Abstract
A sensitive and selective chemiluminescence (CL) sensor based on the peroxidase-like activity of copper nanoclusters was established for the detection of cholesterol. Copper nanoclusters catalyse the CL reaction between luminol and H2O2. Because H2O2 is the oxidative product of cholesterol in the presence of cholesterol oxidase, the oxidation of cholesterol can be quantitatively converted to a CL response by combining the two reactions. The proposed method is simple and can be completed in a few minutes with high sensitivity. Under the optimal conditions, the CL intensity was proportional to the concentration of cholesterol over a wide range of 0.05–10 mM, with a detection limit of 1.5 μM. Furthermore, the method was successfully applied to determine cholesterol in milk powder and human serum with satisfactory accuracy and precision. This method expands the applications of nano-mimic enzymes in the field of CL-based sensors.
Cholesterol, a main lipid in humans, is an important structural component of the cell membrane, where it helps to maintain membrane fluidity and permeability. Because it also plays a vital role as a precursor in the production of Vitamin D and hormones, abnormal cholesterol levels cause certain diseases, such as anaemia, hypolipoproteinaemia, malnutrition hypertension, brain thrombosis, septicaemia and arteriosclerosis1,2,3. In humans, the sources of cholesterol are food and biosynthesis in the liver. Therefore, monitoring the cholesterol levels in food and blood is critical for disease control and prevention. Various analytical methods have been proposed to determine cholesterol content in many foods and biological samples, including liquid chromatograph4,5, spectrophotometric6,7,8, colorimetric9,10,11, electrogenerated chemiluminescence (ECL)12,13, and enzyme-linked immunosorbent assay14,15. The sensing platforms mentioned above has performed well for cholesterol detection, however, limitations still exists in the case of time consumption, low sensitivity and selectivity, and sophisticated instrumentation or standardization difficulties. Accordingly, it would be of great interest to develop cost-effective, ease-to-use and sensitive cholesterol sensor.
Chemiluminescence (CL) has received considerable attention by virtue of its simplicity, low detection limit, wide calibration range, and inexpensive instrumentation. Due to the combination of advantages of CL, Zhang et al. reported a sensor for free cholesterol based on immobilizing cholesterol oxidase onto sol-gel to generate an enzymatic reaction column16. Recently, CL studies have been extended to nanomaterial systems to enhance the inherent sensitivity and expand to novel applications of detection17,18,19. However, only a few nanomaterial-based CL methods are available for the determination of cholesterol. Chen et al. constructed a CL cholesterol sensor based upon the peroxidase-like activity of cupric oxide nanoparticles20. Also, Ehsani et al. relied on the catalytic activity of cupric oxide nanoparticles towards the luminol-H2O2 system using a Box-Behnken design for cholesterol determination21. However, these CL sensors are limited due to the complicated synthesis of the nanomaterials.
Copper nanoclusters (Cu NCs) consisting of several to tens of atoms have recently attracted much attention22,23. Xu et al. has demonstrated that Cu NCs could possess intrinsic peroxidase-like activity24. Compared to natural enzymes, Cu NCs show several advantages, such as ease of preparation, low cost, and high catalytic activity. Additionally, we have found that Cu NCs could greatly enhance the CL of the luminol-H2O2 system in strongly alkaline media25. Moreover, hydrogen peroxide is a product of the cholesterol oxidase-catalysed reaction of cholesterol and oxygen. According to the three aforementioned points, we have established a novel, simple, and sensitive sensor for the detection of cholesterol by combining the highly selective enzymatic reaction with the sensitive chemiluminescence system catalysed by copper nanoclusters in this work.
Experimental
Reagents and materials
A 1.0 × 10−2 mol L−1 stock solution of luminol (3-aminophthalhydrazide) was prepared by dissolving luminol (Sigma) in a 0.1 mol L−1 sodium hydroxide solution and stored at 4 °C. Working solutions of luminol were prepared by diluting the stock solution with ultra-pure water. Bovine serum albumin (BSA) was purchased from Sigma Sangon Biotech Co., Ltd. (Shanghai, China). CuSO4·5H2O, sodium hydroxide, isopropanol, Triton X-100, cholesterol and ChOx were purchased from Sigma-Aldrich Co., Ltd. (USA). A stock solution of cholesterol was prepared by dissolving cholesterol in a mixture of isopropanol and Triton X-100 (1:1, v/v), and the standard cholesterol solutions were diluted with PBS (pH 7.4) and then stored at 4 °C. All of the reagents were used as purchased without purification, and ultra-pure water was used throughout.
