Abstract
Study Objectives:
In neuromuscular disease, non-invasive ventilation (NIV) is indicated if sleep-disordered breathing (SDB) or significant respiratory muscle weakness (RMW) is present. We investigated immediate and long-term effects of NIV on sleep and nocturnal ventilation in patients with late-onset Pompe disease (LOPD).
Methods:
Polysomnography and transcutaneous capnometry were performed in 22 adult patients. If indicated, NIV was initiated the subsequent night and follow-up sleep studies were scheduled. Sleep quality and health-related quality of life (HRQoL) were self-assessed using standard questionnaires.
Results:
Fourteen patients received enzyme replacement therapy (ERT), five patients were treatment-naÏve, and three individuals had previously stopped ERT. Fifteen patients reported symptoms of SDB, all showing abnormal sleep studies. Two patients had obstructive sleep apnea (OSA), three patients showed both OSA and nocturnal hypercapnia, four individuals had nocturnal hypercapnia, and two patients had both OSA and daytime hypercapnia. Four patients showed normal apnea-hypopnea index and CO2 measures but nocturnal tachypnea, orthopnea, and significant RMW were present. Supine forced vital capacity (FVC) and positional drop of FVC were independent predictors of SDB. In patients with SDB, HRQoL was significantly reduced. NIV was initiated in 15 individuals and led to significant improvement of ventilation and oxygenation in the first night of treatment. Follow-up sleep studies revealed stable normoxia and normocapnia without deterioration of sleep outcomes for up to 40 months.
Conclusions:
In LOPD, SDB is common and comprises both hypoventilation and OSA. NIV significantly improves respiration already in the first night of treatment. NIV warrants nocturnal long-term normoventilation without deterioration of sleep quality.
Citation:
Boentert M, Dräger B, Glatz C, Young P. Sleep-disordered breathing and effects of noninvasive ventilation in patients with late-onset Pompe disease. J Clin Sleep Med 2016;12(12):1623–1632.
Keywords: noninvasive ventilation, polysomnography, Pompe disease, respiratory muscle weakness, sleep-disordered breathing
INTRODUCTION
Pompe disease is an autosomal recessive lysosomal storage disorder caused by deficiency of the α-1,4-glucosidase (GAA) enzyme. Glycogen accumulation results in lysosomal dysfunction, autophagy, and progressive tissue damage.1 Early infantile-onset Pompe disease is characterized by quadriplegia and fatal hypertrophic cardiomyopathy if enzyme replacement therapy (ERT) is not available. The term late-onset Pompe disease (LOPD) comprises subtypes with late-infantile, childhood, juvenile, or adult disease manifestation. LOPD lacks major cardiac involvement and is characterized by progressive myopathy of the limb-girdle muscles, the trunk muscles, and the diaphragm.2 Respiratory muscle weakness manifests as nocturnal hypoventilation first, eventually leading to sleep disruption, morning headache, fatigue, and excessive daytime sleepiness.3 In addition, patients may be at risk of obstructive sleep apnea (OSA) due to macroglossia and potential upper airway collapse.4 Sleep-independent symptoms of respiratory muscle weakness include exertional intolerance, speech dyspnea, dyspnea at rest, and orthopnea. The need for noninvasive ventilation (NIV) frequently evolves as the condition progresses. Respiratory decline is reflected by continous decrease of spiromanometric parameters such as forced vital capacity (FVC), maximum inspiratory pressure (MIP), and maximum expiratory pressure (MEP).5,6 Predictors of poor respiratory outcome include male sex, disease duration, and overall neurological impairment.7,8 NIV is indicated if sleep-disordered breathing (SDB), daytime hypercapnia, or clinically significant diaphragmatic weakness is present.9
BRIEF SUMMARY
Current Knowledge/Study Rationale: In patients with late-onset Pompe disease, prevalence of sleep-disordered breathing (SDB) and effects of non-invasive ventilation (NIV) were investigated by means of polysomnography and transcutaneous capnometry. In late-onset Pompe disease, SDB is highly prevalent and may comprise alveolar hypoventilation, obstructive sleep apnea, or both.
Study Impact: Presence of SDB is associated with overall disease status and reduces quality of life. NIV leads to immediate and long-term correction of nocturnal gas exchange.
Mellies et al.10 showed that NIV prospectively normalizes gas exchange in patients with LOPD and respiratory failure. This study was conducted prior to approval of ERT, and enrolled only eight patients with LOPD and daytime hypercapnia. We investigated both immediate and long-term effects of NIV on sleep outcomes and ventilatory measures in a larger cohort of patients with LOPD.
METHODS
Patients
Twenty-two nonrelated patients with late-childhood, juvenile, or adult-onset Pompe disease were admitted to our sleep laboratory for evaluation of nocturnal breathing. None of the patients had been started on NIV or continuous positive airway pressure (CPAP) treatment prior to evaluation. Twelve patients were female, and mean age was 51.9 (15.3) y. In all patients, diagnosis of Pompe disease had been confirmed by either enzyme activity testing, muscle biopsy, sequencing of the GAA gene, or by combination of at least two of these methods. Patients were routinely asked about symptom onset, disease duration, and start of ERT. In addition, patients were asked in detail about symptoms possibly indicating SDB including morning headache, non-restorative sleep, sleep disturbances, or excessive daytime sleepiness. The study was approved by the local ethics committee.
