Besides rod input, rod-driven horizontal cells in zebrafish also receive UV cone input. This means that they process spectral information during photopic conditions and luminance information in scotopic conditions.
Keywords: photoreceptors, horizontal cell, zebrafish, connexins, retina
Abstract
The functional and morphological connectivity between various horizontal cell (HC) types (H1, H2, H3, and H4) and photoreceptors was studied in zebrafish retina. Since HCs are strongly coupled by gap junctions and feedback from HCs to photoreceptors depends strongly on connexin (Cx) hemichannels, we characterized the various HC Cxs (Cx52.6, Cx52.7, Cx52.9, and Cx55.5) in Xenopus oocytes. All Cxs formed hemichannels that were conducting at physiological membrane potentials. The Cx hemichannels differed in kinetic properties and voltage dependence, allowing for specific tuning of the coupling of HCs and the feedback signal from HCs to cones. The morphological connectivity between HC layers and cones was determined next. We used zebrafish expressing green fluorescent protein under the control of Cx promoters. We found that all HCs showed Cx55.5 promoter activity. Cx52.7 promoter activity was exclusively present in H4 cells, while Cx52.9 promoter activity occurred only in H1 cells. Cx52.6 promoter activity was present in H4 cells and in the ventral quadrant of the retina also in H1 cells. Finally, we determined the spectral sensitivities of the HC layers. Three response types were found. Monophasic responses were generated by HCs that contacted all cones (H1 cells), biphasic responses were generated by HCs that contacted M, S, and UV cones (H2 cells), and triphasic responses were generated by HCs that contacted either S and UV cones (H3 cells) or rods and UV cones (H4 cells). Electron microscopy confirms that H4 cells innervate cones. This indicates that rod-driven HCs process spectral information during photopic and luminance information during scotopic conditions.
NEW & NOTEWORTHY
Besides rod input, rod-driven horizontal cells in zebrafish also receive UV cone input. This means that they process spectral information during photopic conditions and luminance information in scotopic conditions.
retinal horizontal cells (HCs) are strongly electrically coupled by gap junctions and receive input from and feed back to photoreceptors (Chalupa and Werner 2003). In this way, HCs generate the surround responses of bipolar cells (BCs), which leads to contrast enhancement (Klaassen et al. 2011). Many species have more than one type of HC, each with a different spectral sensitivity. It has been suggested that these differences in spectral sensitivity of HCs are important for color constancy (Kamermans et al. 1998; Kraaij et al. 1998; Sabbah et al. 2013; Vanleeuwen et al. 2007). The spectral sensitivity of the various HC types strongly depends on the specific connectivity between cones and HCs (Stell et al. 1975). This relation is, however, not fully resolved (Connaughton et al. 2004; Connaughton and Nelson 2010; Kamermans et al. 1991; Kraaij et al. 1998; Li et al. 2009).
Although the various types of HCs in most species have different spectral sensitivities, HCs in cyprinid fish might show the most pronounced spectral coding (Norton et al. 1968; Svaetichin and MacNichol 1958; Tomita 1963; Witkovsky 1967). Monophasic HCs (MHCs) hyperpolarize to light of all visible wavelengths, with a maximal response to middle-wavelength stimuli. Biphasic HCs (BHCs) hyperpolarize to light of short and middle wavelength and depolarize to light of long wavelength. Triphasic HCs (THCs) hyperpolarize to short- and long-wavelength light and depolarize to middle-wavelength light (for review see Kamermans and Spekreijse 1995). Finally, there is a HC type that seems to be driven exclusively by rods (RHC) (Li et al. 2009). HCs can also be classified morphologically. Three HC types connect to cones (H1, H2, and H3), and one connects to rods (H4) (Stell 1975). H1 cells have large roundish somata and short dendrites, whereas H3 cells have small irregular somata and long thin dendrites. H2 cells have intermediate properties. H4 cells have large irregular somata and long thick dendrites (Li et al. 2009). However, these morphological parameters show some overlap.
To account for the spectral sensitivities of HCs in goldfish, Stell and coworkers (Stell et al. 1975; Stell and Lightfoot 1975) suggested that H1 cells are MHCs that are innervated by all cone types (L, M, and S cones), H2 cells are BHCs that are innervated by M and S cones, and H3 cells are THCs that are only innervated by S cones. Such specific connectivity leads to the spectral coding of HCs. Long-wavelength stimulation activates L cones and hyperpolarizes MHCs, which leads to depolarization of the BHCs due to feedback from MHCs to M and S cones. THCs depolarize to middle wavelengths and hyperpolarize to long wavelengths, because of feedback from the BHCs to the S cones.
Part of the Stell model was experimentally confirmed. The depolarizing response to long-wavelength stimuli in BHCs was reduced when feedback from HCs to cones was genetically reduced (Klaassen et al. 2011). However, there are also some predictions made by the Stell model that could not be experimentally confirmed. The Stell model predicts that feedback to S cones induced by long-wavelength stimulation should be inverted relative to feedback induced by middle- and short-wavelength stimulation. Kraaij et al. (1998) tested this prediction directly and found that feedback in S cones did not change sign when moving from short- to long-wavelength stimulation. Second, the spectral sensitivity of the various HC types shows a much larger variability than predicted by the Stell model (Kamermans and Spekreijse 1995). Furthermore, Connaughton and Nelson (2010) described six different spectral HC types in zebrafish. Apart from two types of MHCs, one BHC, and two THCs, they characterized a tetraphasic HC (depolarizing to middle wavelengths and UV light while hyperpolarizing to long- and short-wavelength light). To address these discrepancies, we revisited the question of the correlation between the morphological and the physiological connectivity between cones and HCs.
One reason for these discrepancies might be that the description of the connectivity of HCs with cones in goldfish is mostly based on Golgi impregnated or HRP-filled HCs. Such an approach leads to the connectivity of single HCs with cones. Since HCs are strongly electrically coupled, the spectral sensitivity of HCs is also determined by the other HCs in the network. Therefore, the connectivity between cones and HC layers must be determined.
The zebrafish is especially suited for the analysis of the contacts between cones and HCs because the cones are organized in a regular mosaic, allowing identification of their spectral type. This mosaic is preserved at the level of the synaptic terminals of the cones. Consequently, the tips of HC dendrites innervating these terminals form a regular pattern. L and M cones form double cones and are aligned in double rows in which every pair is flipped when compared with the previous pair. S and UV cones alternate in a single row located between the double-cone rows. In general the S-cone synaptic terminal is located between two L-cone terminals and the UV-cone terminal between two M-cone terminals (Li et al. 2009), although some variability in the position of the S- and UV-cone synaptic terminals has also been described (Li et al. 2012).
The discrepancy between HC spectral sensitivity and HC cone connectivity might be due to differences in the feedback signals generated by the various HC types. Since HC coupling and feedback depend for the major part on connexin (Cx) function (Klaassen et al. 2011), the properties of Cx hemichannels expressed by the various HC types must be determined. Cxs are proteins that form gap-junctional channels and hemichannels. In HCs, they are involved in coupling (Chalupa and Werner 2003) and in the feedback pathway from HCs to cones (Kamermans et al. 2001). In zebrafish a specific group of Cxs exists that are highly related (Eastman et al. 2006; Klaassen et al. 2012). The Cxs in this group have a molecular mass of between 52 and 56 kDa and encompass Cx52.6, Cx52.7, Cx52.9, and Cx55.5. All these Cxs appear to be expressed by HCs (Ciolofan et al. 2007; Dermietzel et al. 2000; Klaassen et al. 2011, 2012; Shields et al. 2007; Zoidl et al. 2004). Cx55.5 is crucial for the feedback signal from HC to cones in zebrafish (Klaassen et al. 2011; Shields et al. 2007). All Cxs seem to be involved in electrical coupling of HCs.
We first set out to functionally characterize these Cxs and determine which HC type expresses the various Cxs. We analyzed the properties of the Cx hemichannels in Xenopus oocytes and found that the current-voltage (I-V) relations of the hemichannels formed by these Cxs fall into two classes. One class shows a rather linear I-V relation (Cx52.6 and Cx52.9), while the other shows a prominent time-dependent reduction of current at negative potentials (Cx55.5 and Cx52.7). Next we cloned the promoter regions of the various Cx genes and generated zebrafish that express green fluorescent protein (GFP) under control of these promoter regions. We found that each HC expresses at least Cx55.5, the major component mediating ephaptic feedback from HCs to cones. The other Cxs show highly specific expression patterns. An unexpected finding was that Cx52.6 was expressed in the whole retina in H4 cells and only in the ventral quadrant of the retina in H1 cells. Using these GFP reporter lines in combination with intracellular recording of the spectral sensitivity of HCs and dye injections, we confirm that H1 cells are MHCs and are innervated by L, M, S, and UV cones and H2 cells are BHCs and are innervated by M, S, and UV cones. However, THCs could either be H3 or H4 cells. H3 cells are innervated by S and UV cones, but, surprisingly, the H4 cells contacted rods and often also UV cones. We discuss whether these results can account for the discrepancies between morphological and physiological connectivity as described in the literature so far.
MATERIALS AND METHODS
Animals
Zebrafish (Danio rerio) were bred and maintained in freshwater aquaria at 28°C under a light regime of 10 h dark:14 h light. Experiments were carried out during the subjective day. All animal experiments were performed under a license obtained from the ethical committee of the Royal Netherlands Academy of Arts and Sciences acting in accordance with the European Communities Council Directive of 24 November 1986 (86/609/EEC). The eyes of the fish were enucleated and the retinas isolated.
Oocyte Recordings
Xenopus laevis oocytes were obtained from EcoCyte Bioscience (Castrop-Rauxel, Germany). With a Nanoject II (Drummond, Broomall, PA), oocytes were injected with 46 nl of a solution containing 100 ng/μl RNA coding for the indicated constructs and 20 ng/μl of an antisense oligonucleotide against Cx38 mRNA in DEPC-treated water. Oocytes injected with 46 nl of a solution containing only the oligonucleotide served as controls. The cells were incubated at 18°C for ∼72 h, after which they were kept at 4°C for at most 60 h. Medium was first refreshed after 72 h and then after each subsequent 24 h. Cells were incubated in a modified Barth solution containing (in mM) 88 NaCl, 1.0 KCl, 0.4 CaCl2, 2.4 NaHCO3, 0.33 Ca(NO3)2, 0.82 MgSO4, 5.0 C6H12O6, and 15.0 HEPES, adjusted to pH 7.6 with 10 M NaOH.
Perfusion solutions contained (in mM) 110 NaCl, 1.3 KCl, 3.0 NaHCO3, 0.9 MgSO4, 0.1 CaCl2, and 19.0 HEPES, adjusted to pH 7.6 with 10 M NaOH. Oocytes were placed in an OPC-1 oocyte perfusion chamber (AutoMate Scientific, Berkeley, CA). A gravity-driven perfusion system was used in combination with a ValveLink8.2 controller (AutoMate Scientific). Electrodes were pulled on a Sutter PC87 puller (Sutter) from GC150TF-10 capillaries with an inner diameter of 1.17 mm and an outer diameter of 1.50 mm (Harvard Apparatus, Edenbridge, UK) to a resistance of 0.2–1 MΩ and filled with 3 M KCl, 10 mM EGTA, and 10 mM HEPES in water, adjusted to pH 7.4 with NaOH. Electrodes were connected to a dual-electrode voltage clamp (OC-725C Oocyte Clamp; Warner Instruments, Hamden, CT) and a CED1401 mkII [Cambridge Electronic Design (CED), Cambridge, UK]. Data acquisition was done with a PC running Signal 3.0 (CED). Cells were kept at a holding potential of −60 mV and stepped for 10 s to potentials varying between −100 mV and 20 mV. Experiments were performed at room temperature. Data analysis was performed with MATLAB (MathWorks, Natick, MA), Excel (Microsoft, Redmond, WA), and Origin Pro 8.0 (OriginLab, Northampton, MA). I-V relations were constructed by averaging over 20 ms around the indicated times in the trace. Means and SEs were calculated for the indicated number of cells.
