Abstract
AIM
To investigate histologic abnormalities in the gastric smooth muscle of patients with diabetes mellitus (DM).
METHODS
Full-thickness gastric specimens were obtained from patients undergoing surgery for gastric cancer. H&E stain and Masson’s Trichrome stain were performed to assess the degree of fibrosis. Immunohistochemical staining using various antibodies was also performed [antibodies against protein gene product 9.5 (PGP9.5), neuronal nitric oxide synthase (nNOS), vasoactive intestinal peptide (VIP), neurokinin-1 (NK1) receptor, c-Kit, and platelet-derived growth factor receptor-alpha, (PDGFRα)]. Immunofluorescent staining and evaluation with confocal microscopy were also conducted.
RESULTS
Twenty-six controls and 35 diabetic patients (21 short-duration patients and 14 long-duration patients) were included. There were no significant differences in basic demographics between the two groups except in mean body mass index (BMI) (higher in the DM group). Proportions of moderate-to-severe intercellular fibrosis in the muscle layer were significantly higher in the DM group than in the control group (P < 0.01). On immunohistochemical staining, c-Kit- and PDGFRα-positive immunoreactivity were significantly decreased in the DM group compared with the control group (P < 0.05). There were no statistically significant differences in PGP9.5, nNOS, VIP, and neurokinin 1 expression. On immunofluorescent staining, cellularity of interstitial cells of Cajal (ICC) was observed to decrease with increasing duration of DM.
CONCLUSION
Our study suggests that increased intercellular fibrosis, loss of ICC, and loss of fibroblast-like cells are found in the smooth muscle of DM patients. These abnormalities may contribute to changes in gastric motor activity in patients with DM.
Keywords: Diabetes mellitus, Interstitial cells of Cajal, Fibroblast-like cell, Gastroparesis, Enteric nerve system
Core tip: In this study, we discovered that increased intercellular fibrosis, loss of interstitial cells of Cajal, and loss of fibroblast-like cells are found in the smooth muscle of diabetes mellitus (DM) patients. These abnormalities may contribute to changes in gastric motor activity in patients with DM.
INTRODUCTION
Diabetes mellitus (DM), a metabolic disease caused by failure of blood sugar control, is a very common disorder, with a prevalence of 8.7% in adults[1]. It is well known that a lack of treatment of DM results in critical damage by causing acute complications (such as diabetic ketoacidosis or nonketotic hyperosmolar coma) along with chronic complications including nephropathy, angiopathy, neuropathy, and ophthalmopathy[2]. It is also known that the majority of DM patients suffer one or more gastrointestinal (GI) symptoms, which involve abdominal pain, early satiety, constipation, diarrhea, nausea, vomiting, and fecal incontinence, and that these symptoms result in a lower quality of life for patients[3-8]. Although the mechanisms of GI complications in DM patients are still not completely understood, GI motor disturbance appears to play a critical role. Because factors including GI smooth muscle, intrinsic or extrinsic enteric nervous system (ENS), and GI hormones are involved in the control of GI motility, it is possible to hypothesize that damage to these factors causes GI dysmotility, and various GI symptoms might occur according to the sites involved[9].
Gastroparesis, a kind of GI complication of DM, is characterized by delayed gastric emptying[10], and occurs as a result of a problem in postprandial gastric contraction activity[9,11,12]. The major symptoms of gastroparesis include postprandial fullness, early satiety, nausea, vomiting, abdominal distension, and abdominal pain. Although many other diseases and circumstances such as medication, connective tissue disorders, neurologic disorders, and tumors can also be related to gastroparesis, DM is the most common cause[3,9,11,13]. In the past, diabetic gastroparesis was regarded as an ambiguous and rare condition that was caused by the irreversible damage of the vagal nervous system, which occurs after an extremely long presence of type 1 DM; however, since the introduction of the “gastric transit time” concept, many studies have been conducted into the pathophysiology of diabetic gastroparesis[5,11,12,14].
For an intact gastric emptying, synergic and appropriate movements of the proximal stomach, distal stomach, pylorus, and small intestine play critical roles. The role of the nervous system, which controls the gastric smooth muscle, is extremely important during gastric emptying[15]. However, recent studies show that the intragastric motor neurotransmission process causing gastric contraction is more complex than a simple process in which the neurotransmitters from nerve endings combine with the receptors of smooth muscle cells (SMC), and it is well known that the interstitial cells of Cajal (ICC) play a very important role during this neurotransmission process[16-20]. Although there is not enough information about the roles in this neurotransmission process, fibroblast-like cells (FLCs) also show network connections to SMC via gap junctions. Therefore, it is reasonable to assume that FLCs perform some role in the GI contraction process[21]. We can estimate the degree of expression of FLCs by immunohistochemical or immunofluorescent staining, because they are widely stained with antibody to platelet-derived growth factor receptor α (PDGFRα)[22].
Research into the pathophysiology of diabetic gastroparesis has hitherto been performed by means of animal models; not only is research using human gastric tissue rare, but the results also do not match well with those of animal models. Therefore, we intend to investigate how SMC, ENS, ICC, and FLCs are affected in DM using human gastric smooth muscle tissue.
MATERIALS AND METHODS
Subjects and tissues
Gastric specimens were obtained from gastric cancer patients who had been admitted to four university hospitals (Keimyung University Dongsan Hospital, Youngnam University Hospital, Kyungpook National University Hospital, Catholic University of Daegu Hospital) in Daegu province, South Korea, for surgery. Shortly after gastrectomy, entire layered tissues of 1 cm × 2 cm in size were taken from areas free of cancer infiltration and used for various microscopic evaluations.
The tissues were taken from fundus, lesser curvature of corpus, and lesser curvature of antrum in the cases of total gastrectomy, and were taken from lesser curvature of corpus, and lesser curvature of antrum in the cases of subtotal gastrectomy (Figure 1). The tissues were obtained shortly after surgery, and removed tissues were fixed in formalin immediately.
Figure 1.
