Abstract
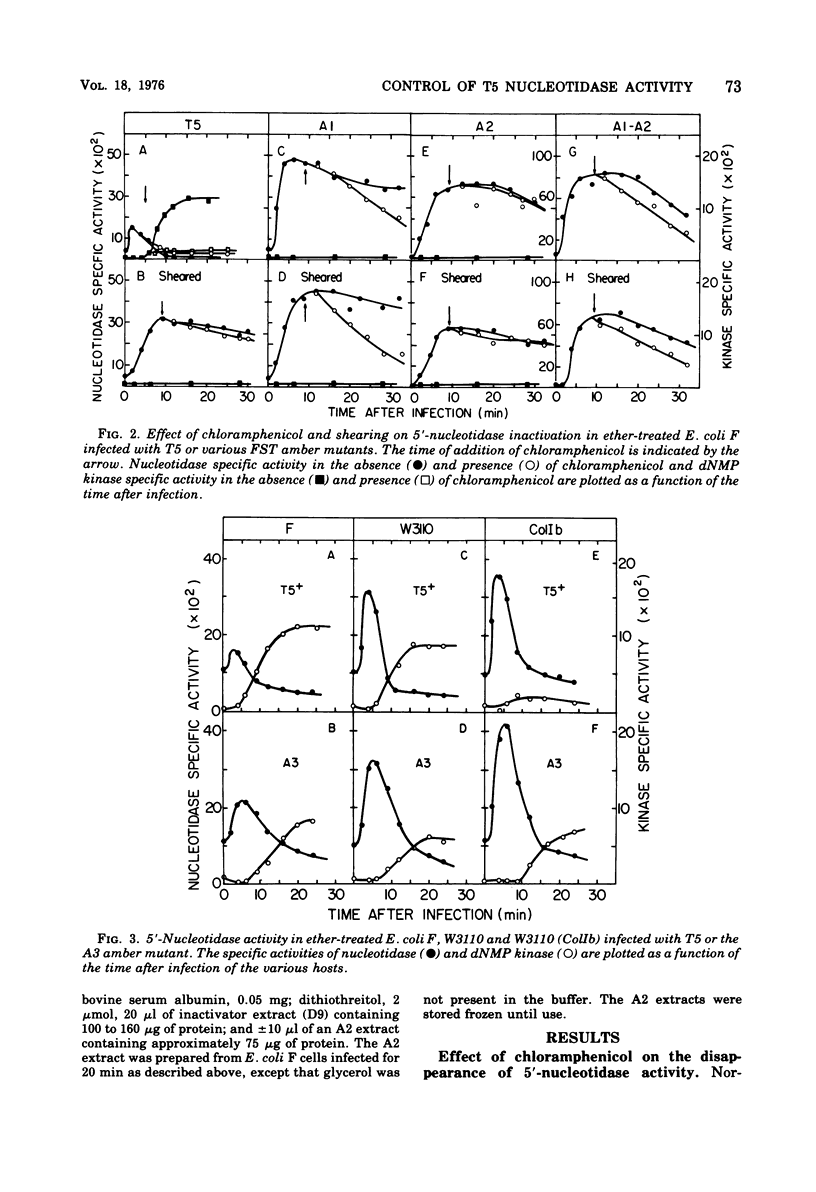
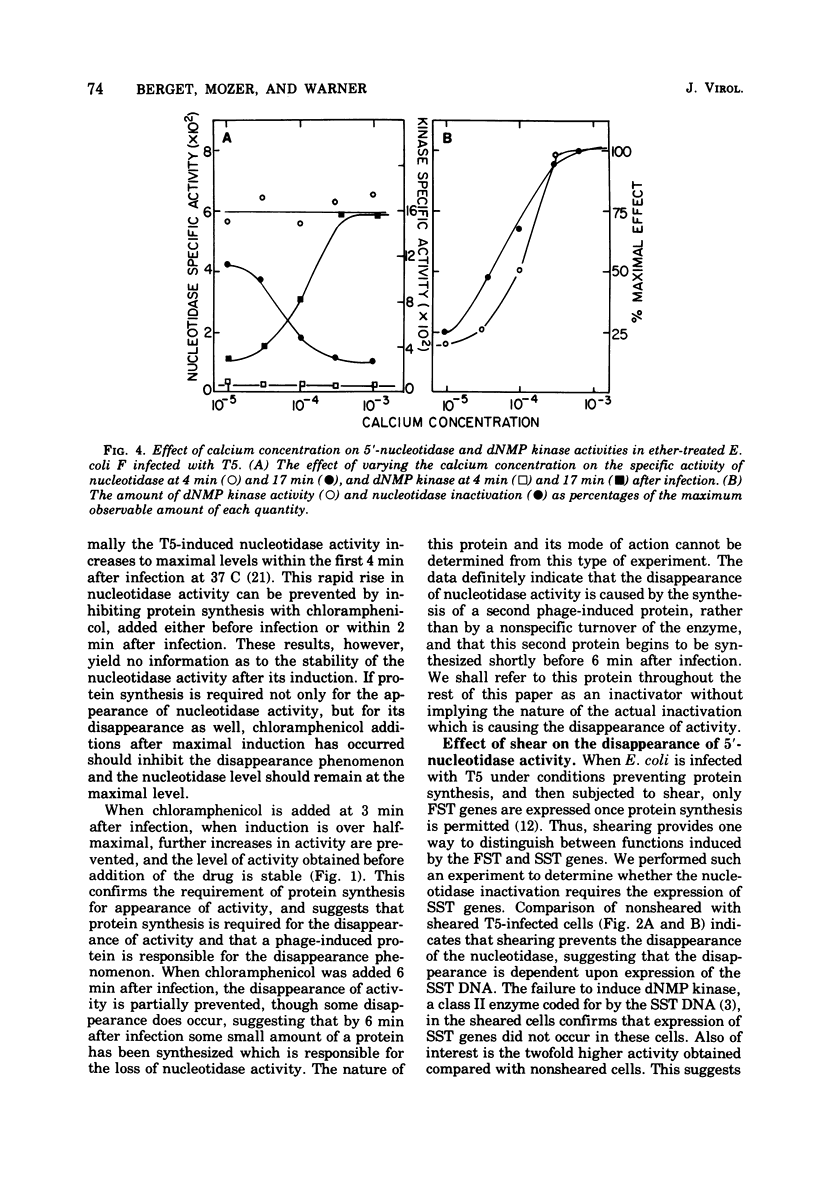
The control of activity of the bacteriophage T5-induced 5'-nucleotidase is dependent upon the amount of T5 parental DNA injected into the cell and expressed. When only the first-step transfer DNA is injected and expressed the amount of 5'-nucleotidase activity observed is two to three times the maximum amount observed after normal T5 infection, and inactivation of the enzyme does not occur. Enzyme inactivation occurs only after the remaining DNA is injected, but only limited expression of this DNA is required. The control of the nucleotidase inactivation process is similar to that for the repair of the nicks in parental DNA, and is probably mediated by a class IIa protein.
Full text
PDF






Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Beckman L. D., Anderson G. C., McCorquodale D. J. Arrangement on the chromosome of the known pre-early genes of bacteriophages T5 and BF23. J Virol. 1973 Nov;12(5):1191–1194. doi: 10.1128/jvi.12.5.1191-1194.1973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beckman L. D., Hoffman M. S., McCorquodale D. J. Pre-early proteins of bacteriophage T5: structure and function. J Mol Biol. 1971 Dec 28;62(3):551–564. doi: 10.1016/0022-2836(71)90155-0. [DOI] [PubMed] [Google Scholar]
- Berget S. M., Warner H. R., McCorquodale D. J. Isolation and partial characterization of bacteriophage T5 mutants deficient in the ability to induce deoxynucleoside monophosphate kinase. J Virol. 1974 Jul;14(1):78–85. doi: 10.1128/jvi.14.1.78-85.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fielding P. E., Lunt M. R. A new deoxyribonuclease activity from bacteria infected with T5 bacteriophage. FEBS Lett. 1969 Nov 12;5(3):214–217. doi: 10.1016/0014-5793(69)80335-2. [DOI] [PubMed] [Google Scholar]
- Hausmann R., Gold M. The enzymatic methylation of ribonucleic acid and deoxyribonucleic acid. IX. Deoxyribonucleic acid methylase in bacteriophage-infected Escherichia coli. J Biol Chem. 1966 May 10;241(9):1985–1994. [PubMed] [Google Scholar]