Generation of Transgenic Fish
The promoters for Cx55.5 and Cx52.6 were cloned previously (Shields et al. 2007). For Cx52.7 a BAC (CH211-153J3, BacPacResourcesCenter) and for Cx52.9 gDNA from TL fish were used as template. Various primer pairs generating amplicons ranging from 1 to 4 kB were tested on promoter activity, and the primer pair generating the smallest amplicon with promoter activity was used. For Cx52.7 these primers were FWD: GGGGACAACTTTGTATAGAAAAGTTGAGATACCACTGGCTGATTGAGCGG, REV: GGGGACTGCTTTTTTGTACAAACTTGCTTCACCGCCACATGTCAGTGCTT, resulting in an 1,843-bp upstream region. For Cx52.9 these primers were FWD: GGGGACAACTTTGTATAGAAAAGTTGGTGGCTGGAAGAGCATCTGCTGCA, REV: GGGGACTGCTTTTTTGTACAAACTTGTCACGCCGGAGGACCCCTGAGAT, generating a 2,747-bp upstream region. The four promoter regions for the various Cxs were used to drive expression of reporters, like GFP or mCherry, with the TOL2 system (Kwan et al. 2007). Plasmid DNA was injected in fertilized zebrafish eggs from a TL strain. Primary injected fish showed mosaic reporter expression in HCs in the retina, but after germline transmission stable lines were created with reporter expression in the full layer of HCs.
Intracellular Recordings and Dye Filling
Dark-adapted zebrafish retinas were mounted with the photoreceptor side up in a recording chamber (Warner) and superfused with saline (concentrations in mM: 102 NaCl, 3 KCl, 2 CaCl2, 1 MgCl2, 5 d-glucose, 28 NaHCO3, 0.2 picrotoxin). The pH was maintained between 7.6 and 7.8 by bubbling with a mixture of CO2 and O2 gas (2.5% CO2-97.5% O2). Intracellular electrodes with a resistance of ∼200 MΩ when filled with 3 M KCl were used. Electrodes were backfilled with 5% neurobiotin dissolved in 200 mM KCl and filled with 3 M KCl. The resistance of these electrodes was >1 GΩ. A World Precision Instruments Intra 767 amplifier (WPI, Sarasota, FL) was used together with a CED 1401 plus ADDA converter (CED) and Signal software (CED). Spot (20 μm) and full-field flashes of light of 500 ms were given with LEDs of 365 nm, 465 nm, 525 nm, and 624 nm (bandwidth of ∼20 nm); the maximal intensity for each wavelength was 8.5 × 1015 quanta m−2s−1. In general, averages of 10 responses were used for analysis. Neurobiotin was injected into the cell by applying depolarizing current pulses. To allow for the dye to diffuse to neighboring cells, retinas were fixated after ∼15 min after the dye injection. Retinas were fixated for 10 min in 4% paraformaldehyde solution pH 6.4 and then in 4% paraformaldehyde solution pH 10.5. Retinas were incubated in 1:500 streptavidin conjugated with Cy3 fluorophore (Jackson Immunoresearch Laboratories) for 2 h in phosphate buffer with 0.3% Triton X. After several washes in PBS, retinas were mounted flat in Vectashield and viewed with a Leica SP5 confocal microscope.
Image Analysis
Confocal microscopy yielded Z stacks of images with green and/or red fluorescent HCs. All optical slices were 1 μm thick. These images were optimized for brightness and contrast with Adobe Photoshop software (Abode Systems, San Jose, CA). The images containing the HC somata were collapsed into a single soma stack image that was used for the quantitative measurements of the HC somata; these stacks contained four to eight optical slices. The images containing the tips of the dendrites, often organized in typical rosettes, were collapsed into a single dendrite stack that was used for the analysis of the connectivity of the fluorescent HCs; these stacks contained two to four slices. The rosettes correlate with the location of the photoreceptor synapses. We defined connectivity as the presence of one or more HC dendrites innervating the synaptic terminal of a photoreceptor. The percentage of labeled HC processes entering a photoreceptor synaptic terminal was estimated by eye. There was variability in the clarity of the preparations, so that individual dendrites could not be counted in all rosettes. To establish connectivity with certainty it was often necessary to examine rosettes in single images of a Z stack.
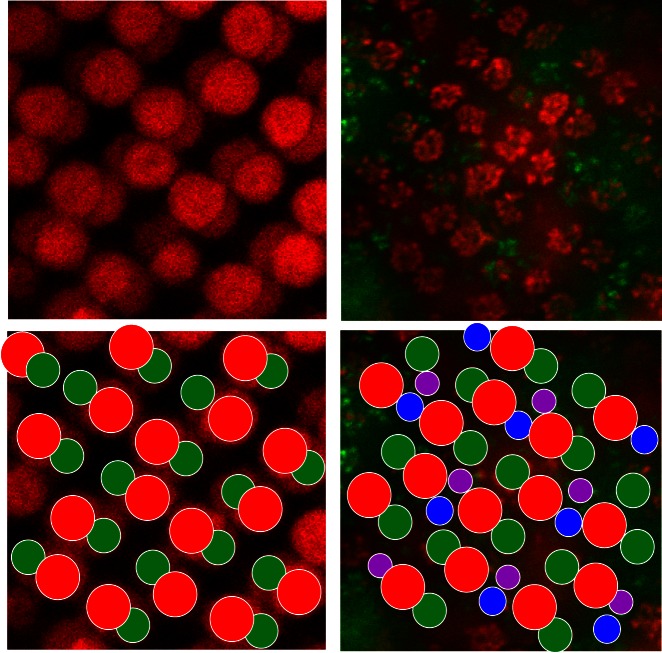
Previous studies have used the regular mosaic to identify photoreceptors (Alvarez-Delfin et al. 2009; Li et al. 2009, 2012; Raymond et al. 2014; Song et al. 2008). We used this regularity to characterize the photoreceptor type to which HCs connected. An example of the mosaic is shown in Fig. 1, in which it is shown that the photoreceptor type can be identified on the basis of the size of the rosette and the location within the mosaic. Double cones (L and M cones) are organized in straight lines, which are alternated by lines with S and UV cones. L-cone synaptic terminals are larger than M-cone terminals, and S- and UV-cone terminals are smaller than M-cone terminals. Rod terminals are the smallest “rosettes,” consisting of only two dendrites.
Fig. 1.
Cone mosaic in Cx55.5:GFP retina with filled H1 network. Confocal images of autofluorescent double cones (left) and the rosettes formed by fluorescent HC dendrites (right, red and green) reveal a regular mosaic, schematized at bottom. The HC dendrites innervate cone synaptic terminals. L- and M-cone terminals (red and green circles) alternate in a double straight line, S- and UV-cone terminals (blue and purple circles) alternate in a single straight line between the double cones. The filled dendrites (red) are from an H1 network, predominantly contacting L and M cones, but filled dendrites in S- and UV-cone terminals can also be seen.
ImageJ software was used to perform measurements on the soma stack images. Pixels with fluorescence above a certain threshold were considered positive. The percentage of positive pixels (of the total) was taken as a measure of the surface covered with fluorescent cells. The software outlined the margins of a soma on the basis of the clustered occurrence of positive pixels. The average soma size was calculated from the soma surfaces of complete somata in the image.
Immunocytochemistry
Light-adapted zebrafish were anesthetized and cervical transected. Zebrafish eyes were fixed for 10 min at room temperature in freshly prepared 0.1 M phosphate-buffered (pH 6.5) 4% formaldehyde, followed by fixation in 0.1 M sodium bicarbonate-buffered 4% formaldehyde (pH 10.4) for 10 min (Eldred et al. 1983). After the eyes were rinsed in 0.1 M phosphate buffer pH 7.4, the anterior eye segment, sclera, and choroid were peeled away. Pieces of retina were incubated free floating for 48 h with primary antibodies against Cx52.9 (1:100) and Cx55.5 (1:3,000) in PBS containing 0.3% Triton X-100 and 5% normal goat serum at room temperature. The antibodies against Cx52.9 and Cx55.5 have been described previously (Klaassen et al. 2011; Shields et al. 2007). After washing (3 × 15 min in PBS), sections were incubated in goat-anti-rabbit Cy3 or goat-anti-chicken Cy3 (1:200) for 1 h at 37°C. After washing three times for 15 min in PBS the pieces of retina were coverslipped with Vectashield. Retinas were observed on an inverted Zeiss Axiovert 100 M microscope equipped with the LSM 510 Meta laser scanning confocal module. Data are presented as a single optical slice, with z thickness of 1.0 μm.
Electron Microscopy
For electron microscopy, zebrafish retinas were fixated as described above. After rinsing in 0.1 M phosphate buffer pH 7.4, tissue was cryoprotected at room temperature in phosphate buffer containing 12.5% sucrose for 30 min and 25% sucrose for 1–2 h. Zebrafish eyes were embedded in Tissue-Tek (Sakura Finetek Europe, Zoeterwoude, The Netherlands) in an aluminum boat and frozen on dry ice. Frozen sections (30–40 μm) were obtained on a freezing microtome and collected in phosphate buffer. Retinal sections were incubated for 96 h with antisera against GFP (Chemicon; 1:200). After rinsing, the sections were incubated in PowerVisionPoly-HRP-Goat Anti-mouse IgG (ImmunoVision Technologies, Daly City, CA). To visualize the peroxidase, the sections were incubated in a Tris·HCl diaminobenzidine solution containing 0.03% H2O2. The diaminobenzidine reaction product was then intensified by a gold-substituted silver peroxidase method (Van den Pol and Gorcs 1986). Sections were rinsed in sodium cacodylate buffer 0.1 M (pH 7.4) and postfixed for 20 min in 1% OsO4 supplemented with 1% potassium ferricyanide in sodium cacodylate buffer 0.1 M (pH 7.4). After rinsing in the sodium cacodylate buffer, the material was dehydrated and embedded in epoxy resin. Ultrathin sections (60 nm) perpendicular to the retina were observed and photographed in a FEI Tecnai 12 electron microscope. Electron micrographs were acquired as TIFF files with IMAGE II cameras. All TIFF files were optimized for brightness and contrast with Photoshop software.
RESULTS
Properties of Connexin Hemichannels
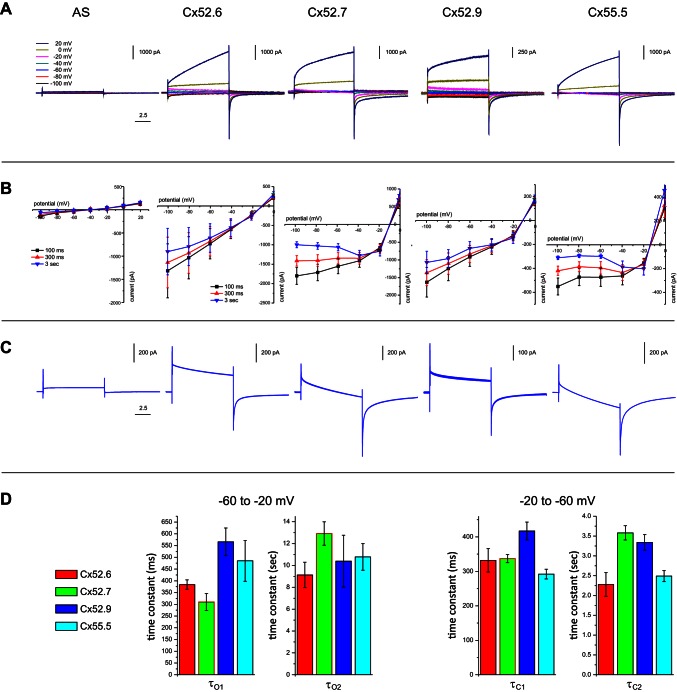
We analyzed the electrophysiological properties of the four zebrafish Cxs in Xenopus oocytes. Cx hemichannel currents were measured 3 days after injection of RNA into the oocytes with a double-electrode voltage-clamp method. Oocytes were injected with RNA encoding for the various Cxs together with Cx38 antisense RNA to block endogenous expression of Cx38. As a control, a series of oocytes was injected only with Cx38 antisense RNA. Figure 2 shows the mean responses of the oocytes to voltage steps of 10 s from the holding potential of −60 mV (Fig. 2A) to potentials ranging from −100 to 20 mV for the different Cxs (see Table 1 for number of oocytes per experiment). Comparison of these current traces with the results for the Cx38 antisense-injected oocytes (n = 8) shows that almost all current under these conditions is mediated by hemichannels, which are formed by the expressed zebrafish Cx (Fig. 2, A and B). All Cxs show a slowly developing outward current when the potential is stepped to positive potentials and only small inward currents when the membrane potential is stepped to negative potentials.