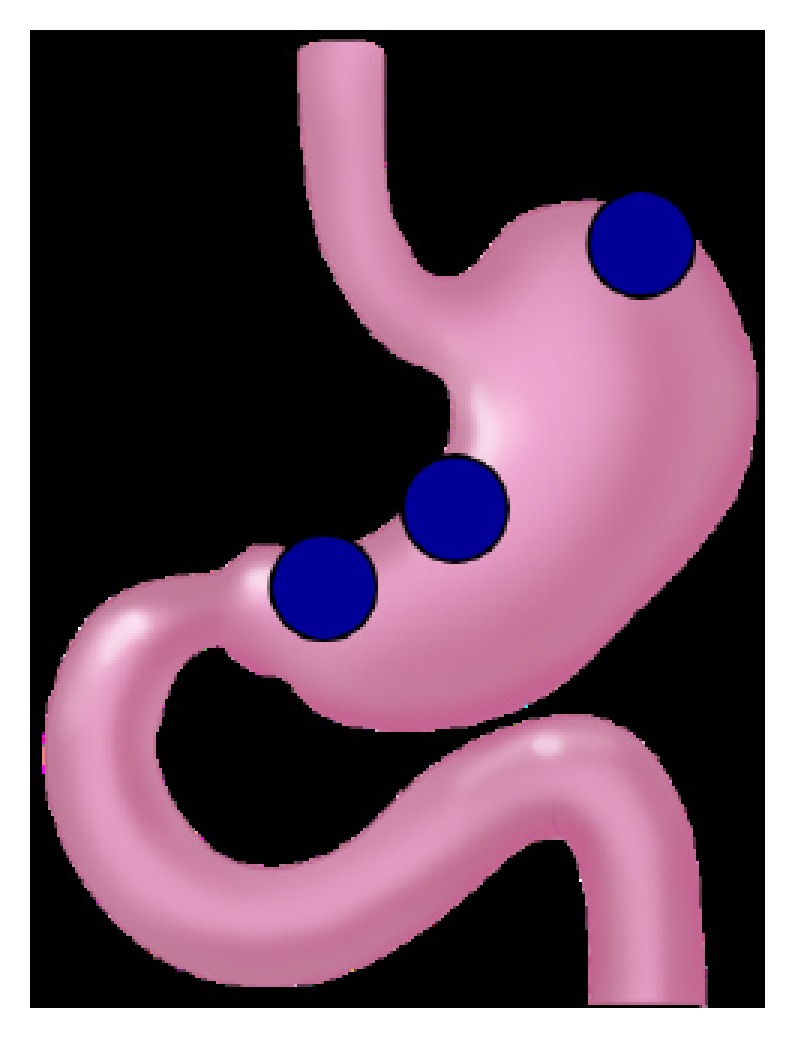
Tissue-sampled sites. Full-thickness tissue samples (2 cm × 1 cm size) were obtained from tumor-free sites in fundus, less curvature of corpus, and less curvature of antrum.
The study protocol was reviewed and approved by the Institutional Review Board at Keimyung University Dongsan Hospital, Daegu, South Korea. A precise explanation of the protocol was given to each patient by a coordinator, and all the patients provided written informed consent before inclusion in this study.
H/E and Masson’s Trichrome stain
Tissue samples were fixed in formalin and embedded in paraffin. Sections (4-μm thick) were stained with H&E (hematoxylin and eosin) and Masson’s Trichrome to evaluate the degree of fibrosis of the muscularis propria layer. Each microscopic evaluation was performed by the same pathologist who was blind to the group to which the patient belonged. The degree of fibrosis was estimated by consulting the criteria which are used for the estimation of hepatic fibrosis in chronic hepatitis[23]: mild fibrosis means minimal fibrosis without bridging; moderate fibrosis means bridging fibrosis without encirclement; severe fibrosis means muscle fiber-encircling fibrosis (Figure 2). The degree of fibrosis was compared between the two groups (no or mild fibrosis vs moderate or severe fibrosis).
Figure 2.
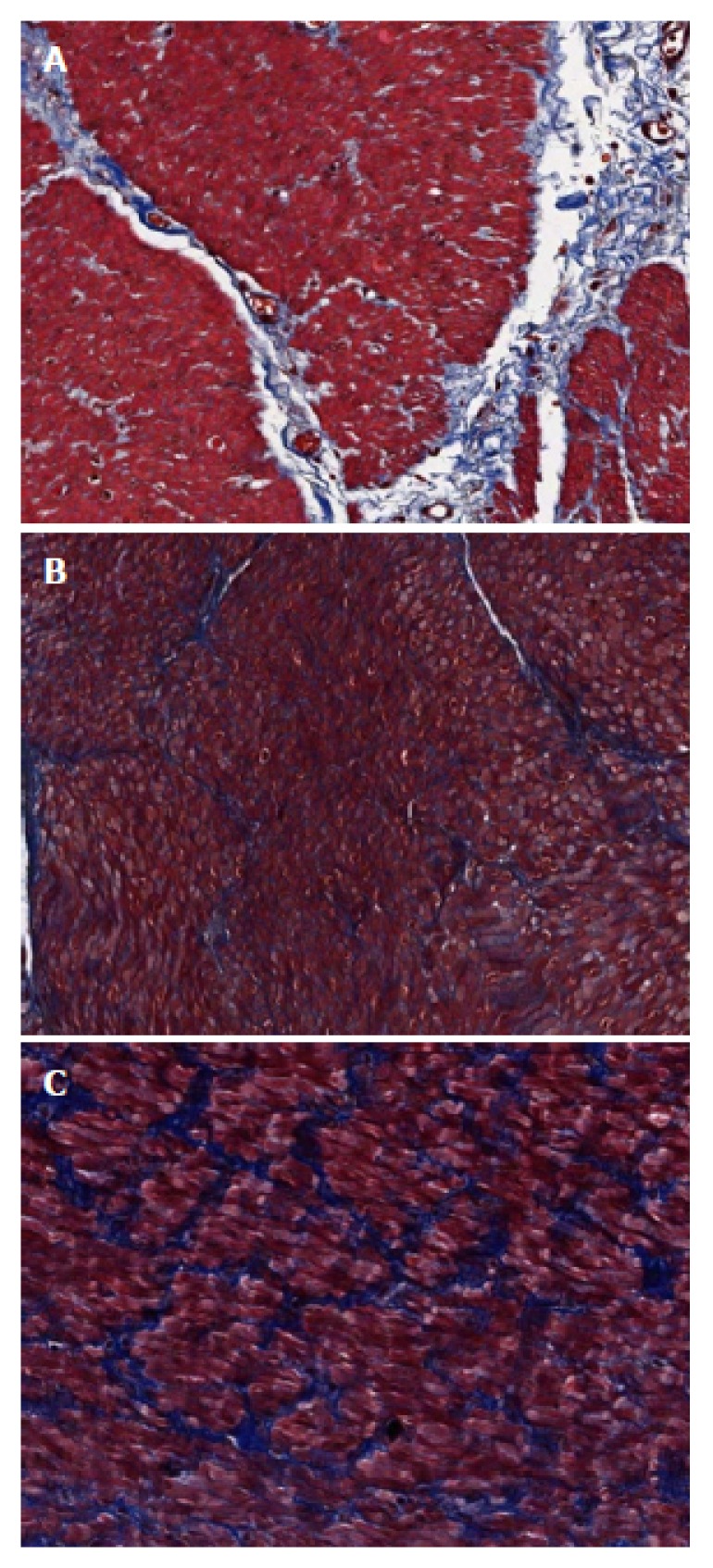
Degree of intercellular fibrosis. A: Mild fibrosis means minimal fibrosis without bridging; B: Moderate fibrosis means bridging fibrosis without encirclement; C: Severe fibrosis means muscle fiber-encircling fibrosis. Masson’s Trichrome stain (× 200).