Questionnaires
For self-assessment of sleep quality patients answered the Pittsburgh Sleep Quality Index (PSQI), which generates a global score and seven component scores reflecting sleep latency, sleep duration, habitual sleep efficiency, sleep disturbances, use of sleep medication, daytime dysfunction, and overall sleep quality.11 Global PSQI score ranges from 0 to 21. A cut-off score of 5 differentiates between “good sleepers” (≤ 5) and “bad sleepers” (> 5). The Epworth Sleepiness Scale (ESS) rates sleep propensity in everyday situations.12 The ESS sum score ranges from 0 to 24, and a score higher than 10 reflects excessive daytime sleepiness. Fatigue was self-assessed using the Fatigue Severity Scale (FSS) which generates a sum score ranging from 1 to 7, with scores higher than 4 and higher than 5 indicating significant or severe fatigue, respectively.13 Health-related quality of life (HRQoL) was assessed using the Medical Outcomes Study 36-Item Short Form (SF-36) questionnaire.14 Physical and mental component summary scales (PCS and MCS) are calculated from the various subscales and transformed to a normalized T-score with a mean of 50 and a standard deviation of 10. Higher scores represent better HRQoL. For self-assessment of motor performance, the Rotterdam 9-Item Handicap Scale was used.15
Clinical Assessment
All patients were ambulatory and performed the 6-min Walk Test (6-MWT) on initial evaluation and on follow-up visits. FVC was obtained in both the upright and the supine position. Predicted FVC was calculated adjusting for age, sex, and body height. MIP and MEP measures were available in only eight patients, and were not included in data analysis.
Sleep Studies
Sleep studies comprised overnight cardiorespiratory polysomnography (PSG) including electroencephalogram, electrooculogram, surface electromyogram, pulse oximetry, nasal pressure and quantitative effort registration, transcutaneous capnometry (Sentec, Therwil, Switzerland), and early morning blood gas analysis using capillary blood from the arterialized earlobe. Sleep stages and sleep-associated events were manually scored according to standard guidelines.16 PSG records were independently validated by both a sleep technologist (CG) and a physician sleep specialist (MB). Sleep-related outcome measures included percentage of sleep stages, sleep efficiency, index of sleep stage changes, arousal index, and time spent awake after onset of sleep. Sleep-related hypoventilation was defined as nocturnal transcutaneous carbon dioxide tension (tcCO2) > 50 mmHg, maximum nocturnal tcCO2 > 10 mmHg above baseline (i.e., ΔtcCO2), or daytime pCO2 > 45 mmHg.9,17 Sleep apnea was defined by an apnea-hypopnea index (AHI) > 5/h total sleep time.18
Ventilator Settings
Patients meeting NIV indication criteria9,17 were started on a bilevel, spontaneous-timed (S/T) ventilation mode with a backup mandatory rate and an averaged tidal volume support. S/T mode was chosen in order to allow spontaneous breathing and increase patient comfort. In the absence of relevant OSA, end-expiratory positive airway pressure (EPAP) was routinely set at 4 cm H2O. Otherwise, EPAP was initially set at 6 cm H2O and titrated, if necessary. Minimal inspiratory positive airway pressure (IPAP) was routinely set at 2 cm H2O above EPAP, and the upper IPAP limit ranged from 15 to 22 cm H2O. Tidal volume was individually titrated between 6 and 10 mL/kg body weight. Mandatory rate was set two breaths per minute (bpm) less than the mean respiratory rate the night before. If buccal weakness was present, oronasal masks were used; otherwise, nasal interfaces were applied. Supplemental oxygen was given to none of the patients. All patients were prescribed a humidifier for use in combination with the NIV device. Long-term adherence to NIV was documented based on device memory data. During the follow-up period, ventilator settings were only modified if significant SDB was detected on control sleep studies.
Follow-up
Follow-up visits comprised full PSG, transcutaneous capnometry, early morning blood gas analysis, spirometry, and the 6-MWT. Patients answered the aforementioned questionnaires. Baseline evaluation and the first night on NIV were defined as T0 and T1, respectively. Control sleep studies were scheduled at 3 to 4 mo after NIV initiation (T2) and, thereafter, every 6 to 9 mo (T3-T6).
Statistical Methods
Statistical data analysis was performed using IBM Statistics for Macintosh version 23.0 (IBM Corp., Armonk, NY). Unless indicated otherwise, results are presented as mean and standard deviation. For comparison between groups, the t test for independent samples was used if data were normally distributed. Categorical variables were analysed using the χ2 test. Spearman correlation coefficient was used for associations between continuous variables. One-way analysis of variance for repeated measures was used to compare outcome parameters across multiple measurements, and p < 0.05 were considered statistically significant.
RESULTS
Patients
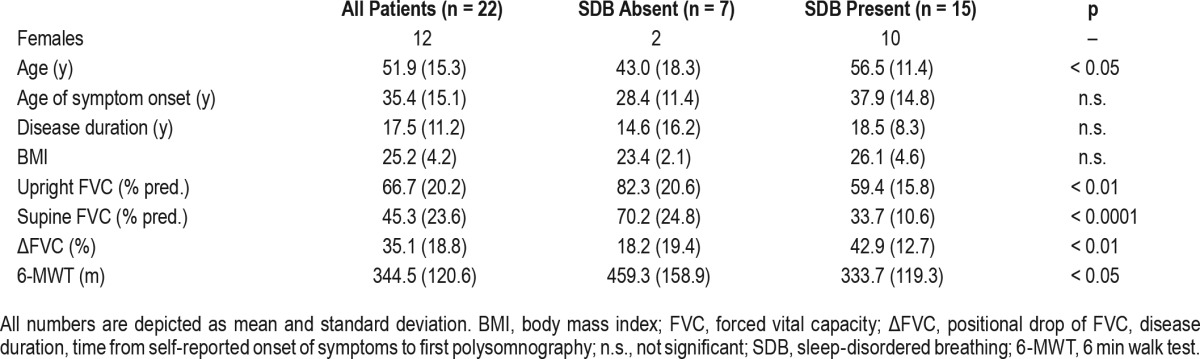
Table 1 depicts demographic and basic clinical data characterizing the initial cohort of 22 patients. Age of self-reported symptom onset ranged from 12 to 63 y, and disease duration was between 4 and 50 y. Comorbidities included arterial hypertension (n = 8) and diabetes (n = 1). None of the patients was taking opioid analgesics or any other medication potentially affecting ventilatory drive. ERT had been initiated in 14 individuals 1 to 34 mo prior to first evaluation, and 5 patients were treatment-naÏve. In three individuals, ERT had been discontinued due to infusion-associated reactions 6 to 22 mo before. In patients with ongoing ERT, mean treatment duration was 4.2 (1.9) y. Infusions were given biweekly at standard dosage (20 mg per kilogram body weight).
