Abstract
Study of the human lung microbiome in the context of pulmonary health and disease is an area of emerging research interest that is being driven by several contributing factors. These factors include increased recognition of the diversity of human-associated microbiota, their roles in health and in diseases associated with chronic inflammation, and advancements in technologies and tools that have facilitated such discoveries about the microbiota in organ systems outside of the lung. Therefore, the overarching goals of lung microbiome research are: to identify and characterize microbial populations associated with the respiratory tract and lungs; to understand their roles in lung health and disease; and, we hope, to allow the development of improved approaches for diagnosing and treating chronic respiratory diseases in which the microbiome has a role. Recent studies of the lung microbiome have yielded a number of interesting findings but also highlighted questions and challenges for researchers and clinicians. In December 2011, the National Heart, Lung, and Blood Institute convened a workshop to identify key issues and areas for further attention or development to advance research on the lung microbiome. Current knowledge and the state of research on the lung and related areas of human microbiome investigation were reviewed and discussed.
Keywords: microbiome, lung, gut
Knowledge about human-associated microbiota, defined as the microorganisms inhabiting specific organ and body system niches, has increased rapidly in recent years. Fueled by the development and application of more sensitive, culture-independent tools for detecting microbes, in particular bacteria, a multitude of studies examining the microbiota in specific body habitats have been performed (1–6). Recent investigations led by members of research consortiums, such as the European Metageno(Biol)mics of the Human Intestinal Tract (MetaHit) project (7) and the National Institutes of Health–sponsored Human Microbiome Project (8, 9), have contributed to our knowledge about the human microbiome. These studies have cataloged human microbiota associated with the skin, oropharynx, gastrointestinal tract, and vagina, and, more recently, the respiratory tract. They also have suggested that the presence or composition of microbiota in a given niche can be a determinant of whether site-specific inflammation or disease is present. The number of microbial cells in the human body exceeds the number of human cells by an order of magnitude (10), so that the estimated microbial gene pool far surpasses that of the host (11). Thus, understanding how the “microbiome”—defined as the totality of microbes, their genomic elements, and their interactions in a given environment—contributes to pathologic or nonpathologic states is of great interest.
In contrast to other organ systems, such as the gastrointestinal tract, investigation of the lung microbiome has emerged only fairly recently. This development represents an extension of longstanding interest in pulmonary disease research to understand potential links between specific microbial exposures and chronic respiratory disease. Few large-scale studies of the lung microbiome have been performed to date. In the Human Microbiome Project, sampling of the anterior nares represents the sole “airway” site, among the five body sites targeted for microbial sequencing (12). Objectives of the Lung HIV Microbiome Project (LHMP), which is sponsored by the National Heart, Lung, and Blood Institute, are to study the respiratory tract microbiome in HIV-infected as well as non–HIV-infected individuals (13). Other recent studies have described the compositions of airway or lung tissue–associated microbiota in healthy individuals and/or in those with existing obstructive lung diseases, such as asthma, chronic obstructive pulmonary disease (COPD), and cystic fibrosis (CF) (14–21). Moreover, correlations between clinical features of disease and characteristics of the lung microbial community have been observed (15, 19, 20, 22). Given the technologies currently available or on the horizon, and the knowledge being gleaned from other areas of human microbiome research, there is great potential to advance understanding of how our respiratory microbiota may contribute to lung disease or health.
The National Heart, Lung, and Blood Institute Division of Lung Diseases convened a workshop entitled “The Role of the Lung Microbiome in Health and Disease” on December 15 and 16, 2011; the objectives were to identify questions and challenges that need further attention to advance research on the lung microbiome. Participants included clinical investigators studying asthma, COPD, CF, and human HIV infection, as well as laboratory scientists conducting investigations of the microbiome and microbiota through use of the approaches of microbiology, genomics, and biostatistics. The workshop covered the following areas: (1) review of current studies of the lung microbiome in health and disease, (2) review of gut microbiome studies that might serve as templates for lung microbiome research, and (3) approaches and methodologies being applied in other microbiome disease research contexts.
Reviewed Topics on the Gut and Lung Microbiome
Portions of the workshop were dedicated to reviewing specific areas of research on the human microbiome, with a primary focus on gut and existing lung microbiome studies. It is important to recognize that studies of bacterial microbiota compose the vast majority of literature to date, whereas far less is known about potential fungal and viral members of the microbiome (23–25).