Fig. 2.
Electrophysiological properties of zebrafish HC Cx hemichannels measured in Xenopus oocytes. A: currents for different 10-s voltage steps in a 2-electrode voltage-clamp experiment measured in oocytes in which different HC Cxs are expressed. AS, Cx38 antisense-injected oocytes. B: current-voltage relations for different HC Cxs, measured at 100 ms, 300 ms, and 3 s after onset of the step. Cx52.6 and Cx52.9 show a similar current-voltage relation, just as Cx52.7 and Cx55.5, which are more rectifying. C: magnified current traces for the voltage step from −60 mV to −20 mV. Cx52.6 and Cx52.9 show a more sustained current, while Cx52.7 and Cx55.5 show a more transient current. To these traces the time constants shown in D were fitted. D: fast and slow time constants for opening (τO1, τO2) and closing (τC1, τC2) of different Cx hemichannels. For statistics see the text and Table 1.
Table 1.
Time constants of hemichannel currents
| Cx52.6 (n = 13) |
Cx52.7 (n = 8) |
Cx52.9 (n = 7) |
Cx55.5 (n = 9) |
ANOVA | |
|---|---|---|---|---|---|
| Channel opening | |||||
| τO1, ms | 384 ± 20 | 310 ± 36 | 566 ± 59 | 485 ± 87 | P = 0.0257; |
| F = 4.074 | |||||
| τO2, s | 9.13 ± 1.16 | 12.92 ± 1.07 | 10.39 ± 2.37 | 10.78 ± 1.22 | P = 0.4074; |
| F = 1.031 | |||||
| AO2/AO1 | 16.73 ± 7.48 | 2.95 ± 0.19 | 5.68 ± 0.46 | 10.42 ± 3.12 | P = 0.0303; |
| F = 3.547 | |||||
| Channel closing | |||||
| τC1, ms | 322 ± 34 | 337 ± 12 | 417 ± 26 | 292 ± 14 | P = 0.0089; |
| F = 4.626 | |||||
| τC2, s | 2.28 ± 0.30 | 3.58 ± 0.18 | 3.34 ± 0.20 | 2.49 ± 0.14 | P = 0.0007; |
| F = 7.356 | |||||
| AC2/AC1 | 0.36 ± 0.07 | 0.56 ± 0.26 | 0.76 ± 0.07 | 0.60 ± 0.11 | P = 0.1768; |
| F = 1.764 |
Values are time constants for n oocytes (mean ± SE).
Under physiological conditions HCs rest at about −40 mV, and when stimulated with light HCs can hyperpolarize to about −70 mV. Therefore, the currents at negative step potentials are physiologically the most relevant. The I-V relations of the currents of Fig. 2A are plotted in Fig. 2B and show that the current mediated by Cx55.5 and Cx52.7 strongly reduces with hyperpolarization, whereas the I–V relations for Cx52.6 and Cx52.9 are almost linear at negative potentials (Fig. 2C).
| (1) |
The time constants of the currents were determined next. A two-exponential function (Eq. 1) was fitted through the curves during the step from the holding potential (−60 mV) to the step potential (−20 mV) (Fig. 2C). This protocol induces channel opening. A large outward current exists immediately after depolarization of the membrane. This current decreases with two time constants. This indicates that the channel inactivates and/or that a secondary inward current is induced by the depolarization. Figure 2D, left, shows the time constants of the currents when stepping from −60 to −20 mV. The short time constant (τO1) differs significantly between the various Cxs (Table 1). Post hoc testing reveals that τO1 of Cx52.6 and Cx52.7 do not differ significantly from each other (P = 0.17). The same holds for Cx52.9 and Cx55.5 (P = 0.44). When these two groups are tested against each other, a significant difference occurs. The hemichannels in the Cx52.6/Cx52.7 group inactivate much faster than the Cx52.9/Cx55.5 group (335 ± 25 ms vs. 523 ± 49 ms; P = 0.0045). The long time constants (τO2) do not differ significantly between the various Cxs (Table 1). The absolute amplitudes are not further analyzed since the Cx expression differed substantially between experiments. Instead, the ratio AO2/AO1 was calculated. The ratios between the amplitude of the slow and the fast exponent for channel opening varied significantly between the various Cxs (Table 1).
Next we looked at the time constants of channel closing (Fig. 2D, right) when stepping from −20 to −60 mV. Directly after the step to −60 mV, a large current occurs. This current decreases with two time constants. This suggests that the channel closes slowly with hyperpolarization. Both the short (τC1) and the long (τC2) time constants differ significantly from each other (Table 1). τC1 of Cx52.6, Cx52.7, and Cx55.5 do not differ significantly from each other (ANOVA, P = 0.62, F = 0.595). τC1 of Cx52.9 is much larger compared with all the other Cxs (Cx52.6 vs. Cx52.9, P = 0.0490; Cx52.7 vs. Cx52.9, P = 0.0084; Cx55.5 vs. Cx52.9, P = 0.0003). Post hoc testing reveals that τC2 of Cx52.6 and Cx55.5 do not differ significantly from each other (P = 0.789). The same holds for Cx52.7 and Cx52.9 (P = 0.390). When these two groups are tested against each other, a significant difference occurs. The Cx52.6/Cx55.5 group is much faster than the Cx52.7/Cx52.9 group (2.32 ± 0.76 s vs. 3.47 ± 0.51 s; P = 0.00002). The ratios of the amplitudes (AC2/AC1) of the fast and the slow exponent for channel closing did not differ significantly between the various Cxs (Table 1).
The main difference between the various Cxs seems to be in the amount of rectification of the I-V relations. Based on this, the four Cxs can be grouped into two categories. Cx52.6 and Cx52.9 have rather linear I-V relations, whereas Cx52.7 and Cx55.5 have a strongly rectifying I-V relation.
Connexin Expression Patterns of Horizontal Cells
Immunocytochemical studies showed that at least Cx55.5 and Cx52.9 were expressed in HCs (Klaassen et al. 2011; Shields et al. 2007). Figure 3, B and C, show antibody labeling for these Cxs in a punctate pattern, representing the gap junctions between HCs. Since the dendrites of the various types of HCs were strongly intermixed in two plexuses (see Supplemental Movie S1),1 it is impossible to determine the Cx expression pattern for each HC type separately on the basis of immunocytochemistry. Therefore, we adopted a strategy in which we expressed GFP under the control of the various Cx promoters. Figure 3A shows an example of GFP expression via the promoter for Cx55.5. Dendrites innervating photoreceptor synaptic terminals show the specific horseshoe shape. Also, on the BC side of the HCs dendrites can be seen together with an axon innervating the inner nuclear layer. The promoter regions for Cx52.6 and Cx55.5 have been described previously (Shields et al. 2007). The promoter regions for Cx52.7 and Cx52.9 were cloned in this study (see materials and methods). GFP-expressing zebrafish lines were generated with these promoters. GFP was expressed in HCs for all of these promoters. We examined the morphology of the somata and the pattern of the dendritic tips of the fluorescent HCs for the different Cxs. We consider the pattern of Cx promoter-driven GFP expression as a valid indication for the expression of the related Cx protein, because the patterns we found were stable over five generations and were equal in various independently generated zebrafish lines.
Fig. 3.
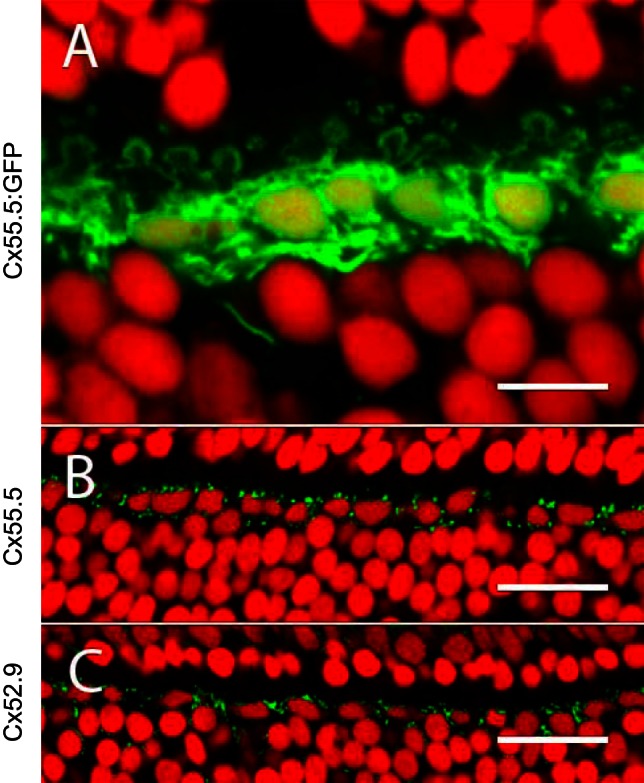
Immunohistochemical localization of zebrafish HC Cxs. A: section of a retina with GFP expression via the Cx55.5 promoter, showing a single layer of HC somata. The horseshoe-shaped structures are dendrites innervating cone synaptic terminals. Nuclei are stained with ethidium bromide (red). B and C: retinal sections stained with antibodies against Cx55.5 and Cx52.9 show punctuate labeling (green) around HCs. Nuclei are stained with ethidium bromide (red). Scale bars: 10 μm (A), 25 μm (B), 25 μm (C).
Cx55.5.
First, we analyzed fluorescence in retinas with GFP expression driven by the promoter for Cx55.5 (Cx55.5:GFP). Figure 4A shows that GFP-labeled HCs in the retinas of the Cx55.5:GFP fish cover the retinal surface completely. Only small areas are not stained. A cross section shows that this is a single layer of HCs (Fig. 3). The soma size of HCs showed a large variability, suggesting that GFP was expressed in multiple HC types. Scrutinizing Fig. 4A reveals that some HCs seem to be labeled more intensely than others. These HCs seem to have slightly smaller somata, with an irregular shape suggestive for H2 cells.
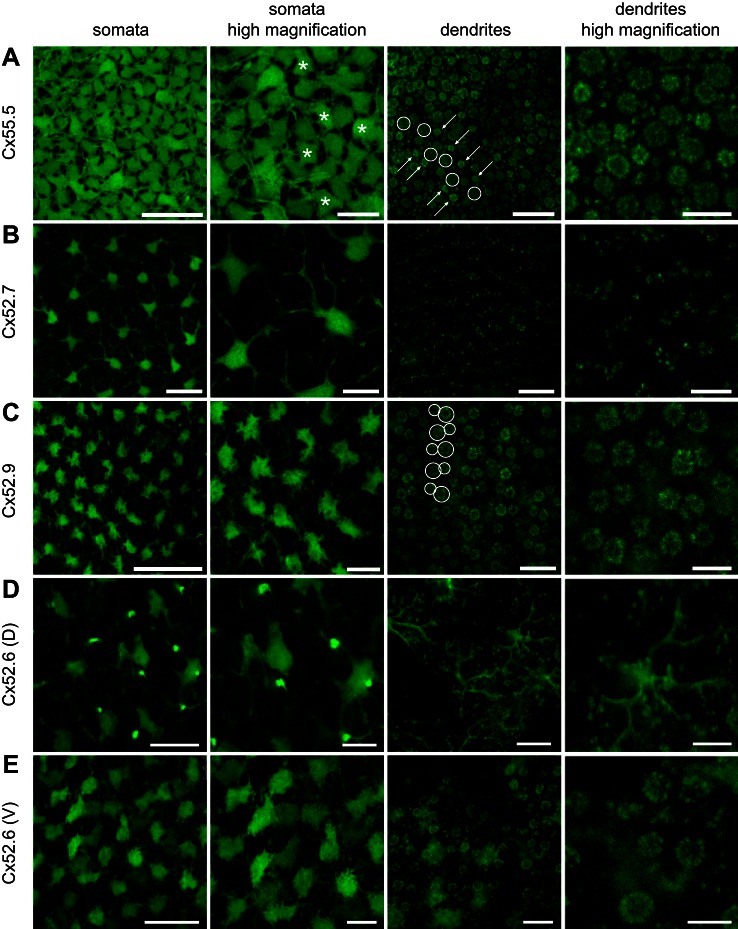
Fig. 4.