Table 1.
Demographic data and clinical characteristics of 22 patients with late-onset Pompe disease.
Respiratory Outcomes (T0)
Table 2 shows mean respiratory indices and nocturnal ventilation measures of the entire cohort. Table S1 (supplemental material) lists ERT status, FVC measurements, respiratory PSG parameters, and self-reported disease duration at T0 along with the specific GAA gene mutations for each patient. Fifteen individuals reported symptoms of SDB such as excessive daytime sleepiness, nonrestorative sleep, or morning headache. Based on PSG and capnometry results alone, either OSA or nocturnal hypoventilation were present in 12 of 22 individuals. One patient with isolated OSA and normal respiratory muscle function (patient #1, Table S1) did not report symptoms of SDB. All 15 symptomatic patients showed SDB, including 2 patients with OSA (AHI > 5/h) and significant upright FVC reduction (< 70% of predicted), 3 patients had OSA and nocturnal hypercapnia, 4 individuals had isolated nocturnal hypercapnia, and two patients had both OSA and daytime hypercapnia (Table S1). In four individuals, AHI and nocturnal CO2 measurements were normal but mean respiratory rate was > 20/min during sleep in the presence of severe orthopnea, FVC reduction (< 70% predicted), and reduced MIP (< 60 cm H2O) (Table S1). In these patients both increased respiratory rate and an upright position in bed were likely to have contributed to the prevention of nocturnal hypercapnia.
Table 2.
Respiratory measures and sleep outcomes on initial polysomnography and immediately after initiation of NIV.
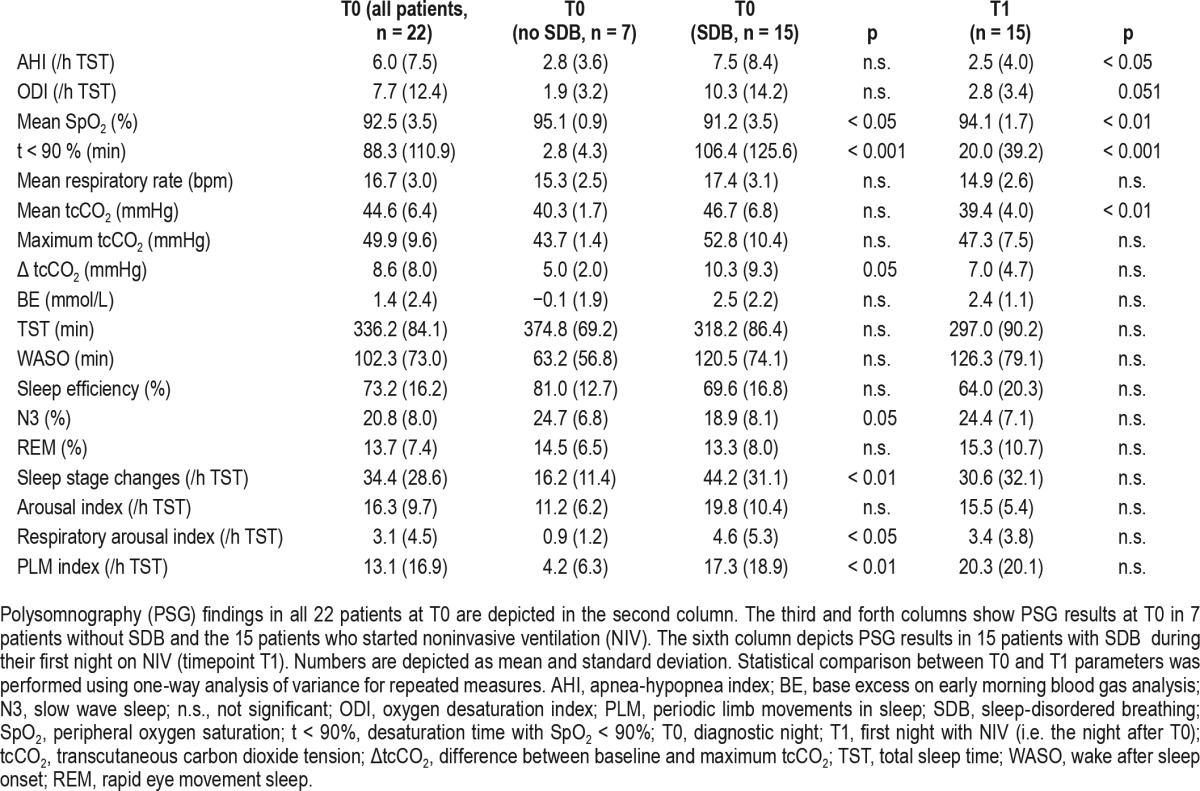
Only 19.3% of all apneas we found were of central origin, and a central apnea index above 5/h was present in none of the patients. In the entire cohort, mean AHI and oxygen desaturation index (ODI) were above normal (Table 2). As expected, patients with a diagnosis of SDB showed worse oxygenation as reflected by both reduced mean oxygen saturation and longer desaturation time.
Sleep Outcomes (T0)
In patients with SDB, various PSG measures of sleep quality were significantly worse compared to individuals with normal nocturnal respiration (Table 2). Significant differences were found with regard to N3 percentage, respiratory arousal index, periodic limb movements in sleep, and the index of sleep stage changes.
Clinical Status and Self-Reported Outcomes (T0)
Eight of 15 patients with SDB were receiving ERT, 4 patients had stopped ERT, and 3 individuals had not yet started ERT. In the entire cohort (n = 22), upright FVC (percentage of predicted, FVC%) was significantly correlated with the Rotterdam 9-Item Handicap Scale score (r = 0.71, p < 0.0001) and with walking distance on the 6-MWT (r = 0.53, p < 0.01). Furthermore, it showed significant negative association with disease duration (r = −0.50, p < 0.01) and age (r = −0.55, p < 0.01). Positional decrease of FVC% was significantly correlated with desaturation time (t < 90%), mean tcCO2, and maximum tcCO2 (r = 0.45, r = 0.44, r = 0.45, each p < 0.05). In addition, supine FVC% and positional decrease of FVC% were independent predictors of SDB when age, BMI, sex, and disease duration were integrated into the linear regression model (data not shown).