Immunohistochemical staining
Sections (4-μm thick) from tissue embedded in a paraffin block were mounted on Superfrost Plus® glass slides (VWR Scientific, West Chester, PA, United States) and incubated at 60 °C for 15 min. Slides were deparaffinized in xylene, rehydrated in graded alcohol, and washed in tap water. Endogenous peroxidase activity was blocked by incubating the sections with 3% H2O2. Slides were placed in a steam cooker that was filled with 10 mmol/L sodium citrate buffer (pH 6.0) for antigen retrieval. After treatment with protein block (DAKO, Carpinteria, CA, United States) for 10 min to block nonspecific protein binding, the rabbit monoclonal or polyclonal antibody for PDGFR-α (sc-338, Santa Cruz Biotechnology, Dallas, TX, United States), neuronal nitric oxide synthase (nNOS) (EP1855Y, Abcam, MA, United States), neurokinin-1 (NK1) receptor (NB100-74469, Nobus, CO, United States), protein gene product 9.5 (PGP9.5) (318A-16, Cell Marque, CA, United States), vasoactive intestinal peptide (VIP) (NB100-6568, Nobus, CO, United States), and c-kit (sc-5535, Santa Cruz Biotechnology, Dallas, TX, United States) were applied for 1 h, respectively. After reaction with a biotinylated anti-mouse antibody for 30 min, antigen-antibody complexes were visualized using a streptavidin-horseradish peroxidase conjugate (DAKO LSAB kit; DAKO, Los Angeles, CA, United States) and diaminobenzidine as a chromogen. Slides were counterstained with Mayer’s hematoxylin for 3-5 min. The results were expressed as stained cell numbers under high magnification (× 400). Each value was calculated from a mean of three different sites.
Immunofluorescent staining
After washing sections (4-μm thick) from tissue embedded in paraffin block with phosphate-buffered saline (PBS, pH 7.4) and 3% dehydroxide solution for 5 min, sections were preincubated with blocking solution (Invitrogen, Carlsbad, CA, United States) for 30 min before being incubated with the anti-ICC (ab5506, Abcam, Cambridge, United Kingdom). After the sections were incubated for 90 min with the primary antibodies, they were washed with PBS again before being incubated with secondary antibody (Alexa Fluor 488 goat anti-rabbit antibody, Invitrogen, CA, United States) for 90 min at 24 °C. After rewashing with PBS, the specimens were counterstained with 4’,6-diamidino-2-phenylindole (DAPI) and mounted. The immunostained tissues were evaluated with confocal laser scanning microscopy (LSM 5 EXCITER; Carl Zeiss, Jena, Germany), using a C-Apochromat objective lens (× 40). Image analysis was completed with LSM software (version 3.98, Carl Zeiss, Jena, Germany). Intensity of fluorescence in the DM group was compared with that in the control group and expressed as a percentage.
Statistical analysis
Values were compared between the DM and control groups. If necessary, subgroup analyses of the DM group between long-term (prevalent 10 or more years) and short-term (prevalent less than 10 years) groups were performed. The SPSS statistical package ver. 20.0 (SPSS Inc., Chicago, IL, United States) was used for statistical analyses. All data are presented as the mean ± SD for continuous variables and as frequency or percentage for categorical variables. Student’s t-test was used for the comparison of continuous variables and a Pearson’s χ2 test for that of categorical variables. P values less than 0.05 were considered statistically significant.
RESULTS
Patient characteristics
From four university hospitals, 61 patients were registered: 39 were male and 22 were female. Thirty-six patients underwent total gastrectomy and 25 received subtotal gastrectomy. The number of patients in the control group was 26 and that of DM patients was 35; among the DM patients, 14 had suffered DM for 10 or more years. Age, gender, and frequency of upper-GI symptoms did not significantly differ between the two groups; however, patients in the DM group had a body mass index (BMI) of 24.2 ± 3.1 kg/m2, which was higher than the average BMI of the control group, which was 22.62 ± 3.0 kg/m2 (P = 0.045) (Table 1).
Table 1.
Patient demographics n (%)
| DM (n = 35) | Control (n = 26) | P value | |
| Age (years) | 62.3 ± 8.9 | 59.7 ± 10.4 | 0.297 |
| Gender | |||
| Male | 24 (68.6) | 15 (57.7) | 0.428 |
| Female | 11 (31.4) | 11 (42.3) | |
| BMI (kg/m2) | 24.2 ± 3.1 | 22.6 ± 3.0 | 0.045 |
| UGI symptoms | |||
| Yes | 26 (74.3) | 22 (84.6) | 0.366 |
| No | 9 (25.7) | 4 (15.4) |
UGI: Upper gastrointestinal; DM: Diabetes mellitus; BMI: Body mass index.
Fibrosis
Frequency of moderate or severe fibrosis appeared to be 80.0% in the antrum, 85.7% in the body, and 81.3% in the fundus for the DM group. However, in the control group, the frequency of moderate or severe fibrosis was 30.8% in the antrum, 42.3% in the body, and 28.6% in the fundus. Therefore, the degree of fibrosis was statistically higher in the DM group in all areas of the stomach (Table 2). However, the DM duration did not affect the degree of fibrosis in any part of the stomach.
