ABSTRACT
To evaluate the bispectral index (BIS) as an indicator of anesthetic depth in Thoroughbred horses, BIS values were measured at multiple stages of sevoflurane anesthesia in five horses anesthetized with guaifenesin and thiopental following premedication with xylazine. There was no significant difference between the BIS values recorded at end-tidal sevoflurane concentrations of 2.8% (median 60 ranging from 47 to 68) and 3.5% (median 71 ranging from 49 to 82) in anesthetized horses. These BIS values during anesthesia were significantly lower (P<0.01) than those in awake horses (median 98 ranging from 98 to 98) or sedated horses (median 92 ranging from 80 to 93). During the recovery phase, the BIS values gradually increased over time but did not significantly increase until the horses showed movement. In conclusion, the BIS value could be useful as an indicator of awakening during the recovery period in horses, as previous reported.
Keywords: anesthetic depth, bispectral index, horse, sevoflurane
During equine surgery, anesthetic depth is mainly evaluated by monitoring the expired gas concentration, heart rate, blood pressure, and eye reflex. However, these indirect indicators do not always accurately reflect the anesthetic depth [16]. Therefore, the adjustment of anesthetic depth depends to a large extent on the experience of the anesthesiologist.
Bispectral index (BIS) monitoring system analyzes electroencephalogram (EEG) to quantify the activity of the central nervous system (CNS) with values from 0 (cortical silence) to 100 (awake) [8, 14, 15, 17]. In the human clinical field, BIS values are widely applied as the indicator of anesthetic depth. The BIS values of approximately 40–60 are reported as necessary to prevent the awakening during surgery [4, 12, 18]. It is also reported that the BIS correctly predicts the degree of CNS depression associated with isoflurane-induced anesthesia [4, 12, 18]. On the other hand, the BIS has been reported as an unreliable indicator of anesthetic depth during propofol or isoflurane anesthesia in horses [6, 19]. Others report that BIS indicates hypnosis levels but does not predict intraoperative movement during halothane and sevoflurane anesthesia in horses [1]. In the horse field, BIS values decrease under general anesthesia and increase in the recovery phase, whereas they cannot be useful as an indicator of anesthetic depth. However, there is no information available regarding use of BIS values under surgical and deep planes of anesthesia in sevoflurane-anesthetized horses. In addition, a direct indicator of anesthetic depth is needed because unpredictable awakening during surgery is not only stressful for the horse but also dangerous for both the horse and operating staff.
The purpose of this study was to validate the BIS as an indicator of anesthetic depth in Thoroughbred horses during sevoflurane anesthesia. In this study, BIS values were measured under sedation, during maintenance of inhalation anesthesia at two different end-tidal sevoflurane concentrations (ETSEVO), and during recovery from anesthesia until the first movements were made.
A protocol for the study was reviewed and approved by the Animal Use and Care Committee and the Animal Welfare and Ethics Committee of the Japan Racing Association’s Miho Training Center (Approved no. 2009-M2). Five Thoroughbred horses were used in this portion of the study (mean ± SD age, 3.2 ± 1.1 years; mean ± SD body weight, 460 ± 18 kg). Food, but not water, was withheld for 12 hr prior to anesthesia. A 14-gauge catheter was placed in the external jugular vein for drug and fluid administration. All horses were premedicated with xylazine (1.0 mg/kg, IV) (Celactar, Bayer, Osaka, Japan), and anesthesia was induced by a rapid injection of 5% guaifenesin (1,000 ml/head, IV) (5% Guaifenesin, Shinyo Pure Chemicals Co., Ltd., Osaka, Japan) with thiopental sodium (approximately 2.0 g/head) (Ravonal, Mitsubishi Tanabe Pharma Co., Osaka, Japan). After the induction of anesthesia, the horses were intubated endotracheally and positioned in lateral recumbency on a padded surgical table. Anesthesia was maintained with sevoflurane (Sevofrane, Maruishi Pharmaceutical Co., Ltd., Osaka, Japan) and oxygen using intermittent positive pressure ventilation (MOK 94, Silver Medical Co., Tokyo, Japan) at a rate of 8 to 12 breaths/min with a peak airway pressure of 25 cmH2O to maintain the arterial carbon dioxide partial pressure (PaCO2) between 45 and 55 mmHg.