Expression of GFP in HCs via different Cx promoters. A: GFP expression in HCs via the promoter of Cx55.5, shown at different magnifications. Somata of different sizes and shapes can be observed, and some irregularly shaped somata appear more brightly fluorescent (asterisks indicate some examples) than others. At the level of the synaptic terminals the HC dendrites in the small S- and UV-cone terminals (arrows) appear more brightly fluorescent than in the large L-cone terminals (circles). Small rod terminals are located between the rosettes of cone terminals. B: GFP expression in HCs via the promoter of Cx52.7, shown at different magnifications. Only a subset of HCs show fluorescence, with large irregular somata and long thick dendrites. The tips of the dendrites of these HCs do not form rosettes but can often be seen in pairs. C: GFP expression in HCs via the promoter of Cx52.9, shown at different magnifications. Only a subset of HCs show fluorescence, with small somata and relatively short dendrites. The tips of the dendrites of these HCs mainly form large rosettes, indicative of L-cone terminals (large circles) and M-cone terminals (smaller circles). D: GFP expression in HCs via the promoter of Cx52.6 in the dorsal 3 quadrants of the retina, shown at different magnifications. Large HCs with irregular somata and long dendrites show fluorescence together with regularly spaced bipolar cells. At the level of the dendrites only punctuate contacts with photoreceptors are visible. E: GFP expression in HCs via the promoter of Cx52.6 in the ventral quadrant of the retina, shown at different magnifications. Only a subset of HCs show fluorescence, with different intensities. The tips of the dendrites form large rosettes and punctuate patterns. In the ventral quadrant no bipolar cells are labeled with GFP. Scale bars, from left to right: 25 μm, 10 μm, 10 μm, and 5 μm.
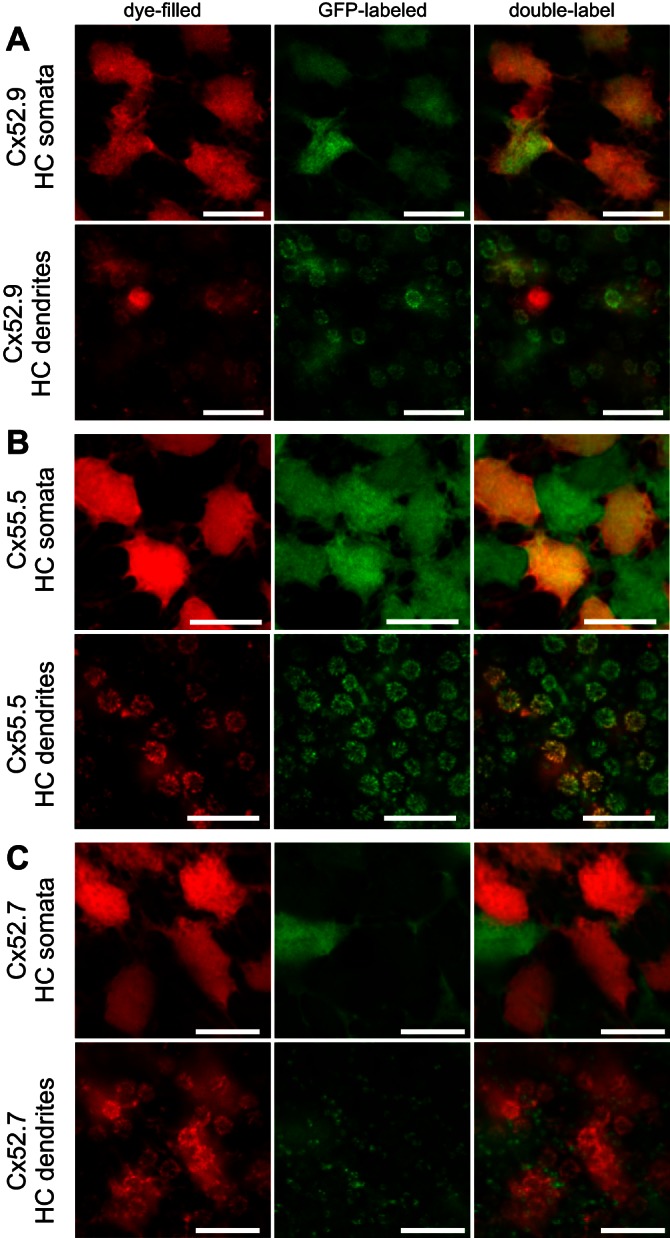
The dendrites of the labeled HCs can be traced back to the photoreceptor synaptic terminals. The tips of the dendrites appear as small structures in the synaptic terminals of the photoreceptors (Fig. 4A). Inside the cone terminals they form rosettes; the multiple synaptic ribbons of cone terminals are organized in a circle with a HC dendrite lateral to each ribbon. Rods have only one synaptic ribbon with two lateral HC dendrites. Figure 4A shows large rosettes, smaller rosettes, and very small structures in a regular mosaic. Since cones are organized in a regular mosaic, the rosettes can be attributed to certain cone types. The largest rosettes represent the HC dendrites in synaptic terminals of L cones. The slightly smaller rosettes represent those of M cones. The L and M cones alternate in successive rows. S and UV cones are aligned in a single row in an alternating fashion. S- and UV-cone terminals are difficult to discriminate on the basis of their morphological appearance (but see below). Often the S-cone rosettes are flanking the L-cone terminals (Li et al. 2012). However, this pattern shows a lot of variability. Rod contacts are scattered between the cone terminals. All the cone and rod synaptic terminals appear to contain GFP-labeled HC dendrites, indicating that Cx55.5 is expressed by all HC types and thus all photoreceptor terminals contain GFP-labeled HC processes.
Cx52.7.
Next, we examined fluorescence in Cx52.7:GFP retinas. In these retinas, GFP was present in HCs of a single type (Fig. 4B). The fluorescent HCs were large (89.5 ± 5.7 μm2, n = 11), with an irregular shape and long thick dendrites. These HCs cover only a relatively small area of the retinal surface (12.2 ± 0.9%), in contrast to the Cx55.5:GFP labeled HCs, which cover the retina almost completely. On the basis of these morphological parameters, these HCs can be classified as H4 cells.
When tracing the dendrites back to the photoreceptors (Fig. 4B), a pattern of scattered puncta appears, indicative of the HC dendrites in the small synaptic terminals of rods. However, one or two dendrites contacting a cone would appear similar to a rod contact in such an analysis. Therefore, we performed an electron microscopic analysis of these retinas. Figure 5, A and B, show that the GFP-labeled dendrites of HCs in this preparation make contacts with rods and additionally with small cone terminals with a limited number of synaptic ribbons. These cones are either S or UV cones. These observations imply that H4 cells are innervated not just by rods but also by S or UV cones.
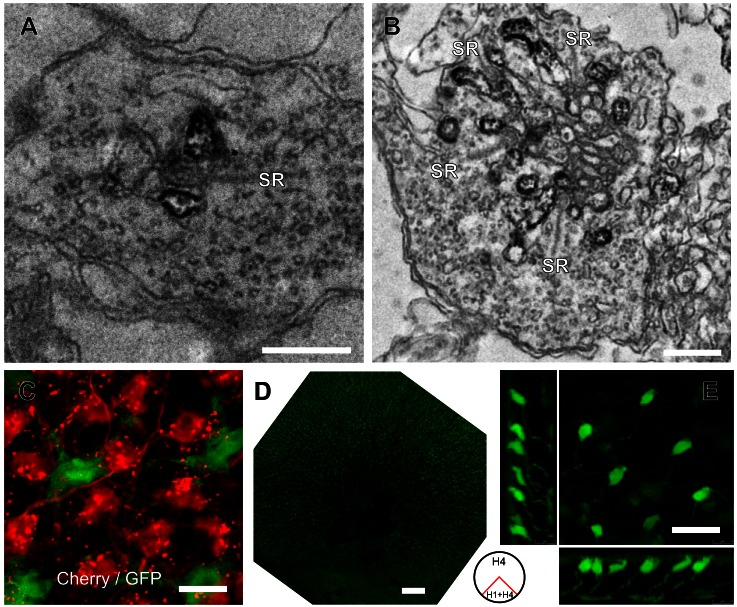
Fig. 5.
Specificity of GFP expression via the Cx52.7, Cx52.9, and Cx52.6 promoters. A: electron microscopic image of a rod synaptic terminal in a Cx52.7:GFP retina. HC dendrites flanking the synaptic ribbon (SR) are stained with an anti-GFP antibody. Scale bar: 500 nm. B: electron microscopic image of a cone synaptic terminal in a Cx52.7:GFP retina. HC dendrites flanking the multiple synaptic ribbons (SR) are stained with an anti-GFP antibody. C: confocal image of a double Cx52.7:GFP and Cx52.9:mCherry transgenic zebrafish shows that there is no overlap between the HC somata showing expression. The expression of mCherry led to some cluttering in the dendrites. D: overview of a Cx52.6:GFP retina. The ventral quadrant shows fluorescence only in HCs (H1 + H4); the dorsal 3 quadrants show fluorescence in H4 HCs and strong fluorescence in a specific type of bipolar cell. E: confocal image of the dorsal region of a Cx52.6:GFP retina, showing the morphology of bipolar cells with green fluorescence. Scale bars: 0.5 μm (A), 0.5 μm (B), 10 μm (C), 200 μm (D), 20 μm (E).
Cx52.9.
GFP labeling in Cx52.9:GFP retinas also appeared in a single HC type (Fig. 4C). These HCs have relatively small somata (55.5 ± 2.8 μm2), have short thin dendrites, and cover only partly the retina (26.4 ± 1.0%). This suggests that these HCs are H1 cells. The tips of dendrites can be found in rosettes but do not seem to make the small scattered contacts that were seen in Cx55.5:GFP and Cx52.7:GFP retinas. This indicates that these HCs contact only cones. The most brightly fluorescent are L- and M-cone rosettes. To exclude that in the retinas of Cx52.7:GFP and Cx52.9:GFP the same HC population was labeled, we generated a double transgenic zebrafish line with expression of Cx52.7:GFP and Cx52.9:mCherry. Figure 5C shows that GFP and mCherry are expressed in the somata of different cells. These results confirm that the promoters for Cx52.7 and Cx52.9 are active in different cell types. Note that unlabeled areas of the size of HC somata exist between the GFP and mCherry labeling, indicating that Cx52.7 and Cx52.9 together do not label all HCs.
Cx52.6.
Next, we analyzed fluorescence in retinas of Cx52.6:GFP fish (Fig. 4, D and E). The fluorescence in these retinas is not homogeneous over the entire retina (Fig. 4, D and E). In the ventral quadrant GFP-labeled HCs covered ∼40.7 ± 1.7% of the retinal surface (n = 6). The somata consisted of two types: one was roundish with short dendrites, suggestive for H1 cells, and the other was large and irregular with thick dendrites, suggestive for H4 cells. On the level of the photoreceptor terminals GFP labeling was found mostly in the larger L- and M-cone terminals and the rod terminals and less in dendrites in the S- and UV-cone terminals. These observations suggest that in the ventral quadrant of the retina the label was present in H1 and H4 cells (Fig. 4E). In the three dorsal quadrants the pattern of GFP expression was very different (Fig. 4D). The average soma size of these HCs was 98.2 ± 7.1 μm2 (n = 7), and the dendritic tips only contacted small structures. Overall the labeling pattern strongly resembled the Cx52.7:GFP retinas. This indicates that in the dorsal three quarters of the retina only H4 cells were labeled (Fig. 4D).