Table 2.
Degree of intercellular fibrosis n (%)
| Degree of fibrosis | DM (n = 35) | Control (n = 26) | P value |
| Antrum | < 0.001 | ||
| Moderate to severe | 28 (80.0) | 8 (30.8) | |
| None or mild | 7 (20.0) | 18 (69.2) | |
| Body | 0.001 | ||
| Moderate to severe | 30 (85.7) | 11 (42.3) | |
| None or mild | 5 (14.3) | 15 (57.7) | |
| Fundus | 0.003 | ||
| Moderate to severe | 13 (81.3) | 6 (28.6) | |
| None or mild | 3 (18.8) | 15 (71.4) |
DM: Diabetes mellitus.
Immunohistochemical staining
When observed at high-power magnification (× 400), the number of c-Kit (+) cells, indicating ICC, appeared to be 11.6 ± 3.6 in the antrum, 12.3 ± 3.8 in the body, and 12.1 ± 3.4 in the fundus of the control group, whereas the antrum, body, and fundus of the DM group yielded c-Kit (+) cell numbers of 8.4 ± 2.9, 8.0 ± 2.8, and 8.4 ± 2.4, respectively. Therefore, the number of c-Kit (+) cells was lower in the DM group than in the control group in all areas of the stomach (P < 0.001) (Table 3; Figure 3A and B).
Table 3.
Results of immunohistochemical stain
| DM (n = 35) | Control (n = 26) | P value | |
| c-Kit | |||
| Antrum | 8.35 ± 2.89 | 11.63 ± 3.64 | 0.001 |
| Body | 7.98 ± 2.84 | 12.29 ± 3.84 | 0.000 |
| Fundus | 8.27 ± 2.40 | 12.16 ± 3.38 | 0.001 |
| PDGFRα | |||
| Antrum | 7.45 ± 1.96 | 7.49 ± 2.58 | 0.965 |
| Body | 6.73 ± 2.37 | 9.13 ± 4.00 | 0.010 |
| Fundus | 5.33 ± 2.73 | 7.16 ± 1.90 | 0.021 |
| PGP9.5 | |||
| Antrum | 18.39 ± 5.16 | 18.64 ± 6.09 | 0.930 |
| Body | 15.47 ± 3.94 | 17.38 ± 4.98 | 0.090 |
| Fundus | 12.89 ± 5.76 | 14.22 ± 5.84 | 0.656 |
| nNOS | |||
| Antrum | 9.01 ± 4.01 | 8.17 ± 3.06 | 0.393 |
| Body | 7.75 ± 2.22 | 8.06 ± 3.79 | 0.976 |
| Fundus | 5.67 ± 2.61 | 6.08 ± 2.42 | 0.747 |
| VIP | |||
| Antrum | 4.73 ± 2.25 | 4.79 ± 2.90 | 0.720 |
| Body | 5.19 ± 2.14 | 5.33 ± 2.40 | 0.952 |
| Fundus | 3.79 ± 2.09 | 5.05 ± 2.94 | 0.256 |
| NK1R | |||
| Antrum | 0.65 ± 0.74 | 0.36 ± 0.38 | 0.253 |
| Body | 0.77 ± 0.78 | 0.59 ± 0.63 | 0.460 |
| Fundus | 0.54 ± 0.40 | 0.57 ± 0.60 | 0.705 |
DM: Diabetes mellitus; PDGFRα: Platelet-derived growth factor receptor α; PGP9.5: Protein gene product 9.5; nNOS: Neuronal nitric oxide synthase; VIP: Vasoactive intestinal peptide; NK1R: Neurokinin 1 receptor.
Figure 3.
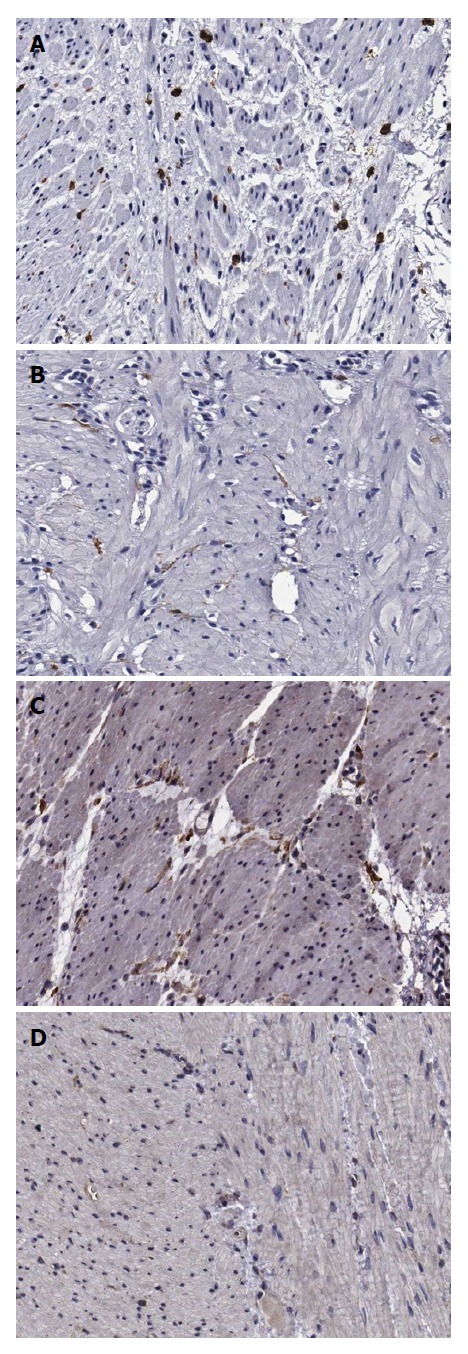
Immunohistochemical staining of interstitial cells of Cajal (upper panel) and platelet-derived growth factor receptor α-positive fibroblast-like cells (lower panel) in the human gastric corpus (× 200). Cellularity of ICC is higher in the control group (A) than in the DM group (B). Cellularity of FLCs is higher in the control group (C) than in the DM group (D). DM: Diabetes mellitus; FLCs: Fibroblast-like cells.
