Abstract
AIM
To evaluate the hepatoprotective effect of lycopene (Ly) on non-alcoholic fatty liver disease (NAFLD) in rat.
METHODS
A rat model of NAFLD was first established by feeding a high-fat diet for 14 wk. Sixty-five rats were randomly divided into normal group, model group and Ly treatment groups. Alanine aminotransferase (ALT), aspartate aminotransferase (AST), triglycerides (TG), total cholesterol (TC) in serum and low density lipoprotein-cholesterol (LDL-C), high density lipoprotein-cholesterol (HDL-C), free fatty acid (FFA), malondialdehyde (MDA), superoxide dismutase (SOD), glutathione (GSH) in liver tissue were evaluated, respectively. While the hepatoprotective effect was also confirmed by histopathological analysis, the expression levels of TNF-α and cytochrome P450 (CYP) 2E1 in rat liver were determined by immunohistochemistry analysis.
RESULTS
A significant decrease was observed in the levels of serum AST (2.07-fold), ALT (2.95-fold), and the blood lipid TG (2.34-fold) and TC (1.66-fold) in the dose of 20 mg/kg Ly-treated rats (P < 0.01), compared to the model group. Pretreatment with 5, 10 and 20 mg/kg of Ly significantly raised the levels of antioxidant enzyme SOD in a dose-dependent manner, to 90.95 ± 9.56, 109.52 ± 11.34 and 121.25 ± 10.68 (P < 0.05, P < 0.01), as compared with the model group. Similarly, the levels of GSH were significantly increased (P < 0.05, P < 0.01) after the Ly treatment. Meanwhile, pretreatment with 5, 10 and 20 mg/kg of Ly significantly reduced MDA amount by 30.87, 45.51 and 54.49% in the liver homogenates, respectively (P < 0.01). The Ly treatment group showed significantly decreased levels of lipid products LDL-C (P < 0.05, P < 0.01), improved HDL-C level and significantly decreased content of FFA, compared to the model group (P < 0.05, P < 0.01). Furthermore, the Ly-treated group also exhibited a down-regulated TNF-α and CYP2E1 expression, decreased infiltration of liver fats and reversed histopathological changes, all in a dose-dependent manner (P < 0.05, P < 0.01).
CONCLUSION
This study suggests that Ly has a protective effect on NAFLD, down-regulates expression of TNF-α, and that CYP2E1 may be one of the action mechanisms for Ly.
Keywords: Lycopene, Antioxidant, Hepatoprotective, Non-alcoholic fatty liver, Cytochrome P450 2E1
Core tip: Lycopene (Ly), a phytochemical belonging to the carotenoid family, is a red-colored pigment, apolar and acyclic carotenoid. The present study was designed to evaluate the possible hepatoprotective effect of Ly on non-alcoholic fatty liver disease (NAFLD) in rat. This study represents the first examination of the effects of Ly on the therapy of NAFLD, and showed down-regulated expression of TNF-α and indicated that CYP2E1 may be one of the action mechanisms for Ly.
INTRODUCTION
Non-alcoholic fatty liver disease (NAFLD) is one of the causes of fatty liver, occurring when fat is deposited (steatosis) in the liver due to causes other than excessive alcohol use. NAFLD is considered to cover a spectrum of liver diseases, including simple steatosis, non-alcoholic steatohepatitis (NASH), liver fibrosis, liver cirrhosis and hepatocellular carcinoma (HCC)[1,2]. Ninety percent of patients with NAFLD show close relation with one or more of the following risk factors: hypertension, dyslipidemia, elevated triglyceride (TG) levels, obesity, insulin resistance, metabolic syndrome, type 2 diabetes mellitus and cardiovascular disease[3]. Currently, the percentage of people with NAFLD is approximately 20% worldwide and 25% in Western countries, making it one of the most dominant causes of chronic liver disease affecting both adults and children[4]. NAFLD is more common in patients with severe diabetes and obesity, mortality and disease evolution to liver fibrosis or liver cirrhosis is increased in old people with NAFLD[5]. Recently, the “two-hit” theory has arisen as a popular mechanism, although the cause of NAFLD remains to be clearly elucidated[6]. Furthermore, there is not any specific drug available for NAFLD, and no drug has yet to be tested in clinical phase III trials. Therefore, no specific therapy can be firmly recommended to the patients with NAFLD[7].
Lycopene (Ly), a phytochemical belonging to carotenoid family, is a red-colored pigment, acyclic and apolar carotenoid[8]. It is abundantly found in red-colored vegetables and fruits, such as tomatoes, papaya, gac fruit, pink grape-fruit, pink guava, carrots and watermelon, with the concentrations ranging from 9 to 42 mg/kg depending on the variety[9]. Ly displays a range of unique and distinct biological properties owing to its acyclic structure, hydrophobicity and large array of conjugated double bonds. Recently, diverse studies have been reported that lycopene exerts powerful antioxidant activity both in vitro and in vivo against the oxidation of proteins, lipids and DNA, and also has the potential of quenching singlet oxygen 100 times more efficiently than vitamin E and 125 times more than glutathione (GSH)[10]. Furthermore, even at low oxygen tension, it can also scavenge peroxyl radicals, inhibiting the process of lipid peroxidation[11]. It is the most efficient quencher of singlet oxygen among all naturally occurring carotenoids[11], and recently it has been in great demand as a food additive and a natural antioxidant. Additionally, Ly also exhibited potent neuroprotective, anti-inflammatory, anti-proliferative, maintenance of normal cell metabolism, cognition enhancing properties, regulating blood lipid metabolism and so on[12-16]. Therefore, with this background, we aimed to investigate the possible beneficial effects and the possible action mechanism of Ly on NAFLD using a rat model system.
