Abstract
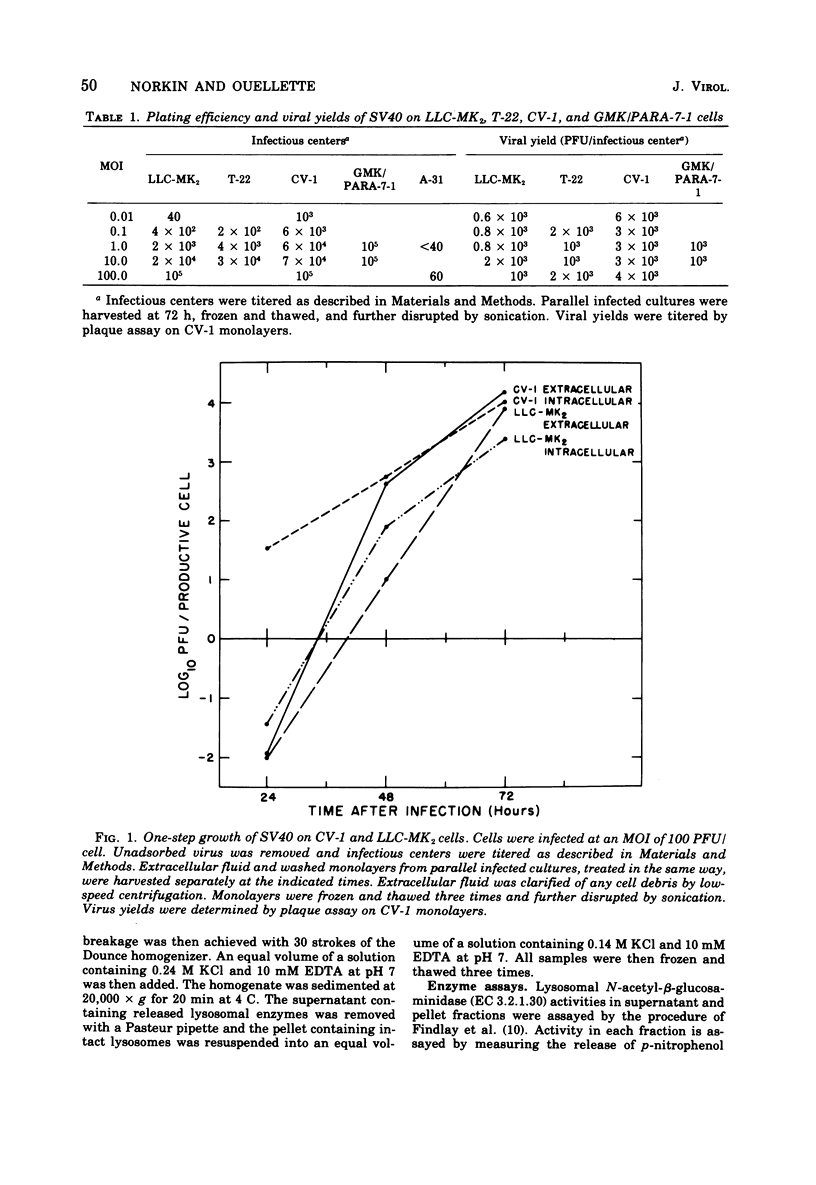
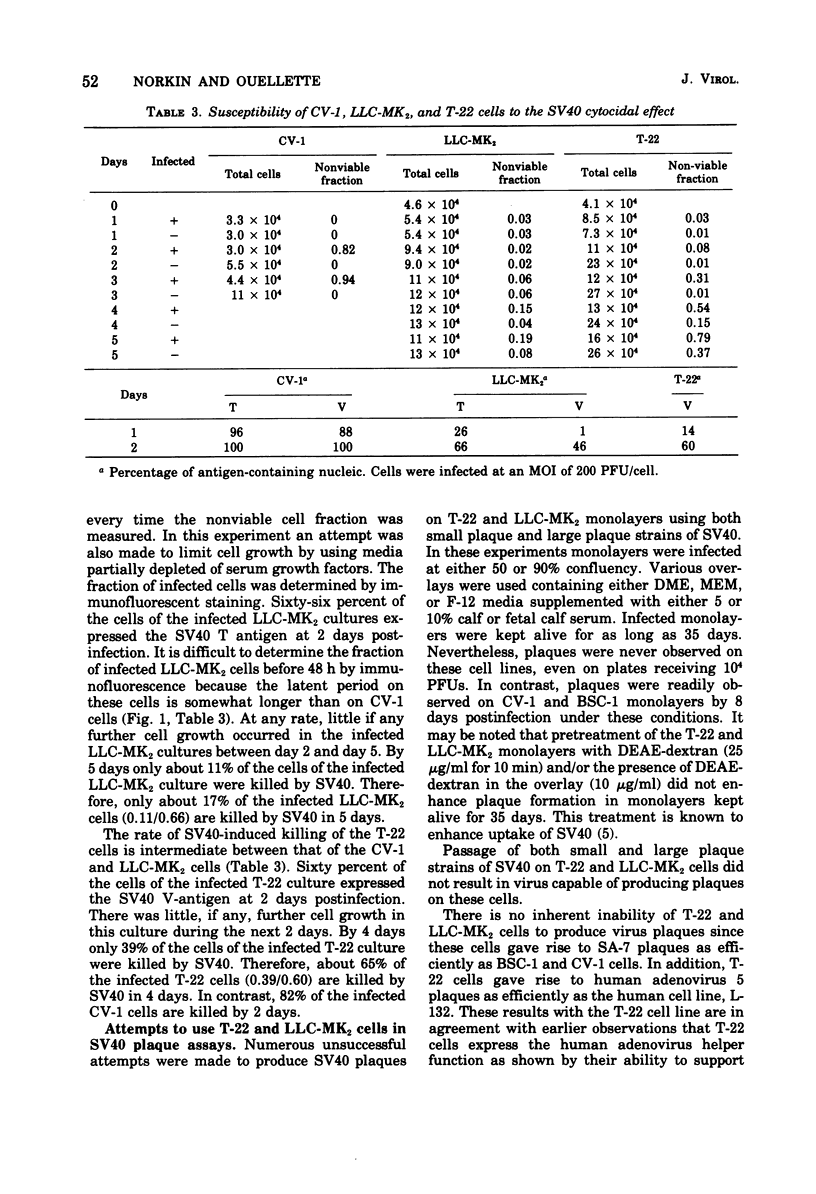
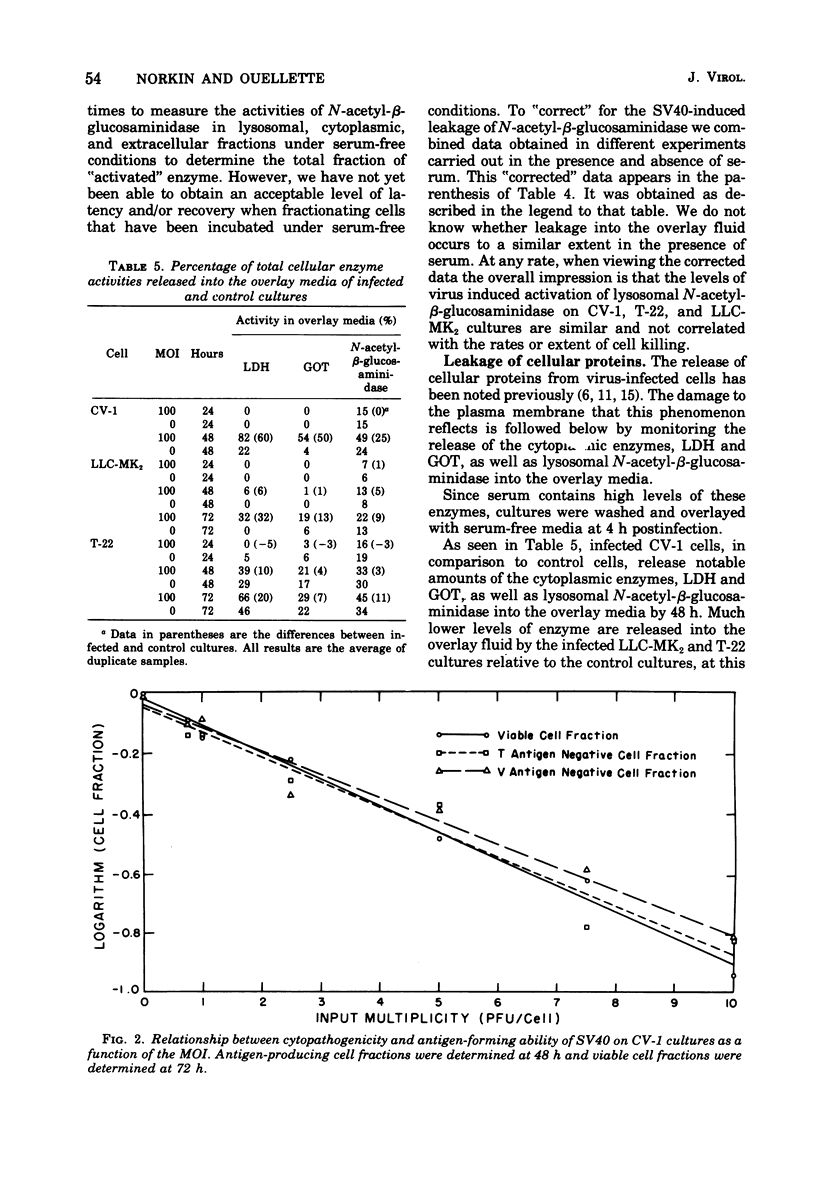
Simian virus 40 (SV40) growth on rhesus kidney cells and on the T-22 line of SV40-transformed green monkey kidney (GMK) cells is largely limited by the low plating efficiency of SV40 on these cells. In addition, a fraction of the rhesus kidney and T-22 cells are resistant to infection by SV40. Nevertheless, 72-h viral yields per infected rhesus kidney and T-22 cell are nearly equivalent to that obtained on normal GMK cells and are independent of the multiplicity of infection. Despite the production of high viral yields, infected rhesus kidney and T-22 cells are killed slowly by SV40. Monolayers of these cells are also refractory to plaque formation by SV40. SV40 induces the release of lysosomal N-acetyl-beta-glucosaminidase into the cytoplasmic fractions of rhesus kidney and T-22 cells to an extent equal to that observed during infection of rapidly killed normal GMK cells. In contrast, damage to the plasma membrane, as indicated by the release of the cellular enzymes lactic dehydrogenase and glutamic oxaloacetic transaminase into the overlay media, occurred to a much greater extent in the normal GMK cells than in the rhesus kidney or T-22 cells. Neither a lysosomal hydrolase mechanism nor viral release appear to be responsible for this phenomenon. The different rates and extents of the SV40 cytocidal process on these cells do not result from the differences in the viral plating efficiency on them.
