Abstract
Study Objectives:
To evaluate the effect of type 1 narcolepsy (NT1) on anthropometric and endocrine features in childhood/adolescence, focusing on patterns and correlates of weight, pubertal development, and growth in treated and untreated patients.
Methods:
We collected anthropometric (height, weight, body mass index (BMI) z-scores), pubertal, metabolic, and endocrine data from 72 NT1 patients at diagnosis and all available premorbid anthropometric parameters of patients from their pediatric files (n = 30). New measurements at 1-y reassessment in patients undergoing different treatments were compared with baseline data.
Results:
We detected a high prevalence of overweight (29.2%), obesity (25%), metabolic syndrome (18.8%), and precocious puberty (16.1%), but no signs of linear growth alterations at diagnosis. According to anthropometric records, weight gain started soon after NT1 onset. At 1-y follow-up reassessment, sodium oxybate treatment was associated with a significant BMI z-score reduction (−1.29 ± 0.30, p < 0.0005) after adjusting for baseline age, sex, sleepiness, and BMI.
Conclusions:
NT1 onset in children/adolescents is associated with rapid weight gain up to overweight/obesity and precocious puberty without affecting growth. In our study, sodium oxybate treatment resulted in a significant weight reduction in NT1 overweight/obese patients at 1-y follow-up.
Citation:
Ponziani V, Gennari M, Pizza F, Balsamo A, Bernardi F, Plazzi G. Growing up with type 1 narcolepsy: its anthropometric and endocrine features. J Clin Sleep Med 2016;12(12):1649–1657.
Keywords: growth, narcolepsy type 1, obesity, precocious puberty, sodium oxybate
INTRODUCTION
Type 1 narcolepsy (NT1) mainly arises during childhood/adolescence1 with hypersomnolence and cataplexy as heralding symptoms.2 Childhood cataplexy differs from the adult form, presenting with remarkable facial muscle weakness (“catapleptic facies”) evident even in the absence of emotional triggers.3,4 Hypnagogic hallucinations and disrupted nocturnal sleep are often present from disease onset, whereas sleep paralysis may appear later during the disease course.5
Hypocretin deficiency is typical of NT1 and probably caused by selective autoimmune destruction of the hypothalamic hypocretin-producing neurons.6,7 Hypocretin deficiency affects not only sleep regulation, but also the neuroendocrine system with abnormalities in energy balance, feeding behavior, glucose metabolism, and modulation of the hypothalamic-pituitary axis.8,9 NT1 patients frequently suffer from obesity,10 type 2 diabetes,11 and central precocious puberty (PP).12 An association with atopic predisposition has also been reported.13 Personality and behavioral14 and psychiatric symptoms15 hinder the correct diagnosis with negative effects on learning, quality of life, and psychological development.16
Treatment studies for NT1 are lacking in the pediatric population and currently the same pharmacological therapies, such as modafinil, sodium oxybate (SO), and venlafaxine17 are used both for adults and children (off-label for venlafaxine).
Although obesity has been associated with NT1 in pediatric patients,18,19 the time gap between NT1 onset and weight gain, and the possible effects of treatment on weight loss20,21 have never been addressed.
BRIEF SUMMARY
Current Knowledge/Study Rationale: Narcolepsy type 1 has been associated with profound metabolic and endocrine alterations in young patients, namely overweight/obesity and precocious puberty. Our study, based on the collaboration between sleep and endocrine pediatric experts, aimed to further investigate whether childhood NT1 has been associated with endocrine disorders such as overweight/obesity and precocious puberty, and the follow up of these comorbidities.
Study Impact: We confirmed the high occurrence of weight increase and pubertal alterations in NT1, and, noteworthy, we showed that clinically significant weight gain begins soon after disease onset, confirming that obesity is an ancillary NT1 symptom. At 1-y follow-up, only sodium oxybate therapy reversed weight gain in overweight and obese NT1 children.
Our study compared the anthropometric, metabolic, and endocrine data collected in a large pediatric NT1 cohort before disease onset, at diagnosis, and at 1-y follow-up.
METHODS
Patients
Seventy-two NT1 patients (35 boys, mean age 11.18 ± 3.13 y) underwent endocrinological evaluation. The findings from 42 patients enrolled in this study were previously reported.12 Ethics committee approval and parental written consent were obtained.
NT1 Diagnosis
Each patient underwent polysomnography followed by multiple sleep latency test (MSLT),5 Pediatric Daytime Sleepiness Scale,22 HLA DQB1*06:02 genotyping, brain magnetic resonance imaging, and whenever possible, cerebrospinal fluid hypocretin-1 (CSF hcrt-1) determination. At discharge, the need for behavioral and off-label pharmacological treatment with SO monotherapy (as first-line approach chosen to address sleepiness, cataplexy and disrupted nocturnal sleep)23,24 or modafinil ± venlafaxine (as a second-line approach designated to manage daytime symptoms)17 were discussed with the family and proposed for each patient. Modafinil was also added if hypersomnolence persisted after SO titration.
Anamnesis
Family history (parents and grandparents) of obesity, type 2 diabetes, dyslipidemia, arterial hypertension, PP, and personal history of allergies were investigated by clinical interview.
Endocrine and Pubertal Assessment
Blood levels of thyroid-stimulating hormone (TSH), free thyroxine (fT4), free triiodothyronine (fT3), adrenocorticotropic hormone (ACTH), serum cortisol, insulin-like growth factor-1 (IGF-1 and IGF-1 z-score), and prolactin (PRL) were collected at 08:00 in patients fasting from midnight onward. Cortisol level at 20:00 was determined on blood samples to evaluate its circadian rhythm. Twenty-four-hour free urine cortisol was also determined. To evaluate growth hormone (GH) pituitary secretion 39 children underwent two stimulating tests with clonidine (0.1 mg/m2 by mouth) and arginine (0.5 g/kg intravenously). Blood samples for GH determination were obtained at baseline and after 30, 60, 90, and 120 min. Pubertal development was established by Breast-Tanner stage 2 in girls and testicular volume ≥ 4 mL in boys (Genital-Tanner stage 2).25,26 When a discrepancy between chronological age and pubertal development was noted, bone age, pelvic ultrasound (in girls), and standard gonadotropin-releasing hormone (GnRH) tests were performed.
Central PP was confirmed in the presence of all of the following: (1) Tanner stage 2 or higher before the age of 8 y in girls or 9 y in boys; (2) plasma luteinizing hormone (LH) levels above 5 mIU/mL after GnRH test and (3) normal brain MRI.