MATERIALS AND METHODS
Materials and reagents
Alanine aminotransferase (ALT), aspartate aminotransferase (AST), TG, total cholesterol (TC), low density lipoprotein-cholesterol (LDL-C), high density lipoprotein-cholesterol (HDL-C), free fatty acid (FFA), malondialdehyde (MDA), superoxide dismutase (SOD) and GSH kits were obtained from Nanjing Jiancheng Bioengineering Institute (Nanjing, China). Protein assay kit was from Zhongshan Institute of Biotechnology (Beijing, China).
Mouse anti-TNF-α, rabbit anti-cytochrome P4502E1 (CYP2E1), horseradish peroxidase (HRP)-conjugated goat anti-mouse IgG and HRP-conjugated goat anti-rabbit IgG antibodies were provided by Proteintech Group, Inc. (Chicago, IL, United States).
Ly (> 95%) was purchased from North China Pharmaceutical Co., Ltd. (Shijiazhuang, China).
The high-fat diet (HFD: 88% basic feed + 10% lard + 2% cholesterol) was prepared in our lab.
Animals
Male Wistar rats (body weight 150 ± 10 g) were obtained from the Experimental Animal Center of Harbin Medical University (China). Six rats were kept in each single polyacrylic cage and were quarantined for 1 wk before the experiments. All animals were housed under standard controlled conditions (temperature: 24 ± 1 °C, humidity: 50% ± 5% and 12 h light/dark cycle), with free access to food and water, and received humane care according to National Institutes of Health Guidelines of the United States (National Research Council of United States, 1996) and the related ethical regulations of Harbin Medical University. Animals were fasted for 12 h before sampling of material.
Experimental design
After acclimatization for 1 wk, 65 Wistar male rats were randomized into 2 groups. Group 1 (normal group) was raised with normal feed (n = 12), and Group 2 (model group) was raised on HFD (n = 53) for consecutive 8 wk. From the 9th wk, all the surviving rats in the model group were further randomly divided into 1 model group and 3 Ly treatment groups, which were given Ly at a dose of 5, 10 or 20 mg/kg/d (n = 12), respectively. The model group was continued on the HFD for 6 wk as before, and the Ly groups were administered orally and continued on the HFD for 6 wk as before.
Rats were sacrificed by cervical dislocation at the end of the experiment, and blood samples of all rats were harvested for serum biochemical markers assay. The fresh liver obtained was weighed to calculate liver coefficient (% = liver weight/body weight × 100). The right liver lobe was fixed in 10% formalin to prepare paraffin sections, and the rest was stored at -80 °C for the other assays.
Serum biochemical markers assay
Serum was collected from blood after centrifugation at 3000 rpm for 10 min at 4 °C. Serum ALT, AST, TG and TC were detected using commercial kits according to the manufacturer’s instructions and using a multifunctional biochemistry analyzer (AU600; Olympus, Tokyo, Japan). The absorbance of ALT and AST was read at 505 nm and the enzyme activity was calculated as U/L. The absorbance of TG and TC was read at 510 nm and the content was calculated as mmol/L.
Measurement of MDA formation in lipid peroxidation
Liver homogenate (10%, w/v) was prepared by homogenizing the liver tissue in 150 mmol/L Tris-HCl buffered saline (pH 7.2) with a polytron homogenizer. The level of MDA in liver tissues was measured at 532 nm with a spectrophotometer (U-2001 Hitachi Ltd., Tokyo, Japan) following the kit protocol from Jiancheng Biological Engineering Institute. The data are expressed as nmol/mg protein of liver tissue.
Measurement of antioxidant and antioxidant enzyme activity
SOD and GSH activity were determined by commercial kit from Jiancheng Biological Engineering Institute following the protocol provided by the manufacturer. The absorbance of the SOD reaction was read at 550 nm and the data are expressed as U/mg protein, while the GSH reaction was read at 420 nm and the enzyme activity was calculated as mg/g protein.
Measurement of liver LDL-C, HDL-C and FFA activity
LDL-C, HDL-C and FFA in liver tissue were measured by commercial kit from Jiancheng Biological Engineering Institute following the protocol provided by the manufacturer. The absorbance of LDL-C, HDL-C and FFA reaction was read at 546 nm and the data are expressed as mmol/L.
Histopathological observation
Liver specimens were fixed overnight in 10% formaldehyde buffer, then embedded in paraffin and cut into 5 μm thickness sections according to the routine procedure. The sections were stained with hematoxylin and eosin (HE) for routine histopathological examination, and examined under a light microscope (BX-50; Olympus) at 200 × magnification for the degree of hepatic steatosis and photographed.
Immunohistochemistry analysis of hepatic TNF-α and CYP2E1
Paraffin-embedded sections (5 μm) were mounted on glass slides, then deparaffinized, incubated in 3% H2O2 for 10 min to quench endogenous peroxidase activity. The sections were stained with mouse anti-TNF-α antibody and rabbit anti-CYP2E1 antibody at 4 °C overnight respectively, after blocking with normal goat serum for 20 min. Then, the sections were incubated with HRP-conjugated goat anti-mouse and HRP-conjugated goat anti-rabbit antibody at 37 °C for 30 min, respectively. The immunoreactive antibodies were visualized by incubation with DAB-H2O2 at room temperature for 10 min. Images were taken at original magnification of 200 × (Olympus BX-50 Microscope and a Leica DMI; Leica Microsystems, Wetzlar, Germany).
Statistical analysis
Data were expressed as mean ± SD and all statistical comparisons were made by means of a one-way ANOVA test followed by Dunett’s t-test. P < 0.05 and < 0.01 were considered statistically significant.