Synthesis of BSA–Cu nanoclusters
BSA modified Cu NCs were prepared in aqueous solution following the previous reported method26. In a typical experiment, 1 mL aqueous CuSO4·5H2O solution (20 mM) was added to BSA solution (5 mL, 15 mg mL−1) under vigorous stirring for 5 min at room temperature. Then, the solution pH was adjusted to 12 by adding NaOH solution and the mixture was allowed to proceed under vigorous stirring at 55 °C for 8 h. The solution was then dialyzed in ultra-pure water for 48 h to remove unreacted Cu2+. The final solution was stored at 4 °C in refrigerator when not in use.
General procedure for CL analysis
The chemiluminescence detection was conducted on a laboratory-built flow injection CL system, consisting of a model IFFM-E flow injection system (Xi’an Remex Co.), a model IFFA-S multifunctional CL detector (Xi’an Remex Co.), and a computer, as shown in Figure S1. Two peristaltic pumps with three channels were used to carry the reactants to the flow cell. One peristaltic pump was used to carry Cu NCs and sample solutions with two channels, and the other pump was used to deliver luminol solution at 1.9 mL/min. The CL signals produced were monitored by a photomultiplier tube and then the output signals were obtained by a computer automatically. Data acquisition and treatment were performed with Remex software running under Windows XP. When the CL system was used to investigate the effects of interference compounds, one peristaltic pump was used to deliver Cu NCs and the mixture of sample and luminol with two channels, and the other pump was used to carry the interference compound solution at 1.9 mL/min.
Quantitative analysis of cholesterol
The cholesterol catalytic reaction was carried out by adding 65 μL of cholesterol (0.05 mM to 10 mM), 25 μL of 30 U ml−1 cholesterol oxidase (ChOx), and 162.5 μL of 0.1 mol/L phosphate buffer solution (pH 7.4) into an EP tube. The mixture was then incubated at 37 °C for 10 min to obtain the testing sample solution. Before CL testing, the sample solutions were diluted 10 times by water. The assay was applied to milk and human serum samples for the determination of cholesterol.
Preparation of samples
Milk powder sample
2.0 g of milk powder was dissolved in 10 mL of KOH/ethanol solution and saponified in a water bath for 1 h. Then, 10 mL water and 20 mL n-hexane were added into the sample solution, and the mixture was centrifuged at 5000 rpm for 5 min. Finally the n-hexane was separated, and the solvent was evaporated under a stream of nitrogen. The residue was redissolved with the previous mixture of isopropanol and Triton X-100.
Human serum sample
For determination of free cholesterol, 0.2 mL of a human serum sample was diluted with 1.8 mL ethanol solution and 2.0 mL water. Each of these 20-fold diluted samples were mixed with 4.0 mL n-hexane and then centrifuged for 5 min. Finally, the n-hexane extract was separated, and the solvent evaporated under a stream of nitrogen. The residue was redissolved with the previous mixture of isopropanol and Triton X-100.
Results and Discussion
Principle for the chemiluminescence detection of cholesterol
The UV-vis and fluorescence spectra of as-prepared Cu NCs are shown in Figures S2 and S3. When the Cu NCs were excited at 325 nm, they showed an emission peak centred at 410 nm. The features of the obtained spectra were in keeping with previous reports, indicating the successful preparation of Cu NCs.
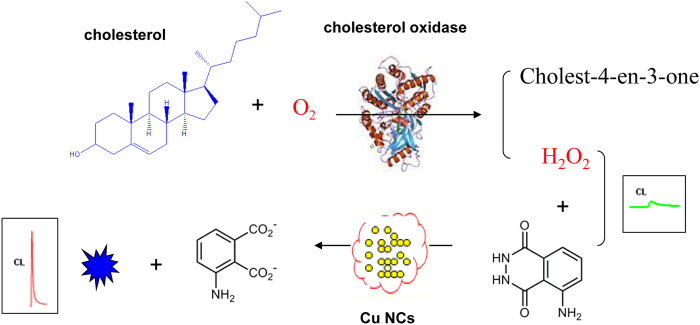
The strategy of this chemiluminescent cholesterol sensor has been suggested to occur in three steps, as shown in Fig. 1. Firstly, H2O2 is generated from the oxidation of cholesterol in the presence of cholesterol oxidase. Secondly, the chemical reaction of luminol with H2O2 is accelerated by the catalytic effect of Cu NCs, resulting in the formation of excited-state 3-aminophthalate anions which emit light (λmax = 425 nm) on relaxation to the ground state (see Equations 1 and 2). Finally, the relationship between the concentration of cholesterol and the intensity of the CL signal was evaluated for the sensor.