Anthropometric and Metabolic Assessment
Height, weight, waist and hip circumferences, body mass index (BMI), BMI percentile,27 and recumbent blood pressure and heart rate were collected by a physician on the first day of hospitalization. Subsequent auxological follow-up was performed at 6 and 12 mo by the same physician (AB) in a dedicated out-patient clinic. A Harpenden stadiometer was used to measure height, and every child was measured three times on the same morning to the nearest completed mm. Body weight was measured in minimal underclothes to the nearest 100 g on accurate and properly calibrated scales. The BMI was calculated as weight divided by square of height (kg/m2). Height, weight, BMI, and BMI z-score were collected at 1-y follow-up in 56 children. Sixteen children have not yet reached the 1-y follow-up because of recent diagnosis.
Fasting blood glucose, basal insulin, total high-density (HDL)/low-density lipoprotein (LDL) cholesterol, triglycerides, oral glucose tolerance test,28 and anti-streptolysin O titer were collected at 08:00 in patients fasting from midnight onward. Normal weight was defined as BMI < 85th percentile, overweight as BMI between 85th and 95th percentile, obesity as BMI > 95th percentile.29 Metabolic syndrome was defined using Cook criteria for children/adolescents30 fulfilled by waist circumference ≥ 90th percentile (sex/age) and at least one of the following criteria: fasting glucose ≥ 110 mg/dL, triglycerides ≥ 110 mg/dL, HDL ≤ 40 mg/dL, and blood pressure ≥ 90th percentile (age/sex/height). The responses to the oral glucose tolerance test were analyzed by calculating the area under the curve (trapezoidal method).
BMI z-score (weight adjusted for height, sex, and age)31 was calculated at baseline in all subjects. At least two BMI z-scores between the age of 3 y and NT1 onset were available for 30 patients from general pediatric records. After the parental decision on treatment, patients were regularly followed up with weekly telephone interviews for 1 mo (SO titration period) and clinical evaluations after 3, 6, and 12 mo.
Statistical Analysis
Continuous and categorical data were explored with descriptive statistics (mean ± standard deviation [SD]) and frequency in the whole population and in patient subgroups. Nonparametric statistical approaches were used for between-group comparisons (Mann-Whitney U or chi-square tests) and for within-subject comparisons (Wilcoxon signed-rank or chi-square tests) for continuous or categorical data, respectively. Multivariate linear regression analyses tested treatments impact on BMI z-score change adjusting for baseline features. A value of p < 0.05 was considered statistically significant.
RESULTS
Mean age at onset of first symptom (either somnolence or cataplexy) was 8.68 ± 2.50 y, mean delay between NT1 onset and diagnosis was 1.70 ± 2.13 y, and 56.5% of the sample had previously received wrong diagnoses. Wrong diagnosis included obesity, unspecified metabolic disorder, unspecified hormonal/ endocrinological disorder, mononucleosis, psychological disorders, depression, psychomotor retardation, attention deficit hyperactivity disorder, encephalitis, epilepsy, chorea, complex tic disorder, myasthenia gravis, myopathy, parasomnia, unspecified hypersomnia, and Kleine-Levin syndrome.
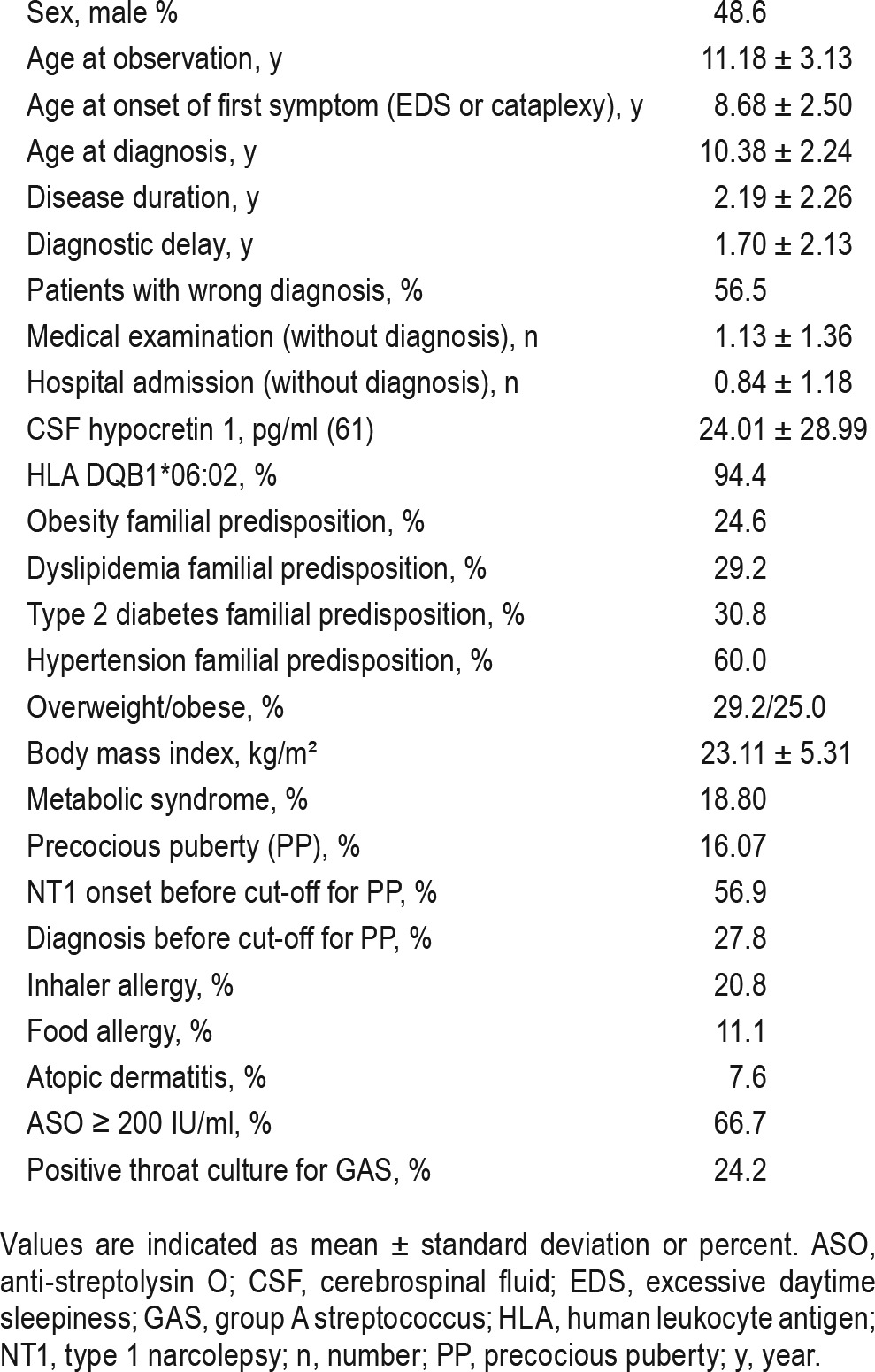
CSF hcrt-1 concentration (61 cases) was 24.01 ± 28.99 pg/mL. HLA DQB1*06:02 allele was present in 68 children (94.4%). Four children with HLA DQB1*06:02 negative geno-type had CSF hcrt-1 concentration below 110 pg/mL.32 MSLT confirmed NT1 (mean sleep latency 4.21 ± 3.24 min, with 4.07 ± 1.07 sleep-onset rapid eye movements periods) in 64 patients; 8 patients with a mean sleep latency above 8 min had CSF hcrt-1 deficiency (Table 1). There were 20.8% of patients with inhaler allergies and 11.1% with food allergies, 7.6% with atopic dermatitis, and 66.7% had high anti-streptolysin O titers (> 200 UI/mL).