A base-apex lead electrocardiogram was used to monitor heart rate (HR) and rhythm. A 20-gauge catheter was placed in the facial artery for measurement of systemic arterial blood pressure. Arterial blood pressures were measured directly through the catheter by a transducer system. Respiratory gas was collected continuously from the circuit end of the endotracheal tube, and ETSEVO was determined by infrared absorption. ETSEVO, HR, systolic arterial blood pressure (SAP), diastolic arterial blood pressure (DAP), and mean arterial blood pressure (MAP) were recorded every 5 min by an anesthesia monitoring system (BP608, Omron Colin Co., Ltd., Tokyo, Japan) until the end of anesthesia. Arterial blood samples were collected every 15 min, and PaCO2, arterial oxygen partial pressure (PaO2), and arterial pH were immediately analyzed by a blood-gas analyzer (ABL800 FLEX, Radiometer Co., Ltd., Tokyo, Japan). Lactated Ringer’s solution (Hartmann’s solution, Nipro Pharma Co., Osaka, Japan) was administered at a rate of approximately 10 ml/kg/hr throughout anesthesia. Dobutamine (Dobutrex, Shionogi & Co., Ltd., Osaka, Japan) was infused to maintain the MAP between 60 and 80 mmHg.
Based on a previous report, BIS values were measured using subdermal spiral needle electrodes (TN204-035, Unique Medical Co., Ltd., Tokyo, Japan) in all horses [19]. These electrodes were placed on the frontal and temporal cephalic regions and connected to a BIS monitor (A-3000, Aspect Medical Systems, Natick, MA, U.S.A.). The BIS values were recorded before administration (awake) and at 5 to 10 min after administration with xylazine (1.0 mg/kg, IV) (sedated). After induction, the ETSEVO was adjusted to 2.8% within 0 min. The ETSEVO was maintained at 2.8% until 45 min after the start of inhalation anesthesia, and BIS values were recorded for 40 to 45 min. The ETSEVO was then maintained at 3.5% until 60 min, and BIS values were recorded for 55 to 60 min. After the endotracheal tube was disconnected from the anesthetic circuit, the horses were immediately transported to a darkened recovery room and allowed to recover without assistance. No horse was given additional drugs prior to recovery. The BIS values at the end of anesthesia, appearance of spontaneous breathing, nystagmus, and movement were recorded.
The mean values for each 5-min BIS recording (awake, sedated, or anesthetized at ETSEVO of 2.8% or 3.5%) were calculated for every horse. The BIS values during anesthesia or recovery were analyzed by one-way repeated-measures analysis of variance (ANOVA). The Tukey-Kramer test for multiple comparisons was applied, when significant differences were identified. The BIS values are presented as median of data from their range, and P<0.05 was considered statistically significant.
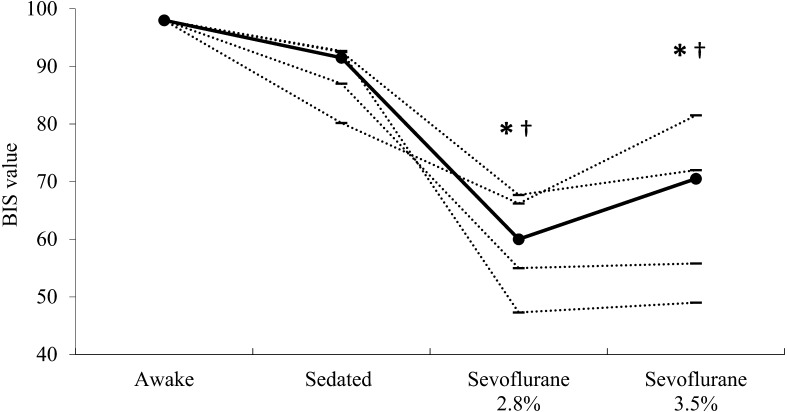
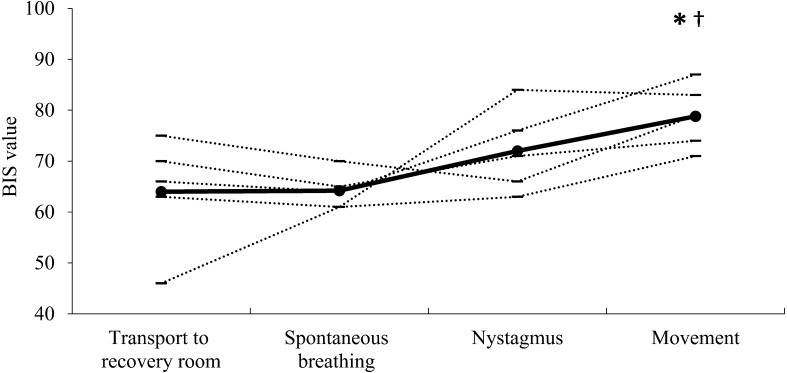
The individual BIS values during awake and sedated states and under anesthesia at ETSEVO of 2.8% and 3.5% are shown in Fig. 1. The median (range) BIS values were 60 (from 47 to 68) and 71 (from 49 to 82) at ETSEVO of 2.8% and 3.5%, respectively, in anesthetized horses. These values were significantly lower during anesthesia (P<0.01) than those in awake horses (median 98 ranging from 98 to 98) or sedated horses (median 92 ranging from 80 to 93). Mean cardiopulmonary values and PaCO2 were maintained within the target values throughout the maintenance period in each horse (data not shown). The individual BIS values during recovery are shown in Fig. 2. The BIS values during movement (median 79 ranging from 71 to 87) were significantly higher (P<0.01) than those at transport to recovery room (median 66 ranging from 46 to 75) and at the appearance of spontaneous breathing (median 64 ranging from 61 to 70).