Figure 1. Principle of the Cu NCs-based chemiluminescence sensor for cholesterol.
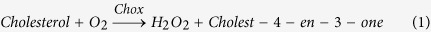 |
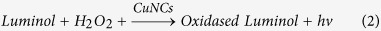 |
Chemiluminescence analysis under different experimental conditions
In our recent work, we studied the possible mechanism of the luminol-H2O2-Cu NC systems using the CL spectra, UV-visible spectroscopy and radical scavengers25. Initially, The O-O bond of H2O2 might be broken up into two OH• radicals via the catalysis of Cu NCs. Next, the OH• radicals are thought to react with the luminol anion and HO2− to form the luminol radical and superoxide radical anion O2•−, which further react with each other to form the excited 3-aminophthalate anion (3-APA*). As a result, the CL signal is enhanced significantly. In this work, H2O2 originated from the oxidation of cholesterol by O2 in the presence of ChOx.
In order to prove the consistency of mechanism, the kinetic curves of CL systems for different situations were considered. A batch method was used to study the reaction of luminol, cholesterol, ChOx and Cu NCs. The addition of Cu NCs to luminol and cholesterol (or ChOx) did not produce light. When cholesterol and ChOx was simultaneously injected into the luminol system without Cu NCs, the CL intensity increased slightly due to the formation of H2O2 (Fig. 2a). Importantly, a remarkable CL enhancement (up to 31-fold in 20 s) was found when Cu NCs were introduced into the luminol-cholesterol/ChOx system, and the light lasted for approximately 200 s (Fig. 2c). Compared with Cu NCs, the CL intensity was only enhanced by approximately 5-fold by a CuSO4 solution (Fig. 2b). These results indicate that the significantly enhanced CL in Fig. 1c is related directly to Cu NCs. The CL spectra of luminol-cholesterol/ChOx in the presence of Cu NCs is shown in Figure S4. The maximum emission wavelength of luminol-cholesterol/ChOx-Cu NCs was found to be about 425 nm, suggesting that the luminophor for the CL system is still the excited 3-aminophthalate anion. Therefore, the addition of cholesterol/ChOx into the luminol system containing Cu NCs does not generate a new luminophor, which is consistent with previous reports.
Figure 2. Kinetic curves of luminol CL system under different conditions.
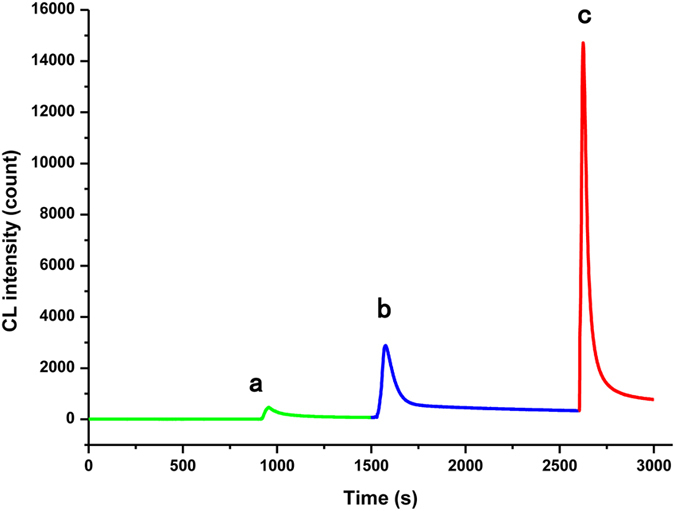
(a) luminol + cholesterol/ChOx; (b) luminol + cholesterol/ChOx + CuSO4; (c) luminol + cholesterol/ChOx + Cu NCs. Luminol solution: 6 × 10−4 mol L−1 (pH 12), Cu NCs : 6.4 mg L−1, ChOx :30 U mL−1. cholesterol :10 mM, Cu2+: 20 mM.