Table 1.
Demographic, endocrine, and immunological characteristics from 72 children with type 1 narcolepsy.
Endocrine and Pubertal Assessment
No thyroid or adrenal gland alterations were detected. Indeed, mean ± SD blood values of TSH, ACTH, serum 08:00 and 20:00 cortisol were 2.08 ± 1.11 μUI/mL, 24.89 ± 13.77 pg/mL, 116.0 ± 60.95 ng/mL, and 28.87 ± 16.40 ng/mL, respectively. Prolactin blood levels were normal (mean value 15.13 ± 7.19 ng/mL). IGF-1 z-score was within normal range in all patients (mean value 0.26 ± 1.01, range −1.27 to 2.27). Among the 39 patients tested with the arginine and clonidine tests, 23 (5 with normal weight, 18 overweight/obese) had blunted GH concentration (below 8 ng/mL) in at least one determination. We used the 8 ng/mL cutoff as indicated by Italian guidelines.33
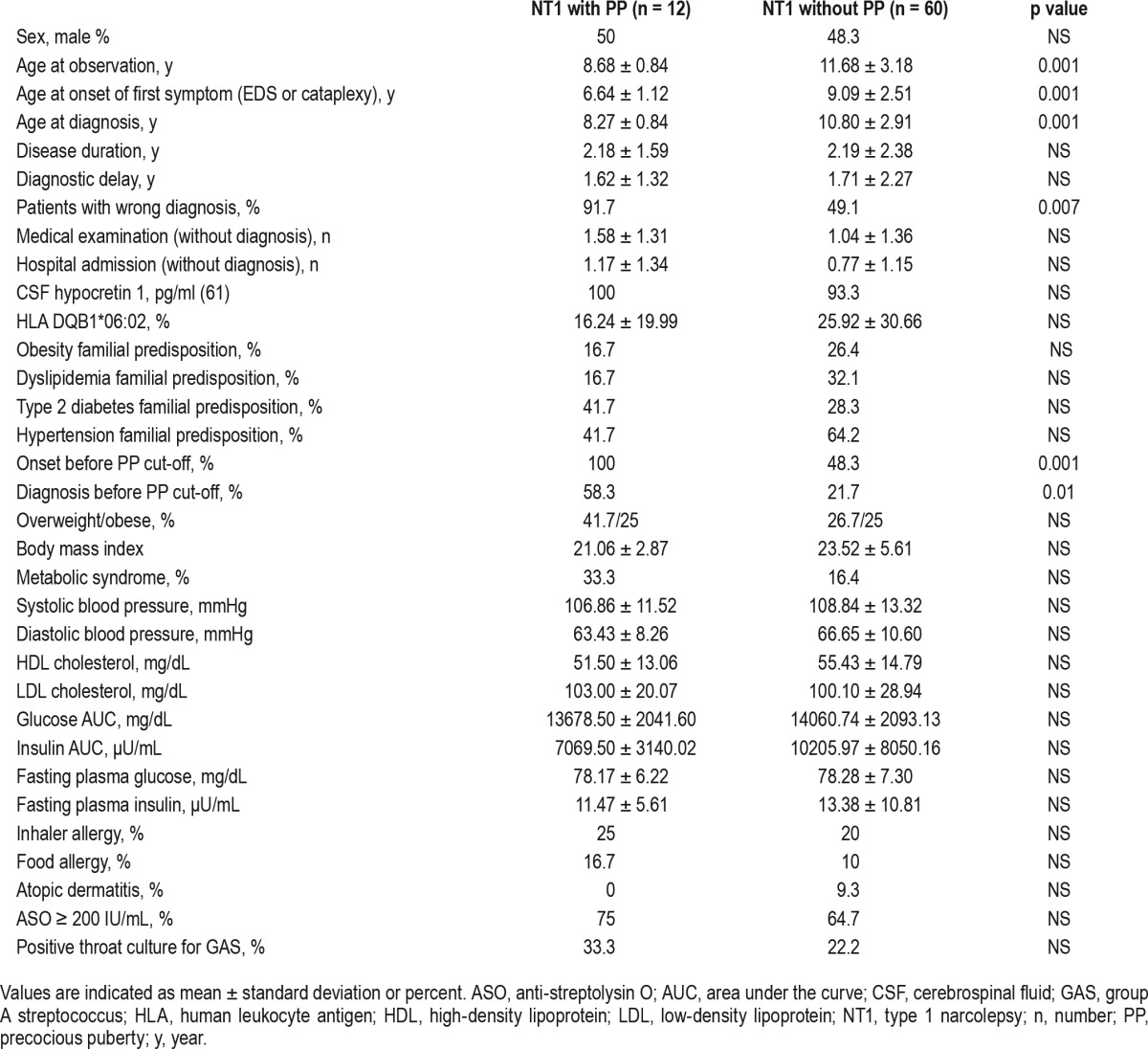
Twenty-five children were prepubertal, 35 were pubertal (menarche mean age 11.17 ± 1.13 y), and 12 (16.07%, 6 girls) had PP, fulfilling the criteria mentioned in the Methods section. None of these children had parents with PP. Eleven PP children underwent GnRH analogous treatment. Comparing children with (n = 12) and without (n = 60) PP, no differences in sleep-related parameters, overweight/obesity, allergies, family history, or metabolic characteristics were detected. However, children with PP more frequently received wrong diagnoses (Table 2: 91.7% vs. 49.1%, p = 0.007).
Table 2.
Comparison between NT1 children with and without precocious puberty.
