Abstract
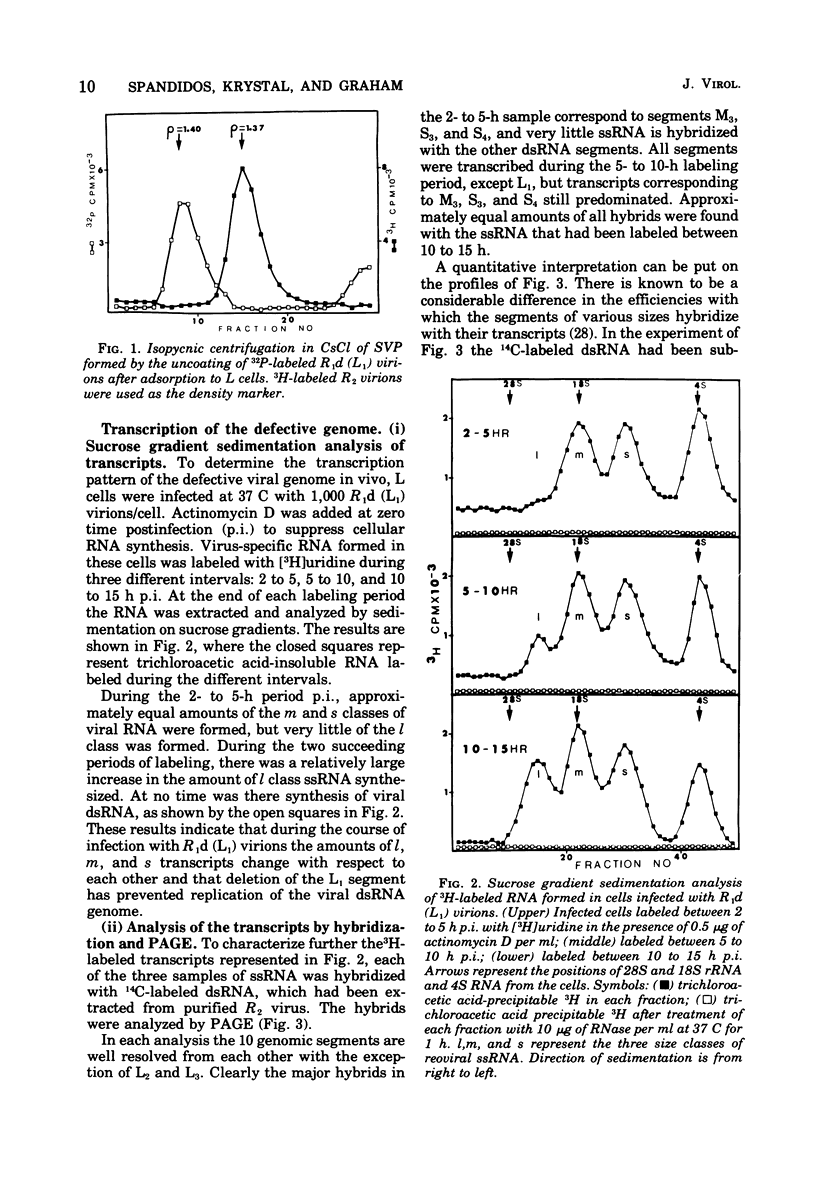
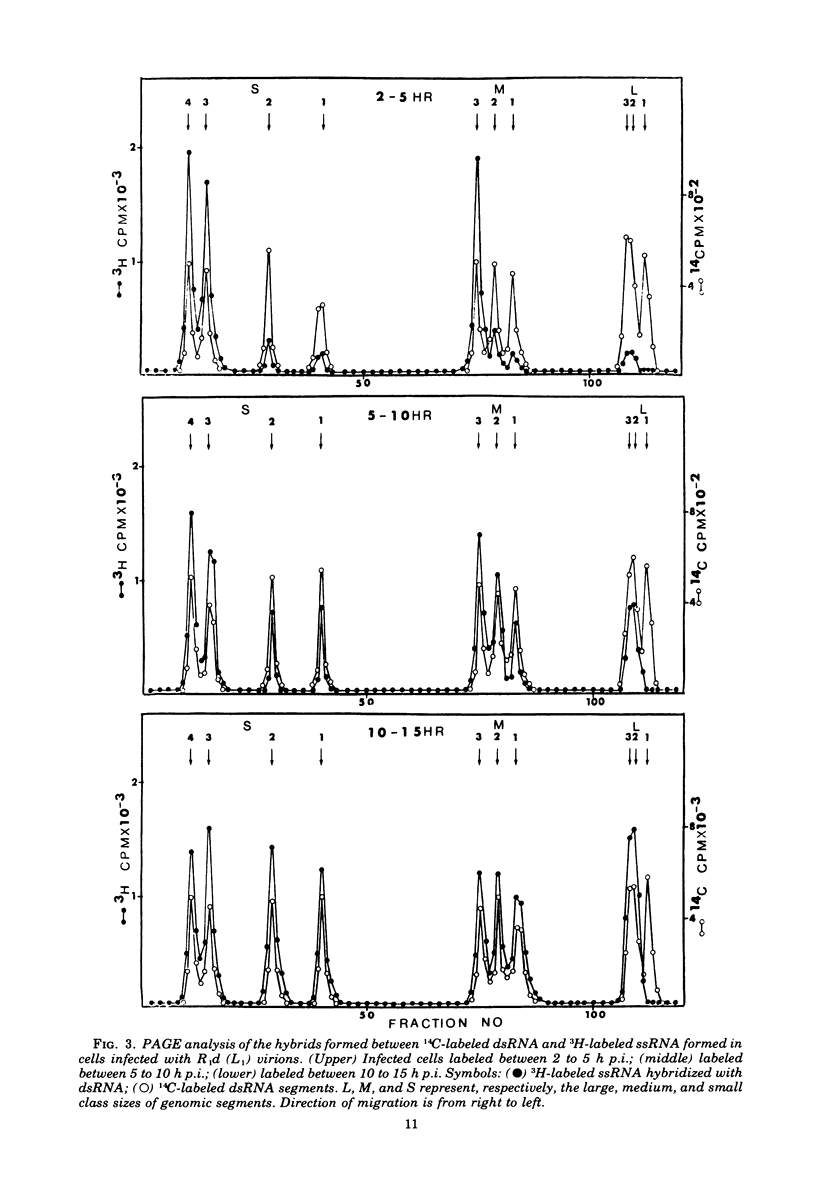
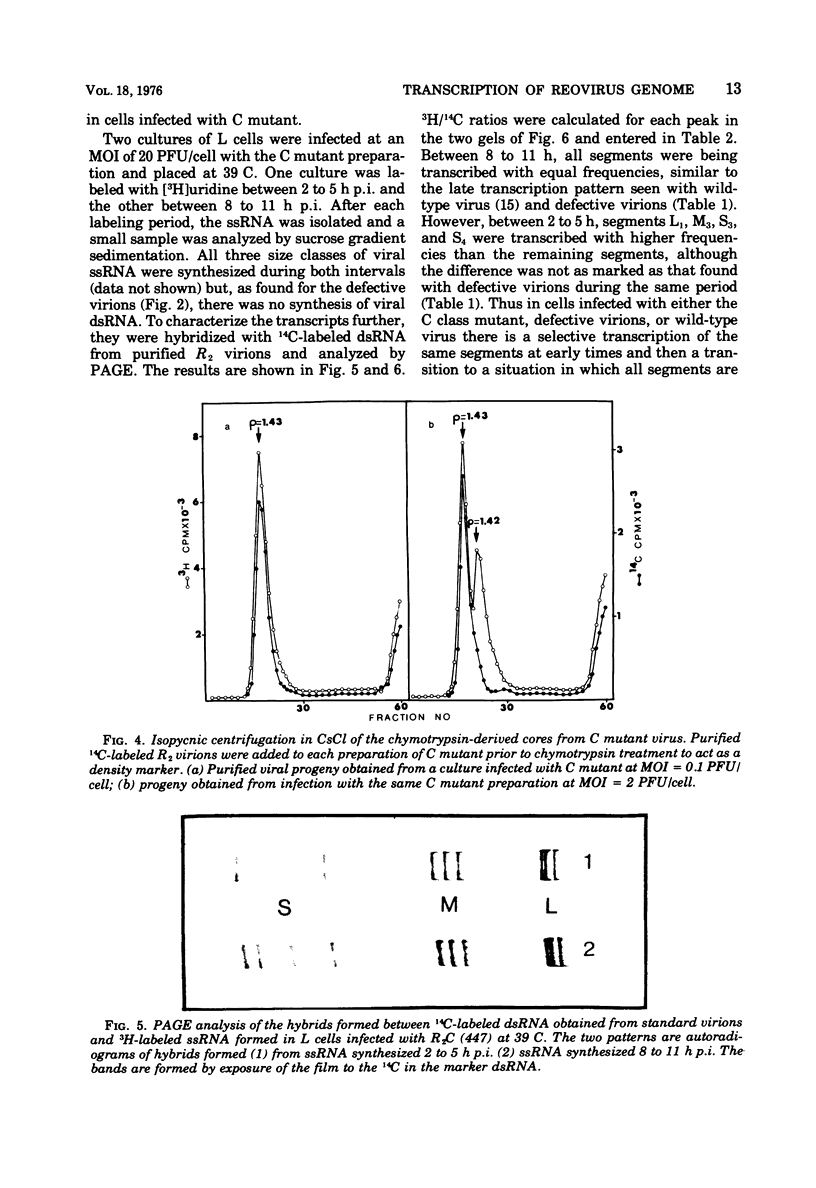
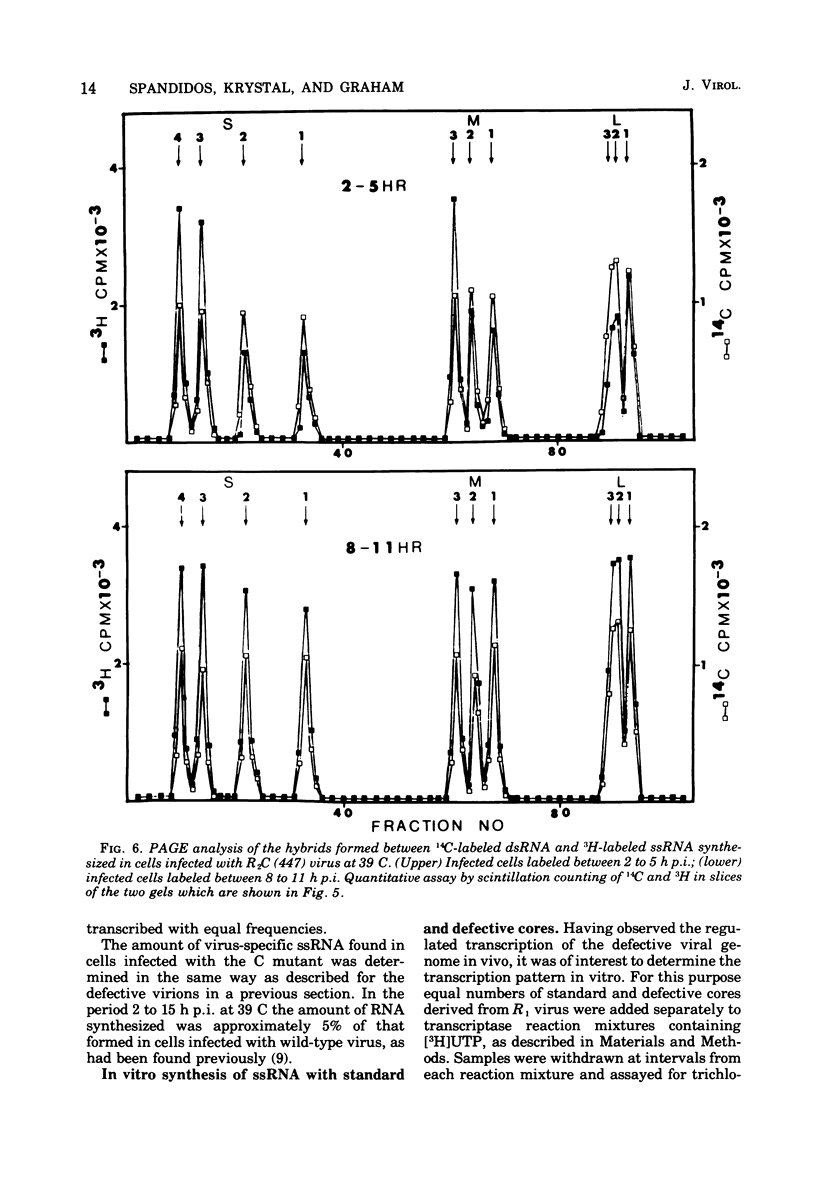
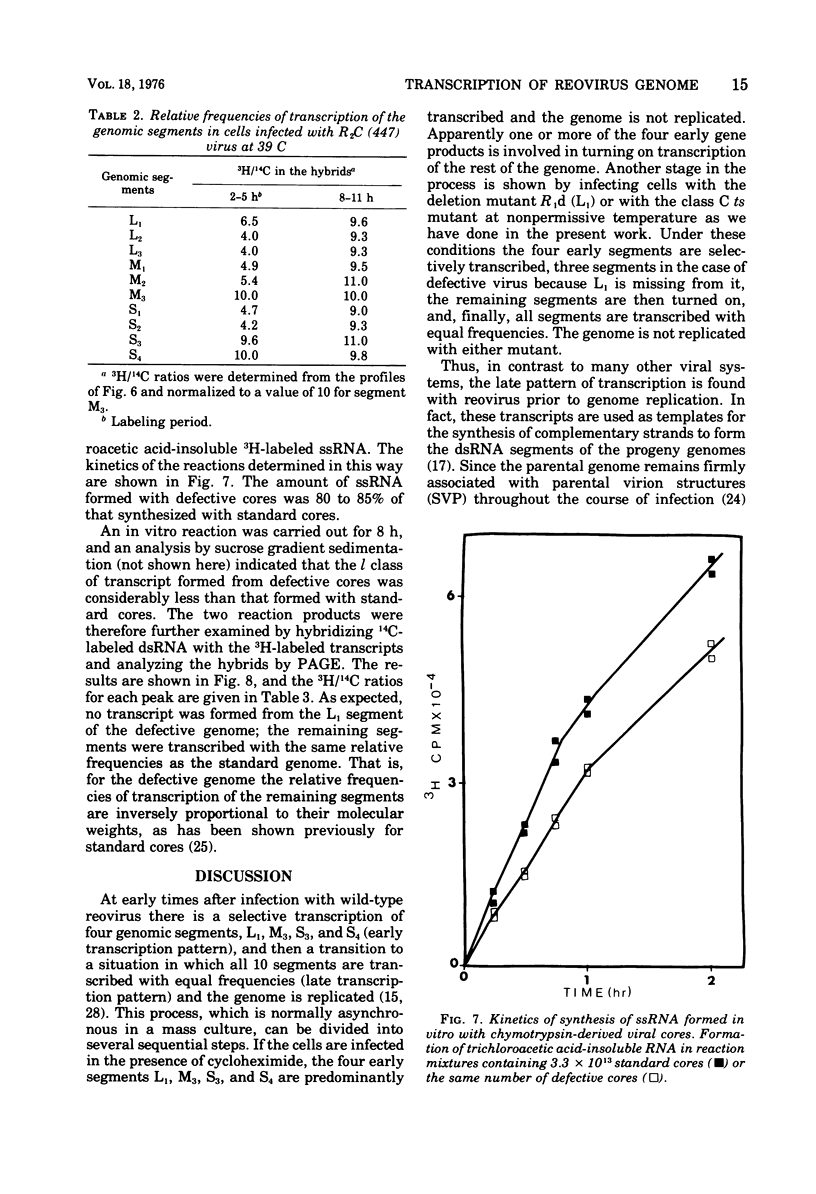
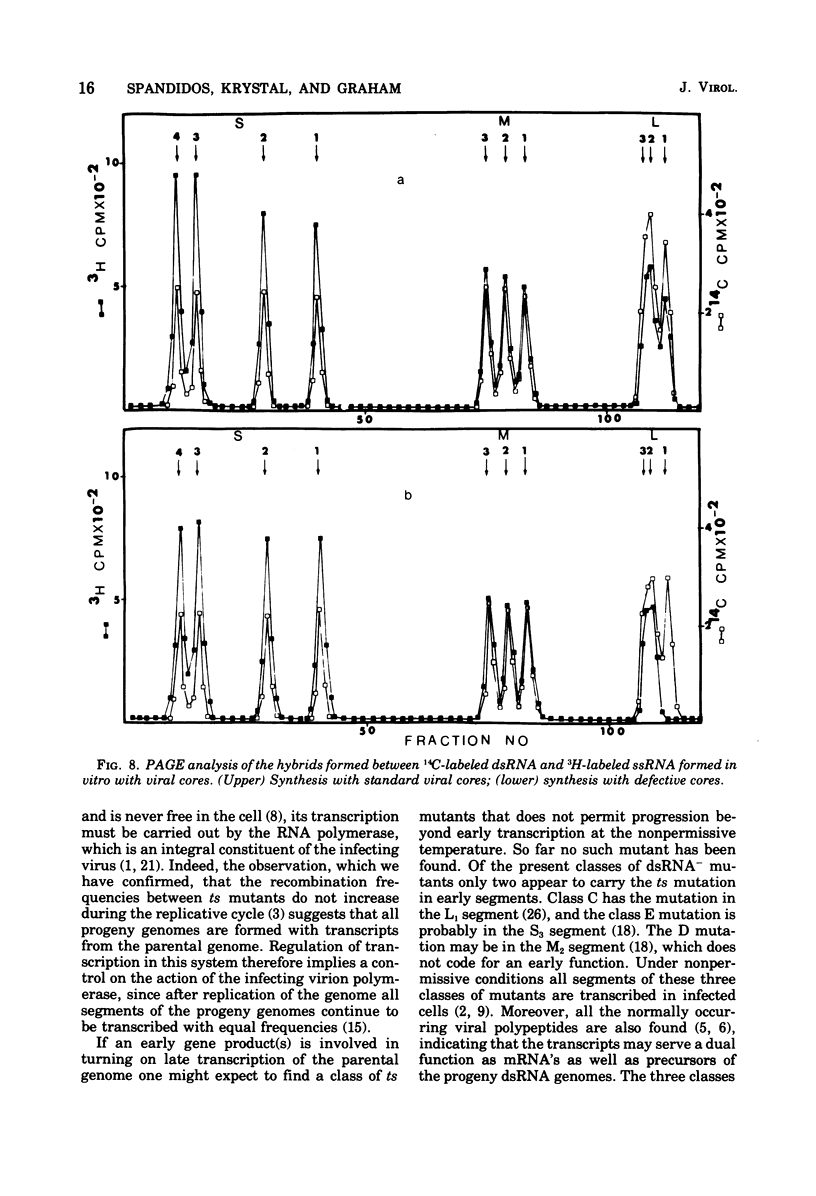
Defective reovirus, which lacks the largest (L1) of the 10 double-stranded (ds) RNA genomic segments, attaches to L cells and is uncoated in the same way as reovirus. The defective genome does