- Hendrickson H. E., McCorquodale D. J. Genetic and physiological studies of bacteriophage T5. 2. The relationship between phage DNA synthesis and protein synthesis in T5-infected cells. Biochem Biophys Res Commun. 1971 May 21;43(4):735–740. doi: 10.1016/0006-291x(71)90677-2. [DOI] [PubMed] [Google Scholar]
- Herman R. C., Moyer R. W. In vivo repair of bacteriophage t5 DNA: an assay for viral growth control. Virology. 1975 Aug;66(2):393–407. doi: 10.1016/0042-6822(75)90212-3. [DOI] [PubMed] [Google Scholar]
- Herman R. C., Moyer R. W. In vivo repair of the single-strand interruptions contained in bacteriophage T5 DNA. Proc Natl Acad Sci U S A. 1974 Mar;71(3):680–684. doi: 10.1073/pnas.71.3.680. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LANNI Y. T. Invasion by bacteriophage T5. III. Stages revealed by changes in susceptibility of early complexes to abortive infection. Virology. 1961 Oct;15:127–135. doi: 10.1016/0042-6822(61)90229-x. [DOI] [PubMed] [Google Scholar]
- Lanni Y. T. First-step-transfer deoxyribonucleic acid of bacteriophage T5. Bacteriol Rev. 1968 Sep;32(3):227–242. doi: 10.1128/br.32.3.227-242.1968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lanni Y. Functions of two genes in the first-step-transfer DNA of bacteriophage T5. J Mol Biol. 1969 Aug 28;44(1):173–183. doi: 10.1016/0022-2836(69)90412-4. [DOI] [PubMed] [Google Scholar]
- McCorquodale D. J., Lanni Y. T. Patterns of protein synthesis in Escherichia coli infected by amber mutants in the first-step-transfer DNA of T5. J Mol Biol. 1970 Feb 28;48(1):133–143. doi: 10.1016/0022-2836(70)90224-x. [DOI] [PubMed] [Google Scholar]
- Mizobuchi K., Anderson G. C., McCorquodale D. J. Abortive infection by bacteriophage BF23 due to the colicin Ib factor. I. Genetic studies of nonrestricted and amber mutants of bacteriophage BF23. Genetics. 1971 Jul;68(3):323–340. doi: 10.1093/genetics/68.3.323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moyer R. W., Buchanan J. M. Effect of calcium ions on synthesis of T5-specific proteins. J Biol Chem. 1970 Nov 25;245(22):5897–5903. [PubMed] [Google Scholar]