Fig. 1.
Bispectral index (BIS) values determined from EEG of healthy horses before (awake) and after (sedated) premedication with xylazine and at 2.8% and 3.5% of the end-tidal sevoflurane concentration (n=5, respectively). Horizontal lines indicate values for individual horses; filled-in circles represent the median value for each state. *Significantly different (P<0.01) from the median value for awake horses. †Significantly different (P<0.01) from the median value for the sedated horses.
Fig. 2.
Mean bispectral index (BIS) values at transport to the recovery room and at the appearance of spontaneous breathing, nystagmus and movement (n=5, respectively). Horizontal lines indicate values for individual horses; filled-in circles represent the median value for each state. *Significantly different (P<0.01) from the median value at transport to the recovery room. †Significantly different (P<0.01) from the median value at the appearance of spontaneous breathing.
In humans, BIS values are used as an index of the depth of sevoflurane anesthesia. In addition, BIS values and sevoflurane concentrations correlate closely with sedation score [10, 19]. However, it is reported that the BIS values correlate only with ETSEVO below 1.5% [13]. In this study, the BIS values of horses under anesthesia at ETSEVO of 2.8% and 3.5% were significantly lower than those of horses that were awake or under sedation. However, there were no significant a dose-related decrease in the BIS values at the two different end-tidal concentrations unless these concentrations correspond to surgical and deep planes of anesthesia. This result is similar to the previous report in isoflurane-anesthetized horses [6]. It was speculated that several factors, including EEG patterns during inhalation anesthesia, the algorithm used for BIS measurements, and anesthetic induction agents, could be associated with the paradoxical changes in the BIS values in the present study.
A paradoxical increase in BIS has also been detected with increasing isoflurane concentrations in humans [3]. Others have reported that the BIS increased paradoxically as the ETSEVO increased from 3.0 to 4.0% in children aged 6 months to 12 years [11]. Detsch et al. [3] reported that a paradoxical increase in BIS could be related to continuous pre-burst EEG patterns consisting of high-frequency activity. Haga et al. [6] reported that the increase in BIS observed in horses anesthetized at 1.9% isoflurane, compared with 1.4%, could be a general effect of isoflurane that develops prior to a high degree of burst suppression. In this study, BIS increased, but not significantly, in horses anesthetized at 3.5% sevoflurane compared with 2.8%. In horses anesthetized with sevoflurane, a paradoxical increase in BIS similar to that in previous reports might occur [3, 6, 11]. It was hypothesized that the BIS might not be accurate depending on the concentration of inhalation anesthetics.