Patients with SDB were significantly older than patients without SBD, and disease duration was slightly longer in this subgroup (Table 1). Walking distance on the 6-MWT was shorter in patients with SDB (p = 0.05). As expected, FVC% in both the upright and supine position was significantly lower in individuals with SDB.
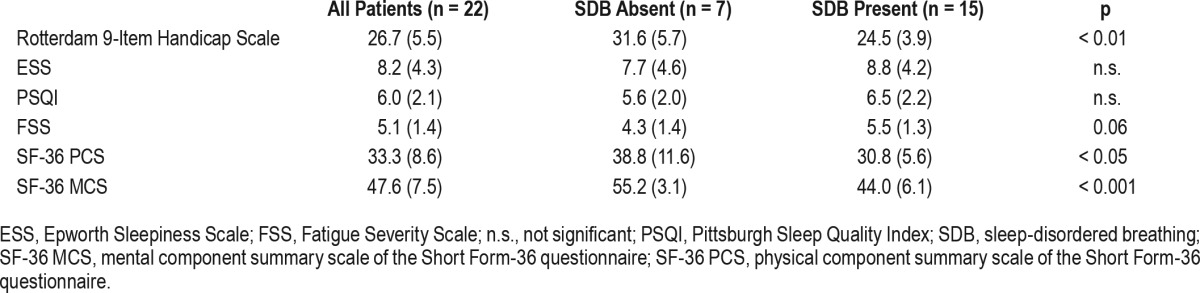
Table 3 shows self-rated questionnaire scores for motor handicap, fatigue, hypersomnolence, sleep quality, and HRQoL in the initial cohort. Seven patients reported excessive daytime sleepiness (ESS score > 10), 12 patients specified impaired sleep quality (PSQI global score > 5), and 17 participants complained of fatigue as reflected by a FSS score higher than 4. Sleep propensity, impairment of sleep quality, and fatigue did not differ significantly between patients with and without SDB. However, self-rated motor performance and both physical and mental HRQoL scores were significantly lower in patients subsequently started on NIV. Interestingly, the ESS, PSQI, and FSS sum scores were significantly higher in females than in male patients, and the SF-36 physical component summary scale was lower in women with LOPD than in men (ESS: 10.3 vs. 6.3, PSQI: 7.2 vs. 5.0, FSS: 6.1 vs. 4.1, SF-36 PCS: 29.0 vs. 38.5, all p < 0.05).
Table 3.
Self-rated neurological impairment, daytime sleep propensity, sleep quality, fatigue, and health-related quality of life in 22 patients with late-onset Pompe disease.
Short-Term Effects of NIV (T1)
NIV was started in 15 patients with symptomatic SDB. Ventilator settings were chosen as described previously. At T1, NIV was tolerated for at least 3 h by all 15 patients. Respiratory measures and objective sleep outcomes at T1 are depicted in Tables 2 and 3, respectively. The majority of apneas and hypopneas were effectively suppressed by NIV, and oxygenation was enhanced in the first night of treatment in all patients. AHI, mean SaO2, t < 90%, and mean tcCO2 showed significant improvement (Table 2). In patients with either OSA or nocturnal hypercapnia on sleep studies, findings were similar (data not shown). In addition, ODI and basal tcCO2 were significantly improved in this subgroup (ODI: 13.6 vs. 3.2, basal tcCO2: 42.5 vs. 36.9, both p < 0.05). Objective sleep quality measures did not significantly improve from T0 to T1. However, percentage of sleep stages N3 and rapid eye movement (REM) were increased and sleep stage change index was decreased with NIV (Table 3).
Long-Term Follow-up (T2 – T6)
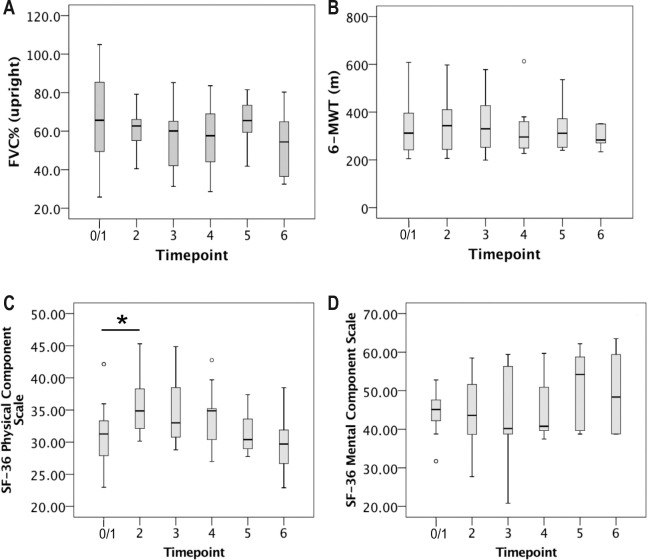
Full data sets from 22 patients were available at T0. Fifteen patients were started on NIV the following night (T1). One patient subsequently declined NIV after several attempts due to impregnable mask intolerance, and one patient was lost to follow-up. Thus, 13 patients underwent sleep studies at T2. At subsequent follow-up visits, the number of patients decreased to 11 at T3, 9 at T4, 8 at T5, and 6 at T6. Length of the follow-up period ranged from 3 to 40 mo. Unscheduled follow-up visits due to acute respiratory deterioration or any other reason were necessary in none of the patients. Mean upright FVC (% predicted, FVC%) declined between T0/1 and T6 but differences were not significant (Figure 1A). Mean walking distance on the 6-MWT did not significantly change over time either (Figure 1B). For both FVC% and 6-MWT results, analysis of variance for repeated measures was used to compare subsequent timepoints. With regard to FVC% and 6-MWT walking distance, the individual change rate per month was calculated for each patient depending on length of follow-up (see Table S2, supplemental material). Mean annual decline of FVC% was 1.7% in the 12 individuals who were followed for more than 1 y. In the same subgroup, mean annual decline of the 6-min walking distance was 2.7 m. Results were compared between patients with and without ERT, but differences were not significant (data not shown).