Anthropometric and Metabolic Characteristics
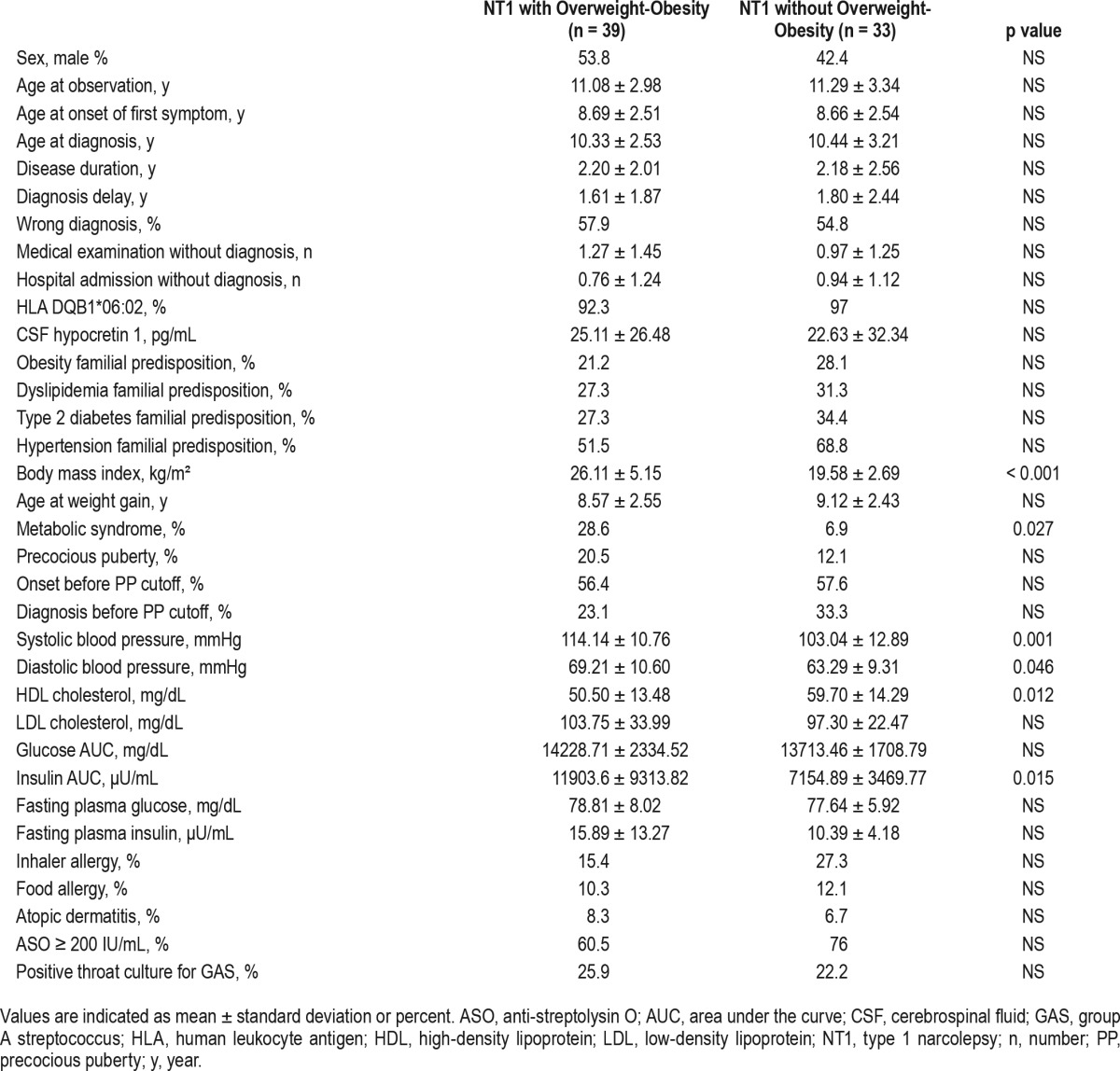
All children were of regular height (mean height z-score 0.72 ± 1.07) with a projected height within the range of their target height. There were 29% and 25% of patients who were overweight and obese, respectively, and 18.8% had metabolic syndrome. The overweight/obese children showed worse MSLT performances than those with normal weight (Table 3). Overweight/obese cases had higher diastolic (69.21 ± 10.60 vs. 63.29 ± 9.31 mmHg, p = 0.046) and systolic (114.14 ± 10.76 vs. 103.04 ± 12.89 mmHg, p = 0.001) blood pressure, lower HDL cholesterol (50.50 ± 13.48 vs. 59.70 ± 14.29 mg/dL, p = 0.012), higher insulin area under the curve values (11903.6 ± 9313.82 vs. 7154.89 ± 3469.77 μU/mL, p = 0.015), and higher prevalence of metabolic syndrome (28.6% vs. 6.9%, p = 0.027) when compared to a normal-weight cohort. No significant differences emerged in family history, allergies or PP prevalence (Table 4).
Table 3.
Comparison of sleep-related parameters between normal weight and overweight/obese NT1 children.
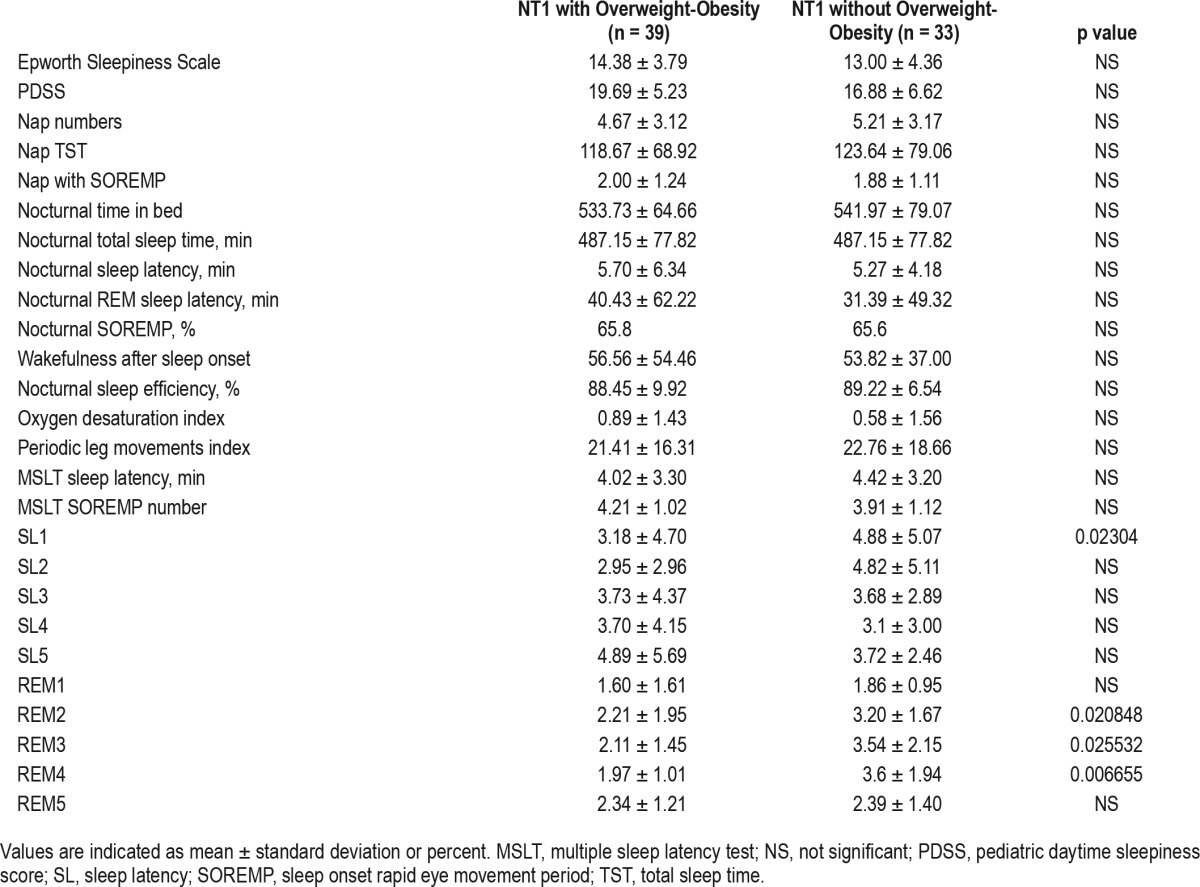
Table 4.