RESULTS
Effects of Ly on body weight and liver coefficient
After 8 wk of HFD feeding, the body weights of rats in the model group were significantly increased compared to that of rats in the control group (P < 0.01, Figure 1A). Meanwhile, after Ly treatment for 6 wk, the gain in body weight for the rats in the 10 and 20 mg/kg Ly-treated groups was lower than that for the rats in the model group (P < 0.01, Figure 1A), which indicated that Ly treatment could inhibit the occurrence of obesity in HFD-administrated rats. Furthermore, consistent with these modifications, the liver coefficient was also reduced markedly in the Ly-treated rats (P < 0.05, P < 0.01, Figure 1B), compared to the control group.
Figure 1.
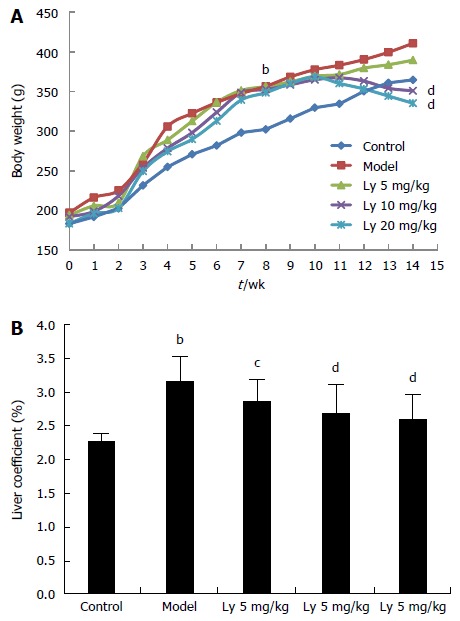
Effects of lycopene on body weight (A) and liver coefficient (B). bP < 0.01 vs control group; cP < 0.05, dP < 0.01 vs model group. Ly: Lycopene.
Effect of Ly on serum ALT and AST levels
Serum levels of AST and ALT indirectly reflects the failure of liver function. As shown in Table 1, serum AST (2.67-fold) and ALT (3.66-fold) activities were significantly increased after the administration of HFD, as compared with the normal group (P < 0.01). Compared with the model group, the levels of AST and ALT were significantly decreased in a dose-dependent manner after Ly treatment (5, 10 and 20 mg/kg) (P < 0.05, P < 0.01, Table 1).
Table 1.
Effect of lycopene on serum liver function markers and blood lipid levels
| Group | ALT (IU/L) | AST (IU/L) | TG (mmol/L) | TC (mmol/L) |
| Control | 16.72 ± 2.62 | 60.65 ± 6.28 | 0.52 ± 0.04 | 0.81 ± 0.06 |
| Model | 61.25 ± 13.55b | 162.17 ± 35.53b | 1.38 ± 0.21b | 3.04 ± 0.72b |
| Ly 5 mg/kg | 30.90 ± 3.84c | 95.91 ± 13.65c | 1.02 ± 0.10 | 2.31 ± 0.24 |
| Ly 10 mg/kg | 26.33 ± 2.06d | 88.53 ± 9.18d | 0.75 ± 0.06c | 2.00 ± 0.12c |
| Ly 20 mg/kg | 20.77 ± 3.52d | 78.44 ± 9.79d | 0.59 ± 0.03d | 1.83 ± 0.15d |
Data are expressed as mean ± SD (n = 12) for each group.
P < 0.01 vs control group;
P < 0.05,
P < 0.01 vs model group. Ly: Lycopene; ALT: Alanine aminotransferase; AST: Aspartate aminotransferase; TG: Triglycerides; TC: Total cholesterol.
Effects of Ly on blood lipid levels
HFD-induced NAFLD provoked a marked incremental change in TC and TG levels compared with those in the normal group (P < 0.01, Table 1), which indicates the successful establishment of the NAFLD model in rats. However, after Ly exposure, the concentrations of both TC and TG in blood were remarkably decreased in dose-dependent manners, as compared to the NAFLD model group (P < 0.05, P < 0.01, Table 1). All of these findings indicate that Ly exerts obvious lipid-lowering effects against NAFLD.
Effects of Ly on liver tissue SOD, GSH and MDA levels
The levels of liver antioxidant activities of SOD and GSH were measured due to the oxidative stress exhibited in the development of NAFLD[17]. SOD and GSH are capable of scavenging the lipid hydroperoxides, lipid peroxide radicals and other products which are toxic metabolites of NAFLD. Therefore, our study measured the contents of SOD, GSH and MDA in liver tissue of rats. From Table 2, we can clearly see the significant differences between the HFD-treated model group and the normal group for the levels of SOD and GSH, which were largely decreased (P < 0.01, Table 2) in the HFD-treated group compared with that of normal group. However, pretreatment with 5, 10 and 20 mg/kg of Ly significantly raised the levels of the antioxidant enzyme SOD in a dose-dependent manner, to 90.95 ± 9.56, 109.52 ± 11.34 and 121.25 ± 10.68 respectively (P < 0.05, P < 0.01, Table 2), as compared with the model group. Similarly, the levels of GSH were significantly increased by treatment with 10 and 20 mg/kg of Ly (P < 0.05, P < 0.01, Table 2).
Table 2.
Effect of lycopene on liver antioxidant enzyme-specific activities, antioxidant and lipid peroxidation levels
| Group | SOD (U/mgprot) | GSH (mg/gprot) | MDA (nmol/mgprot) |
| Control | 131.42 ± 16.24 | 6.76 ± 1.54 | 3.46 ± 1.11 |
| Model | 77.70 ± 7.63b | 2.55 ± 0.78b | 7.58 ± 3.10b |
| Ly 5 mg/kg | 90.95 ± 9.56c | 2.68 ± 1.26 | 5.24 ± 1.46d |
| Ly 10 mg/kg | 109.52 ± 11.34d | 3.76 ± 0.91c | 4.13 ± 1.13d |
| Ly 20 mg/kg | 121.25 ± 10.68d | 4.79 ± 1.51d | 3.45 ± 1.39d |
Data are expressed as mean ± SD (n = 12) for each group.