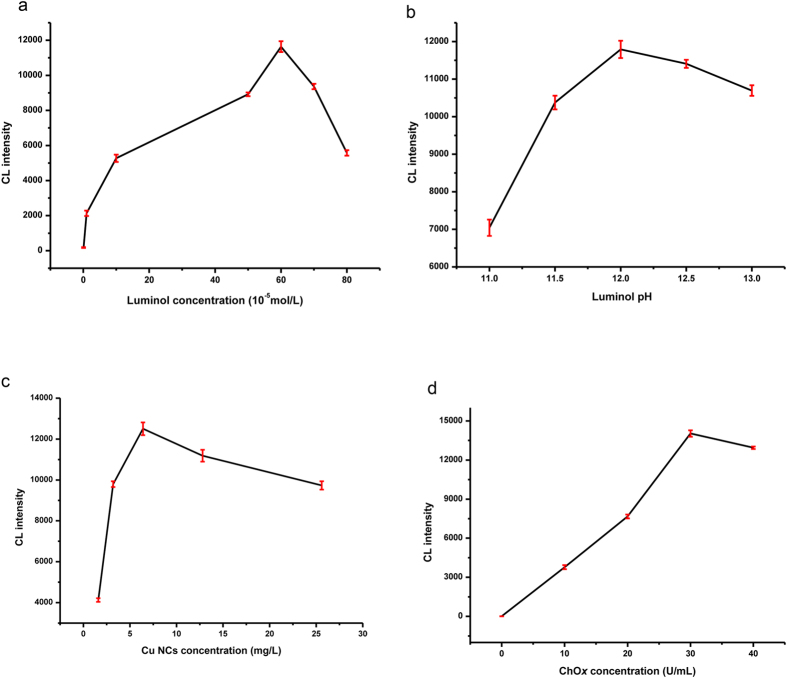
Optimization of the experimental conditions
The cholesterol sensor was investigated under varying concentrations of luminol, ChOx, and Cu NCs as well as luminol solutions of differing pH. As shown in Fig. 3, the CL intensity increased with increasing luminol concentration from 1 × 10−6 to 6 × 10−4 mol L−1 (Fig. 3a), but a higher concentration of luminol led to self-absorption of the emitted radiation and a decrease in the CL intensity. Because the pH of the luminol solution is a key factor in the generation of CL, the effect of solution pH values from 11 to 13 on the sensor performance was studied (Fig. 3b). At pH values lower than 12, the CL intensity increased with increasing pH, but the opposite trends was observed at pH values higher than 12. The effect of the Cu NC concentration was also tested (Fig. 3c), and the optimized concentration was found to be 6.4 mg L−1 Cu NCs. ChOx plays an important role in forming H2O2, which leads to CL emission. Therefore, the impact of the ChOx concentration was studied over the range of 0–40 U ml−1, and it was found that the CL intensity increased with increasing concentration of enzyme (Fig. 3d). However, considering the CL intensity and reagent consumption, the optimized conditions for the CL system were as follows: 6 × 10−4 mol L−1 luminol in NaOH solution (pH 12) with 6.4 mg L−1 Cu NCs and 30 U mL−1 ChOx.
Figure 3. Effects of different concentration of reagents and pH on the CL intensity.
Cholesterol detection based on chemiluminescence sensor
The CL sensor was evaluated under the optimal experimental conditions described above by detecting standard cholesterol solutions. As shown in Fig. 4, the CL intensity was found to increase with increasing cholesterol concentrations. The linear calibration range encompassed over 3 orders of magnitude from 0.05 mM to 10 mM with a relatively low detection limit of 1.5 μM (LOD, S/N = 3), and the regression equation was I = 1366.2c + 343.8 with a correlation coefficient R = 0.9993 (n = 8) (where c is the cholesterol concentration in mM). The relative standard deviation (RSD) was 2.4% for 1 mM cholesterol (n = 11). Furthermore, the performance of the proposed CL sensor was compared with previously reported cholesterol assays in terms of LOD and linear range (Table 1). It could be observed that the proposed CL sensor showed a high sensitivity for the determination of cholesterol.
Figure 4. The linear calibration plot of the CL reaction for cholesterol detection.