- Moyer R. W., Fu A. S., Szabo C. Regulation of bacteriophage T5 development by ColI factors. J Virol. 1972 May;9(5):804–812. doi: 10.1128/jvi.9.5.804-812.1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pai S. H., Rahmsdorf H. J., Ponta H., Hirsch-Kauffmann M., Herrlich P., Schweiger M. Protein kinase of bacteriophage T7. 2. Properties, enzyme synthesis in vitro and regulation of enzyme synthesis and activity in vivo. Eur J Biochem. 1975 Jun 16;55(1):305–314. doi: 10.1111/j.1432-1033.1975.tb02164.x. [DOI] [PubMed] [Google Scholar]
- Rahmsdorf H. J., Pai S. H., Ponta H., Herrlich P., Roskoski R., Jr, Schweiger M., Studier F. W. Protein kinase induction in Escherichia coli by bacteriophage T7. Proc Natl Acad Sci U S A. 1974 Feb;71(2):586–589. doi: 10.1073/pnas.71.2.586. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sakaki Y. Inactivation of the ATP-dependent DNase of Escherichia coli after infection with double-stranded DNA phages. J Virol. 1974 Dec;14(6):1611–1612. doi: 10.1128/jvi.14.6.1611-1612.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Szabo C., Dharmgrongartama B., Moyer R. W. The regulation of transcription in bacteriophage T5-infected Escherichia coli. Biochemistry. 1975 Mar 11;14(5):989–997. doi: 10.1021/bi00676a018. [DOI] [PubMed] [Google Scholar]
- Warner H. R., Drong R. F., Berget S. M. Early events after infection of Escherichia coli by bacteriophage T5. Induction of a 5'-nucleotidase activity and excretion of free bases. J Virol. 1975 Feb;15(2):273–280. doi: 10.1128/jvi.15.2.273-280.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zweig M., Rosenkranz H. S., Morgan C. Development of coliphage T5: ultrastructural and biochemical studies. J Virol. 1972 Mar;9(3):526–543. doi: 10.1128/jvi.9.3.526-543.1972. [DOI] [PMC free article] [PubMed] [Google Scholar]