However, the average PDGFRα (+) cell numbers in the control group were found to be 7.5 ± 2.6 in the antrum, 9.1 ± 4.0 in the body, and 7.2 ± 1.9 in the fundus, whereas the antrum, body, and fundus of the DM group yielded PDGFRα (+) cell numbers of 7.5 ± 2.0, 6.7 ± 2.4, and 5.3 ± 2.7, respectively. Therefore, fewer PDGFRα (+) cells were found in the body (P = 0.010) and the fundus (P = 0.021) of the DM group compared to those of the control group (Table 3; Figure 3C and D).
There were no significant differences between both groups with regard to degree of expression of PGP9.5, nNOS, VIP, or NK1 receptor in any areas of stomach (Table 3).
Immunofluorescent staining
Immunofluorescence intensity of c-Kit (+) cells was 100.0% ± 13.2% in the control group, 64.1% ± 0.7% in the DM group of < 10 years’ duration, and 36.1% ± 5.1% in the DM group with > 10 years’ duration. Therefore, with increasing DM duration, the density of c-Kit (+) cells appeared to decrease (Figure 4).
Figure 4.
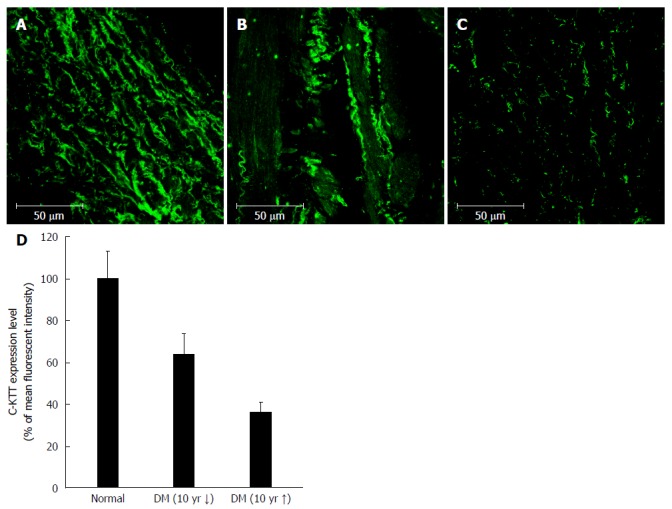
Immunofluorescent staining of interstitial cells of Cajal in the human gastric corpus. Panels (A), (B), and (C) show representative images from the control, DM of < 10 years’ duration, and DM of > 10 years’ groups, respectively. Cellularity of ICC decreases with increasing duration of DM (D). DM: Diabetes mellitus; ICC: Interstitial cells of Cajal.
DISCUSSION
In this research, the effects of DM on SMC, ENS, ICC, and FLCs (all related to GI motility) were investigated. Results showed that DM patients have excessive amounts of fibrosis in their gastric smooth muscles; decreased density of ICC and PDGFRα was also found in these patients.
Because formerly reported studies related to the gastric smooth muscle were achieved by means of animal experimentation and only a few studies used human gastric tissue, human gastric smooth muscle samples excised during cancer surgery were used in this study.
The tissues used in this study were taken from regions isolated from the cancer foci. Although the duration of gastric cancer was not consistent in each patient and the possibility that the cancer itself might affect the structure of adjacent smooth muscle cannot be disregarded, we included cancer surgery cases because it is difficult in practice to obtain gastric smooth muscle tissue samples from cases other than these[16,24].
Extrinsic nerve (such as vagus or sympathetic nerve) dysfunction[25,26], ICC dysfunction[16,19,27], intrinsic enteric nerve dysfunction[16,28], and smooth muscle dysfunction itself[29] have been consistently suggested as factors that affect gastric motility disorder during the causation of gastroparesis in DM patients. In this study, markers for these components were also observed by means of immunohistochemical and immunofluorescent staining.
Although several reports indicate that DM patients showing gastroparesis also show dysfunction of extrinsic nerve cells[25,26], this could not be observed in this study because the tissues excised were too small. Because it is unrealistic to stain entire specimens for observation, it seems more appropriate to measure pancreatic or gastric secretory function after stimulation of the vagal nerve to evaluate the function of the gastric extrinsic nervous system.
Results from previous studies regarding changes in the gastric smooth muscle in DM patients are not consistent. Several studies report the degeneration and fibrosis of smooth muscle[30]; however, one report demonstrated no relationship of early DM with fibrosis[31]. These results suggest that fibrosis of the gastric smooth muscle might indicate an advanced state of diabetic complication. In our study, the ratio of moderate or severe fibrosis was significantly higher in the DM group than in the control group, but there was no difference according to the prevalent duration of DM. This result suggests that fibrosis of the gastric smooth muscle begins during the early stage of DM.
Since the role of ICC is accepted to be extremely critical for proper GI motility, several GI motility disorders have been confirmed to be caused by ICC damage[32-34]. The pathophysiology of diabetic gastroparesis has also been established to involve damage to ICC, not only in animal experiments but also in a human study[27]. This damage includes both a decrease in the number of ICC and microstructural abnormality[35]. Recent studies have shown that Ano-1 is the most important protein involved in the electrophysiological role of ICC, and that abnormalities in Ano-1 are involved in the development of diabetic gastroparesis[36]. In this study, we also proved that the number of ICC is decreased in all gastric areas of DM patients, and that these numbers are more severely decreased in long-term cases of DM. However, we could not investigate the degree of Ano-1 expression and microstructural abnormality of ICC; further investigations into this are necessary.
FLCs that express PDGFRα are interstitial cells that are assumed to have a particular role in GI motility and are connected to SMC through gap junctions. Although located very close to ICC, ultrastructural and functional aspects of FLCs are distinct from those of ICC[21]. Located very close to nerve endings, FLCs are considered to have a role in neurotransmission, especially within purinergic neurotransmission[37,38]. In this study, whereas a decrease in FLCs was observed in the gastric body and fundus of the DM group, no difference in the numbers of FLCs was observed in the antrum of the DM group compared to that of the control group. Considering that the fundus and upper body of the stomach play important roles in gastric accommodation through postprandial relaxation, it can be hypothesized that the damage in gastric accommodation caused by the FLCs decrease might be the major element causing gastroparesis in DM patients. Further research will be necessary after considering the functional aspects of FLCs.
