in glaucoma patients and healthy controls. Ophthalmology. 2011;118(1):52–59. doi: 10.1016/j.ophtha.2010.05.016. [DOI] [PubMed] [Google Scholar]
- 45.Coudrillier B, Boote C, Quigley HA, Nguyen TD. Scleral anisotropy and its effects on the mechanical response of the optic nerve head. Biomech Model Mechanobiol. 2013;12(5):941–963. doi: 10.1007/s10237-012-0455-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Norman RE, Flanagan JG, Sigal IA, Rausch SM, Tertinegg I, Ethier CR. Finite element modeling of the human sclera: influence on optic nerve head biomechanics and connections with glaucoma. Exp Eye Res. 2011;93(1):4–12. doi: 10.1016/j.exer.2010.09.014. [DOI] [PubMed] [Google Scholar]
- 47.Eilaghi A, Flanagan JG, Simmons CA, Ethier CR. Effects of scleral stiffness properties on optic nerve head biomechanics. Ann Biomed Eng. 2010;38(4):1586–1592. doi: 10.1007/s10439-009-9879-7. [DOI] [PubMed] [Google Scholar]
- 48.Fazio MA, Grytz R, Morris JS, Bruno L, Girkin CA, Downs JC. Human scleral structural stiffness increases more rapidly with age in donors of African descent compared to donors of European descent. Invest Ophthalmol Vis Sci. 2014;55(11):7189–7198. doi: 10.1167/iovs.14-14894. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Murienne BJ, Jefferys JL, Quigley HA, Nguyen TD. The effects of glycosaminoglycan degradation on the mechanical behavior of the posterior porcine sclera. Acta Biomater. 2015;12:195–206. doi: 10.1016/j.actbio.2014.10.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Kimball EC, Nguyen C, Steinhart MR, Nguyen TD, Pease ME, Oglesby EN, Oveson BC, Quigley HA. Experimental scleral cross-linking increases glaucoma damage in a mouse model. Exp Eye Res. 2014;128:129–140. doi: 10.1016/j.exer.2014.08.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Ayyalasomayajula A, Park RI, Simon BR, Vande Geest JP. A porohyperelastic finite element model of the eye: the influence of stiffness and permeability on intraocular pressure and optic nerve head biomechanics. Comput Methods Biomech Biomed Engin. 2016;19(6):591–602. doi: 10.1080/10255842.2015.1052417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Yamanari M, Nagase S, Fukuda S, Ishii K, Tanaka R, Yasui T, Oshika T, Miura M, Yasuno Y. Scleral birefringence as measured by polarization-sensitive optical coherence tomography and ocular biometric parameters of human eyes in vivo. Biomed Opt Express. 2014;5(5):1391–1402. doi: 10.1364/BOE.5.001391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Ho LC, Sigal IA, Jan NJ, Squires A, Tse Z, Wu EX, Kim SG, Schuman JS, Chan KC. Magic angle-enhanced MRI of fibrous microstructures in sclera and cornea with and without intraocular pressure loading. Invest Ophthalmol Vis Sci. 2014;55(9):5662–5672. doi: 10.1167/iovs.14-14561. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Norman RE, Flanagan JG, Rausch SM, Sigal IA, Tertinegg I, Eilaghi A, Portnoy S, Sled JG, Ethier CR. Dimensions of the human sclera: thickness measurement and regional changes with axial length. Exp Eye Res. 2010;90(2):277–284. doi: 10.1016/j.exer.2009.11.001. [DOI] [PubMed] [Google Scholar]
- 55.Ho LC, Conner IP, Do CW, Kim SG, Wu EX, Wollstein G, Schuman JS, Chan KC. In vivo assessment of aqueous humor dynamics upon chronic ocular hypertension and hypotensive drug treatment using gadolinium-enhanced MRI. Invest Ophthalmol Vis Sci. 2014;55(6):3747–3757. doi: 10.1167/iovs.14-14263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Chan KC, Fu QL, Hui ES, So KF, Wu EX. Evaluation of the retina and optic nerve in a rat model of chronic glaucoma using in vivo manganese-enhanced magnetic resonance imaging. Neuroimage. 2008;40(3):1166–1174. doi: 10.1016/j.neuroimage.2008.01.002. [DOI] [PubMed] [Google Scholar]
- 57.Chan KC, So KF, Wu EX. Proton magnetic resonance spectroscopy revealed choline reduction in the visual cortex in an experimental model of chronic glaucoma. Exp Eye Res. 2009;88(1):65–70. doi: 10.1016/j.exer.2008.10.002. [DOI] [PubMed] [Google Scholar]
- 58.Pijanka JK, Coudrillier B, Ziegler K, Sorensen T, Meek KM, Nguyen TD, Quigley HA, Boote C. Quantitative mapping of collagen fiber orientation in non-glaucoma and glaucoma posterior human sclerae. Invest Ophthalmol Vis Sci. 2012;53(9):5258–5270. doi: 10.1167/iovs.12-9705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Roberts MD, Grau V, Grimm J, Reynaud J, Bellezza AJ, Burgoyne CF, Downs JC. Remodeling of the connective tissue microarchitecture of the lamina cribrosa in early experimental glaucoma. Invest Ophthalmol Vis Sci. 2009;50(2):681–690. doi: 10.1167/iovs.08-1792. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Palko JR, Iwabe S, Pan X, Agarwal G, Komaromy AM, Liu J. Biomechanical properties and correlation with collagen solubility profile in the posterior sclera of canine eyes with an ADAMTS10 mutation. Invest Ophthalmol Vis Sci. 2013;54(4):2685–2695. doi: 10.1167/iovs.12-10621. [DOI] [PMC free article] [PubMed] [Google Scholar]