The algorithm for measuring BIS values was originally developed using human EEG and might produce potential errors in the evaluation of anesthetic depth. The BIS values considered indicative of hypnosis in humans were 40–65 for general anesthesia [9]. On the other hand, it was reported that the mean ± SD BIS value in sevoflurane-anesthetized canines at ETSEVO of 1.0 MAC was 73 ± 5 [5]. As with canines, the BIS values in this study were somewhat higher than those in humans. The possible causes of this difference in BIS values were suggested to be the differences in size, density, and shape of their skulls and nervous tissues [1, 7, 14]. Unfortunately, the reliabilities of BIS values were not confirmed because a signal quality index (SQI) and the suppression rate (SR) were not recorded for each BIS measurement in the present study. However, it was considered that the device’s algorithm is less capable of deriving accurate BIS values when applied to horses, as with previous reports [1].
Finally, the longer lasting anesthetic effects of the induction agents might have had an influence on anesthetic depth when the BIS values were measured at the ETSEVO of 2.8% and 3.5% in the horses. Because both thiopental sodium and guaifenesin bind to the gamma-aminobutyric acid A receptors in the central nervous system and produce unconsciousness or muscle relaxation, it is considered that they have effects that cause variability in BIS values. In this study, the BIS in the 3.5% sevoflurane group was measured 10 min after measurement of that in the 2.8% group. Consequently, the effect of induction agents on BIS was lower in the 3.5% group compared with the 2.8% group. This was why there were no significant differences between the BIS values at ETSEVO of 2.8% and 3.5%.
BIS values and sevoflurane concentrations below 1.5% correlate with the sedation score in humans [13]. This indicates that BIS values during the recovery phase can be useful as an indicator of awakening because of elimination of sevoflurane from the body. In this study, BIS values increased gradually over time during the recovery period; the BIS values at the time of movement were significantly higher than those at transport to the recovery room and at the appearance of spontaneous breathing. It was suggested that the BIS could predict awakening in the recovery phase after sevoflurane anesthesia. These results are similar to those of previous reports for horses recovering from sevoflurane [1] or propofol anesthesia [19]. On the other hand, the rising trend of BIS values differed among individuals. It was likely that the increase in BIS during the recovery from anesthesia was related to an increase in electromyographic (EMG) power. In humans, increasing EMG power is known to increase BIS values [2]. However, EMG power, SQI, and SR were not measured in this study. Therefore, the present study could not confirm that the increase in BIS value during recovery was produced by changes in EEG alone or in combination with changes in EMG power. Furthermore, the study was considered to have limitations because the EMG power may have been involved in the increase in BIS values during the recovery phase. To evaluate anesthetic depth precisely, it may be important for anesthesiologists to monitor the changes in BIS values carefully along with EMG power, SQI, and SR.
It is considered that the present study might show both good and bad aspects of BIS values in the evaluation of anesthetic depth in horses anesthetized with sevoflurane, although it included several limitations, as mentioned above. In conclusion, the BIS value could be useful as an indicator of awakening in horses during the recovery period, similar to previous reports in horses anesthetized with isoflurane [6] and propofol [19].