not replicate in the cells, but it is transcribed. During the first 5 h after infection, three of the genomic segments, M3, S3, and S4, are more frequently transcribed than the remaining six segments. During the succeeding 5 h, there is a transition to a situation in which all nine segments are transcribed at the same relative frequencies. Since the class C ts mutation has been allocated to the L1 segment (Spandidos and Graham, 1975) the transcription of the C mutant genome was investigated in cells infected with it at the nonpermissive temperature, at which the parental genome does not replicate. Genomic segments L1, M3, S3, and S4 are predominantly transcribed at early times, and later all 10 segments are transcribed with the same relative frequencies. Transcription of the defective viral genome and the C mutant genome is therefore regulated in the same way as previously found for wild-type virus (Nonoyama, Millward, and Graham, 1974), and the regulation is independent of genome replication. Apparently the L1 segment function is involved in dsRNA synthesis but not in regulating the early to late transcription. It is suggested that a cellular repressor may be involved in this regulation and that derepression might be effected by one of the early viral gene products. Virion transcriptase activity was studied in vitro with cores prepared by chymotrypsin digestion of purified defective and standard virions. For both genomes the relative frequencies of transcription of the dsRNA segments are inversely proportional to their molecular weights. These results can be accounted for in a model that postulates each segment to be transcribed independently of the other. The same model with certain restrictions can describe the in vivo transcription of the viral genome.