The damage caused to not only the extrinsic nervous system but also the intrinsic ENS in the diabetic animal model has long been investigated and has led to the elucidation of the impairment of nonadrenergic noncholinergic neurotransmission, impaired post-receptor response to adrenalin[39,40], and especially impaired NO-mediated neurotransmission[33,41]. One study showed the impairment of several kinds of neurotransmitters including nNOS in colonic smooth muscle of DM patients[42], while another report showed decreased expression of both nNOS and NK-1 in the gastric smooth muscle of DM patients[16]. Therefore, reduced expression of nNOS was anticipated in this research as well; however, no difference in nNOS expression was observed between the DM group and control group. The result also did not exhibit any difference between the two groups with regard to expressions of NK-1 receptor, PGP9.5 (neuronal marker), and VIP. Further investigation using immunofluorescence may be helpful in providing more clarity.
There are several limitations of our study. First, the symptom intensity and serial glucose level of each patient from the DM group were not analyzed. Because DM patients do not always show symptoms of gastroparesis, further study for the identification of pathologic factors associated with the presence or degree of symptoms will be necessary. Second, physiologic studies for investigation of gastric smooth muscle function and mechanism of muscular fibrosis were not performed. Additional studies on how the pathologic abnormalities observed in this study and gastric smooth muscle dysfunction affect each other might be helpful in the discovery of the mechanism of gastric dysmotility and the subsequent symptoms. Lastly, according to recent animal studies, the differentiation process of macrophages plays an important role in the causation of diabetic gastroparesis[43,44]; however, experiments on this process could not be performed in this study. Because very little research into the role of the macrophage differentiation process in the causation of diabetic gastroparesis has been performed in human tissue, future study on this topic is needed.
Despite these limitations and the necessity for future research, this study is valuable because abundant human tissues were used to identify effects on SMC, ICC, and FLCs in DM patients and the findings considered the prevalent duration of DM.
COMMENTS
Background
Gastroparesis, a kind of gastrointestinal (GI) complication of diabetes mellitus (DM), is characterized by delayed gastric emptying, and occurs as a result of a problem in postprandial gastric contraction activity. Although many other diseases and circumstances such as medication, connective tissue disorders, neurologic disorders, and tumors can also be related to gastroparesis, DM is the most common cause. Although the mechanisms of diabetic gastroparesis are still not completely understood, gastric motor disturbance appears to play a critical role. Because factors including gastric smooth muscle, intrinsic or extrinsic enteric nervous system (ENS), and GI hormones are involved in the control of gastric motility, it is possible to hypothesize that damage to these factors causes gastric dysmotility and gastroparetic symptoms.
Research frontiers
Research into the pathophysiology of diabetic gastroparesis has hitherto been performed by means of animal models; not only is research using human gastric tissue rare, but the results also do not match well with those of animal models.
Innovations and breakthroughs
This is a unique study that investigated the histologic abnormalities in the gastric smooth muscle of patients with DM using human gastric tissues.
Applications
Increased intercellular fibrosis, loss of interstitial cells of Cajal (ICC), and loss of fibroblast-like cells were found in the gastric smooth muscle of DM patients. These findings suggest that changes in gastric motor activity in patients with DM may be caused by these abnormalities.
Terminology
The ICC is a kind of interstitial cell that is located in the GI tract. Many ICC communicating with each other form network systems and serve as electrical pacemakers. As a result, spontaneous electrical slow waves are generated in the GI tract. Since the role of ICC was accepted to be extremely critical for proper GI motility, several GI motility disorders have been confirmed to be caused by ICC damage.
Peer-review
In this study the authors aimed to investigate histologic abnormalities in the gastric smooth muscle of patients with DM and showed that DM patients have excessive amounts of fibrosis on their gastric smooth muscles, which may contribute to changes in gastric motor activity in patients with DM. They used histologic and staining techniques to identify the proposed changes of tissue samples. Since most of the published findings have been obtained from animal research, using human tissues makes this study distinguished and important.
Footnotes
Manuscript source: Invited manuscript
Specialty type: Gastroenterology and hepatology
Country of origin: South Korea
Peer-review report classification
Grade A (Excellent): A
Grade B (Very good): B, B
Grade C (Good): C
Grade D (Fair): 0
Grade E (Poor): 0
Institutional review board statement: This study was approved by Keimyung University School of Medicine/Dongsan Hospital Institutional Review Board.
Conflict-of-interest statement: Not declared.
Data sharing statement: No additional data are available.
Peer-review started: June 23, 2016
First decision: September 21, 2016
Article in press: October 31, 2016
P- Reviewer: Gazouli M, Hussain SAR, Ozdemir S, Panchu P S- Editor: Qi Y L- Editor: Logan S E- Editor: Liu WX
References