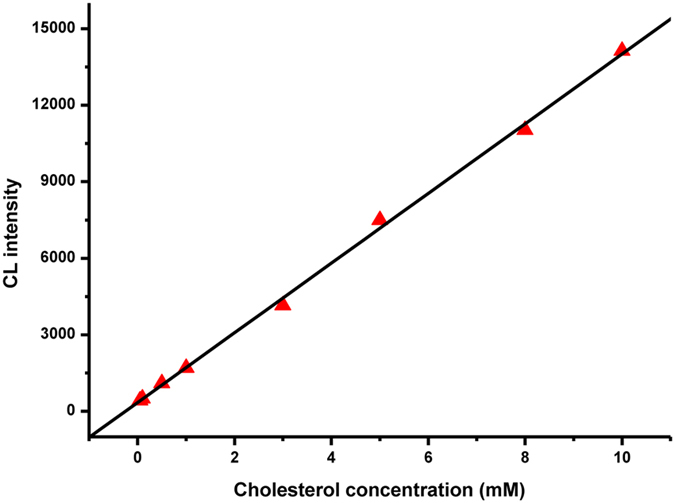
Table 1. Comparison of the analytical performance of proposed method with others.
| Method | Material | Linear range (mM) | Detecton limit (mM) |
|---|---|---|---|
| Colorimetry11 | ZnO NPs-CNTs | 0.5–500a | 0.2a |
| Chronoamper-ometry27 | ChOx-CS/Hb-CS | 0.01–0.6 | 0.0095 |
| Amperometry28 | Pt-ZnO Nanospheres | 2.78–12.2 | 0.5 |
| CV29 | (PDDA-[MWCNTs-ChOx]5) | 0.02–1 | 0.03 |
| ECL130 | Ag NPs | 1–700 | 0.65 |
| ECL231 | Au NPs | 0.033–1 | 0.0011 |
| ECL332 | CdSeTe/ZnS QD | 0.25–5 | Not given |
| CL121 | CuO NPs | 0.025–7.17 | 0.0064 |
| CL2 | Cu NCs | 0.05–10 | 0.0015 |
anmol/L.
Selectivity and stability of the chemiluminescence sensor
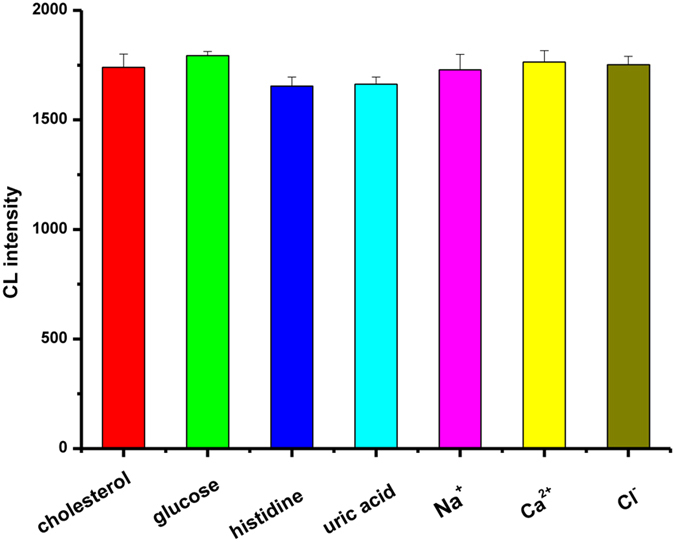
Selectivity and stability are important parameters for examining the performance of a sensor. To study the selectivity of the method towards the detection of cholesterol, a standard solution of 1 mM cholesterol was subjected to varying amounts of possible interferents. The tolerable limit was considered as a relative error less than the 5% level. As shown in Fig. 5, interferents, such as the organic molecules glucose, histidine, and uric acid and the inorganic ions Na+, Ca2+, and Cl−, had a negligible effect on the detection of 1 mM cholesterol, demonstrating that ChOx has a selectivity for cholesterol catalysis. The stability of the sensor was also studied by measuring the change in the CL signal at regular intervals of 2 days for 2 weeks. The CL signal decreased to 90% after 1 week and 75% after 2 weeks. These results show that the sensor has good selectivity and stability.