P < 0.01 vs control group;
P < 0.05,
P < 0.01 vs model group. Ly: Lycopene; SOD: Superoxide dismutase; GSH: Glutathione; MDA: Malondialdehyde.
MDA, an end-product of the breakdown of polyunsaturated fatty acids and related esters, is an important index of lipid peroxidation in many organ homogenates[17]. Administration of HFD caused a significant increase in MDA concentration (2.19-fold), as compared with the normal group (P < 0.01, Table 2). However, pretreatment with 5, 10 and 20 mg/kg of Ly significantly reduced the MDA amount by 30.87, 45.51 and 54.49% in the liver homogenates respectively (P < 0.01, Table 2).
Effects of Ly on LDL-C, HDL-C and FFA levels in liver tissue
Levels of lipid products were significantly increased after 8 wk of HFD feeding in the model group compared to the control group (P < 0.01, Table 3). Results showed that LDL-C was significantly increased in the model group compared with the normal group (P < 0.01, Table 3) and dramatically decreased in the Ly-treatment group, as compared with that in the model group (P < 0.05, P < 0.01, Table 3). In contrast, HDL-C level was significantly decreased at the end of the experiment, and Ly treatment significantly improved the HDL-C level, as compared with that in the model group (P < 0.05, P < 0.01, Table 3). Similarly, the concentration of FFA was remarkably increased after HFD administration, and pretreatment with Ly significantly decreased the content of FFA in a dose-dependent manner (P < 0.05, P < 0.01, Table 3).
Table 3.
Effect of lycopene on low density lipoprotein-cholesterol, high density lipoprotein-cholesterol and free fatty acid levels in liver tissue
| Group | LDL-C (mmol/L) | HDL-C (mmol/L) | FFA (mmol/L) |
| Control | 0.34 ± 0.08 | 0.98 ± 0.10 | 0.82 ± 0.13 |
| Model | 2.48 ± 0.13b | 0.55 ± 0.02b | 2.03 ± 0.15b |
| Ly 5 mg/kg | 1.32 ± 0.10c | 0.70 ± 0.04c | 1.73 ± 0.12 |
| Ly 10 mg/kg | 0.95 ± 0.05d | 0.80 ± 0.05d | 1.56 ± 0.10c |
| Ly 20 mg/kg | 0.62 ± 0.08d | 0.87 ± 0.05d | 1.34 ± 0.08d |
Data are expressed as mean ± SD (n = 12) for each group.
P < 0.01 vs control group;
P < 0.05,
P < 0.01 vs model group. Ly: Lycopene; LDL-C: Low density lipoprotein-cholesterol; HDL-C: High density lipoprotein-cholesterol; FFA: Free fatty acid.
Histopathological changes in the liver tissue
Observed with the naked eye, the livers of the control group were deep red, moist, glossy and resilient (Figure 2AI), while those of the model group showed yellow necrotic foci, grey-red color, loss of luster and tumescent (Figure 2AII). However, in Ly-treated rats, the liver injury was attenuated dramatically in a dose-dependent manner (Figure 2AIII-V).
HE-stained sections are shown in Figure 2B. Under the photomicroscope, liver sections from the normal control group showed normal lobular architecture, liver cells with well-preserved cytoplasm and well-defined nucleus (Figure 2BI). Meanwhile liver sections from the model group showed full fat vacuoles in lobule cells, infiltration of inflammatory cells, cell swelling and lipid degeneration in the central region of the lobules (Figure 2BII). Furthermore, in the liver sections of the Ly-treated group, inflammatory response and lipid degeneration were remarkably alleviated, as compared with the model group, and the liver cell volume became smaller, the fat droplet number was reduced and the hepatic lobules were clearly delineated (Figure 2BIII-V).
Figure 2.
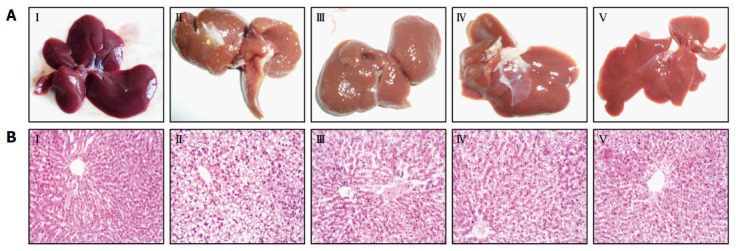
Appearance of rat liver tissue (A) and histopathological examination by HE (B, 200 ×). I: Control group; II: Model group; III: Ly 5 mg/kg group; IV: Ly 10 mg/kg group; V: Ly 20 mg/kg group. Ly: Lycopene.
Effect of Ly on immunohistochemistry analysis of hepatic TNF-α and CYP2E1
The immunohistochemistry (IHC) analysis of liver tissue showed no TNF-α expression in the normal group (Figure 3AI), but increased expression of TNF-α in the HFD-model group (Figure 3AII). After pretreatment with Ly (5, 10 and 20 mg/kg), TNF-α expression decreased in a dose-dependent manner, but remained higher than that in the normal group (Figure 3AIII-V). Quantification of the positive expression of TNF-α is shown in Figure 3AVI. The results are presented as mean ± SD of (n = 12). bP < 0.01 was significantly different from the normal group; cP < 0.05 and dP < 0.01 were significantly different from the model group, respectively.