Comparison between NT1 children with and without overweight/obesity.
Anthropometric Characteristics before NT1 Onset
We analyzed anthropometric characteristics in a 2-y period before NT1 onset in the subset of 30 patients for whom written medical records were available. The within-subject comparison of three values of mean BMI z-score included the first one 2 y before NT1 onset (Time-2), referred to a mean age of 5.61 ± 2.49 y, the second one 1 y before NT1 (Time-1) referred to a mean age of 6.64 ± 2.59 y, and the third one at the time of diagnosis (T0). Weight gain started close to NT1 onset with significant changes from T-1 to T0. Overweight/obesity increased from 16.7% at T-2 and 16.6% at T-1 to 50.0% at T0 (Figure 1).
Figure 1. Body mass index (BMI) z-scores until NT1 onset.
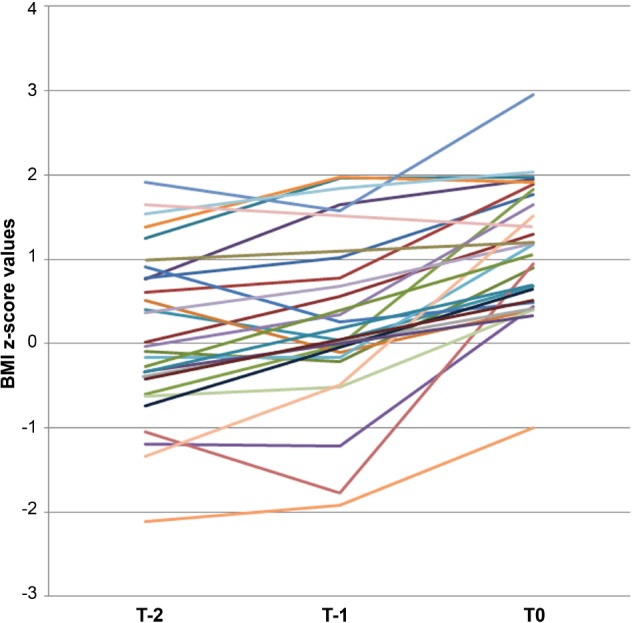
The graph shows the individual time course of BMI z scores obtained through general pediatric records available in 30 patients at a mean age of 5.61 ± 2.49 y (T-2), and 6.64 ± 2.59 y (T-1), and at diagnosis (T0) with evidence of a significant increase occurring between T-1 and T0 around NT1 onset.
1-Year Follow-Up
Fifty-six children were reevaluated after 1 y. Children who for different reasons were not included in the follow-up had no significant differences from the others at NT1 diagnosis time. Thirty-five were treated with SO alone (n = 16) or in association with modafinil (n = 19), 14 with modafinil, and 7 were untreated. No statistical differences of BMI or BMI z-scores (Kruskal-Wallis test, p = 0.197 and p = 0.301 respectively) were found between the four groups (untreated, treated with SO, treated with modafinil, treated with SO and modafinil) at baseline. All had a regular height after 1 y. Mean ± SD of growth velocity calculated from diagnosis time to follow-up was 1.84 ± 5.93. Thirteen patients had a growth velocity lower than 2 SD, but all of these had already started pubertal development at diagnosis. Patients treated with SO had a lower BMI z-score at follow-up compared to baseline (0.50 ± 1.23 vs. 1.19 ± 1.00, p < 0.0005) and obesity decreased from 31.42% to 17.14%, and overweight from 25.71% to 17.14% in this group. Patients without SO showed comparable BMI z-scores at baseline and at follow-up (Table 5).
Table 5.
Comparison between children with (Group 1) and without (Group 2) sodium oxybate therapy.
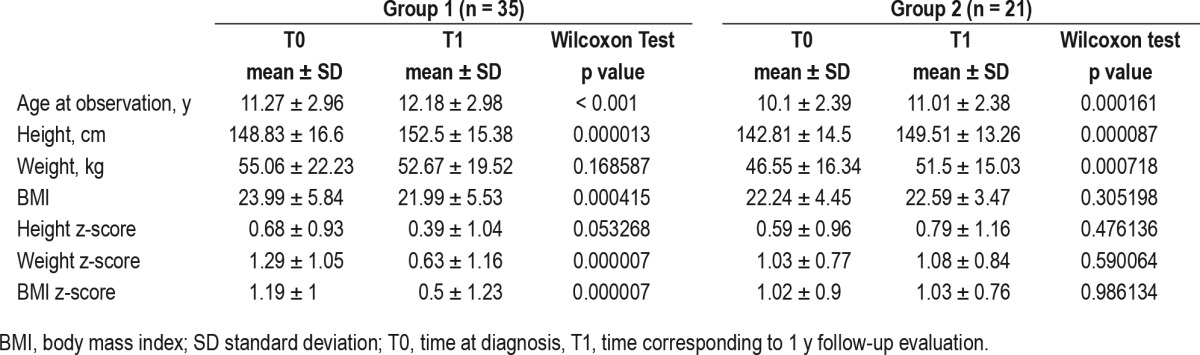
In order to establish the effect of treatment on weight, we calculated the BMI z-score difference (outcome) and applied a linear logistic regression considering treatment type as predictors (SO, modafinil, and combined therapy versus no treatment as reference group). Correcting for baseline features (Model 1; age, sex, BMI z-score, disease duration), and also for sleepiness level (Model 2; Pediatric Daytime Sleepiness Scale), only SO monotherapy maintained a significant effect (Table 6, Figure 2).
Table 6.
Effect of treatments on body mass index z-score change.
Figure 2. Body mass index (BMI) z-scores before NT1 onset and after one year of therapy.