In addition, focusing down in the retina reveals label in a subset of BCs (Fig. 4D and Fig. 5E). These cells were evenly spaced and had somata close to the HC layer with axons ending in the deeper layers of the inner plexiform layer (Fig. 5E), indicating that this is a single type of BC. The dendrites of these cells contacted multiple photoreceptor terminals, but we are unable to tell which photoreceptors. Since in zebrafish ON- and OFF-layers are not clearly separated, it is difficult to classify these cells (Connaughton et al. 2004; Li et al. 2012). These cells are not discussed further in this report.
In summary, analysis of Cx promoter-driven GFP expression in HCs shows that various HC types express different combinations of Cxs (Table 2). H1 cells express Cx55.5, Cx52.9, and Cx52.6 but the latter only in the ventral part of the retina. H2 and H3 cells express Cx55.5, while H4 cells express Cx55.5, Cx52.7, and Cx52.6. However, very weak GFP expression driven by the other Cx promoters cannot be excluded for all HCs.
Table 2.
Morphological properties of GFP-expressing retinas
| Promoter Driving GFP Expression |
|||||
|---|---|---|---|---|---|
| Cx55.5 | Cx52.7 | Cx52.9 | Cx52.6 Ventral | Cx52.6 Dorsal | |
| Small roundish somata | + | − | + | + | − |
| Small irregular somata | ++ | − | ± | − | − |
| Large irregular somata | ++ | ++ | − | ++ | ++ |
| Short dendrites | ++ | − | + | ++ | − |
| Long dendrites | ++ | ++ | − | ++ | ++ |
| Dendritic terminals | L, M, S, UV cones and rods | S or UV cones and rods | L, M, S, UV cones | L, M, S, UV cones and rods | S or UV cones and rods |
| HC type | H1, H2, H3, H4 | H4 | H1 | H1, H4 | H4 |
The different GFP-expressing retinas show typical and specific GFP fluorescence. ++, Bright green fluorescence; +, green fluorescence; ±, variable weak green fluorescence; −, no fluorescence.
Determining Connectivity Between HCs and Photoreceptors
To correlate the connectivity between photoreceptors and the various HC layers, responses of HCs to full-field flashes of light of different wavelengths (365 nm, 465 nm, 525 nm, and 624 nm) were obtained in retinas with GFP-expressing HCs. Spectral response profiles of HCs could be classified into three groups (Fig. 6): 1) cells that hyperpolarized to all wavelengths with a maximal response to 525-nm light (n = 27); 2) cells that hyperpolarized to 465-nm and 525-nm light but depolarized to 624-nm light (n = 12); and 3) cells that had a maximal hyperpolarization to 365-nm and 465-nm light (n = 16). Responses to 525-nm or 624-nm light were highly variable in this group.
Fig. 6.
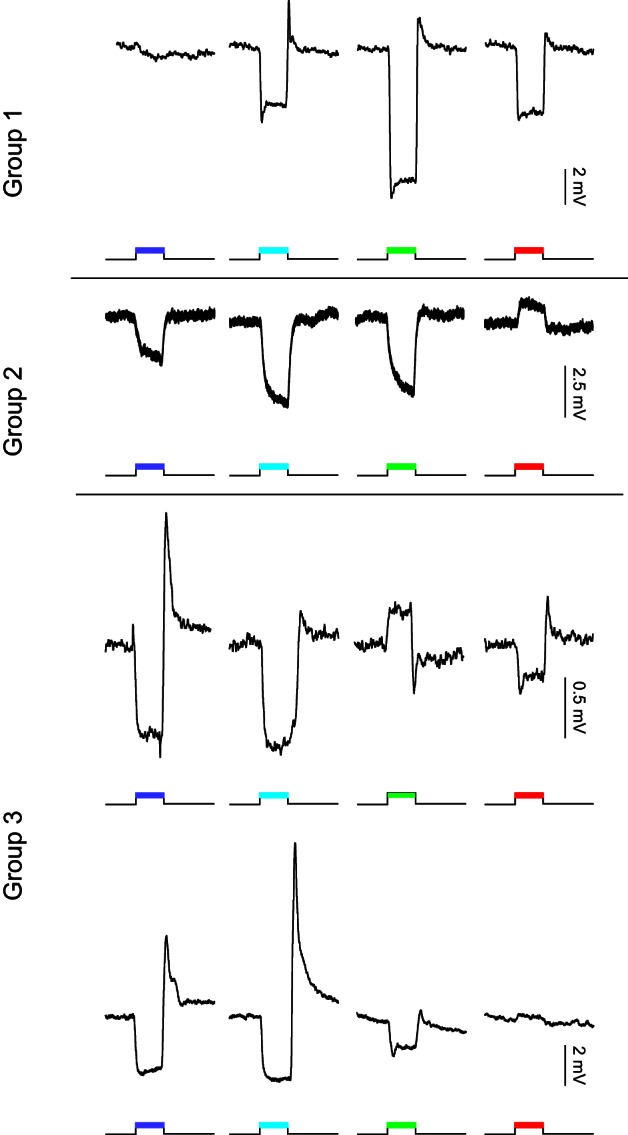
Examples of spectral response profiles of recorded zebrafish HCs. Group 1: monophasic profiles with hyperpolarizing responses to red (624 nm), green (525 nm), and blue (465 nm) light and barely a response to UV light (365 nm). Group 2: biphasic profiles with depolarizing responses to red (624 nm) light and hyperpolarizing responses to green (525 nm) and blue (465 nm) light. Responses to UV light (365 nm) are small and variable and most often hyperpolarizing. Group 3: triphasic-like profiles are variable but have in common the large hyperpolarizing responses to short-wavelength light (365 and 465 nm). Responses to green (525 nm) and red (624 nm) light are variable in this group.
After recording, HCs were filled with neurobiotin, fixated, incubated with streptavidin-Cy3, and analyzed on a Leica SP5 confocal microscope. Morphological properties of the filled HCs were analyzed and correlated with the GFP expression pattern and the spectral responses of the HCs. Only preparations in which both the physiological recording and the dye filling were of sufficient quality were used for analysis of the connectivity.
Group 1.
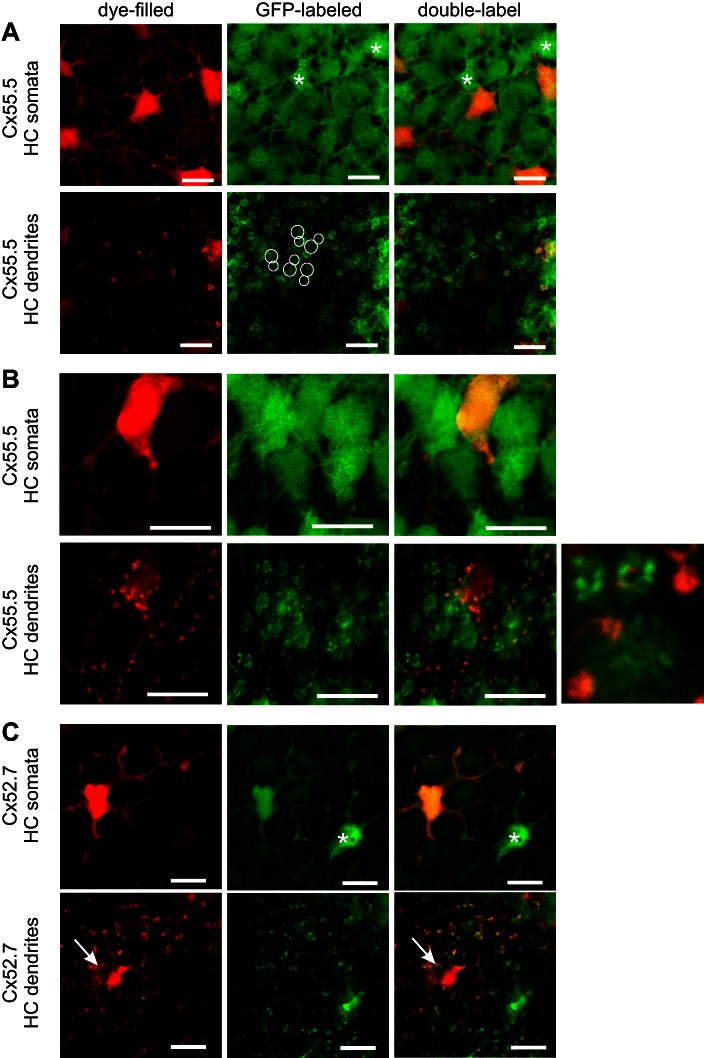
The HCs (n = 17) in this group hyperpolarized to all stimulus wavelengths, with a maximal response to 525-nm light (Fig. 6). The neurobiotin-filled cells had regular somata with relatively short dendrites. On average the soma size was 67.5 ± 3.6 μm2 (n = 16). Figure 7A shows complete overlap of filled HCs with the GFP fluorescence in the Cx52.9:GFP retina, suggesting that these HCs were of the morphological H1 type. In this preparation one cone was accidentally damaged by the electrode and filled with neurobiotin. This cell in Fig. 7A shows the red fluorescent Cy3 labeling but no GFP labeling.
Fig. 7.
Morphological characteristics of monophasic HCs. A: filled HCs with a monophasic response profile (red) in a Cx52.9:GFP retina (green) showing complete overlap, indicating H1-type HCs. Accidently a cone was filled, showing red without green. At the level of the dendrites, large rosettes indicative of L- and M-cone terminals show overlap of red and green. Scale bars: 10 μm. B: filled HCs with a monophasic response profile (red) in a Cx55.5:GFP retina (green) showing an alternating pattern of red and green HCs. At the level of the dendrites, large rosettes indicative of L- and M-cone terminals are red, while smaller rosettes indicative of S- and UV-cone terminals are bright green. Scale bars: 10 μm. C: filled HCs with a monophasic response profile (red) in a Cx52.7:GFP retina (green) show no overlap between red and green HCs, indicating that the filled HCs are not H4-type HCs. At the level of the dendrites, large rosettes indicative of L- and M-cone terminals are red, while dendrites in rod synaptic terminals are green. Scale bars: 10 μm.
To study the connections these HCs made with photoreceptors, we filled HCs in the Cx55.5:GFP zebrafish, since in these fish all HC dendrites innervating all the cone and rod synaptic terminals are labeled with GFP (Fig. 7B and Fig. 4A). This allowed us to estimate the contacts made by the various HCs with the different types of cones (Table 3). The dendritic tips of the neurobiotin-filled HCs made large and aligned contacts in double rows, indicating that these HCs contacted double (L and M) cones (Fig. 7). Filled dendrites were visible in only one of the smaller cone terminals, of either S or UV cones (most clear in Fig. 7B). In only one retina were contacts between the filled HCs and both small cone terminals (S and UV cones) observed. All dendrites in the L-cone rosettes were labeled, while only part of the dendritic tips in a rosette of M cones were labeled, suggesting that H1 cells are the only HCs contacting L cones. In the presumed UV cones only a few dendrites per rosette were labeled (Fig. 7B). HCs from group 1 made no contacts with rods as is indicated by the lack of overlap between neurobiotin and GFP in the Cx52.7:GFP fish (Fig. 7C). Overall, cells from group 1 seemed to be H1 cells contacting L, M, and S, and/or UV cones. These cells hyperpolarized to all stimulating wavelengths and are therefore MHCs.
Table 3.
Connectivity of photoreceptors and HCs
| MHC |
BHC |
THC |
RHC |
|||||
|---|---|---|---|---|---|---|---|---|
| Contacted photoreceptors | Labeled dendrites | Contacted photoreceptors | Labeled dendrites | Contacted photoreceptors | Labeled dendrites | Contacted photoreceptors | Labeled dendrites | |
| L cone | 100% | 100% | 0% | 0% | 0% | 0% | 0% | 0% |
| M co ne | 100% | 50–80% | 100% | 40–70% | 0% | 0% | 0% | 0% |
| S cone | 0–50% | 0–10% | 100% | 50–80% | 100% | 50–70% | 0% | 0% |
| UV cone | 80–100% | 0–40% | 100% | 50–80% | 100% | 20–40% | 0–80% | 20–40% |
| Rod | 0% | 0% | 0% | 0% | 0% | 0% | 100% | 100% |
| Summary contacts | L, M, S, UV | M, S, UV | S, UV | UV, rods | ||||
Left column under each HC type shows estimate of % of cones contacted by each HC type, which is often 100% or 0%, but not always. Right column shows estimate of % of dendrites within a synaptic terminal originating from a certain type of photoreceptor.