In Figure 3B, the normal liver expressed the lowest amount of CYP2E1 (Figure 3BI). The HFD-model group showed significantly higher expression of CYP2E1, as compared with the controls (P < 0.01, Figure 3BII). Meanwhile the Ly-treated group (5, 10 and 20 mg/kg) showed markedly decreased CYP2E1 expression (Figure 3BIII-V). Quantification of the positive expression of CYP2E1 is shown in Figure 3BVI. The results are presented as mean ± SD (n = 12). bP < 0.01 was significantly different from the control group; cP < 0.05 and dP < 0.01 were significantly different from the model group, respectively.
Figure 3.
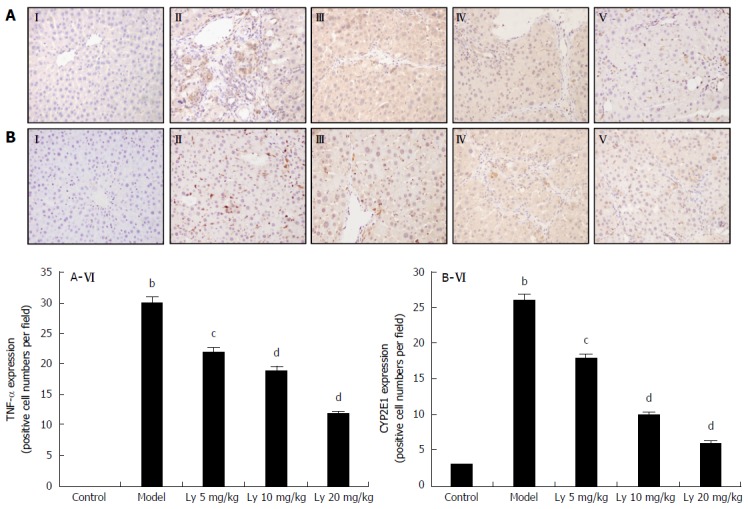
Representative photographs of immunological histological chemistry examination (200 ×). A: TNF-α; B: CYP2E1. I: Control group; II: Model group; III: Ly 5 mg/kg group; IV: Ly 10 mg/kg group; V: Ly 20 mg/kg group; VI: Quantification of TNF-α (A-VI) and CYP2E1 (B-VI) stained cells. The results are expressed as mean ± SD of 12 rats. bP < 0.01 vs control group; cP < 0.05, dP < 0.01 vs model group. Ly: Lycopene.
DISCUSSION
NAFLD is defined by hepatic fat deposition in the absence of excessive alcohol intake, which is also associated with the insulin resistance (IR) and metabolic syndrome[18-20]. Generally, NAFLD is defined as a concentration of hepatic TG exceeding 5% liver weight, and often exhibits a histological spectrum ranging from simple steatosis to NASH. NASH is characterized by hepatocellular damage, fibrogenesis and lobular necro-inflammation[21,22], which may evolve to hepatic cirrhosis and HCC[23,24]. Although HFD-induced NAFLD animal models need a lengthy feeding period, they are more close to human NAFLD in pathophysiology, including induced obesity, IR and hepatic steatosis in mice or rats[25]. Emotional disorders or poor diet with the key points of blood stasis and phlegm is regarded as the etiology of NAFLD, and these etiologies are related to the organs of liver, spleen and kidney, according to the traditional medicine theory[26]. Thus, promoting blood circulation to remove meridian obstruction, reducing phlegm, removing dampness and liver-kidney-tonifying are an effective approach to treatment of NAFLD. However, at present, although tremendous effort has made in prevention of NAFLD by clinicians and researchers alike, there are no approved treatment drugs for NAFLD. Hence, developing and exploring a novel agent to delay or reverse the pathogenesis progression in NAFLD are very important objectives.
Ly is a natural pigment, synthesized by plants and microorganisms. Red fruits and vegetables are the most common sources of Ly, which exhibits the highest antioxidant activity among all dietary carotenoids. Furthermore, the Mediterranean dietary pattern, which includes proportionally high consumption of vegetables and fruits with Ly, has shown notable benefits for NAFLD patients[27,28]. Therefore, nowadays, the potential role of Ly in human health is beginning to be recognized, and the most important health benefits are hypothesized to occur through their ability to protect against oxidative damage[29,30]. In vitro studies have demonstrated that Ly is an effective antioxidant and radical scavenger[31,32]. Ly is the most potent singlet oxygen quencher among natural carotenoids, due to its high number of conjugated dienes[33], and recent studies have shown that Ly is at least two times as active as β-carotene in protecting lymphocytes for NO2· radical-induced membrane damage[34,35], which indicates that Ly is the most potent scavenger of ROS among other major dietary carotenoids[36,37]. In addition, Ly was shown to protect human LDL against photosensitized oxidative damage[32]. Thus, based on the benefits of Ly, the aim of the present study was to explore the effect of Ly in prevention of HFD-induced NAFLD in a rat model. To the best of our knowledge, this is the first time research has attempted to explore the potent effects of Ly on HFD-induced NAFLD rats.
In the present study, compared to a normal control group, it was demonstrated that the liver coefficient and the levels of serum ALT, AST, TG and TC were significantly increased, the levels of LDL-C and FFA in liver were markedly increased, and HDL-C was markedly reduced in HFD-induced NAFLD model rats. Pretreatment with Ly showed that Ly is able to inhibit the incremental changes in ALT and AST, to decrease the TG, TC, LDL-C and FFA levels, and to increase the HDL-C level. In addition, the histopathological changes from microscopy observation correlated with the examination of liver function. The centrilobular hepatic necrosis, ballooning degeneration, fatty change and infiltrating lymphocytes were observed in NAFLD model group. Treatment with Ly prevented these histopathological changes in rats induced with HFD. Thus, these results suggested that the inhibition of the elevation of liver function markers, obvious lipid-lowering and liver damage may related to the protective effect of Ly against HFD-induced NAFLD. Moreover, Ly enhanced the activities of SOD, increased GSH and diminished MDA against the HFD-induced NAFLD in these animals, suggesting that the activity of antioxidants may play a role in the mechanism of its hepatoprotective effects.