Figure 5. The interference effect of 1 mM glucose, 0.1 mM histidine, 0.11 mM uric acid, 10 mM Na+, 10 mM Ca2+ and 10 mM Cl− in the detection of 1 mM cholesterol in 0.1 mol/L phosphate buffer solution (pH 7.4).
Application of the chemiluminescence sensor
To explore potential applications of the method, the content of cholesterol in commercial milk and human serum samples was determined. The milk and human serum samples were first pre-treated according to previous reports20. Next, recovery experiments were carried out to evaluate the practical applicability of the method by adding a known amount of cholesterol to the real samples. As shown in Table 2, the recoveries of the spiked milk samples and human serum samples ranged from 91.0% to 103.9% with the RSD less than 5.0%, indicating that the CL sensor is reliable for the detection of cholesterol in real samples.
Table 2. Evaluation of cholesterol determination in milk and human serum samples by standard addition method.
| Samples | Added (mM ) | Found (mM ) | Recovery (%) | RSD (%) |
|---|---|---|---|---|
| Milk sample 1 | 0 | 0.071 | — | 2.5 |
| 0.1 | 0.162 | 91.0 | 2.4 | |
| 0.5 | 0.540 | 93.8 | 3.0 | |
| Milk sample 2 | 0 | 0.062 | — | 2.0 |
| 0.1 | 0.156 | 94.0 | 3.5 | |
| 0.5 | 0.646 | 98.0 | 2.8 | |
| Serum sample 1 | 0 | 1.850 | — | 4.3 |
| 5 | 6.981 | 102.3 | 4.5 | |
| 10 | 11.963 | 101.1 | 3.2 | |
| Serum sample 2 | 0 | 1.197 | — | 3.7 |
| 5 | 6.390 | 103.9 | 4.2 | |
| 10 | 11.120 | 99.2 | 4.0 |
(n = 3).
Conclusion
In summary, we have constructed a novel chemiluminescent cholesterol sensor based on the peroxidase-like activity of copper nanoclusters. The sensor showed good performance for cholesterol detection with the advantages of high sensitivity, selectivity, stability, an acceptable linear range of 0.05–10 mM and a relatively low detection limit (1.5 μM). Moreover, the method was successfully applied to the determination of cholesterol in milk and human serum samples. This work is also expected to widen the application of enzyme-catalysed chemiluminescence reactions in bioanalysis.
Additional Information
How to cite this article: Xu, S. et al. A novel chemiluminescence sensor for sensitive detection of cholesterol based on the peroxidase-like activity of copper nanoclusters. Sci. Rep. 6, 39157; doi: 10.1038/srep39157 (2016).
Publisher’s note: Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Material
Acknowledgments
This work is supported by National agricultural product quality safety risk assessment (GJFP201601006).
Footnotes
Author Contributions S.-J.X. and Y.-Q.W. designed the research and prepared all figures. S.-J.X. and D.-Y.Z. performed the experiments. M.K. and D.F. analyzed the data. The other authors commented on the manuscript. All authors wrote and reviewed the manuscript.
References
- Andrade I., Santos L. & Ramos F. Advances in analytical methods to study cholesterol metabolism: the determination of serum noncholesterol sterols. Biomed Chromatogr 27, 1234–1242, doi: 10.1002/bmc.2840 (2013). [DOI] [PubMed] [Google Scholar]
- Brown H. H., Zlatkis A., Zak B. & Boyle A. J. Rapid procedure for determination of free serum cholesterol. Analytical Chemistry 26, 397–399 (1954). [Google Scholar]
- Aravamudhan S., Ramgir N. S. & Bhansah S. Electrochemical biosensor for targeted detection in blood using aligned Au nanowires. Sensor Actuat B-Chem 127, 29–35, doi: 10.1016/j.snb.2007.07.008 (2007). [DOI] [Google Scholar]