Full text
PDF






Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Borsa J., Graham A. F. Reovirus: RNA polymerase activity in purified virions. Biochem Biophys Res Commun. 1968 Dec 30;33(6):895–901. doi: 10.1016/0006-291x(68)90396-3. [DOI] [PubMed] [Google Scholar]
- Cross R. K., Fields B. N. Temperature-sensitive mutants of reovirus type 3: studies on the synthesis of viral RNA. Virology. 1972 Dec;50(3):799–809. doi: 10.1016/0042-6822(72)90434-5. [DOI] [PubMed] [Google Scholar]
- Fields B. N., Joklik W. K. Isolation and preliminary genetic and biochemical characterization of temperature-sensitive mutants of reovirus. Virology. 1969 Mar;37(3):335–342. doi: 10.1016/0042-6822(69)90217-7. [DOI] [PubMed] [Google Scholar]
- Fields B. N., Laskov R., Scharff M. D. Temperature-sensitive mutants of reovirus type 3: studies on the synthesis of viral peptides. Virology. 1972 Oct;50(1):209–215. doi: 10.1016/0042-6822(72)90361-3. [DOI] [PubMed] [Google Scholar]
- Fields B. N., Raine C. S., Baum S. G. Temperature-sensitive mutants of reovirus type 3: defects in viral maturation as studied by immunofluorescence and electron microscopy. Virology. 1971 Mar;43(3):569–578. doi: 10.1016/0042-6822(71)90282-0. [DOI] [PubMed] [Google Scholar]
- Fields B. N. Temperature-sensitive mutants of reovirus type 3 features of genetic recombination. Virology. 1971 Oct;46(1):142–148. doi: 10.1016/0042-6822(71)90013-4. [DOI] [PubMed] [Google Scholar]
- Gillies S., Bullivant S., Bellamy A. R. Viral RNA polymerases: electron microscopy of reovirus reaction cores. Science. 1971 Nov 12;174(4010):694–696. doi: 10.1126/science.174.4010.694. [DOI] [PubMed] [Google Scholar]
- Gomatos P. J. RNA synthesis in reovirus-infected L929 mouse fibroblasts. Proc Natl Acad Sci U S A. 1967 Oct;58(4):1798–1805. doi: 10.1073/pnas.58.4.1798. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ito Y., Joklik W. K. Temperature-sensitive mutants of reovirus. I. Patterns of gene expression by mutants of groups C, D, and E. Virology. 1972 Oct;50(1):189–201. doi: 10.1016/0042-6822(72)90359-5. [DOI] [PubMed] [Google Scholar]
- Ito Y., Joklik W. K. Temperature-sensitive mutants of reovirus. II. Anomalous electrophoretic migration of certain hybrid RNA molecules composed of mutant plus strands and wild-type minus strands. Virology. 1972 Oct;50(1):202–208. doi: 10.1016/0042-6822(72)90360-1. [DOI] [PubMed] [Google Scholar]
- Lau R. Y., Van Alstyne D., Berckmans R., Graham A. F. Synthesis of reovirus-specific polypeptides in cells pretreated with cycloheximide. J Virol. 1975 Sep;16(3):470–478. doi: 10.1128/jvi.16.3.470-478.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matsuhisa T., Joklik W. K. Temperature-sensitive mutants of reovirus. V. Studies on the nature of the temperature-sensitive lesion of the group C mutant ts447. Virology. 1974 Aug;60(2):380–389. doi: 10.1016/0042-6822(74)90333-x. [DOI] [PubMed] [Google Scholar]
