Abstract
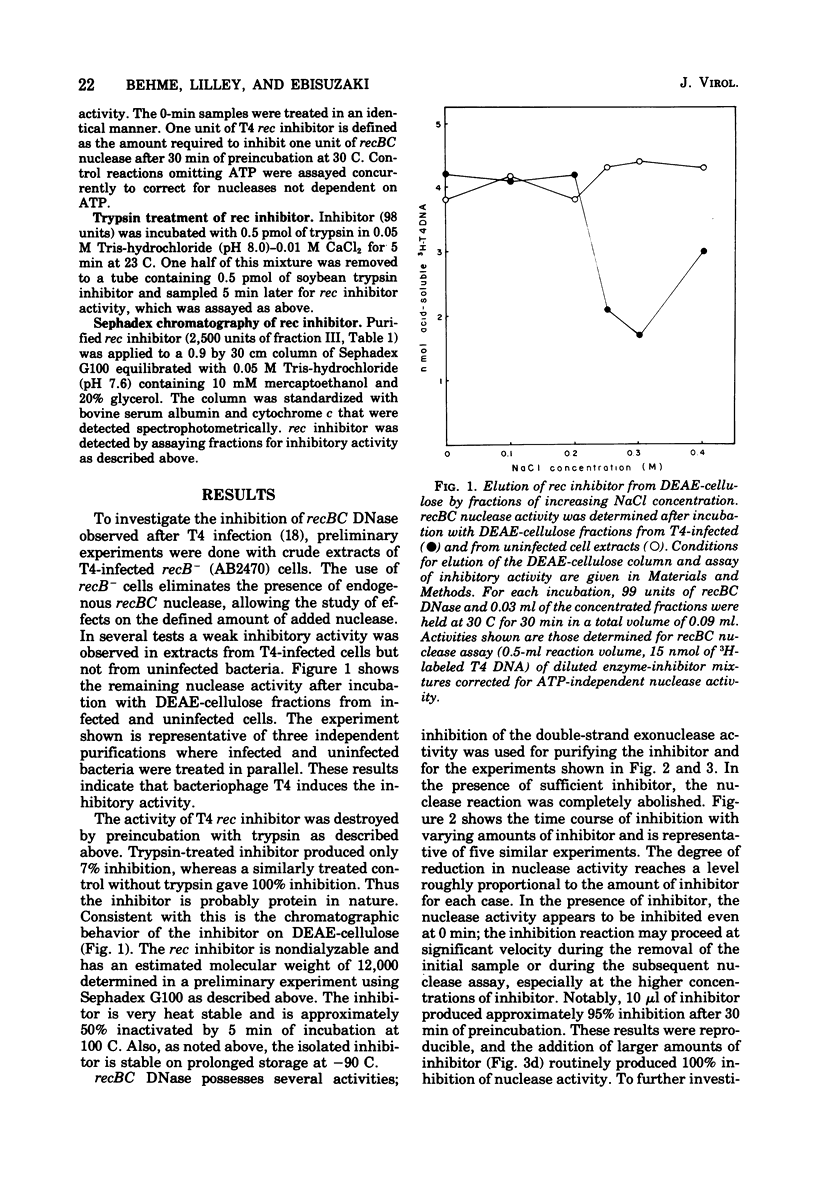
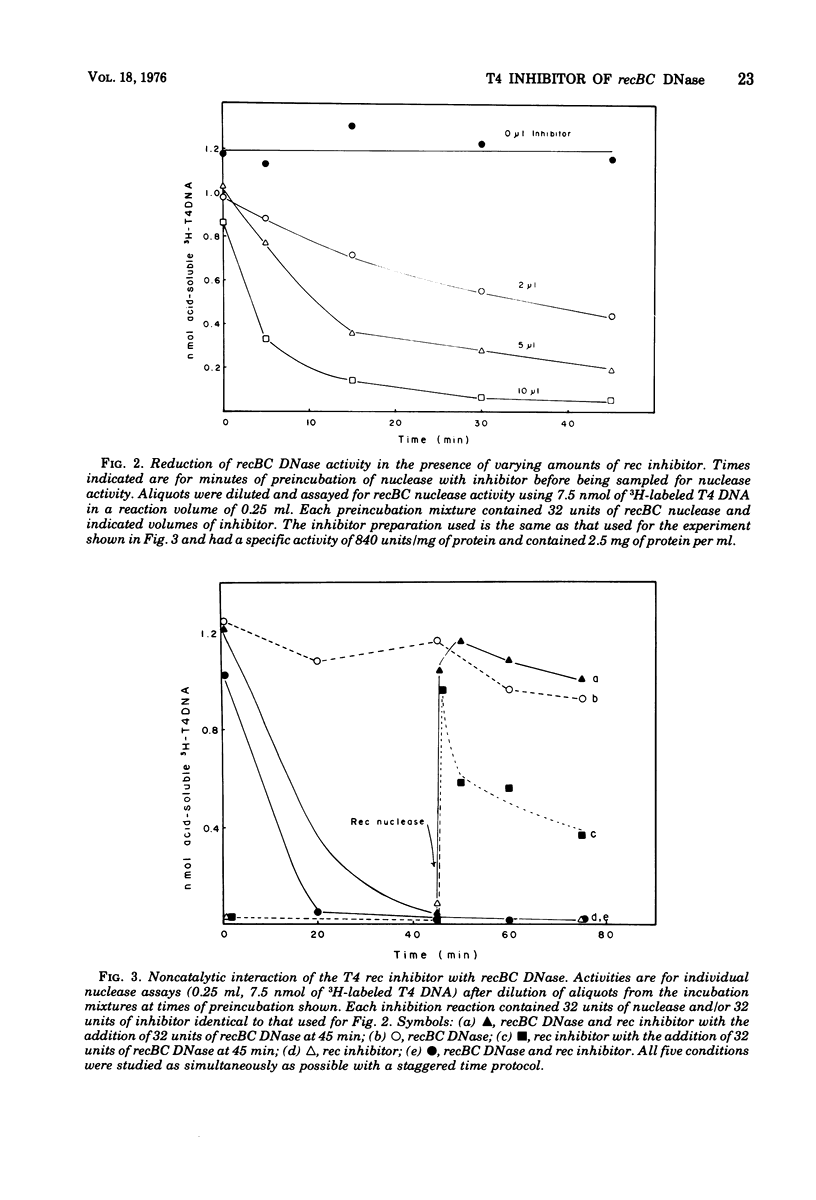
Infection by bacteriophage T4 has previously been shown to cause a rapid inhibition of the host recBC DNase, an ATP-dependent DNase that is required for genetic recombination in Escherichia coli. We report here the partial purification of a protein ("T4 rec inhibitor") from extracts of T4-infected cells and some characteristics of the in vitro inhibition reaction with purified inhibitor and recBC nuclease. This inhibitory activity could not be purified from extracts of uninfected E. coli. Both the ATP-dependent exonuclease and DNA-dependent ATPase activities of recBC DNase are inhibited by T4 rec inhibitor. Experiments suggest that the inhibitor interacts with the nuclease in a stoichiometric manner. The biological significance of this inhibition is discussed with respect to control reactions in phage-infected cells.