Full text
PDF






Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- ALLISON A. C., SANDELIN K. Activation of lysosomal enzymes in virus-infected cells and its possible relationship to cytopathic effects. J Exp Med. 1963 Jun 1;117:879–887. doi: 10.1084/jem.117.6.879. [DOI] [PMC free article] [PubMed] [Google Scholar]
- BABLANIAN R., EGGERS H. J., TAMM I. STUDIES ON THE MECHANISM OF POLIOVIRUS-INDUCED CELL DAMAGE. I. THE RELATION BETWEEN POLIOVIRUS,-INDUCED METABOLIC AND MORPHOLOGICAL ALTERATIONS IN CULTURED CELLS. Virology. 1965 May;26:100–113. doi: 10.1016/0042-6822(65)90030-9. [DOI] [PubMed] [Google Scholar]
- Bablanian R. Studies on the mechanism of vaccinia virus cytopathic effects: effect of inhibutors of RNA and protein synthesis on early virus-induced cell damage. J Gen Virol. 1970 Feb;6(2):221–230. doi: 10.1099/0022-1317-6-2-221. [DOI] [PubMed] [Google Scholar]
- Barbanti-Brodano G., Swetly P., Koprowski H. Superinfection of simian virus 40-transformed permissive cells with simian virus 40. J Virol. 1970 Nov;6(5):644–651. doi: 10.1128/jvi.6.5.644-651.1970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blackman K. E., Bubel H. C. Poliovirus-induced Cellular Injury. J Virol. 1969 Sep;4(3):203–208. doi: 10.1128/jvi.4.3.203-208.1969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bowers W. E., Finkenstaedt J. T., de Duve C. Lysosomes in lymphoid tissue. I. The measurement of hydrolytic activities in whole homogenates. J Cell Biol. 1967 Feb;32(2):325–337. doi: 10.1083/jcb.32.2.325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Butel J. S., Richardson L. S., Melnick J. L. Variation in properties of SV40-transformed simian cell lines detected by superinfection with SV40 and human adenoviruses. Virology. 1971 Dec;46(3):844–855. doi: 10.1016/0042-6822(71)90085-7. [DOI] [PubMed] [Google Scholar]
- FINDLAY J., LEVVY G. A., MARSH C. A. Inhibition of glycosidases by aldonolactones of corresponding configuration. 2. Inhibitors of beta-N-acetylglucosaminidase. Biochem J. 1958 Jul;69(3):467–476. doi: 10.1042/bj0690467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- GILBERT V. E. ENZYME RELEASE FROM TISSUE CULTURES AS AN INDICATOR OF CELLULAR INJURY BY VIRUSES. Virology. 1963 Dec;21:609–616. doi: 10.1016/0042-6822(63)90234-4. [DOI] [PubMed] [Google Scholar]
- GINSBERG H. S. Biological and biochemical basis for cell injury by animal viruses. Fed Proc. 1961 Jul;20:656–660. [PubMed] [Google Scholar]
- Guskey L. E., Smith P. C., Wolff D. A. Patterns of cytopathology and lysosomal enzyme release in poliovirus-infected HEp-2 cells treated with either 2-(alpha-hydroxybenzyl)-benzimidazole or guanidine HCl. J Gen Virol. 1970 Jan;6(1):151–161. doi: 10.1099/0022-1317-6-1-151. [DOI] [PubMed] [Google Scholar]
- Haase A. T., Levy H., Baron S., Kasel J. A. Mengovirus-induced cytopathic effect in L-cells: protective effect of interferon. J Virol. 1969 Oct;4(4):490–495. doi: 10.1128/jvi.4.4.490-495.1969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Katzman J., Wilson D. E. Newcastle disease virus-induced plasma membrane damage. J Gen Virol. 1974 Jul;24(1):101–113. doi: 10.1099/0022-1317-24-1-101. [DOI] [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- SWEET B. H., HILLEMAN M. R. The vacuolating virus, S.V. 40. Proc Soc Exp Biol Med. 1960 Nov;105:420–427. doi: 10.3181/00379727-105-26128. [DOI] [PubMed] [Google Scholar]
- Shiroki K., Shimojo H. Transformation of green monkey kidney cells by SV40 genome: the establishment of transformed cell lines and the replication of human adenoviruses and SV40 in transformed cells. Virology. 1971 Jul;45(1):163–171. doi: 10.1016/0042-6822(71)90123-1. [DOI] [PubMed] [Google Scholar]
- Studzinski G. P., Gierthy J. F., Cholon J. J. An autoradiographic screening test for mycoplasmal contamination of mammalian cell cultures. In Vitro. 1973 May-Jun;8(6):466–472. doi: 10.1007/BF02615948. [DOI] [PubMed] [Google Scholar]