Figure 1. Upright forced vital capacity (FVC), walking performance, and health-related quality of life between T1 and T6.
Mean upright forced vital capacity (% predicted) (A), mean 6-min walking test (6-MWT) walking distance (B), and self-reported quality of life (C,D) over time in patients with late-onset Pompe disease on noninvasive ventilation (NIV). Number of patients was 15 at T0 (no NIV), 13 at T2, 11 at T3, 9 at T4, 8 at T5, and 6 at T6. Boxes depict mean, lower (Q1) and upper quartile (Q3). Whiskers represent data falling n between Q1 and Q1 – 1.5 × interquartile range (IQR) or Q3 and Q3 + 1.5 × IQR, respectively. Statistical comparison between subsequent timepoints was performed by means of analysis of variance for repeated measures. Asterisks show statistically significant changes between timepoints (*p < 0.05).
Mean adherence to NIV increased from 69.5% (34.0) at T2 (n = 13) to 83.5% (18.3) at T6 (n = 6), and mean duration of NIV usage per night increased from 4.5 (2.2) h at T2 to 6.8 (1.4) h at T6. Differences were not significant. In the six patients with the longest follow-up period, adherence to NIV stayed constantly high (T2: 89.8% (4.0), T6: 86.2% (8.6)) and mean ventilator usage per night had increased by 2.2 h from T2 to T6 (p = 0.06).
Ventilation Outcomes (T2 – T6)
In none of the patients did ventilation mode have to be changed. All patients who had initially been equipped with a nasal interface were switched to oronasal masks due to oral air leakage. Adjustment of either pressure settings or mandatory rate was performed if SDB recurred, albeit the mask was optimally fitted or if correction of the interface did not lead to normalization of ventilation measures.
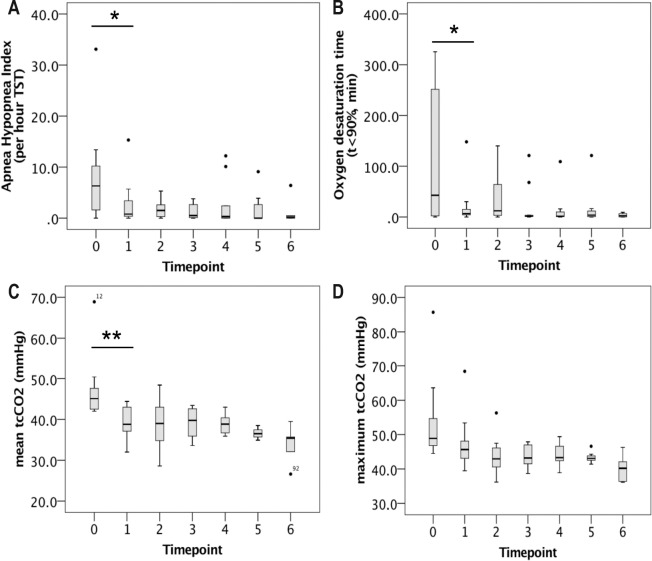
Figure 2 depicts AHI, oxygenation, and ventilation parameters on follow-up sleep studies in patients on nocturnal NIV. Mean duration of oxygen desaturation (t < 90%) constantly decreased from T1 to T6 (20.4 min vs. 3.5 min, not significant, n.s.). After titration of EPAP in all patients who still showed obstructive events at T1, upper airway obstruction as reflected by an AHI > 5/h recurred in two patients at T4 and one individual at T5 and T6, respectively. Maximum tcCO2 exceeded 50 mmHg in three subjects at T1 and one patient at T2, requiring adjustment of mandatory minute ventilation. Subsequently, normocapnia was present at daytime and during the night in all ventilated patients.
Figure 2. Long-term nocturnal ventilation outcomes in patients with late-onset Pompe disease started on noninvasive ventilation (NIV).
Number of patients was 15 at T0 (no NIV), 13 at T2, 11 at T3, 9 at T4, 8 at T5, and 6 at T6. Panels show apnea-hypopnea index (A), oxygen desaturation time (B), mean tcCO2 (C), and maximum tcCO2 (D). Single data points represent outliers. Asterisks show statistically significant changes between timepoints (*p < 0.05, **p < 0.01). Statistical comparison between timepoints was performed by means of analysis of variance for repeated measures. tcCO2, transcutaneous carbon dioxide tension; TST, total sleep time (in hours).
Sleep Outcomes (T2 – T6)
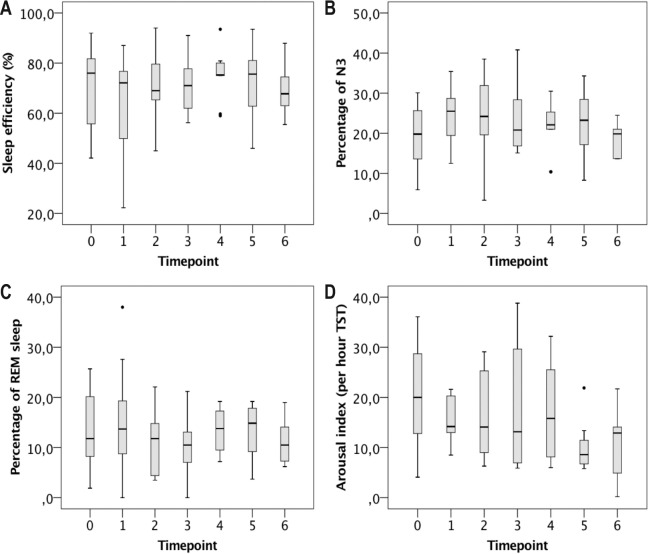
Polysomnographic measures of sleep quality did not show significant alterations on subsequent sleep studies (Figure 3). Mean percentage of sleep stage N3 remained near physiological values until T6. Mean percentage of REM sleep was markedly reduced at T0 and stayed subnormal throughout the entire follow-up period.
Figure 3. Long-term sleep outcomes in patients with late-onset Pompe disease started on non-invasive ventilation (NIV).