TNF-α is a central proinflammatory cytokine, which is associated with a variety of physiological and pathological conditions, including cytotoxicity, growth stimulation, immune-modulation and pro-inflammatory activity. In addition, TNF-α is produced predominantly by the monocyte macrophage lineage in liver, and the main population of this lineage is Kupffer cells. Thus, increased TNF-α production by activated Kupffer cells may be responsible for NAFLD. Furthermore, the most current studies have indicated that inhibition of TNF-α could decrease the content of hepatic fatty storage in the HFD-induced NAFLD model[38]. In our study, the effects of TNF-α in damaged liver was evaluated by IHC. Compared to the normal group, rats treated with HFD showed up-regulated expression of TNF-α, while pretreatment with Ly led to down-regulated expression of TNF-α compared to the HFD-model group.
The isoform 2E1 of CYP is one of the most potent microsome cytochromes to generate ROS, and it is involved in the metabolism of isoniazid and the mediation of its hepatotoxicity[39], which has been exhibited to be invariably increased in the livers of NAFLD patients[40]. In this study, the expression of CYP2E1 in the HFD-model group was observed to be increased, while the Ly treatment group showed a significant down-regulation of its expression, especially in the high-dose Ly-treated group.
In conclusion, oral administration of Ly improved lipid profiles and remarkably decreased the levels of serum AST, ALT, TG and TC, alleviated the levels of liver LDL-C and FFA, increased the activities of antioxidant enzymes (GSH, SOD) and reduced the lipid peroxides in liver (MDA) in NAFLD model rats. Further, the Ly-treated group also showed down-regulated expression of TNF-α and CYP2E1, decreased liver fats infiltration and improved histopathological changes, all in dose-dependent manners. The increased antioxidant enzyme levels and the decreased lipid peroxides contents are suggested to be important mechanisms of Ly in preventing the development of liver damage induced by HFD.
COMMENTS
Background
Non-alcoholic fatty liver disease (NAFLD) is one of the causes of fatty liver, which encompasses a spectrum of liver diseases, including simple steatosis, non-alcoholic steatohepatitis (NASH), liver fibrosis, liver cirrhosis and hepatocellular carcinoma (HCC). Until now, there is not any specific drug available, and no drug has currently been tested in clinical phase III trials. Lycopene (Ly), a phytochemical belonging to the carotenoid family, is a red-colored pigment, apolar and acyclic carotenoid. Ly exhibits a range of distinct and unique biological properties owing to its acyclic structure, hydrophobicity and large array of conjugated double bonds. A recent report showed that the Mediterranean dietary pattern, which includes proportionally high consumption of vegetables and fruits with Ly, has notable benefits for NAFLD patients. Thus, with this background, we aimed to investigate the possible beneficial effects and the possible action mechanism of Ly on NAFLD in a rat model system.
Research frontiers
No specific drug has been tested in clinical phase III trials for NAFLD to date, and there are few research studies of the hepatoprotective effects of Ly.
Innovations and breakthroughs
This study represents the first investigation of the effects of Ly as a therapy of NAFLD, and showed that down-regulated expression of TNF-α and CYP2E1 may be one of the action mechanisms for Ly.
Applications
This study suggests that Ly has a protective effect on NAFLD, which is very important for the future development of a potent NAFLD drug.
Terminology
NAFLD is one of the causes of fatty liver, defined as biopsy-proven hepatic steatosis. It covers a spectrum of liver diseases, including simple steatosis, NASH, liver fibrosis, liver cirrhosis and HCC. Recently, many NAFLD drug research studies have focused on the traditional Chinese medicines.
Peer-review
This is a meaningful study, in which the effects of “lycopene” were examined on an NAFLD rat model. The results are very important and suggest that Ly exerts a protective effect on NAFLD through down-regulation of TNF-α and CYP2E1 expression.
Footnotes
Manuscript source: Unsolicited manuscript
Specialty type: Gastroenterology and hepatology
Country of origin: China
Peer-review report classification
Grade A (Excellent): A
Grade B (Very good): B
Grade C (Good): 0
Grade D (Fair): 0
Grade E (Poor): 0
Institutional review board statement: The study was reviewed and approved by Harbin Medical University Institutional Review Board, Harbin, China.
Institutional animal care and use committee statement: All procedures involving rats in this manuscript were reviewed and approved by the Institutional Animal Care and Use Committee on the Ethics of Animal Experiments of Harbin Medical University (HMU, Protocol Number: 20150301).
Conflict-of-interest statement: We have no financial relationships to disclose.
Data sharing statement: No additional data are available.