- 1.Shi Y, Hu FB. The global implications of diabetes and cancer. Lancet. 2014;383:1947–1948. doi: 10.1016/S0140-6736(14)60886-2. [DOI] [PubMed] [Google Scholar]
- 2.Mokdad AH, Ford ES, Bowman BA, Nelson DE, Engelgau MM, Vinicor F, Marks JS. Diabetes trends in the U.S.: 1990-1998. Diabetes Care. 2000;23:1278–1283. doi: 10.2337/diacare.23.9.1278. [DOI] [PubMed] [Google Scholar]
- 3.Talley NJ, Young L, Bytzer P, Hammer J, Leemon M, Jones M, Horowitz M. Impact of chronic gastrointestinal symptoms in diabetes mellitus on health-related quality of life. Am J Gastroenterol. 2001;96:71–76. doi: 10.1111/j.1572-0241.2001.03350.x. [DOI] [PubMed] [Google Scholar]
- 4.Bytzer P, Talley NJ, Leemon M, Young LJ, Jones MP, Horowitz M. Prevalence of gastrointestinal symptoms associated with diabetes mellitus: a population-based survey of 15,000 adults. Arch Intern Med. 2001;161:1989–1996. doi: 10.1001/archinte.161.16.1989. [DOI] [PubMed] [Google Scholar]
- 5.Koch KL. Diabetic gastropathy: gastric neuromuscular dysfunction in diabetes mellitus: a review of symptoms, pathophysiology, and treatment. Dig Dis Sci. 1999;44:1061–1075. doi: 10.1023/a:1026647417465. [DOI] [PubMed] [Google Scholar]
- 6.Horowitz M, Fraser R. Disordered gastric motor function in diabetes mellitus. Diabetologia. 1994;37:543–551. doi: 10.1007/BF00403371. [DOI] [PubMed] [Google Scholar]
- 7.Enck P, Rathmann W, Spiekermann M, Czerner D, Tschöpe D, Ziegler D, Strohmeyer G, Gries FA. Prevalence of gastrointestinal symptoms in diabetic patients and non-diabetic subjects. Z Gastroenterol. 1994;32:637–641. [PubMed] [Google Scholar]
- 8.Feldman M, Schiller LR. Disorders of gastrointestinal motility associated with diabetes mellitus. Ann Intern Med. 1983;98:378–384. doi: 10.7326/0003-4819-98-3-378. [DOI] [PubMed] [Google Scholar]
- 9.Horváth VJ, Izbéki F, Lengyel C, Kempler P, Várkonyi T. Diabetic gastroparesis: functional/morphologic background, diagnosis, and treatment options. Curr Diab Rep. 2014;14:527. doi: 10.1007/s11892-014-0527-8. [DOI] [PubMed] [Google Scholar]
- 10.Kassander P. Asymptomatic gastric retention in diabetics (gastroparesis diabeticorum) Ann Intern Med. 1958;48:797–812. doi: 10.7326/0003-4819-48-4-797. [DOI] [PubMed] [Google Scholar]
- 11.Thazhath SS, Jones KL, Horowitz M, Rayner CK. Diabetic gastroparesis: recent insights into pathophysiology and implications for management. Expert Rev Gastroenterol Hepatol. 2013;7:127–139. doi: 10.1586/egh.12.82. [DOI] [PubMed] [Google Scholar]
- 12.Chang J, Rayner CK, Jones KL, Horowitz M. Diabetic gastroparesis-backwards and forwards. J Gastroenterol Hepatol. 2011;26 Suppl 1:46–57. doi: 10.1111/j.1440-1746.2010.06573.x. [DOI] [PubMed] [Google Scholar]
- 13.Jung HK, Choung RS, Locke GR, Schleck CD, Zinsmeister AR, Szarka LA, Mullan B, Talley NJ. The incidence, prevalence, and outcomes of patients with gastroparesis in Olmsted County, Minnesota, from 1996 to 2006. Gastroenterology. 2009;136:1225–1233. doi: 10.1053/j.gastro.2008.12.047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Vittal H, Farrugia G, Gomez G, Pasricha PJ. Mechanisms of disease: the pathological basis of gastroparesis--a review of experimental and clinical studies. Nat Clin Pract Gastroenterol Hepatol. 2007;4:336–346. doi: 10.1038/ncpgasthep0838. [DOI] [PubMed] [Google Scholar]
- 15.Horowitz M, Dent J. Disordered gastric emptying: mechanical basis, assessment and treatment. Baillieres Clin Gastroenterol. 1991;5:371–407. doi: 10.1016/0950-3528(91)90034-x. [DOI] [PubMed] [Google Scholar]
- 16.Iwasaki H, Kajimura M, Osawa S, Kanaoka S, Furuta T, Ikuma M, Hishida A. A deficiency of gastric interstitial cells of Cajal accompanied by decreased expression of neuronal nitric oxide synthase and substance P in patients with type 2 diabetes mellitus. J Gastroenterol. 2006;41:1076–1087. doi: 10.1007/s00535-006-1909-8. [DOI] [PubMed] [Google Scholar]
- 17.Horváth VJ, Vittal H, Lörincz A, Chen H, Almeida-Porada G, Redelman D, Ordög T. Reduced stem cell factor links smooth myopathy and loss of interstitial cells of cajal in murine diabetic gastroparesis. Gastroenterology. 2006;130:759–770. doi: 10.1053/j.gastro.2005.12.027. [DOI] [PubMed] [Google Scholar]
- 18.James AN, Ryan JP, Crowell MD, Parkman HP. Regional gastric contractility alterations in a diabetic gastroparesis mouse model: effects of cholinergic and serotoninergic stimulation. Am J Physiol Gastrointest Liver Physiol. 2004;287:G612–G619. doi: 10.1152/ajpgi.00431.2003. [DOI] [PubMed] [Google Scholar]
- 19.Ordög T, Takayama I, Cheung WK, Ward SM, Sanders KM. Remodeling of networks of interstitial cells of Cajal in a murine model of diabetic gastroparesis. Diabetes. 2000;49:1731–1739. doi: 10.2337/diabetes.49.10.1731. [DOI] [PubMed] [Google Scholar]
- 20.Ward SM, Morris G, Reese L, Wang XY, Sanders KM. Interstitial cells of Cajal mediate enteric inhibitory neurotransmission in the lower esophageal and pyloric sphincters. Gastroenterology. 1998;115:314–329. doi: 10.1016/s0016-5085(98)70198-2. [DOI] [PubMed] [Google Scholar]
- 21.Horiguchi K, Komuro T. Ultrastructural observations of fibroblast-like cells forming gap junctions in the W/W(nu) mouse small intestine. J Auton Nerv Syst. 2000;80:142–147. doi: 10.1016/s0165-1838(00)00089-8. [DOI] [PubMed] [Google Scholar]
- 22.Iino S, Nojyo Y. Immunohistochemical demonstration of c-Kit-negative fibroblast-like cells in murine gastrointestinal musculature. Arch Histol Cytol. 2009;72:107–115. doi: 10.1679/aohc.72.107. [DOI] [PubMed] [Google Scholar]
- 23.Desmet VJ, Gerber M, Hoofnagle JH, Manns M, Scheuer PJ. Classification of chronic hepatitis: diagnosis, grading and staging. Hepatology. 1994;19:1513–1520. [PubMed] [Google Scholar]
- 24.Min YW, Hong YS, Ko EJ, Lee JY, Min BH, Sohn TS, Kim JJ, Rhee PL. Impairment of the proximal to distal tonic gradient in the human diabetic stomach. Neurogastroenterol Motil. 2014;26:229–236. doi: 10.1111/nmo.12253. [DOI] [PubMed] [Google Scholar]