- Nonoyama M., Millward S., Graham A. F. Control of transcription of the reovirus genome. Nucleic Acids Res. 1974 Mar;1(3):373–385. doi: 10.1093/nar/1.3.373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nonoyama M., Watanabe Y., Graham A. F. Defective virions of reovirus. J Virol. 1970 Aug;6(2):226–236. doi: 10.1128/jvi.6.2.226-236.1970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schonberg M., Silverstein S. C., Levin D. H., Acs G. Asynchronous synthesis of the complementary strands of the reovirus genome. Proc Natl Acad Sci U S A. 1971 Feb;68(2):505–508. doi: 10.1073/pnas.68.2.505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schuerch A. R., Joklik W. K. Temperature-sensitive mutants of reovirus. IV. Evidence that anomalous electrophoretic migration behavior of certain double-stranded RNA hybrid species is mutant group-specific. Virology. 1973 Nov;56(1):218–229. doi: 10.1016/0042-6822(73)90301-2. [DOI] [PubMed] [Google Scholar]
- Schuerch A. R., Matsuhisa T., Joklik W. K. Temperature-sensitive mutants of reovirus. VI. Mutant ts 447 and ts 556 particles that lack either one or two genome RNA segments. Intervirology. 1974;3(1-2):36–46. doi: 10.1159/000149740. [DOI] [PubMed] [Google Scholar]
- Shatkin A. J., LaFiandra A. J. Transcription by infectious subviral particles of reovirus. J Virol. 1972 Oct;10(4):698–706. doi: 10.1128/jvi.10.4.698-706.1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shatkin A. J., Sipe J. D. RNA polymerase activity in purified reoviruses. Proc Natl Acad Sci U S A. 1968 Dec;61(4):1462–1469. doi: 10.1073/pnas.61.4.1462. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Silverstein S. C., Schonberg M., Levin D. H., Acs G. The reovirus replicative cycle: conservation of parental RNA and protein. Proc Natl Acad Sci U S A. 1970 Sep;67(1):275–281. doi: 10.1073/pnas.67.1.275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith R. E., Zweerink H. J., Joklik W. K. Polypeptide components of virions, top component and cores of reovirus type 3. Virology. 1969 Dec;39(4):791–810. doi: 10.1016/0042-6822(69)90017-8. [DOI] [PubMed] [Google Scholar]
- Spandidos D. A., Graham A. F. Complementation between temperature-sensitive and deletion mutants of reovirus. J Virol. 1975 Dec;16(6):1444–1452. doi: 10.1128/jvi.16.6.1444-1452.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spandidos D. A., Graham A. F. Complementation of defective reovirus by ts mutants. J Virol. 1975 Apr;15(4):954–963. doi: 10.1128/jvi.15.4.954-963.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Watanabe Y., Millward S., Graham A. F. Regulation of transcription of the Reovirus genome. J Mol Biol. 1968 Aug 28;36(1):107–123. doi: 10.1016/0022-2836(68)90223-4. [DOI] [PubMed] [Google Scholar]
- Zweerink H. J., Joklik W. K. Studies on the intracellular synthesis of reovirus-specified proteins. Virology. 1970 Jul;41(3):501–518. doi: 10.1016/0042-6822(70)90171-6. [DOI] [PubMed] [Google Scholar]