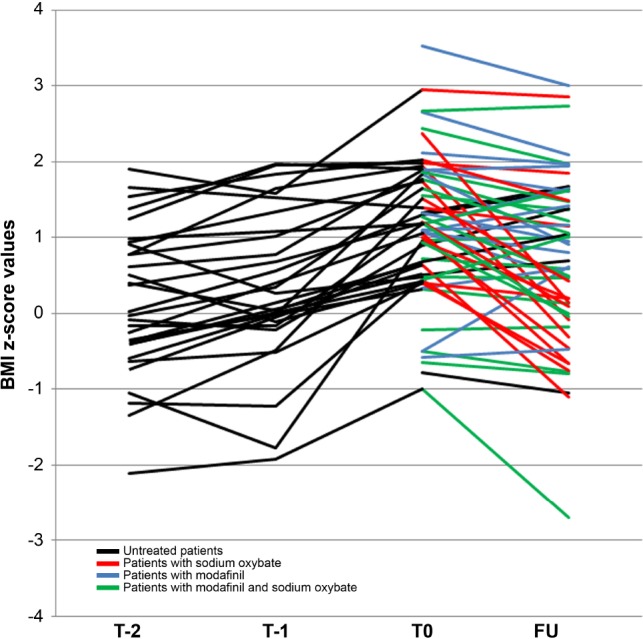
The graph shows the individual evolution of BMI z-scores before diagnosis (T-2, T-1), at diagnosis (T0), and at 1-year follow-up (FU). Different treatments (labeled by color codes) were used after diagnosis with sodium oxybate inducing a significant reduction of BMI z-score.
DISCUSSION
Investigating weight and growth changes in NT1 children before disease onset, at diagnosis, and at 1-y follow-up, we confirmed the high occurrence of overweight/obesity among this population. Our study also demonstrated the temporal correlation between weight gain and NT1 onset, and the positive effect of SO treatment on weight.
The prevalence rates of both inhaler and food allergies were similar to those of other studies34 with a negligible difference from the general population.35,36
The high prevalence of wrong diagnosis in patients with comorbid central PP and the diagnostic delay highlight the challenge of diagnosing NT1 also in pediatric populations and suggest obesity should be considered an ancillary though potentially misleading NT1 symptom.
Twenty-three children had GH levels after stimulation37 below the current cutoff for GH deficiency,33 suggesting an effect on pituitary GH secretion, despite normal IGF1 levels. This finding allows us to speculate that BMI alterations per se can modify the GH response to stimulation tests,38 and the NT1-nocturnal sleep disruption might be responsible for the altered GH secretion to stimulation tests. Further studies on spontaneous circadian GH secretion in NT1 children will hopefully add knowledge to the role and cause of GH-modified secretion during puberty.
A subgroup of patients (25.4%) had a growth velocity lower than the expected value, but they were all pubertal at diagnosis and growth velocity is known to slow down in the final phase of pubertal development.39 Further studies could clarify whether NT1 patients had a final height penalized by a PP or anticipated puberty or dysregulated GH secretion.
The high prevalence of PP12 (16.07% vs. 0.015%) was sex equivalent,40 as opposed to the nearly 10:1 female to male ratio in idiopathic central PP, suggesting a causal role of NT1. Interestingly, the menarche occurred at a mean age of 11.17 ± 1.13 y in NT1 pubertal girls, approximately 1 y before the mean menarche age in Italian adolescents.41 This signal of PP or early puberty points to a complex and unknown interplay of hypocretinergic system and hypothalamus-pituitary regulation, particularly GnRH secretion, requiring further studies on the issue.42
Changes in eating behavior and basal metabolism,43 and autonomic dysregulation,44 altering the circadian rhythm of GH45 and TSH46 secretion, have been invoked to explain the association between NT1 and obesity.17,18 Even if our study could not explain why NT1 causes overweight (29.2%) and obesity (25%) in a clinical population free from major medical comorbidities, we did confirm higher prevalence rates than in the general Italian population (overweight 20.9%, obesity 9.8%).47 General pediatric records showed a stable overweight-obesity prevalence (roughly 17%) before NT1 onset peaking up to 50% at diagnosis, proving that obesity appears closely after NT1 onset. Overweight and obese children17 showed a greater prevalence of metabolic alterations, with a higher risk of hypertension, insulin resistance, lower HDL cholesterol, and overall higher prevalence of metabolic syndrome compared to normal weight NT1 children. PP and overweight/obesity are not mutually associated in NT1, but NT1 onset at a younger age is an intrinsic risk factor for PP.
Our study provided new data on NT1 clinical history and the effect of SO on weight and BMI. As already reported in adults with NT1, SO induced weight loss in obese/overweight children without causing normal-weight children to be underweight. We may only speculate that lipolysis stimulation,48 GH-circadian rhythm reconstruction,49 potential changes in sympathetic activity,50 changes in eating behavior, or the recovery of diurnal daytime motor activity and nocturnal slow-wave sleep may all be associated with the positive effect of SO on weight loss in these patients.
Although our study suffers from limitations regarding the not-randomized intervention, and the small number of patients with available medical records (30 of 72), we highlight the absence of significant differences between the treatment subgroups of NT1 patients. Despite performing comparisons between relatively small groups of patients for both premorbid and follow-up weight changes, the data were numerically sufficient to reach statistical significance and answer our research questions on the time course of weight changes, NT1 onset, and follow-up with and without treatment. Another important study limitation is the absence of a control group. Further randomized studies are necessary to address the links between NT1, obesity, and PP, and to understand how SO treatment affects weight balance.
CONCLUSIONS
We confirm that NT1 is associated with obesity and PP when appearing at a young age. It is not totally clear if linear growth could be affected in the long term. SO treatment promotes weight loss, especially in overweight/obese patients.
DISCLOSURE STATEMENT
This was not an industry supported study. Virginia Ponziani, Monia Gennari, Fabio Pizza, Antonio Balsamo, Filippo Bernardi have no conflicts of interest to disclose; Giuseppe Plazzi has served on the advisory board for UCB pharma, Bioproject, and Jazz pharmaceuticals. Disclosure of any off-label or investigational use: sodium oxybate (Xyrem), modafinil (Provigil), and venlafaxine were administered off-label because these drugs are not currently licensed for children with narcolepsy. The research setting was the Pediatric and Pediatric Endocrine Units, S. Orsola-Malpighi University Hospital, Bologna, Italy; Department of Biomedical and NeuroMotor Sciences, University of Bologna, Italy.
ACKNOWLEDGMENTS
The authors are indebted to all the study participants and their families, namely the Italian association of narcoleptic patients (Associazione Italiana Narcolettici e Ipersonni, Onlus; http://www.narcolessia.it/). Without their contributions, this study would not have been possible.