References
- 1.Belda E., Blissitt K.J., Duncan J.C., Laredo F.G., Escobar Gil de Montes M., Clutton R.E. 2010. The bispectral index during recovery from halothane and sevoflurane anaesthesia in horses. Vet. Anaesth. Analg. 37: 25–34. [DOI] [PubMed] [Google Scholar]
- 2.Bruhn J., Bouillon T.W., Shafer S.L. 2000. Electromyographic activity falsely elevates the bispectral index. Anesthesiology 92: 1485–1487. [DOI] [PubMed] [Google Scholar]
- 3.Detsch O., Schneider G., Kochs E., Hapfelmeier G., Werner C. 2000. Increasing isoflurane concentration may cause paradoxical increases in the EEG bispectral index in surgical patients. Br. J. Anaesth. 84: 33–37. [DOI] [PubMed] [Google Scholar]
- 4.Glass P.S., Bloom M., Kearse L., Rosow C., Sebel P., Manberg P. 1997. Bispectral analysis measures sedation and memory effects of propofol, midazolam, isoflurane, and alfentanil in healthy volunteers. Anesthesiology 86: 836–847. [DOI] [PubMed] [Google Scholar]
- 5.Greene S.A., Benson G.J., Tranquilli W.J., Grimm K.A. 2002. Relationship of canine bispectral index to multiples of sevoflurane minimal alveolar concentration, using patch or subdermal electrodes. Comp. Med. 52: 424–428. [PubMed] [Google Scholar]
- 6.Haga H.A., Dolvik N.I. 2002. Evaluation of the bispectral index as an indicator of degree of central nervous system depression in isoflurane-anesthetized horses. Am. J. Vet. Res. 63: 438–442. [DOI] [PubMed] [Google Scholar]
- 7.Hans P., Dewandre P.Y., Brichant J.F., Bonhomme V. 2005. Comparative effects of ketamine on Bispectral Index and spectral entropy of the electroencephalogram under sevoflurane anaesthesia. Br. J. Anaesth. 94: 336–340. [DOI] [PubMed] [Google Scholar]
- 8.Johansen J.W. 2006. Update on bispectral index monitoring. Best Pract. Res. Clin. Anaesthesiol. 20: 81–99. [DOI] [PubMed] [Google Scholar]
- 9.Johansen J.W., Sebel P.S., Sigl J.C. 2000. Clinical impact of hypnotic-titration guidelines based on EEG bispectral index (BIS) monitoring during routine anesthetic care. J. Clin. Anesth. 12: 433–443. [DOI] [PubMed] [Google Scholar]
- 10.Katoh T., Suzuki A., Ikeda K. 1998. Electroencephalographic derivatives as a tool for predicting the depth of sedation and anesthesia induced by sevoflurane. Anesthesiology 88: 642–650. [DOI] [PubMed] [Google Scholar]
- 11.Kim H.S., Oh A.Y., Kim C.S., Kim S.D., Seo K.S., Kim J.H. 2005. Correlation of bispectral index with end-tidal sevoflurane concentration and age in infants and children. Br. J. Anaesth. 95: 362–366. [DOI] [PubMed] [Google Scholar]
- 12.Olofsen E., Dahan A. 1999. The dynamic relationship between end-tidal sevoflurane and isoflurane concentrations and bispectral index and spectral edge frequency of the electroencephalogram. Anesthesiology 90: 1345–1353. [DOI] [PubMed] [Google Scholar]
- 13.Rinaldi S., Consales G., De Gaudio A.R. 2007. State entropy and bispectral index: correlation with end tidal sevoflurane concentrations. Minerva Anestesiol. 73: 39–48. [PubMed] [Google Scholar]
- 14.Rosow C., Manberg P.J. 2001. Bispectral index monitoring. Anesthesiol. Clin. North America 19: 947–966, xi. [DOI] [PubMed] [Google Scholar]
- 15.Sigl J.C., Chamoun N.G. 1994. An introduction to bispectral analysis for the electroencephalogram. J. Clin. Monit. 10: 392–404. [DOI] [PubMed] [Google Scholar]
- 16.Struys M.M., Jensen E.W., Smith W., Smith N.T., Rampil I., Dumortier F.J., Mestach C., Mortier E.P. 2002. Performance of the ARX-derived auditory evoked potential index as an indicator of anesthetic depth: a comparison with bispectral index and hemodynamic measures during propofol administration. Anesthesiology 96: 803–816. [DOI] [PubMed] [Google Scholar]
- 17.Tirel O., Wodey E., Harris R., Bansard J.Y., Ecoffey C., Senhadji L. 2008. Variation of bispectral index under TIVA with propofol in a paediatric population. Br. J. Anaesth. 100: 82–87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Vernon J.M., Lang E., Sebel P.S., Manberg P. 1995. Prediction of movement using bispectral electroencephalographic analysis during propofol/alfentanil or isoflurane/alfentanil anesthesia. Anesth. Analg. 80: 780–785. [DOI] [PubMed] [Google Scholar]
- 19.Yamashita K., Akashi N., Katayama Y., Uchida Y., Umar M.A., Itami T., Inoue H., Sams R.A., Muir W.W. 3rd. 2009. Evaluation of bispectral index (BIS) as an indicator of central nervous system depression in horses anesthetized with propofol. J. Vet. Med. Sci. 71: 1465–1471. [DOI] [PubMed] [Google Scholar]