- Mariutti L. R. B., Nogueira G. C. & Bragagnolo N. Optimization and validation of analytical conditions for cholesterol and cholesterol oxides extraction in chicken meat using response surface methodology. J Agr Food Chem 56, 2913–2918, doi: 10.1021/jf0735432 (2008). [DOI] [PubMed] [Google Scholar]
- Lin Y. T., Wu S. S. & Wu H. L. Highly sensitive analysis of cholesterol and sitosterol in foods and human biosamples by liquid chromatography with fluorescence detection. J Chromatogr A 1156, 280–287, doi: 10.1016/j.chroma.2007.01.091 (2007). [DOI] [PubMed] [Google Scholar]
- Odo J. et al. Spectrophotometric Determination of Hydrogen Peroxide, Glucose, Uric Acid, and Cholesterol Using Peroxidase-like Activity of an Fe(III) Complex of Thiacalix[4]arenetetrasulfonate Attached to an Anion-exchanger. Analytical Sciences 29, 1041–1048 (2013). [DOI] [PubMed] [Google Scholar]
- Arya S. K., Datta M., Singh S. P. & Malhotra B. D. Biosensor for total cholesterol estimation using N-(2-aminoethyl)-3-aminopropyltrimethoxysilane self-assembled monolayer. Anal Bioanal Chem 389, 2235–2242, doi: 10.1007/s00216-007-1655-7 (2007). [DOI] [PubMed] [Google Scholar]
- Pineiro-Avila G., Salvador A. & de la Guardia M. Flow injection determination of free and total cholesterol in animal greases using enzymes in non-aqueous media. Analyst 123, 999–1003, doi: 10.1039/A800622i (1998). [DOI] [PubMed] [Google Scholar]
- Li R. M., Xiong C., Xiao Z. Y. & Ling L. S. Colorimetric detection of cholesterol with G-quadruplex-based DNAzymes and ABTS(2-). Anal Chim Acta 724, 80–85, doi: 10.1016/j.aca.2012.02.015 (2012). [DOI] [PubMed] [Google Scholar]
- Qureshi R. N., Kok W. T. & Schoenmakers P. J. Fractionation of human serum lipoproteins and simultaneous enzymatic determination of cholesterol and triglycerides. Anal Chim Acta 654, 85–91, doi: 10.1016/j.aca.2009.06.060 (2009). [DOI] [PubMed] [Google Scholar]
- Hayat A., Haider W., Raza Y. & Marty J. L. Colorimetric cholesterol sensor based on peroxidase like activity of zinc oxide nanoparticles incorporated carbon nanotubes. Talanta 143, 157–161, doi: 10.1016/j.talanta.2015.05.051 (2015). [DOI] [PubMed] [Google Scholar]
- Huan J. et al. Amplified solid-state electrochemiluminescence detection of cholesterol in near-infrared range based on CdTe quantum dots decorated multiwalled carbon nanotubes@reduced graphene oxide nanoribbons. Biosens Bioelectron 73, 221–227, doi: 10.1016/j.bios.2015.06.004 (2015). [DOI] [PubMed] [Google Scholar]
- Zhang M. H. et al. A cathodic electrogenerated chemiluminescence biosensor based on luminol and hemin-graphene nanosheets for cholesterol detection. Rsc Adv 2, 4639–4641, doi: 10.1039/c2ra20374j (2012). [DOI] [Google Scholar]
- Karimi S., Ghourchian H., Rahimi P. & Rafiee-Pour H. A. A nanocomposite based biosensor for cholesterol determination. Anal Methods-Uk 4, 3225–3231, doi: 10.1039/c2ay25826a (2012). [DOI] [Google Scholar]
- Shen J. & Liu C. C. Development of a screen-printed cholesterol biosensor: Comparing the performance of gold and platinum as the working electrode material and fabrication using a self-assembly approach. Sensor Actuat B-Chem 120, 417–425, doi: 10.1016/j.snb.2006.02.035 (2007). [DOI] [Google Scholar]
- Hu Y. F. & Zhang Z. J. Determination of free cholesterol based on a novel flow-injection chemiluminescence method by immobilizing enzyme. Luminescence 23, 338–343, doi: 10.1002/bio.1042 (2008). [DOI] [PubMed] [Google Scholar]
- He Y. & Cui H. Fabrication of Luminol and Lucigenin Bifunctionalized Gold Nnanoparticles/Graphene Oxide Nanocomposites with Dual-Wavelength Chemiluminescence. J Phys Chem C 116, 12953–12957, doi: 10.1021/jp303304z (2012). [DOI] [Google Scholar]