Number of patients was 15 at T0 (no NIV), 13 at T2, 11 at T3, 9 at T4, 8 at T5, and 6 at T6. Panels show sleep efficiency (A), percentage of N3 (B), percentage of rapid eye movement (REM) sleep (C), and arousal index (D). Single data points represent outliers. Statistical comparison between timepoints was performed by means of analysis of variance for repeated measures. N3, slow wave sleep; TST, total sleep time.
Self-Reported Patient Outcomes (T2 – T6)
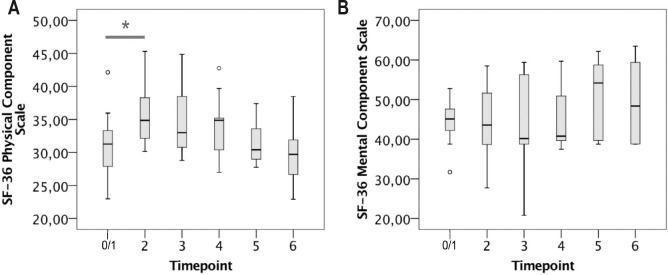
At T0, patients with SDB specified significantly worse HRQoL than individuals without SDB. Three months after initiation of nocturnal NIV (T2), the PCS score of the SF-36 questionnaire showed significant improvement (Figure 4A). Subsequently, the PCS score appeared to decline again over time but differences were not significant. The MCS score showed no significant short-term and long-term alterations (Figure 4B). Sleep quality as self-assessed by using the PSQI was reduced in 9 of 15 patients at T0 and showed a tendency to deteriorate over time. At T6, all patients were “bad sleepers” according to the PSQI, and the PSQI global score was higher than 5 at each timepoint (data not shown). Mean ESS sum score and the number of patients with an ESS sum score of 11 or higher did not change significantly between T0/1 and T6 (data not shown). Mean FSS sum score decreased between T0 and T2 (n.s.) but stayed above 4 throughout the observational period without significant change (data not shown).
Figure 4. SF-26 physical and mental component scales in patients with LOPD and NIV in the long run.
Number of patients was 15 at T0 (prior to NIV initiation), 13 at T2, 11 at T3, 9 at T4, 8 at T5, and 6 at T6.
DISCUSSION
Respiratory muscle weakness is a clinical hallmark of LOPD, putting patients at risk of substantial symptom burden, respiratory complications, and premature death. Thus, NIV remains one of the most important therapeutic interventions. NIV, if indicated, has become an integral part of clinical recommendations for comprehensive care of patients with LOPD.19–21 Indication criteria for noninvasive ventilatory support in patients with neuromuscular disease are based on clinical symptoms, reduction of FVC, daytime pCO2 levels, and the presence of SDB.22 Our study impressively shows that SDB is common in LOPD, and confirms previous reports on the close intercorrelation between respiratory muscle weakness and SDB in this condition.5 As a limitation, it has to be taken into account that patients admitted for sleep studies represent a preselected population. In LOPD, diaphragmatic dysfunction may sometimes precede but often parallels limb girdle weakness7,8 as shown here by a significant correlation between FVC and walking distance on the 6-MWT. In addition, our study supports that supine FVC and positional drop of FVC are independent predictors of nocturnal hypoventilation.5,23,24 To date, little is known about the prevalence of OSA in patients with LOPD. Macroglossia appears to be a common finding, possibly predisposing to upper airway obstruction.4,25,26 In our study, OSA was initially present in 8 of 22 patients, which by far exceeds its prevalence in the normal population.27 Whereas it seems controversial whether isolated OSA in patients with LOPD is adequately treated with CPAP, bilevel ventilation should be started in any patient with signs or symptoms of respiratory muscle weakness, and concomitant OSA necessitates careful titration of pressure settings. In LOPD, symptoms of respiratory muscle weakness are common and correlate with self-reported sleep quality, fatigue, and impaired daytime performance.3 Our study underlines that the presence of SDB may be associated with increased fatigue and significantly reduces HRQoL in both physical and mental aspects.
This study shows that NIV significantly improves oxygenation and ventilation already in the first night of treatment. NIV warrants nocturnal normoventilation in the long term, and adjustment of ventilator settings is only rarely required based on regular follow-up sleep studies. Sleep outcomes did not significantly improve in the first night on NIV in our cohort, although a tendency of N3 and REM sleep stage enhancement was apparent. Objective sleep quality is most likely to be improved by NIV in individuals with marked sleep disruption due to SDB (Figure S1, supplemental material).
Our findings are in line with few previous PSG-based studies in patients with infantile and late-onset Pompe disease. In children, OSA and hypoventilation are highly prevalent, and NIV sustainably corrects gas exchange.28,29 In contrast to these two reports, we did not find central apneas to be of any relevance in adult patients. Our results confirm one previous study that applied NIV to eight patients with LOPD and advanced hypercapnic respiratory failure.10 In this prospective study, improvement of gas exchange was also stable, albeit further deterioration of respiratory muscle function was present.
Our study is limited by the fact that duration of follow-up varied substantially between patients. Thus, long-term data are still based on small sample sizes, making general conclusions difficult. This obstacle makes it impossible to discriminate the effects of either ERT or NIV on disease progression and respiratory muscle function. In addition, two of the ventilated patients in our cohort re-started ERT after successful desensitization, and one individual stopped ERT due to an anaphylactic reaction shortly after NIV had been initiated. Because diaphragmatic dysfunction is associated with age, longer disease duration, and worse motor performance, it is obvious that the 15 patients started on NIV in this study were older and more severely affected than unselected patients with LOPD. In our opinion this explains best why several patients receiving ERT showed a much higher decline of both FVC% and 6-MWT walking distance than previously described even in untreated patients.30 Compared to the Late-Onset Treatment Study population,30 mean age was 17 y higher in our cohort. In addition, duration of follow-up was up to 40 mo (mean 25.3 mo), a circumstance that can potentially unmask decreasing effectiveness of ERT after prolonged treatment. This issue has also been raised by a previous study showing decline of mean FVC% and 6-min walking distance after 36 mo of ERT.31
In our study, self-reported quality of life was significantly improved 3 mo after initiation of NIV. It seems surprising that the SF-36 PCS score appeared to decrease again between timepoints T2 and T6. However, we previously showed that physical HRQoL is more reduced in patients using NIV than in patients without home ventilatory support,3 which may be attributed to various aspects such as older age, more rapid functional deterioration with more advanced disease, and the use of long-term positive pressure ventilation itself.