ABBREVIATIONS
- ACTH
adrenocorticotropic hormone
- ASO
anti-streptolysin O titers
- BMI
body mass index
- CSF hcrt-1
cerebrospinal fluid hypocretin-1
- fT3
free triiodothyronine
- fT4
free thyroxine
- GH
growth hormone
- GnRH
gonadotropin-releasing hormone
- HDL
high-density lipoprotein
- HLA
human leucocyte antigen
- IGF-1
insulin-like growth factor-1
- LDL
low-density lipoprotein
- LH
luteinizing hormone
- MRI
magnetic resonance imaging
- MSLT
multiple sleep latency test
- NT1
narcolepsy type 1
- PP
precocious puberty
- PRL
prolactin
- SD
standard deviation
- SO
sodium oxybate
- TSH
thyroid-stimulating hormone
REFERENCES
- 1.Yoss RE, Daly DD. Narcolepsy in children. Pediatrics. 1960;25:1025–33. [PubMed] [Google Scholar]
- 2.Peterson PC, Husain AM. Pediatric narcolepsy. Brain Dev. 2008;30:609–23. doi: 10.1016/j.braindev.2008.02.004. [DOI] [PubMed] [Google Scholar]
- 3.Plazzi G, Pizza F, Palaia V, et al. Complex movement disorders at disease onset in childhood narcolepsy with cataplexy. Brain. 2011;134:3480–92. doi: 10.1093/brain/awr244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Serra L, Montagna P, Mignot E, Lugaresi E, Plazzi G. Cataplexy features in childhood narcolepsy. Mov Disord. 2008;23:37–41. doi: 10.1002/mds.21965. [DOI] [PubMed] [Google Scholar]
- 5.American Academy of Sleep Medicine. International classification of sleep disorders. 3rd ed. Darien, IL: American Academy of Sleep Medicine; 2014. [Google Scholar]
- 6.Peyron C, Faraco J, Rogers W, et al. A mutation in a case of early onset narcolepsy and a generalized absence of hypocretin peptides in human narcoleptic brains. Nat Med. 2000;6:991–7. doi: 10.1038/79690. [DOI] [PubMed] [Google Scholar]
- 7.Partinen M, Saarenpää-Heikkilä O, Ilveskoski I, et al. Increased incidence and clinical picture of childhood narcolepsy following the 2009 H1N1 pandemic vaccination campaign in Finland. PLoS One. 2012;7:e33723. doi: 10.1371/journal.pone.0033723. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Peyron C, Tighe DK, van den Pol AN, et al. Neurons containing hypocretin (orexin) project to multiple neuronal systems. J Neurosci. 1998;18:9996–10015. doi: 10.1523/JNEUROSCI.18-23-09996.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Tsujino N, Sakurai T. Orexin/hypocretin: a neuropeptide at the interface of sleep, energy homeostasis, and reward system. Pharmacol Rev. 2009;61:162–76. doi: 10.1124/pr.109.001321. [DOI] [PubMed] [Google Scholar]
- 10.Dahmen N, Bierbrauer J, Kasten M. Increased prevalence of obesity in narcoleptic patients and relatives. Eur Arch Psychiatry Clin Neurosci. 2001;251:85–9. doi: 10.1007/s004060170057. [DOI] [PubMed] [Google Scholar]
- 11.Honda Y, Doi Y, Ninomiya R, Ninomiya C. Increased frequency of non-insulin-dependent diabetes mellitus among narcoleptic patients. Sleep. 1986;981:254–9. doi: 10.1093/sleep/9.1.254. [DOI] [PubMed] [Google Scholar]
- 12.Poli F, Pizza F, Mignot E, et al. High prevalence of precocious puberty and obesity in childhood narcolepsy with cataplexy. Sleep. 2013;36:175–81. doi: 10.5665/sleep.2366. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Martìnez-Orozco FJ, Vicario Jl, Villalibre-Valderrey I, De Andrés C, Fernàndez-Arquero M, Peraita-Adrados R. Narcolepsy with cataplexy and comorbid immunopathological diseases. J Sleep Res. 2014;23:414–9. doi: 10.1111/jsr.12143. [DOI] [PubMed] [Google Scholar]
- 14.Kotagal S. Narcolepsy in children. Semin Pediatr Neurol. 1996;3:36–43. doi: 10.1016/s1071-9091(96)80027-1. [DOI] [PubMed] [Google Scholar]
- 15.Guilleminault C, Pelayo R. Narcolepsy in prepubertal children. Ann Neurol. 1998;43:135–42. doi: 10.1002/ana.410430125. [DOI] [PubMed] [Google Scholar]
- 16.Thorpy MJ, Krieger AC. Delayed diagnosis of narcolepsy: characterization and impact. Sleep Med. 2014;15:502–7. doi: 10.1016/j.sleep.2014.01.015. [DOI] [PubMed] [Google Scholar]
- 17.Aran A, Einen M, Lin L, Plazzi G, Nishino S, Mignot E. Clinical and therapeutic aspects of childhood narcolepsy-cataplexy: a retrospective study of 51 children. Sleep. 2010;33:1457–64. doi: 10.1093/sleep/33.11.1457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Schuld A, Hebebrand J, Geller F, Pollmacher T. Increased body-mass index in patients with narcolepsy. Lancet. 2000;355:1274–5. doi: 10.1016/S0140-6736(05)74704-8. [DOI] [PubMed] [Google Scholar]
- 19.Kotagal S, Krahn LE, Slocumb N. A putative link between childhood narcolepsy and obesity. Sleep Med. 2004;5:147–50. doi: 10.1016/j.sleep.2003.10.006. [DOI] [PubMed] [Google Scholar]
- 20.Mansukhani MP, Kotagal S. Sodium oxybate in the treatment of childhood narcolepsy-cataplexy: a retrospective study. Sleep Med. 2012;13:60–10. doi: 10.1016/j.sleep.2011.10.032. [DOI] [PubMed] [Google Scholar]
- 21.Lecendreux M, Poli F, Oudiette D, et al. Tolerance and efficacy of sodium oxybate in childhood narcolepsy with cataplexy: a retrospective study. Sleep. 2012;35:709–11. doi: 10.5665/sleep.1836. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Drake C, Nickel C, Burduvali E, Roth T, Jefferson C, Badia P. The Pediatric Daytime Sleepiness Scale (PDSS): sleep habits and school outcomes in middle-school children. Sleep. 2003;26:455–8. [PubMed] [Google Scholar]
- 23.Robinson DM, Keating GM. Sodium oxybate: a review of its use in the management of narcolepsy. CNS Drugs. 2007;21:337–54. doi: 10.2165/00023210-200721040-00007. [DOI] [PubMed] [Google Scholar]
- 24.Murali H, Kotogal S. Off-label treatment of severe childhood narcolepsycataplexy with sodium oxybate. Sleep. 2006;29:1025–9. doi: 10.1093/sleep/29.8.1025. [DOI] [PubMed] [Google Scholar]