Peer-review started: June 13, 2016
First decision: July 29, 2016
Article in press: October 10, 2016
P- Reviewer: Abenavol L, Lee HC S- Editor: Gong ZM L- Editor: Filipodia E- Editor: Wang CH
References
- 1.Masarone M, Federico A, Abenavoli L, Loguercio C, Persico M. Non alcoholic fatty liver: epidemiology and natural history. Rev Recent Clin Trials. 2014;9:126–133. doi: 10.2174/1574887109666141216111143. [DOI] [PubMed] [Google Scholar]
- 2.Wilkins T, Tadkod A, Hepburn I, Schade RR. Nonalcoholic fatty liver disease: diagnosis and management. Am Fam Physician. 2013;88:35–42. [PubMed] [Google Scholar]
- 3.Schuppan D, Schattenberg JM. Non-alcoholic steatohepatitis: pathogenesis and novel therapeutic approaches. J Gastroenterol Hepatol. 2013;28 Suppl 1:68–76. doi: 10.1111/jgh.12212. [DOI] [PubMed] [Google Scholar]
- 4.Keating SE, Hackett DA, George J, Johnson NA. Exercise and non-alcoholic fatty liver disease: a systematic review and meta-analysis. J Hepatol. 2012;57:157–166. doi: 10.1016/j.jhep.2012.02.023. [DOI] [PubMed] [Google Scholar]
- 5.Satapathy SK, Sanyal AJ. Epidemiology and Natural History of Nonalcoholic Fatty Liver Disease. Semin Liver Dis. 2015;35:221–235. doi: 10.1055/s-0035-1562943. [DOI] [PubMed] [Google Scholar]
- 6.Day CP, James OF. Steatohepatitis: a tale of two “hits”? Gastroenterology. 1998;114:842–845. doi: 10.1016/s0016-5085(98)70599-2. [DOI] [PubMed] [Google Scholar]
- 7.European Association for the Study of the Liver (EASL), European Association for the Study of Diabetes (EASD), European Association for the Study of Obesity (EASO) EASL-EASD-EASO Clinical Practice Guidelines for the management of non-alcoholic fatty liver disease. J Hepatol. 2016;64:1388–1402. doi: 10.1016/j.jhep.2015.11.004. [DOI] [PubMed] [Google Scholar]
- 8.Engelmann NJ, Clinton SK, Erdman JW. Nutritional aspects of phytoene and phytofluene, carotenoid precursors to lycopene. Adv Nutr. 2011;2:51–61. doi: 10.3945/an.110.000075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Visioli F, Riso P, Grande S, Galli C, Porrini M. Protective activity of tomato products on in vivo markers of lipid oxidation. Eur J Nutr. 2003;42:201–206. doi: 10.1007/s00394-003-0415-5. [DOI] [PubMed] [Google Scholar]
- 10.Mein JR, Lian F, Wang XD. Biological activity of lycopene metabolites: implications for cancer prevention. Nutr Rev. 2008;66:667–683. doi: 10.1111/j.1753-4887.2008.00120.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Stahl W, Sies H. Carotenoids and flavonoids contribute to nutritional protection against skin damage from sunlight. Mol Biotechnol. 2007;37:26–30. doi: 10.1007/s12033-007-0051-z. [DOI] [PubMed] [Google Scholar]
- 12.Kumar P, Kalonia H, Kumar A. Lycopene modulates nitric oxide pathways against 3-nitropropionic acid-induced neurotoxicity. Life Sci. 2009;85:711–718. doi: 10.1016/j.lfs.2009.10.001. [DOI] [PubMed] [Google Scholar]
- 13.Datta S, Jamwal S, Deshmukh R, Kumar P. Beneficial effects of lycopene against haloperidol induced orofacial dyskinesia in rats: Possible neurotransmitters and neuroinflammation modulation. Eur J Pharmacol. 2016;771:229–235. doi: 10.1016/j.ejphar.2015.12.032. [DOI] [PubMed] [Google Scholar]
- 14.Sachdeva AK, Chopra K. Lycopene abrogates Aβ(1-42)-mediated neuroinflammatory cascade in an experimental model of Alzheimer’s disease. J Nutr Biochem. 2015;26:736–744. doi: 10.1016/j.jnutbio.2015.01.012. [DOI] [PubMed] [Google Scholar]
- 15.Maiani G, Castón MJ, Catasta G, Toti E, Cambrodón IG, Bysted A, Granado-Lorencio F, Olmedilla-Alonso B, Knuthsen P, Valoti M, et al. Carotenoids: actual knowledge on food sources, intakes, stability and bioavailability and their protective role in humans. Mol Nutr Food Res. 2009;53 Suppl 2:S194–S218. doi: 10.1002/mnfr.200800053. [DOI] [PubMed] [Google Scholar]
- 16.Ried K, Fakler P. Protective effect of lycopene on serum cholesterol and blood pressure: Meta-analyses of intervention trials. Maturitas. 2011;68:299–310. doi: 10.1016/j.maturitas.2010.11.018. [DOI] [PubMed] [Google Scholar]
- 17.Yen FL, Wu TH, Lin LT, Lin CC. Hepatoprotective and antioxidant effects of Cuscuta chinensis against acetaminophen-induced hepatotoxicity in rats. J Ethnopharmacol. 2007;111:123–128. doi: 10.1016/j.jep.2006.11.003. [DOI] [PubMed] [Google Scholar]
- 18.Marchesini G, Brizi M, Morselli-Labate AM, Bianchi G, Bugianesi E, McCullough AJ, Forlani G, Melchionda N. Association of nonalcoholic fatty liver disease with insulin resistance. Am J Med. 1999;107:450–455. doi: 10.1016/s0002-9343(99)00271-5. [DOI] [PubMed] [Google Scholar]
- 19.Fabbrini E, Mohammed BS, Magkos F, Korenblat KM, Patterson BW, Klein S. Alterations in adipose tissue and hepatic lipid kinetics in obese men and women with nonalcoholic fatty liver disease. Gastroenterology. 2008;134:424–431. doi: 10.1053/j.gastro.2007.11.038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Cohen JC, Horton JD, Hobbs HH. Human fatty liver disease: old questions and new insights. Science. 2011;332:1519–1523. doi: 10.1126/science.1204265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Kleiner DE, Brunt EM, Van Natta M, Behling C, Contos MJ, Cummings OW, Ferrell LD, Liu YC, Torbenson MS, Unalp-Arida A, et al. Design and validation of a histological scoring system for nonalcoholic fatty liver disease. Hepatology. 2005;41:1313–1321. doi: 10.1002/hep.20701. [DOI] [PubMed] [Google Scholar]