To conclude, NIV may not ostensibly delay further neurological and respiratory impairment in patients with LOPD but appears to sustainably improve ventilation without additional decrease of objective sleep quality.
DISCLOSURE STATEMENT
This study was supported by Genzyme GmbH, Neu-Isenburg, Germany. Drs. Boentert, Dräger, and Young received speaker honoraria, travel grants, and research grants by Genzyme GmbH, Neu-Isenburg, Germany, and Genzyme Europe B. V., Naarden, The Netherlands. In addition, Drs. Boentert and Young received speaker honoraria by Heinen+Löwenstein GmbH, Bad Ems, Germany. C. Glatz has indicated no financial conflicts of interest.
ACKNOWLEDGMENTS
Author contributions: C. Glatz and M. Boentert were responsible for data collection and independent re-evaluation of PSG recordings. Drs. Boentert and Dräger performed the statistical analyses. Drs. Boentert and Young prepared the manuscript.
ABBREVIATIONS
- 6-MWT
six minutes walk test
- AHI
apnea-hypopnea index
- BE
base excess
- BMI
body mass index
- bpm
breaths per minute
- CPAP
continous positive airway pressure
- EPAP
expiratory positive airway pressure
- ERT
enzyme replacement therapy
- ESS
Epworth sleepiness scale
- FVC
forced vital capacity
- FSS
fatigue severity scale
- GAA
α-1,4-glucosidase
- HRQoL
health-related quality of life
- IPAP
inspiratory positive airway pressure
- LOPD
late-onset Pompe disease
- MCS
mental component scale (SF-36)
- MEP
maximum expiratory pressure
- MIP
maximum inspiratory pressure
- N3
slow wave sleep
- NIV
non-invasive ventilation
- n.s.
not significant
- ODI
oxygen desaturation index
- OSA
obstructive sleep apnea
- PCS
physical component scale (SF-36)
- PLM
periodic limb movements
- PSG
polysomnography
- PSQI
Pittsburgh sleep quality index
- REM sleep
rapid eye movement sleep
- RMW
respiratory muscle weakness
- SDB
sleep-disordered breathing
- SF-36
short form-36 questionnaire
- SpO2
peripheral oxygen saturation
- S/T mode
spontaneous timed mode
- tcCO2
transcutaneous carbon dioxide tension
- TST
total sleep time
- WASO
wake after sleep onset
REFERENCES
- 1.Fukuda T, Roberts A, Ahearn M, et al. Autophagy and lysosomes in Pompe disease. Autophagy. 2006;2:318–20. doi: 10.4161/auto.2984. [DOI] [PubMed] [Google Scholar]
- 2.Bembi B, Cerini E, Danesino C, et al. Diagnosis of glycogenosis type II. Neurology. 2008;71:S4–11. doi: 10.1212/WNL.0b013e31818da91e. [DOI] [PubMed] [Google Scholar]
- 3.Boentert M, Karabul N, Wenninger S, et al. Sleep-related symptoms and sleep-disordered breathing in adult Pompe disease. Eur J Neurol. 2015;22:369–76. doi: 10.1111/ene.12582. [DOI] [PubMed] [Google Scholar]
- 4.Dubrovsky A, Corderi J, Lin M, Kishnani PS, Jones HN. Expanding the phenotype of late-onset Pompe disease: tongue weakness: a new clinical observation. Muscle Nerve. 2011;44:897–901. doi: 10.1002/mus.22202. [DOI] [PubMed] [Google Scholar]
- 5.Mellies U, Ragette R, Schwake C, Baethmann M, Voit T, Teschler H. Sleep-disordered breathing and respiratory failure in acid maltase deficiency. Neurology. 2001;57:1290–5. doi: 10.1212/wnl.57.7.1290. [DOI] [PubMed] [Google Scholar]
- 6.ATS/ERS Statement on respiratory muscle testing. Am J Respir Crit Care Med. 2002;166:518–624. doi: 10.1164/rccm.166.4.518. [DOI] [PubMed] [Google Scholar]
- 7.Van der Beek NA, Hagemans ML, Reuser AJ, et al. Rate of disease progression during long-term follow-up of patients with late-onset Pompe disease. Neuromuscul Disord. 2009;19:113–7. doi: 10.1016/j.nmd.2008.11.007. [DOI] [PubMed] [Google Scholar]
- 8.van der Beek NA, van Capelle CI, van der Velden-van Etten KI, et al. Rate of progression and predictive factors for pulmonary outcome in children and adults with Pompe disease. Mol Genet Metab. 2011;104:129–36. doi: 10.1016/j.ymgme.2011.06.012. [DOI] [PubMed] [Google Scholar]
- 9.Windisch W, Walterspacher S, Siemon K, Geiseler J, Sitter H. Guidelines for non-invasive and invasive mechanical ventilation for treatment of chronic respiratory failure. Published by the German Society for Pneumology (DGP) Pneumologie. 2010;64:640–52. doi: 10.1055/s-0030-1255558. [DOI] [PubMed] [Google Scholar]
- 10.Mellies U, Stehling F, Dohna-Schwake C, Ragette R, Teschler H, Voit T. Respiratory failure in Pompe disease: treatment with noninvasive ventilation. Neurology. 2005;64:1465–7. doi: 10.1212/01.WNL.0000158682.85052.C0. [DOI] [PubMed] [Google Scholar]
- 11.Buysse DJ, Reynolds CF, 3rd, Monk TH, Berman SR, Kupfer DJ. The Pittsburgh Sleep Quality Index: a new instrument for psychiatric practice and research. Psychiatry Res. 1989;28:193–213. doi: 10.1016/0165-1781(89)90047-4. [DOI] [PubMed] [Google Scholar]
- 12.Johns MW. A new method for measuring daytime sleepiness: the Epworth sleepiness scale. Sleep. 1991;14:540–5. doi: 10.1093/sleep/14.6.540. [DOI] [PubMed] [Google Scholar]