- 25.Marshall WA, Tanner JM. Variations in the pattern of pubertal changes in girls. Arch Dis Child. 1969;44:291–303. doi: 10.1136/adc.44.235.291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Marshall WA, Tanner JM. Variations in the pattern of pubertal changes in boys. Arch Dis Child. 1970;45:13–23. doi: 10.1136/adc.45.239.13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Cacciari E, Milani S, Balsamo A, et al. Italian cross-sectional growth charts for height, weight and BMI (2 to 20 yr) Endocrinol Invest. 2006;29:581–93. doi: 10.1007/BF03344156. [DOI] [PubMed] [Google Scholar]
- 28.American Diabetes Association. Type 2 diabetes in children and adolescents. Diabetes Care. 2000;23:381–9. doi: 10.2337/diacare.23.3.381. [DOI] [PubMed] [Google Scholar]
- 29.Wang R. Cross-national comparison of childhood obesity: the epidemic and the relationship between obesity and socioeconomic status. Int J Epidemiol. 2001;30:1129–36. doi: 10.1093/ije/30.5.1129. [DOI] [PubMed] [Google Scholar]
- 30.Cook S, Weitzman M, Auinger P, Nguyen M, Dietz WH. Prevalence of a metabolic syndrome phenotype in adolescents: findings from the third National Health and Nutrition Examination Survey, 1988-1994. Arch Pediatr Adolesc Med. 2003;157:821–7. doi: 10.1001/archpedi.157.8.821. [DOI] [PubMed] [Google Scholar]
- 31.Kakinami L, Henderson M, Chiolero A, et al. Identifying the best body mass index metric to assess adiposity change in children. Arch Dis Child. 2014;99:1020–4. doi: 10.1136/archdischild-2013-305163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Han F, Lin L, Schormair B, et al. HLA DQB1*06:02 negative narcolepsy with hypocretin/orexin deficiency. Sleep. 2014;37:160–8. doi: 10.5665/sleep.4066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. www.agenziafarmaco.gov.it/it/content/nota-39.
- 34.Aydinoz S, Huang YS, Gozal D, Inocente CO, Franco P, Kheirandish-Gozal L. Allergies and disease severity in childhood narcolepsy: preliminary findings. Sleep. 2015;38:1981–4. doi: 10.5665/sleep.5254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Rona RJ, Keil T, Summers C, et al. The prevalence of food allergy: a meta-analysis. J Allergy Clin Immunol. 2007;120:683–6. doi: 10.1016/j.jaci.2007.05.026. [DOI] [PubMed] [Google Scholar]
- 36.Schultz-Larsen F, Hanifin J. Epidemiology of atopic dermatitis. Immunol Allergy Clin N Am. 2002;22:1–24. [Google Scholar]
- 37.Ghigo E, Bellone J, Aimaretti G, et al. Reliability of provocative tests to assess growth hormone secretory status. Study in 472 normally growing children. J Clin Endocrinol Metab. 1996;81:3323–7. doi: 10.1210/jcem.81.9.8784091. [DOI] [PubMed] [Google Scholar]
- 38.Barret J, Maranda L, Nwosu BU. The relationship between subnormal peak-stimulated growth hormone levels and auxological characteristics in obese children. Front Endocrinol (Lausanne) 2014;5:35. doi: 10.3389/fendo.2014.00035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Rothenbuhler A, Linglart A, Bougneres P. A randomized pilot trail of growth hormone with anastrozole versus growth hormone alone, starting at the very end of puberty in adolescents with idiopatic short stature. Int J Pediatr Endocrinol. 2015;2015:4. doi: 10.1186/1687-9856-2015-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Soriano-Giullen L, Argente J. Central precocious puberty: epidemiology, etiology, diagnosis and treatment. An Pediatr (Barc) 2011;74:336.e1–336. doi: 10.1016/j.anpedi.2010.11.003. [DOI] [PubMed] [Google Scholar]
- 41.Rigon F, De Sanctis V, Bernasconi S, et al. Menstrual pattern and menstrual disorders among adolescents: an update of the Italian data. Ital J Pediatr. 2012;38:38. doi: 10.1186/1824-7288-38-38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Skrapits K, Kanti V, Savanyú Z, et al. Lateral hypothalamic orexin and melanin-concentrating hormone neurons provide direct input to gonadotropin-releasing hormone neurons in the human. Front Cell Neurosci. 2015;9:348. doi: 10.3389/fncel.2015.00348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Chabas D, Foulon C, Gonzales J, et al. Eating disorder and metabolism in narcoleptic patients. Sleep. 2007;30:1267–73. doi: 10.1093/sleep/30.10.1267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Plazzi G, Moghadam KK, Maggi LS, et al. Autonomic disturbance in narcolepsy. Sleep Med Rev. 2011;15:187–96. doi: 10.1016/j.smrv.2010.05.002. [DOI] [PubMed] [Google Scholar]
- 45.Overeem S, Kok SW, Lammers GJ, et al. Somatotropic axis in hypocretindeficient narcoleptic humans: altered circadian distribution of GH-scretory events. Am J Physiol Endocrinol Metab. 2003;284:E641–7. doi: 10.1152/ajpendo.00421.2002. [DOI] [PubMed] [Google Scholar]
- 46.Kok SW, Roelfsema F, Overeem S, et al. Altered setting of the pituitary-thyroid ensemble in hypocretin-deicient narcoleptic men. Am J Physiol Endocrinol Metab. 2005;288:E89. doi: 10.1152/ajpendo.00327.2004. [DOI] [PubMed] [Google Scholar]
- 47. www.epicentro.iss.it/okkioallasalute/sintesideirisultati2014.
- 48.Donjacour CE, Aziz NA, Overeem S, Kalsbeek A, Pijl H, Lammers GJ. Glucose and fat metabolism in narcolepsy and the effect of sodium oxybate: a hyperinsulinemic-euglycemic clamp study. Sleep. 2014;37:795–801. doi: 10.5665/sleep.3592. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Donjacour CE, Aziz NA, Roelfsema F, et al. Effect of sodium oxybate on GH secretion in narcolepsy patients and healthy controls. Am J Physiol Endocrinol Metab. 2011;300:E1069–75. doi: 10.1152/ajpendo.00623.2010. [DOI] [PubMed] [Google Scholar]
- 50.Donadio V, Liguori R, Vandi S, et al. Lower wake resting sympathetic and cardiovascular activities in narcolepsy with cataplexy. Neurology. 2014;83:1080–6. doi: 10.1212/WNL.0000000000000793. [DOI] [PubMed] [Google Scholar]