Group 2.
The HCs (n = 8) in this group hyperpolarized to short-wavelength light stimuli and depolarized to long-wavelength light stimuli (Fig. 6). These HCs had irregularly shaped somata with short dendrites. In the Cx55.5:GFP retinas the neurobiotin-filled cells correspond with the brightly fluorescent GFP cells, which form an alternating pattern with less bright green cells (Fig. 8A). Figure 8B shows that these cells were not H1 cells, since there was no overlap of the neurobiotin label with the GFP label in the Cx52.9:GFP retinas. Analyzing the connectivity of the HCs with photoreceptors in the Cx55.5:GFP retinas, it becomes clear that they contact all cone synaptic terminals except the larger ones (Fig. 8). These data show that these HCs contact M, S, and UV cones. Interestingly, one HC in this group depolarized to light of 365 nm, while the other seven HCs in this group either hyperpolarized to 365-nm light or showed no response to this wavelength, suggesting that they receive predominantly S-cone input and a variable amount of UV-cone input. The amplitude of the depolarization to 624-nm light was always on the same order of magnitude as the hyperpolarizing response to 525-nm light.
Fig. 8.
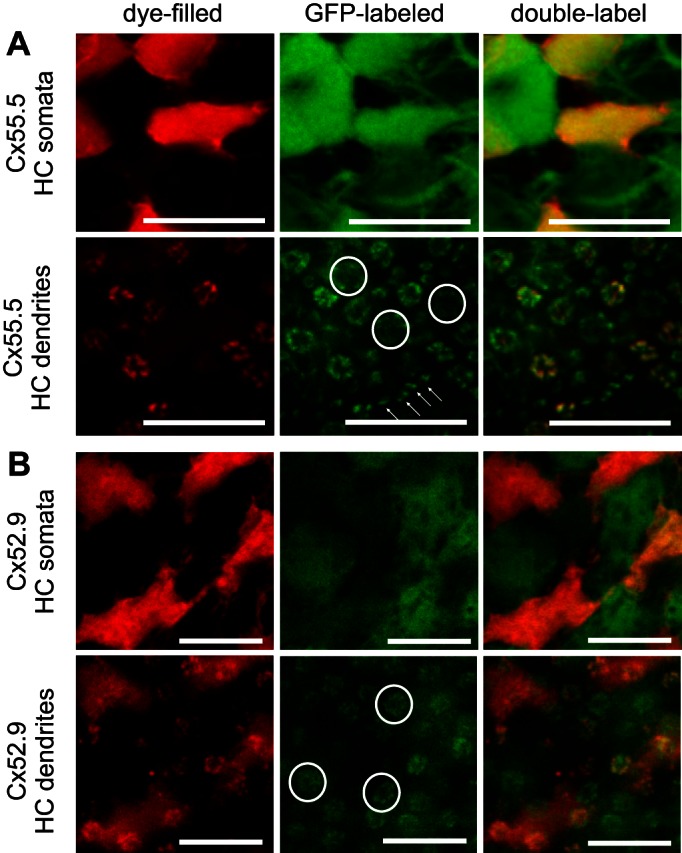
Morphological characteristics of biphasic HCs. A: filled HCs with a biphasic response profile (red) in a Cx55.5:GFP retina (green) showing an overlap of red and bright green HCs, indicating that the filled HCs are H2-type HCs. At the level of the dendrites, small rosettes show overlap of red and green fluorescence, while the largest rosettes (circles, indicating L-cone terminals) and dendrites inside rod terminals (arrows) were not filled. Scale bars: 10 μm. B: filled HCs with a biphasic response profile (red) in a Cx52.9:GFP retina (green) showing an alternating pattern of red and green fluorescent HCs, indicating that the filled HCs are not H1 type HCs. The largest rosettes (circles, indicating the L-cone terminals) show green but not red fluorescence, indicating innervation from H1-type HCs but not innervation from H2-type HCs. Scale bars: 10 μm.
Overall, cells in group 2 appeared to be H2 cells contacting M, S, and UV cones. These cells had a biphasic spectral sensitivity with depolarizing responses in the long wavelength part of the spectrum and thus correspond with the BHCs.
Group 3.
The HCs (n = 8) in this group hyperpolarized to short-wavelength light and depolarized or did not show any response to long-wavelength light stimuli. The absence of a clear triphasic response behavior in some cells might have been due to the adaptational state of the retina. HCs in this group showed variable morphology. These HCs were much wider spaced than the H1 and H2 cells. Some cells had H3 morphology. The somata of these HCs were small and irregularly shaped, but the dendrites were longer than the H2 cells (Fig. 9A). These cells contacted S cones and UV cones, but one more than the other.
Fig. 9.
Morphological characteristics of triphasic HCs. A: filled HCs with a triphasic response profile (red) in a Cx55.5:GFP retina (green). Bright green fluorescent HCs are H2-type HCs (asterisks indicate examples), and red somata show no overlap with these cells. At the level of the dendrites, small rosettes show overlap of red and bright green fluorescence, while the large rosettes of L cones (large circles) and M cones (small circles) were not filled (not red). No rod connectivity is visible, suggesting that these are H3-type HCs. Scale bars: 10 μm. B: filled HCs with a triphasic response profile (red) in a Cx55.5:GFP retina (green) showing no overlap of red and bright green HCs, indicating that the filled HCs are not H2-type HCs. At the level of the dendrites only punctuate labeling of filled dendrites can be seen, while large rosettes only show GFP labeling. Dendrites in rod synaptic terminals show overlap and also some dendrites inside smaller cone terminals (S or UV cones), suggesting that these are H4-type HCs. The inset shows a higher magnification of an S- and UV-cone terminal (bright green), an L-cone terminal, and 3 rod terminals. Scale bars: 10 μm. C: filled HCs with a triphasic response profile (red) in a Cx52.7:GFP retina (green) show complete overlap, indicating that the filled HCs are H4-type HCs. At the level of the dendrites punctuate labeling of filled dendrites can be seen, indicative of HC dendrites in rod synaptic terminals. One single H4-type HC appears not to be coupled to the other H4-type HCs (asterisk). Accidently a cone (arrow) was filled, showing red without green. Scale bars: 10 μm.
Other HCs had H4 morphology, also with long but thicker dendrites, and with larger irregularly shaped somata (Fig. 9, B and C). Recordings and injections in the Cx52.7:GFP fish confirmed that some cells in this group were H4 cells (Fig. 9C). The dendrites of these HCs contacted predominantly rods and one of the two small cone terminals, either S or UV cones (Fig. 9B). In two of six preparations, the cone contacts were absent and only rod contacts were observed.
Since the H4 cells contact predominantly one cone type and respond strongly to UV light, we concluded that the cones contacted by the H4 cells are UV cones. The fluorescent appearance of the two small cone terminals differs slightly. One has a starlike appearance, whereas the fluorescence in the other is more diffuse. Since the H4 cell contacts mostly the starlike terminals, we concluded that these terminals were the UV cones. Following this logic, we estimated the connectivity of the HCs with the cones. Table 3 summarizes these morphological observations about the connectivity between photoreceptors and HCs based on the pattern of HC dendrites innervating cone and rod synaptic terminals. We estimated the fraction of terminals containing dendrites from a certain HC type (contacted photoreceptors, Table 3), but we also estimated, when possible, the fraction of dendrites within a terminal originating from a certain HC type (labeled dendrites, Table 3). These estimates are represented in Table 3 with intervals indicating the variability between preparations.
L cones only innervate MHCs; the dendrites within L-cone terminals originate 100% from MHCs. M cones innervate MHCs and BHCs, and the number of dendrites from MHCs inside M-cone terminals is usually a bit larger than the number of dendrites from BHCs. S-cone terminals contains equal amounts of BHC and THC dendrites and sometimes a few dendrites from MHCs. UV cones innervate all HC types, but the largest number of dendrites inside the UV-cone terminal originate from BHCs. Rods only connect to RHCs.
DISCUSSION
In this study we examined the connectivity of HCs with photoreceptors and its relation with the spectral sensitivity of HCs. Zebrafish retina contains four different types of HC, called H1–H4. HCs express four different Cxs. We investigated the biophysical properties of the hemichannels that are formed by these four Cxs, and it appeared that Cx52.6 and Cx52.9 have relatively linear I-V relations while the current through the Cx52.7 and Cx55.5 hemichannels reduces with time at negative membrane potentials. The currents could be fitted best with a sum of two exponential functions, indicating complex Cx-specific activation and inactivation kinetics.
To determine which of those Cxs was expressed in which HC type and how this relates to the connectivity of HCs with the cones and their spectral sensitivity, zebrafish were generated that express GFP driven by the promoters of the four Cxs. These fish lines showed very different and specific patterns of GFP expression. The Cx55.5 promoter drove GFP expression in all HC types, the Cx52.7 drove GFP expression only in H4 cells, the Cx52.9 promoter drove GFP expression only in H1 cells, and the Cx52.6 promoter drove GFP expression in H1 and H4 cells. This specificity was used to determine the connectivity of physiologically classified HCs with the cones. Three groups of response profiles were found. One group of HCs showed only hyperpolarizing responses to light of different wavelengths and consisted of H1 cells contacting L, M, and UV cones and occasionally S cones. A second group of HCs showed hyperpolarizing responses to short- and middle-wavelength light stimulation and depolarizing responses to long-wavelength light stimulation and consisted of H2 cells contacting M, S, and UV cones. The third group showed the most variability in responses but was characterized by large hyperpolarizing responses in response to short wavelengths. In this group responses to middle-wavelength light were hyperpolarizing, depolarizing, or absent and responses to long-wavelength light were also either hyperpolarizing, depolarizing, or absent, but always small. This group of short-wavelength-dominated HCs consisted of H3 cells contacting mostly S cones and H4 cells contacting rods and UV cones.
Properties of Connexin Hemichannels
On the basis of the properties of their I-V relations, the four Cx-hemichannels could be grouped into two categories. Cx52.6 and Cx52.9 have linear I-V relations, while the hemichannel current of Cx52.7 and Cx55.5 reduced strongly at negative membrane potentials. This division was unexpected, since on the basis of homology of the amino acid sequences, Cx55.5 and Cx52.9 should form one group and Cx52.6 and Cx52.7 the other.
The voltage-clamp analysis revealed that both the hyperpolarization-induced and depolarization-induced hemichannel currents could be fitted best with a sum of two exponentials: one very slow process (time constant of a few seconds) and one a bit faster (time constant of ∼380 ms). The very slow process has been seen by other researchers as well and has been attributed to the activation or inactivation of a Ca2+-dependent Cl− current [ICl(Ca)] known to exist in Xenopus oocytes (Barish 1983; Gillo et al. 1989). This current is activated by an influx of Ca2+ and depends on the intracellular Ca2+ concentration. This current slowly develops as the intracellular Ca2+ concentration builds up. Since the Cx55.5 and Cx52.7 hemichannels show a larger slow component than the Cx52.6 and Cx52.9 hemichannels (see Fig. 2), one could speculate that the latter Cxs are less permeable for Ca2+ than the first two. Such a difference between Cxs in Ca2+ permeability has been shown previously by Verselis and coworkers (Sanchez et al. 2010).
This enhanced Ca2+ permeability of Cx55.5 opens the interesting possibility of an interaction between Cx hemichannels and Panx1 channels in the HC dendrites. At the resting membrane potential of HCs, Cx55.5 hemichannels are open, mediating a current into the HCs. This current will make the synaptic cleft slightly negative and influences the Ca2+ channels of cones in an ephaptic manner (Kamermans et al. 2001). In addition, the Cx55.5 hemichannels will allow Ca2+ to flow into the HC, increasing the Ca2+ concentration in the HC dendritic tips. Panx1 channels are located at the HC dendritic tips as well. Since Panx1 channels are gated by intracellular Ca2+ (Locovei et al. 2006; Prochnow et al. 2009), opening of these channels will lead to ATP release. This leads to acidification of the synaptic cleft and inhibition of the Ca2+ channels of the cones (Vroman et al. 2014). Upon hyperpolarization, the conductance of the Cx55.5 hemichannels will reduce and thus the Ca2+ influx will reduce. Panx1 channels will also close in a voltage-dependent manner, but in addition their closure might be enhanced by the reduction of the Ca2+ concentration in the HC dendrites. This will lead to a reduction of the release of ATP and resulting acidification of the synaptic cleft. This mechanism would enhance negative feedback from HCs to cones.