- 13.Krupp LB, LaRocca NG, Muir-Nash J, Steinberg AD. The fatigue severity scale. Application to patients with multiple sclerosis and systemic lupus erythematosus. Arch Neurol. 1989;46:1121–3. doi: 10.1001/archneur.1989.00520460115022. [DOI] [PubMed] [Google Scholar]
- 14.Ware JE, Jr, Sherbourne CD. The MOS 36-item short-form health survey (SF-36). I. Conceptual framework and item selection. Med Care. 1992;30:473–83. [PubMed] [Google Scholar]
- 15.Merkies IS, Schmitz PI, Van Der Meche FG, Samijn JP, Van Doorn PA. Psychometric evaluation of a new handicap scale in immune-mediated polyneuropathies. Muscle Nerve. 2002;25:370–7. doi: 10.1002/mus.10045. [DOI] [PubMed] [Google Scholar]
- 16.Berry RB, Brooks R, Gamaldo CE, Harding SM, Lloyd RM, Marcus CL, Vaughn BV for the American Academy of Sleep Medicine. Darien, IL: American Academy of Sleep Medicine; 2015. The AASM Manual for the scoring of Sleep and Associated Events: Rules, Terminology and Technical Specifications, Version 2.2. www.aasmnet.org. [Google Scholar]
- 17.AAHCP/AARC/AACP/AAP/ASDA/ATS/NAMDRC Clinical indications for noninvasive positive pressure ventilation in chronic respiratory failure due to restrictive lung disease, COPD, and nocturnal hypoventilation - a consensus conference report. Chest. 1999;116:521–34. doi: 10.1378/chest.116.2.521. [DOI] [PubMed] [Google Scholar]
- 18.American Academy of Sleep Medicine. International classification of sleep disorders. 3rd ed. Darien, IL: American Academy of Sleep Medicine; 2014. [Google Scholar]
- 19.Ambrosino N, Confalonieri M, Crescimanno G, Vianello A, Vitacca M. The role of respiratory management of Pompe disease. Respir Med. 2013;107:1124–32. doi: 10.1016/j.rmed.2013.03.004. [DOI] [PubMed] [Google Scholar]
- 20.Bembi B, Cerini E, Danesino C, et al. Management and treatment of glycogenosis type II. Neurology. 2008;71:S12–36. doi: 10.1212/WNL.0b013e31818da93f. [DOI] [PubMed] [Google Scholar]
- 21.Cupler EJ, Berger KI, Leshner RT, et al. Consensus treatment recommendations for late-onset Pompe disease. Muscle Nerve. 2012;45:319–33. doi: 10.1002/mus.22329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Windisch W, Brambring J, Budweiser S, et al. [Non-invasive and invasive mechanical ventilation for treatment of chronic respiratory failure. S2-Guidelines published by the German Medical Association of Pneumology and Ventilatory Support] Pneumologie. 2010;64:207–40. doi: 10.1055/s-0029-1243978. [DOI] [PubMed] [Google Scholar]
- 23.Prigent H, Orlikowski D, Laforet P, et al. Supine volume drop and diaphragmatic function in adults with Pompe disease. Eur Respir J. 2012;39:1545–6. doi: 10.1183/09031936.00169011. [DOI] [PubMed] [Google Scholar]
- 24.Johnson EM, Roberts M, Mozaffar T, Young P, Quartel A, Berger KI. Pulmonary function tests (maximum inspiratory pressure, maximum expiratory pressure, vital capacity, forced vital capacity) predict ventilator use in late-onset Pompe disease. Neuromuscul Disord. 2016;26:136–45. doi: 10.1016/j.nmd.2015.11.009. [DOI] [PubMed] [Google Scholar]
- 25.Carlier RY, Laforet P, Wary C, et al. Whole-body muscle MRI in 20 patients suffering from late onset Pompe disease: involvement patterns. Neuromuscul Disord. 2011;21:791–9. doi: 10.1016/j.nmd.2011.06.748. [DOI] [PubMed] [Google Scholar]
- 26.Nuckton TJ, Glidden DV, Browner WS, Claman DM. Physical examination: Mallampati score as an independent predictor of obstructive sleep apnea. Sleep. 2006;29:903–8. doi: 10.1093/sleep/29.7.903. [DOI] [PubMed] [Google Scholar]
- 27.Young T, Palta M, Dempsey J, Skatrud J, Weber S, Badr S. The occurrence of sleep-disordered breathing among middle-aged adults. N Engl J Med. 1993;328:1230–5. doi: 10.1056/NEJM199304293281704. [DOI] [PubMed] [Google Scholar]
- 28.Kansagra S, Austin S, DeArmey S, Kazi Z, Kravitz RM, Kishnani PS. Longitudinal polysomnographic findings in infantile Pompe disease. Am J Med Genet A. 2015;167A:858–61. doi: 10.1002/ajmg.a.37007. [DOI] [PubMed] [Google Scholar]
- 29.Kansagra S, Austin S, DeArmey S, Kishnani PS, Kravitz RM. Polysomnographic findings in infantile Pompe disease. Am J Med Genet A. 2013;161A:3196–200. doi: 10.1002/ajmg.a.36227. [DOI] [PubMed] [Google Scholar]
- 30.van der Ploeg AT, Clemens PR, Corzo D, et al. A randomized study of alglucosidase alfa in late-onset Pompe's disease. N Engl J Med. 2010;362:1396–406. doi: 10.1056/NEJMoa0909859. [DOI] [PubMed] [Google Scholar]
- 31.Regnery C, Kornblum C, Hanisch F, et al. 36 months observational clinical study of 38 adult Pompe disease patients under alglucosidase alfa enzyme replacement therapy. J Inher Metab Dis. 2012;35:837–45. doi: 10.1007/s10545-012-9451-8. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.