- 22.Day CP. From fat to inflammation. Gastroenterology. 2006;130:207–210. doi: 10.1053/j.gastro.2005.11.017. [DOI] [PubMed] [Google Scholar]
- 23.Bugianesi E, Leone N, Vanni E, Marchesini G, Brunello F, Carucci P, Musso A, De Paolis P, Capussotti L, Salizzoni M, et al. Expanding the natural history of nonalcoholic steatohepatitis: from cryptogenic cirrhosis to hepatocellular carcinoma. Gastroenterology. 2002;123:134–140. doi: 10.1053/gast.2002.34168. [DOI] [PubMed] [Google Scholar]
- 24.Liu YL, Patman GL, Leathart JB, Piguet AC, Burt AD, Dufour JF, Day CP, Daly AK, Reeves HL, Anstee QM. Carriage of the PNPLA3 rs738409 C & gt; G polymorphism confers an increased risk of non-alcoholic fatty liver disease associated hepatocellular carcinoma. J Hepatol. 2014;61:75–81. doi: 10.1016/j.jhep.2014.02.030. [DOI] [PubMed] [Google Scholar]
- 25.Carabelli J, Burgueño AL, Rosselli MS, Gianotti TF, Lago NR, Pirola CJ, Sookoian S. High fat diet-induced liver steatosis promotes an increase in liver mitochondrial biogenesis in response to hypoxia. J Cell Mol Med. 2011;15:1329–1338. doi: 10.1111/j.1582-4934.2010.01128.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Dong H, Lu FE, Zhao L. Chinese herbal medicine in the treatment of nonalcoholic fatty liver disease. Chin J Integr Med. 2012;18:152–160. doi: 10.1007/s11655-012-0993-2. [DOI] [PubMed] [Google Scholar]
- 27.Federico A, Zulli C, de Sio I, Del Prete A, Dallio M, Masarone M, Loguercio C. Focus on emerging drugs for the treatment of patients with non-alcoholic fatty liver disease. World J Gastroenterol. 2014;20:16841–16857. doi: 10.3748/wjg.v20.i45.16841. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Godos J, Federico A, Dallio M, Scazzina F. Mediterranean diet and nonalcoholic fatty liver disease: molecular mechanisms of protection. Int J Food Sci Nutr. 2016 doi: 10.1080/09637486.2016.1214239. Epub ahead of print: 1-10. [DOI] [PubMed] [Google Scholar]
- 29.Stahl W, Sies H. Lycopene: a biologically important carotenoid for humans? Arch Biochem Biophys. 1996;336:1–9. doi: 10.1006/abbi.1996.0525. [DOI] [PubMed] [Google Scholar]
- 30.Gerster H. The potential role of lycopene for human health. J Am Coll Nutr. 1997;16:109–126. doi: 10.1080/07315724.1997.10718661. [DOI] [PubMed] [Google Scholar]
- 31.Clinton SK. Lycopene: chemistry, biology, and implications for human health and disease. Nutr Rev. 1998;56:35–51. doi: 10.1111/j.1753-4887.1998.tb01691.x. [DOI] [PubMed] [Google Scholar]
- 32.Rao AV, Fleshner N, Agarwal S. Serum and tissue lycopene and biomarkers of oxidation in prostate cancer patients: a case-control study. Nutr Cancer. 1999;33:159–164. doi: 10.1207/S15327914NC330207. [DOI] [PubMed] [Google Scholar]
- 33.Miller NJ, Sampson J, Candeias LP, Bramley PM, Rice-Evans CA. Antioxidant activities of carotenes and xanthophylls. FEBS Lett. 1996;384:240–242. doi: 10.1016/0014-5793(96)00323-7. [DOI] [PubMed] [Google Scholar]
- 34.Böhm F, Tinkler JH, Truscott TG. Carotenoids protect against cell membrane damage by the nitrogen dioxide radical. Nat Med. 1995;1:98–99. doi: 10.1038/nm0295-98. [DOI] [PubMed] [Google Scholar]
- 35.Tinkler JH, Böhm F, Schalch W, Truscott TG. Dietary carotenoids protect human cells from damage. J Photochem Photobiol B. 1994;26:283–285. doi: 10.1016/1011-1344(94)07049-0. [DOI] [PubMed] [Google Scholar]
- 36.Mortensen A, Skibsted LH. Relative stability of carotenoid radical cations and homologue tocopheroxyl radicals. A real time kinetic study of antioxidant hierarchy. FEBS Lett. 1997;417:261–266. doi: 10.1016/s0014-5793(97)01297-0. [DOI] [PubMed] [Google Scholar]
- 37.Mortensen A, Skibsted LH, Sampson J, Rice-Evans C, Everett SA. Comparative mechanisms and rates of free radical scavenging by carotenoid antioxidants. FEBS Lett. 1997;418:91–97. doi: 10.1016/s0014-5793(97)01355-0. [DOI] [PubMed] [Google Scholar]
- 38.Gao HY, Huang J, Wang HY, Du XW, Cheng SM, Han Y, Wang LF, Li GY, Wang JH. Protective effect of Zhuyeqing liquor, a Chinese traditional health liquor, on acute alcohol-induced liver injury in mice. J Inflamm (Lond) 2013;10:30. doi: 10.1186/1476-9255-10-30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Yang P, Zeng Q, Cao WC, Wang YX, Huang Z, Li J, Liu C, Lu WQ. Interactions between CYP2E1, GSTZ1 and GSTT1 polymorphisms and exposure to drinking water trihalomethanes and their association with semen quality. Environ Res. 2016;147:445–452. doi: 10.1016/j.envres.2016.03.009. [DOI] [PubMed] [Google Scholar]
- 40.Weltman MD, Farrell GC, Hall P, Ingelman-Sundberg M, Liddle C. Hepatic cytochrome P450 2E1 is increased in patients with nonalcoholic steatohepatitis. Hepatology. 1998;27:128–133. doi: 10.1002/hep.510270121. [DOI] [PubMed] [Google Scholar]