The time constants of channel inactivation after a step from −60 mV to −20 mV and of channel closing after a step from −20 mV to −60 mV are all roughly between 300 and 500 ms. Subtle differences between the various Cxs are present. Cx55.5 and Cx52.9 have slightly longer time constants for channel inactivation than Cx52.6 and Cx52.7. For channel closing the time constants for all Cx are more similar than for channel opening, with the exception of Cx52.9, which is the slowest. These results indicate that the Cx hemichannels are not static pores but that they change their conductance with a time constant of 300–500 ms.
HCs feed back to cones via two pathways: one involving Cx hemichannels and one involving Panx1 channels. The Cx pathway is ephaptic and ultrafast. The other is dependent on ATP release, which induces pH changes in the synaptic cleft and has a time constant of ∼200 ms (Vroman et al. 2014). We now show that Cx hemichannels inactivate with a time constant on the same order of magnitude, meaning that the Cx hemichannel feedback will reduce with time while the Panx1-mediated feedback will slowly increase. Thus fast ephaptic feedback appears to dominate the early part of the response, while the Panx1/ATP feedback seems to dominate the sustained part of feedback. The exact ratio of ephaptic vs. Panx1/ATP feedback during these different time periods is still unknown.
The GFP-promoter study suggests that H2 and H3 cells express only Cx55.5 while the H1 cell express in addition to Cx55.5 also Cx52.6 and Cx52.9 and H4 cells express Cx52.6 and Cx52.7. This opens the possibility that H1 and H4 cells tune the ephaptic feedback signal per cone type. By expressing Cx hemichannels that inactivate less or have different time constants, the ratio of ephaptic feedback vs. Panx1/ATP feedback could be modulated. However, since H2 and H3 cells express only Cx55.5, variation in spectral coding of H2 and H3 cells is unlikely to result from differential expression of Cxs in HCs. In this study only properties of homomeric hemichannels formed by one Cx were investigated, but it is possible that H1 and H4 cells express channels that are formed by more than one Cx. These heteromeric hemichannels might have intermediate properties.
Connectivity Between Horizontal Cells and Photoreceptors
Morphological study of the connectivity between cones and HCs in zebrafish retina has been carried previously out by Song et al. (2008) and Li et al. (2009). They studied connectivity of individual HCs, which were labeled by DiI. Their data show some variability, because the labeling of individual HCs does not always allow classification of individual synaptic terminals on the basis of the regular cone mosaic. Our results largely confirm their conclusions, but the variability in the connectivity of L and M cones was not found. Our analysis shows with greater certainty that H1 cells connect to L cones and H2 cells connect to L and M cones. The variability in the connectivity of S and UV cones was also observed in our study (Table 3). The DiI labeling did not allow observation of rod HC dendrites innervating cone synaptic terminals.
When the morphological connectivity pattern we have described is compared with the model-derived inputs to the HCs in the study of Connaughton and Nelson (2010), discrepancies occur mostly in the S-cone and UV-cone ratios. Connaughton and Nelson (2010) determined the input strength to the various HCs on the basis of the response amplitude. In their phenomenological model, cone inputs could either hyperpolarize or depolarize HCs with light stimulation and no feedback pathways were included. In reality, the depolarizing responses are most likely generated via negative feedback from HCs to cones. When we only consider the hyperpolarizing cone inputs to HCs, Connaughton and Nelson provide the scheme in Table 4.
Table 4.
Presumed connectivity between cones and HCs as proposed by Connaughton and Nelson (2010)
| L1 | L2 | BHC | THC Blue | THC UV | TetraHC | |
|---|---|---|---|---|---|---|
| L cone | 52% | 76% | 0% | 11% | 13% | 22% |
| M cone | 48% | 22% | 57% | 0% | 0% | 0% |
| S cone | 0% | 0% | 40% | 73% | 3% | 78% |
| UV cone | 0% | 2% | 2% | 16% | 85% | 0% |
These percentages are deduced from the results of the model of Connaughton and Nelson (2010), representing the relative contributions of each cone type to hyperpolarizing responses.
The main differences between the connectivity derived by Connaughton and Nelson (2010) and the connectivity derived in this paper are that Connaughton and Nelson found 1) the absence of S-cone input to the H1 cells, 2) a small amount of UV-cone input to the H2 cells, and 3) the absence of UV-cone input to the rod-driven HCs. Especially the connectivity between HCs and the S and UV cones appears to be rather variable, which might have given rise to the various physiological HC subtypes identified by Connaughton and Nelson.
It is intriguing that the H4 cells receive input from UV cones and rods. This suggests a special relation between these two classes of photoreceptors. Recently it was shown that mutations in specific zebrafish genes with a role in cone development cause a phenotype in which UV cones in the retina are replaced by rods (Alvarez-Delfin et al. 2009; Duval et al. 2014). This developmental relation between rods and UV cones is interesting with respect to our finding that H4-type HCs connect with rods and also with UV cones.
Horizontal Cell Spectral Coding
The mechanisms underlying the spectral sensitivity of HCs have been studied extensively during the past decades. Stell et al. (1975) proposed a cascade model to account for the spectral coding of HCs in goldfish. In general the Stell model describes the spectral coding of HCs rather well. However, as discussed in the introduction, a number of discrepancies between the Stell model and the actual measurements exist. The Stell model is a model based on morphology. Various studies have been conducted to test the Stell model, and various variations on the Stell model have been proposed (Connaughton and Nelson 2010; Djamgoz 1984; Djamgoz et al. 1985; Hashimoto et al. 1988; Kamermans et al. 1991; Li et al. 2009). All those studies were pure electrophysiological studies or pure morphological studies or relied on the connectivity of individually stained HCs. Despite extensive research, discrepancies between the model predictions and the actually measured responses remained.
In this study we revisited this classic question and determined the connectivity between HC layers and photoreceptors. The connectivity and responses of H1- and H2-type HCs in zebrafish are rather similar to those in goldfish. Exceptions are that the H1 cells receive UV-cone input and limited S-cone input and that H2 cells receive both S- and UV-cone input. The most striking difference, however, is the finding that rod-driven HCs (H4) can receive input from UV cones. In the light-adapted retina, these H4 cells have a triphasic spectral sensitivity, resembling that of H3 cells. The spectral profiles of the H3- and H4-type HCs are highly variable, which may have been the reason that Connaughton and Nelson (2010) identified six physiological HC types in zebrafish. They added three new classes of cells: THC blue, THC UV, and tetraphasic HCs. The cells they found in these three groups together appear to be the group 3 cells described in the present study. It seems likely that their THC blue cells were actually H3 cells, while the THC UV cells may have been H4 cells. The tetraphasic cell identified by them is most likely a H3 cell with no or very limited UV-cone input. Similarly, the difference between the H1–L1 and the H1–L2 of Connaughton seems to be the variable relative input from the S and UV cones.
In all studies dealing with the spectral sensitivity of HCs, highly variable results have been obtained. Could the variability be related to differences between retinal localizations? The retinas with fluorescent HCs express GFP via one of the Cx promoters. The fluorescent HCs of Cx55.5, Cx52.9, and Cx52.7 and the pattern of their dendritic tips showed a relatively uniform distribution across the retina. The ventral quadrant of the Cx52.6:GFP retina, however, showed a different pattern from the rest of the retina. This implies that in respect to some properties there are ventral-dorsal differences in the zebrafish retina. Although we did not study this, it is possible that part of the observed variability in the short-wavelength-dominated HCs (THCs) is caused by the different regions of the retina that we recorded from; it is also possible that the rod-driven HCs only receive UV-cone input in a certain region of the retina.
Mechanism Underlying Spectral Coding of Horizontal Cells
The depolarizing responses in HCs are generated by feedback from other HCs to cones. If a HC does not receive a direct input from a specific cone type, then the HC might depolarize for wavelengths that stimulate that particular cone type due to feedback from the other HCs to the other cone types. This explanation accounts for the depolarizing responses of BHCs to long-wavelength light stimulation and of THCs to middle-wavelength light stimulation. We sometimes observed depolarizing response in BHCs to UV light stimulation. Such responses can be accounted for by argumentation similar to that for the other depolarizing responses, i.e., these particular BHCs did not receive a strong UV-cone input.
The question remains of how the hyperpolarizing responses to long-wavelength light stimulation occur in THCs. For instance, the hyperpolarizing response to red light in the THC is suggested to occur because of feedback from the BHC to the S cones. With red light stimulation the BHC will depolarize and thus send an inverted feedback signal to the S cones. However, such inverted feedback signals have not been found (Kraaij et al. 1998). Furthermore, when scrutinizing the depolarizing responses one observes that the depolarizing responses are often preceded by a hyperpolarizing transient. One would expect that such a transient response would also be visible in the hyperpolarizing responses of the THC due to red light stimulation. However, this is not the case (see Fig. 6 and Connaughton and Nelson 2010). These observations lead to the suggestion that the hyperpolarizing responses to red light in the THC are mediated via another mechanism.
Vroman and Kamermans (2015) suggested that glutamate spillover from L cones to S- and UV-cone terminals might be the origin of the hyperpolarizing responses of THCs to long-wavelength light stimulation. In the dark, L, M, S, and UV cones release glutamate continuously, causing spillover. When stimulated with long-wavelength light L cones will hyperpolarize and reduce their glutamate release. Consequently, the glutamate concentration around the synaptic terminal of adjacent UV and S cones will reduce, leading to a reduction of the glutamate concentration sensed by the THCs. This will induce the hyperpolarizing response observed in the THC cells without requiring a sign-reversed negative feedback response in the S cones. Such a scenario would solve a long-standing controversy in retinal research and shows that morphological and functional connectivity may not always fully overlap.
Another option would be that the dendrites of various HCs present inside a single cone terminal might interact directly with each other. In the triad in the synaptic terminal, HC dendrites end lateral to the synaptic ribbon. Sometimes, it appears that the two HC dendrites belong to different HC types: one dendrite of an H2 HC and one dendrite of an H1 HC. Long-wavelength light stimulation hyperpolarizes the H1 cell. This hyperpolarization leads to an increase in the current flowing through the Cx hemichannels at the tips of these HCs, making the potential in the synaptic cleft slightly more negative. This will be sensed by glutamate receptors on the dendrite of the other HC in the following way. Since the reversal potential of the glutamate-gated channels is around 0 mV and HCs rest at about −40 mV, the driving force for the glutamate-gated current is about −40 mV. When the potential deep in the synaptic cleft becomes slightly negative, the driving force for the glutamate-gated current will reduce, leading to a hyperpolarization of the H2 cell. So this means that dendrites of HCs in the same synaptic invagination are slightly coupled. This coupling will be most prominent in conditions where the cone is not stimulated by light.
What is the consequence of the UV-cone input to H4 cells? The H4 cells will behave rather similar to the H3 cells in photopic conditions. The H4 cell will feed back to rods, inducing a surround response via the rod synaptic terminals introducing a surround response in the rod pathway under photopic conditions. In scotopic conditions the contact between the H4 cells and the UV cones will result in a rod-driven surround response in the cone pathway. Such pathways have also been identified in the mouse (Szikra et al. 2014). This cross talk between the cone and the rod pathway illustrates one of the “design principles” of the retina: it codes information as efficiently as possible, using the least amount of energy. One way to achieve this is to use all available neurons in any adaptational state (Atick et al. 1992; Srinivasan et al. 1982). Our results and those of Szikra et al. (2014) suggest that the retina reuses the neurons dedicated to rod vision during nighttime to mediate cone-driven surround responses in daytime and vice versa.
GRANTS
This work was supported by grants from ZonMW and ALW.
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the author(s).