- Zhang Z. F., Cui H., Lai C. Z. & Liu L. J. Gold nanoparticle-catalyzed luminol chemiluminescence and its analytical applications. Analytical Chemistry 77, 3324–3329, doi: 10.1021/ac050036f (2005). [DOI] [PubMed] [Google Scholar]
- Cui H., Zhang Z. F., Shi M. J., Xu Y. & Wu Y. L. Light emission of gold nanoparticles induced by the reaction of bis(2,4,6-trichlorophenyl) oxalate and hydrogen peroxide. Analytical Chemistry 77, 6402–6406, doi: 10.1021/ac050882q (2005). [DOI] [PubMed] [Google Scholar]
- Hong L., Liu A. L., Li G. W., Chen W. & Lin X. H. Chemiluminescent cholesterol sensor based on peroxidase-like activity of cupric oxide nanoparticles. Biosens Bioelectron 43, 1–5, doi: 10.1016/j.bios.2012.11.031 (2013). [DOI] [PubMed] [Google Scholar]
- Chaichi M. J. & Ehsani M. Determination of Glucose and Cholesterol Using a Novel Optimized Luminol- CuO Nanoparticles-H2O2 Chemiluminescence Method by Box-Behnken Design. J Fluoresc 25, 861–870, doi: 10.1007/s10895-015-1566-5 (2015). [DOI] [PubMed] [Google Scholar]
- Shang L., Dong S. J. & Nienhaus G. U. Ultra-small fluorescent metal nanoclusters: Synthesis and biological applications. Nano Today 6, 401–418, doi: 10.1016/j.nantod.2011.06.004 (2011). [DOI] [Google Scholar]
- Hu X. et al. Recent advances in the analytical applications of copper nanoclusters. TrAC Trends in Analytical Chemistry 77, 66–75, doi: 10.1016/j.trac.2015.12.013 (2016). [DOI] [Google Scholar]
- Hu L. Z. et al. Copper nanoclusters as peroxidase mimetics and their applications to H2O2 and glucose detection. Anal Chim Acta 762, 83–86, doi: 10.1016/j.aca.2012.11.056 (2013). [DOI] [PubMed] [Google Scholar]
- Xu S. J., Chen F. N., Deng M. & Sui Y. Y. Luminol chemiluminescence enhanced by copper nanoclusters and its analytical application. Rsc Adv 4, 15664–15670, doi: 10.1039/c4ra00516c (2014). [DOI] [Google Scholar]
- Goswami N. et al. Copper Quantum Clusters in Protein Matrix: Potential Sensor of Pb2+ Ion. Analytical Chemistry 83, 9676–9680, doi: 10.1021/ac202610e (2011). [DOI] [PubMed] [Google Scholar]
- Zhao C. Z., Wan L., Jiang L., Wang Q. & Jiao K. Highly sensitive and selective cholesterol biosensor based on direct electron transfer of hemoglobin. Analytical Biochemistry 383, 25–30, doi: 10.1016/j.ab.2008.08.022 (2008). [DOI] [PubMed] [Google Scholar]
- Ahmad M., Pan C. F., Gan L., Nawaz Z. & Zhu J. Highly Sensitive Amperometric Cholesterol Biosensor Based on Pt-Incorporated Fullerene-like ZnO Nanospheres. J Phys Chem C 114, 243–250, doi: 10.1021/jp9089497 (2010). [DOI] [Google Scholar]
- Manjunatha R. et al. Direct electrochemistry of cholesterol oxidase on MWCNTs. J Electroanal Chem 651, 24–29, doi: 10.1016/j.jelechem.2010.11.009 (2011). [DOI] [Google Scholar]
- Nantaphol S., Chailapakul O. & Siangproh W. A novel paper-based device coupled with a silver nanoparticle-modified boron-doped diamond electrode for cholesterol detection. Anal Chim Acta 891, 136–143, doi: 10.1016/j.aca.2015.08.007 (2015). [DOI] [PubMed] [Google Scholar]
- Zhang M. H. et al. A biosensor for cholesterol based on gold nanoparticles-catalyzed luminol electrogenerated chemiluminescence. Biosens Bioelectron 32, 288–292, doi: 10.1016/j.bios.2011.12.008 (2012). [DOI] [PubMed] [Google Scholar]
- Stewart A. J. et al. A Cholesterol Biosensor Based on the NIR Electrogenerated-Chemiluminescence (ECL) of Water-Soluble CdSeTe/ZnS Quantum Dots. Electrochim Acta 157, 8–14, doi: 10.1016/j.electacta.2015.01.073 (2015). [DOI] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.