- 25.Guy RJ, Dawson JL, Garrett JR, Laws JW, Thomas PK, Sharma AK, Watkins PJ. Diabetic gastroparesis from autonomic neuropathy: surgical considerations and changes in vagus nerve morphology. J Neurol Neurosurg Psychiatry. 1984;47:686–691. doi: 10.1136/jnnp.47.7.686. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Duchen LW, Anjorin A, Watkins PJ, Mackay JD. Pathology of autonomic neuropathy in diabetes mellitus. Ann Intern Med. 1980;92:301–303. doi: 10.7326/0003-4819-92-2-301. [DOI] [PubMed] [Google Scholar]
- 27.He CL, Soffer EE, Ferris CD, Walsh RM, Szurszewski JH, Farrugia G. Loss of interstitial cells of cajal and inhibitory innervation in insulin-dependent diabetes. Gastroenterology. 2001;121:427–434. doi: 10.1053/gast.2001.26264. [DOI] [PubMed] [Google Scholar]
- 28.Harberson J, Thomas RM, Harbison SP, Parkman HP. Gastric neuromuscular pathology in gastroparesis: analysis of full-thickness antral biopsies. Dig Dis Sci. 2010;55:359–370. doi: 10.1007/s10620-009-1071-2. [DOI] [PubMed] [Google Scholar]
- 29.Pasricha PJ, Pehlivanov ND, Gomez G, Vittal H, Lurken MS, Farrugia G. Changes in the gastric enteric nervous system and muscle: a case report on two patients with diabetic gastroparesis. BMC Gastroenterol. 2008;8:21. doi: 10.1186/1471-230X-8-21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Ejskjaer NT, Bradley JL, Buxton-Thomas MS, Edmonds ME, Howard ER, Purewal T, Thomas PK, Watkins PJ. Novel surgical treatment and gastric pathology in diabetic gastroparesis. Diabet Med. 1999;16:488–495. doi: 10.1046/j.1464-5491.1999.00086.x. [DOI] [PubMed] [Google Scholar]
- 31.Grover M, Farrugia G, Lurken MS, Bernard CE, Faussone-Pellegrini MS, Smyrk TC, Parkman HP, Abell TL, Snape WJ, Hasler WL, et al. Cellular changes in diabetic and idiopathic gastroparesis. Gastroenterology. 2011;140:1575–1585.e8. doi: 10.1053/j.gastro.2011.01.046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Sanders KM, Salter AK, Hennig GW, Koh SD, Perrino BA, Ward SM, Baker SA. Responses to enteric motor neurons in the gastric fundus of mice with reduced intramuscular interstitial cells of cajal. J Neurogastroenterol Motil. 2014;20:171–184. doi: 10.5056/jnm.2014.20.2.171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Kim SJ, Park JH, Song DK, Park KS, Lee JE, Kim ES, Cho KB, Jang BK, Chung WJ, Hwang JS, et al. Alterations of colonic contractility in long-term diabetic rat model. J Neurogastroenterol Motil. 2011;17:372–380. doi: 10.5056/jnm.2011.17.4.372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Farrugia G. Interstitial cells of Cajal in health and disease. Neurogastroenterol Motil. 2008;20 Suppl 1:54–63. doi: 10.1111/j.1365-2982.2008.01109.x. [DOI] [PubMed] [Google Scholar]
- 35.Faussone-Pellegrini MS, Grover M, Pasricha PJ, Bernard CE, Lurken MS, Smyrk TC, Parkman HP, Abell TL, Snape WJ, Hasler WL, et al. Ultrastructural differences between diabetic and idiopathic gastroparesis. J Cell Mol Med. 2012;16:1573–1581. doi: 10.1111/j.1582-4934.2011.01451.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Mazzone A, Bernard CE, Strege PR, Beyder A, Galietta LJ, Pasricha PJ, Rae JL, Parkman HP, Linden DR, Szurszewski JH, et al. Altered expression of Ano1 variants in human diabetic gastroparesis. J Biol Chem. 2011;286:13393–13403. doi: 10.1074/jbc.M110.196089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Kurahashi M, Mutafova-Yambolieva V, Koh SD, Sanders KM. Platelet-derived growth factor receptor-α-positive cells and not smooth muscle cells mediate purinergic hyperpolarization in murine colonic muscles. Am J Physiol Cell Physiol. 2014;307:C561–C570. doi: 10.1152/ajpcell.00080.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Blair PJ, Rhee PL, Sanders KM, Ward SM. The significance of interstitial cells in neurogastroenterology. J Neurogastroenterol Motil. 2014;20:294–317. doi: 10.5056/jnm14060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Imaeda K, Takano H, Koshita M, Yamamoto Y, Joh T, Suzuki H. Electrical properties of colonic smooth muscle in spontaneously non-insulin-dependent diabetic rats. J Smooth Muscle Res. 1998;34:1–11. doi: 10.1540/jsmr.34.1. [DOI] [PubMed] [Google Scholar]
- 40.Perdue MH, Davison JS. Altered regulation of intestinal ion transport by enteric nerves in diabetic rats. Am J Physiol. 1988;254:G444–G449. doi: 10.1152/ajpgi.1988.254.3.G444. [DOI] [PubMed] [Google Scholar]
- 41.Takahashi T, Nakamura K, Itoh H, Sima AA, Owyang C. Impaired expression of nitric oxide synthase in the gastric myenteric plexus of spontaneously diabetic rats. Gastroenterology. 1997;113:1535–1544. doi: 10.1053/gast.1997.v113.pm9352855. [DOI] [PubMed] [Google Scholar]
- 42.Chandrasekharan B, Anitha M, Blatt R, Shahnavaz N, Kooby D, Staley C, Mwangi S, Jones DP, Sitaraman SV, Srinivasan S. Colonic motor dysfunction in human diabetes is associated with enteric neuronal loss and increased oxidative stress. Neurogastroenterol Motil. 2011;23:131–138, e26. doi: 10.1111/j.1365-2982.2010.01611.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Muller PA, Koscsó B, Rajani GM, Stevanovic K, Berres ML, Hashimoto D, Mortha A, Leboeuf M, Li XM, Mucida D, et al. Crosstalk between muscularis macrophages and enteric neurons regulates gastrointestinal motility. Cell. 2014;158:300–313. doi: 10.1016/j.cell.2014.04.050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Choi KM, Kashyap PC, Dutta N, Stoltz GJ, Ordog T, Shea Donohue T, Bauer AJ, Linden DR, Szurszewski JH, Gibbons SJ, et al. CD206-positive M2 macrophages that express heme oxygenase-1 protect against diabetic gastroparesis in mice. Gastroenterology. 2010;138:2399–2409, 2409.e1. doi: 10.1053/j.gastro.2010.02.014. [DOI] [PMC free article] [PubMed] [Google Scholar]