AUTHOR CONTRIBUTIONS
L.J.K. and M.K. conceived and designed research; L.J.K., W.d.G., J.B.v.A., and J.K. performed experiments; L.J.K., W.d.G., J.K., and M.K. analyzed data; L.J.K. and M.K. interpreted results of experiments; L.J.K., J.K., and M.K. prepared figures; L.J.K. and M.K. drafted manuscript; L.J.K., W.d.G., J.K., and M.K. edited and revised manuscript; L.J.K., W.d.G., J.B.v.A., J.K., and M.K. approved final version of manuscript.
Supplementary Material
ACKNOWLEDGMENTS
We thank Dr. Georg Zoidl for his critical comments on the manuscript.
Present address of L.J. Klaassen: Faculty of Science, Vrije Universiteit, De Boelelaan 1081, 1081 HV Amsterdam, The Netherlands.
Footnotes
Supplemental Material for this article is available online at the Journal website.
REFERENCES
- Alvarez-Delfin K, Morris AC, Snelson CD, Gamse JT, Gupta T, Marlow FL, Mullins MC, Burgess HA, Granato M, Fadool JM. Tbx2b is required for ultraviolet photoreceptor cell specification during zebrafish retinal development. Proc Natl Acad Sci USA 106: 2023–2028, 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Atick JJ, Li Z, Redlich AN. Understanding retinal color coding from first principles. Vision Res 4: 559–572, 1992. [Google Scholar]
- Barish ME. A transient calcium-dependent chloride current in the immature Xenopus oocyte. J Physiol 342: 309–325, 1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chalupa LM, Werner JS. The Visual Neurosciences. Cambridge, MA: MIT Press, 2003. [Google Scholar]
- Ciolofan C, Lynn BD, Wellershaus K, Willecke K, Nagy JI. Spatial relationships of connexin36, connexin57 and zonula occludens-1 in the outer plexiform layer of mouse retina. Neuroscience 148: 473–488, 2007. [DOI] [PubMed] [Google Scholar]
- Connaughton VP, Graham D, Nelson R. Identification and morphological classification of horizontal, bipolar, and amacrine cells within the zebrafish retina. J Comp Neurol 477: 371–385, 2004. [DOI] [PubMed] [Google Scholar]
- Connaughton VP, Nelson R. Spectral responses in zebrafish horizontal cells include a tetraphasic response and a novel UV-dominated triphasic response. J Neurophysiol 104: 2407–2422, 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dermietzel R, Kremer M, Paputsoglu G, Stang A, Skerrett IM, Gomes D, Srinivas M, Janssen-Bienhold U, Weiler R, Nicholson BJ, Bruzzone R, Spray DC. Molecular and functional diversity of neural connexins in the retina. J Neurosci 20: 8331–8343, 2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Djamgoz MB. Electrophysiological characterization of the spectral sensitivities of horizontal cells in cyprinid fish retina. Vision Res 24: 1677–1687, 1984. [DOI] [PubMed] [Google Scholar]
- Djamgoz MB, Downing JE, Wagner HJ. The cellular origin of an unusual type of S-potential: an intracellular horseradish peroxidase study in a cyprinid fish retina. J Neurocytol 14: 469–486, 1985. [DOI] [PubMed] [Google Scholar]
- Duval MG, Oel AP, Allison WT. gdf6a is required for cone photoreceptor subtype differentiation and for the actions of tbx2b in determining rod versus cone photoreceptor fate. PLoS One 9: e92991, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eastman SD, Chen TH, Falk MM, Mendelson TC, Iovine MK. Phylogenetic analysis of three complete gap junction gene families reveals lineage-specific duplications and highly supported gene classes. Genomics 87: 265–274, 2006. [DOI] [PubMed] [Google Scholar]
- Eldred WD, Zucker C, Karten HJ, Yazulla S. Comparison of fixation and penetration enhancement techniques for use in ultrastructural immunocytochemistry. J Histochem Cytochem 31: 285–292, 1983. [DOI] [PubMed] [Google Scholar]
- Gillo B, Landau EM, Moriarty TM, Roberts JL, Sealfon SC. A novel calcium-dependent chloride current in Xenopus oocytes injected with brain messenger RNA. J Physiol 417: 47–61, 1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hashimoto Y, Harosi FI, Ueki K, Fukurotani K. Ultra-violet-sensitive cones in the color-coding systems of cyprinid retinas. Neurosci Res 8: S81–S95, 1988. [DOI] [PubMed] [Google Scholar]
- Kamermans M, Fahrenfort I, Schultz K, Janssen-Bienhold U, Sjoerdsma T, Weiler R. Hemichannel-mediated inhibition in the outer retina. Science 292: 1178–1180, 2001. [DOI] [PubMed] [Google Scholar]
- Kamermans M, Kraaij DA, Spekreijse H. The cone/horizontal cell network: a possible site for color constancy. Vis Neurosci 15: 787–797, 1998. [DOI] [PubMed] [Google Scholar]
- Kamermans M, Spekreijse H. Spectral behavior of cone-driven horizontal cells in teleost retina. Prog Retin Eye Res 14: 313–360, 1995. [Google Scholar]
- Kamermans M, van Dijk BW, Spekreijse H. Color opponency in cone-driven horizontal cells in carp retina. Aspecific pathways between cones and horizontal cells. J Gen Physiol 97: 819–843, 1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klaassen LJ, Fahrenfort I, Kamermans M. Connexin hemichannel mediated ephaptic inhibition in the retina. Brain Res 1487: 25–38, 2012. [DOI] [PubMed] [Google Scholar]
- Klaassen LJ, Sun Z, Steijaert MN, Bolte P, Fahrenfort I, Sjoerdsma T, Klooster J, Claassen Y, Shields CR, Ten Eikelder HM, Janssen-Bienhold U, Zoidl G, McMahon DG, Kamermans M. Synaptic transmission from horizontal cells to cones is impaired by loss of connexin hemichannels. PLoS Biol 9: e1001107, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kraaij DA, Kamermans M, Spekreijse H. Spectral sensitivity of the feedback signal from horizontal cells to cones in goldfish retina. Vis Neurosci 15: 799–808, 1998. [DOI] [PubMed] [Google Scholar]
- Kwan KM, Fujimoto E, Grabher C, Mangum BD, Hardy ME, Campbell DS, Parant JM, Yost HJ, Kanki JP, Chien CB. The Tol2kit: a multisite gateway-based construction kit for Tol2 transposon transgenesis constructs. Dev Dyn 236: 3088–3099, 2007. [DOI] [PubMed] [Google Scholar]
- Li YN, Matsui JI, Dowling JE. Specificity of the horizontal cell-photoreceptor connections in the zebrafish (Danio rerio) retina. J Comp Neurol 516: 442–453, 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li YN, Tsujimura T, Kawamura S, Dowling JE. Bipolar cell-photoreceptor connectivity in the zebrafish (Danio rerio) retina. J Comp Neurol 520: 3786–3802, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Locovei S, Wang J, Dahl G. Activation of pannexin 1 channels by ATP through P2Y receptors and by cytoplasmic calcium. FEBS Lett 580: 239–244, 2006. [DOI] [PubMed] [Google Scholar]
- Norton AL, Spekreijse H, Wolbarsht ML, Wagner HG. Receptive field organization of the S-potential. Science 160: 1021–1022, 1968. [DOI] [PubMed] [Google Scholar]
- Prochnow N, Hoffmann S, Vroman R, Klooster J, Bunse S, Kamermans M, Dermietzel R, Zoidl G. Pannexin1 in the outer retina of the zebrafish, Danio rerio. Neuroscience 162: 1039–1054, 2009. [DOI] [PubMed] [Google Scholar]
- Raymond PA, Colvin SM, Jabeen Z, Nagashima M, Barthel LK, Hadidjojo J, Popova L, Pejaver VR, Lubensky DK. Patterning the cone mosaic array in zebrafish retina requires specification of ultraviolet-sensitive cones. PLoS One 9: e85325, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sabbah S, Zhu C, Hornsby MA, Kamermans M, Hawryshyn CW. Feedback from horizontal cells to cones mediates color induction and may facilitate color constancy in rainbow trout. PLoS One 8: e66216, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sanchez HA, Mese G, Srinivas M, White TW, Verselis VK. Differentially altered Ca2+ regulation and Ca2+ permeability in Cx26 hemichannels formed by the A40V and G45E mutations that cause keratitis ichthyosis deafness syndrome. J Gen Physiol 136: 47–62, 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shields CR, Klooster J, Claassen Y, Ul-Hussain M, Zoidl G, Dermietzel R, Kamermans M. Retinal horizontal cell-specific promoter activity and protein expression of zebrafish connexin 52.6 and connexin 55.5. J Comp Neurol 501: 765–779, 2007. [DOI] [PubMed] [Google Scholar]
- Song PI, Matsui JI, Dowling JE. Morphological types and connectivity of horizontal cells found in the adult zebrafish (Danio rerio) retina. J Comp Neurol 506: 328–338, 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Srinivasan MV, Laughlin SB, Dubs A. Predictive coding: a fresh view of inhibition in the retina. Proc R Soc Lond B Biol Sci 216: 427–459, 1982. [DOI] [PubMed] [Google Scholar]
- Stell WK. Horizontal cell axons and axon terminals in goldfish retina. J Comp Neurol 159: 503–520, 1975. [DOI] [PubMed] [Google Scholar]
- Stell WK, Lightfoot DO. Color-specific interconnections of cones and horizontal cells in the retina of the goldfish. J Comp Neurol 159: 473–502, 1975. [DOI] [PubMed] [Google Scholar]
- Stell WK, Lightfoot DO, Wheeler TG, Leeper HF. Goldfish retina: functional polarization of cone horizontal cell dendrites and synapses. Science 190: 989–990, 1975. [DOI] [PubMed] [Google Scholar]
- Svaetichin G, MacNichol EF. Retinal mechanisms for chromatic and achromatic vision. Ann NY Acad Sci 74: 385–404, 1958. [DOI] [PubMed] [Google Scholar]
- Szikra T, Trenholm S, Drinnenberg A, Juettner J, Raics Z, Farrow K, Biel M, Awatramani GB, Clark DA, Sahel JA, da Silveira RA, Roska B. Rods in daylight act as relay cells for cone-driven horizontal cell-mediated surround inhibition. Nat Neurosci 17: 1728–1735, 2014. [DOI] [PubMed] [Google Scholar]
- Tomita T. Electrical activity in the vertebrate retina. J Opt Soc Am 53: 49–57, 1963. [DOI] [PubMed] [Google Scholar]
- Van den Pol AN, Gorcs T. Synaptic relationships between neurons containing vasopressin, gastrin-releasing peptide, vasoactive intestinal polypeptide, and glutamate decarboxylase immunoreactivity in the suprachiasmatic nucleus: dual ultrastructural immunocytochemistry with gold-substituted silver peroxidase. J Comp Neurol 252: 507–521, 1986. [DOI] [PubMed] [Google Scholar]
- Vanleeuwen MT, Joselevitch C, Fahrenfort I, Kamermans M. The contribution of the outer retina to color constancy: a general model for color constancy synthesized from primate and fish data. Vis Neurosci 24: 277–290, 2007. [DOI] [PubMed] [Google Scholar]
- Vroman R, Kamermans M. Feedback-induced glutamate spillover enhances negative feedback from horizontal cells to cones. J Physiol 593: 2927–2940, 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vroman R, Klaassen LJ, Howlett MH, Cenedese V, Klooster J, Sjoerdsma T, Kamermans M. Extracellular ATP hydrolysis inhibits synaptic transmission by increasing pH buffering in the synaptic cleft. PLoS Biol 12: e1001864, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Witkovsky P. A comparison of ganglion cell and S-potential response properties in carp retina. J Neurophysiol 30: 546–561, 1967. [DOI] [PubMed] [Google Scholar]
- Zoidl G, Bruzzone R, Weickert S, Kremer M, Zoidl C, Mitropoulou G, Srinivas M, Spray DC, Dermietzel R. Molecular cloning and functional expression of zfCx52.6: a novel connexin with hemichannel-forming properties expressed in horizontal cells of the zebrafish retina. J Biol Chem 279: 2913–2921, 2004. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.