Full text
PDF



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Barbour S. D., Clark A. J. Biochemical and genetic studies of recombination proficiency in Escherichia coli. I. Enzymatic activity associated with recB+ and recC+ genes. Proc Natl Acad Sci U S A. 1970 Apr;65(4):955–961. doi: 10.1073/pnas.65.4.955. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Debreceni N., Behme M. T., Ebisuzaki K. A DNA-dependent ATPase from E. coli infected with bacteriophaget4. Biochem Biophys Res Commun. 1970 Oct 9;41(1):115–121. doi: 10.1016/0006-291x(70)90476-6. [DOI] [PubMed] [Google Scholar]
- Enquist L. W., Skalka A. Replication of bacteriophage lambda DNA dependent on the function of host and viral genes. I. Interaction of red, gam and rec. J Mol Biol. 1973 Apr 5;75(2):185–212. doi: 10.1016/0022-2836(73)90016-8. [DOI] [PubMed] [Google Scholar]
- Goff C. G. Chemical structure of a modification of the Escherichia coli ribonucleic acid polymerase alpha polypeptides induced by bacteriophage T4 infection. J Biol Chem. 1974 Oct 10;249(19):6181–6190. [PubMed] [Google Scholar]
- Goldmark P. J., Linn S. Purification and properties of the recBC DNase of Escherichia coli K-12. J Biol Chem. 1972 Mar 25;247(6):1849–1860. [PubMed] [Google Scholar]
- Horvitz H. R. Control by bacteriophage T4 of two sequential phosphorylations of the alpha subunit of Escherichia coli RNA polymerase. J Mol Biol. 1974 Dec 25;90(4):727–738. doi: 10.1016/0022-2836(74)90536-1. [DOI] [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- Neidhardt F. C., Marchin G. L., McClain W. H., Boyd R. F., Earhart C. F. Phage-induced modification of valyl-tRNA synthetase. J Cell Physiol. 1969 Oct;74(2 Suppl):87+–87+. doi: 10.1002/jcp.1040740408. [DOI] [PubMed] [Google Scholar]
- Nobrega F. G., Rola F. H., Pasetto-Nobrega M., Oishi M. Adenosine triphosphatase associated with adenosine triphosphate-dependent deoxyribonuclease (recB-recC enzyme-E. coli-ATP to phosphodiester hydrolysis ratio-DNA-dependent ATPase activity). Proc Natl Acad Sci U S A. 1972 Jan;69(1):15–19. doi: 10.1073/pnas.69.1.15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oishi M. An ATP-dependent deoxyribonuclease from Escherichia coli with a possible role in genetic recombination. Proc Natl Acad Sci U S A. 1969 Dec;64(4):1292–1299. doi: 10.1073/pnas.64.4.1292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sakaki Y. Inactivation of the ATP-dependent DNase of Escherichia coli after infection with double-stranded DNA phages. J Virol. 1974 Dec;14(6):1611–1612. doi: 10.1128/jvi.14.6.1611-1612.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sakaki Y., Karu A. E., Linn S., Echols H. Purification and properties of the gamma-protein specified by bacteriophage lambda: an inhibitor of the host RecBC recombination enzyme. Proc Natl Acad Sci U S A. 1973 Aug;70(8):2215–2219. doi: 10.1073/pnas.70.8.2215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tanner D., Oishi M. The effect of bacteriophage T4 infection on an ATP-dependent deoxyribonuclease in Escherichia coli. Biochim Biophys Acta. 1971 Feb 11;228(3):767–769. doi: 10.1016/0005-2787(71)90747-7. [DOI] [PubMed] [Google Scholar]
- Tomizawa J., Ogawa H. Structural genes of ATP-dependent deoxyribonuclease of Escherichia coli. Nat New Biol. 1972 Sep 6;239(88):14–16. doi: 10.1038/newbio239014a0. [DOI] [PubMed] [Google Scholar]
- Unger R. C., Clark A. J. Interaction of the recombination pathways of bacteriophage lambda and its host Escherichia coli K12: effects on exonuclease V activity. J Mol Biol. 1972 Oct 14;70(3):539–548. doi: 10.1016/0022-2836(72)90558-x. [DOI] [PubMed] [Google Scholar]
- Wackernagel W. An improved spheroplast assay for lambda-DNA and the influence of the bacterial genotype on the transfection rate. Virology. 1972 Apr;48(1):94–103. doi: 10.1016/0042-6822(72)90117-1. [DOI] [PubMed] [Google Scholar]
- Wackernagel W., Hermanns U. Inhibition of exonuclease V after infection of E. coli by bacteriophage T7. Biochem Biophys Res Commun. 1974 Sep 23;60(2):521–527. doi: 10.1016/0006-291x(74)90271-x. [DOI] [PubMed] [Google Scholar]
- Wright M., Buttin G. Les mécanismes de dégradation enzymatique du chromosome bactérien et leur régulation. Bull Soc Chim Biol (Paris) 1969;51(10):1373–1383. [PubMed] [Google Scholar]