Gut Microbiome
Extensive literature exists on the human gut microbiome, and many reviews are available (26–31). Broadly, studies of the gut microbiome have encompassed characterization of differences in microbiota composition in states of health or disease, effects of diet or therapeutics on the gut microbiota (32), and metagenomics-based studies of the gut microbiome to investigate the pool of putative gene functions (11). Historically, among the earliest established and widely used culture-independent approaches to study bacterial communities in many environments are those based on analysis of the 16S ribosomal RNA (rRNA) biomarker gene. A conserved gene present in all bacteria and archaea species, 16S rRNA-based analyses enable phylogenetic classification of detected organisms based on polymorphisms in hypervariable sequence regions. Several large 16S rRNA sequence databases exist (e.g., Ribosomal Database Project; Greengenes) (33).
Through a different approach, metagenomics (34) is a way to characterize the pan-genomes present in a sample via shotgun sequencing of all DNA present and has more recently been applied to study the microbiome (11).
Commensal gut microbiota are essential for normal immune system development and contribute also to homeostatic and metabolic functions in the host (29). The indigenous gut microbiota also can serve to resist colonization against specific enteric pathogens (35). Perturbation of the gut microbiota, as occurs in mice that are germ-free or have been treated with systemic antibiotics, is associated with altered immune responses, decreased gut peristalsis, lower body temperature (36), altered sleep–wake cycle (37), changes in serum cholesterol (38), and increased susceptibility to infection. Thus, the gut microbiome exerts effects both locally and distally via mechanisms such as generated metabolites (38) or induced immunoregulatory responses. Feeding of select organisms to manipulate the composition of gut microbiota can lead to different immune responses, depending on the individual or group of organisms used (39). Administration of products derived from specific bacterial species, including those considered to be probiotic, can lead to an expansion in relative numbers of regulatory T cells, which in turn has been associated with attenuated airway inflammation and reduced airway hyperresponsiveness (40, 41).
Lung Microbiome
By contrast, literature on the respiratory microbiome in health and disease is much less extensive (42, 43). Relatively few studies applying contemporary techniques for microbial community profiling, such as next-generation sequencing or microarray platforms, exist on the microbiome of other respiratory compartments, such as the naso-oropharynx (44, 45) or lower airways.
In a study of six healthy individuals examined by detailed sampling of the upper and lower airways and through use of 16S rRNA-based pyrosequencing, the composition of lower airway microbiota appeared indistinguishable from that of the upper respiratory tract (supraglottic) samples (14). Bioburden as determined from 16S rRNA quantitative polymerase chain reaction (PCR) was observed to diminish along the tracheobronchial tree. Rare lung-specific bacterial sequences could be identified but were low-level and not broadly shared between individuals. In contrast, differences in lower airway microbiota composition have been noted between healthy subjects and those with obstructive lung diseases like asthma and COPD (17, 19). Given the anatomical continuity between the upper and lower respiratory tracts, obtaining lower respiratory samples for microbiome studies in living research subjects clearly presents challenges. Similar sampling concerns have been ascribed to analysis of stool specimens for inferring variability in microbiome biology from different sections of the lower GI tract.
Recent studies of the lung microbiome have revealed other findings as well. Variation within the bronchial tree in lung microbiota composition has been described among subjects with asthma, COPD, and CF (15, 17, 46), including heterogeneity in microbiota composition within the same lobe of the lung examined in COPD lung explants (15). In addition, both microbial community diversity and distinct community compositions have been correlated with features of clinical disease, such as airway hyperresponsiveness in asthma (19) or the severity of airflow obstruction in COPD or CF (15, 20–22).
A recent study of human lung transplant recipients identified markedly higher bacterial burden in lung allografts compared with nontransplant control lungs (4). Both community structure and the types and relative proportions of bacteria differed from healthy subjects. Many transplant recipients showed outgrowth in lungs of specific bacterial lineages, often including anaerobic lineages not sampled by traditional respiratory culture. Long-term lung transplant failure has been linked in part to microbial factors, and whether aspects of community structure or specific bacteria identifiable by culture-independent methods are associated with transplant outcome is currently not known. This study also suggested that unbiased comprehensive microbiome approaches could add useful information to traditional culture in assessing lung infection.
It is important to note that different 16S rRNA-based techniques have been applied to study bacterial microbiota composition in current lung microbiome studies. These include platforms such as terminal restriction fragment length polymorphism analysis, traditional 16S rRNA clone library generation with Sanger sequencing, 454-pyrosequencing, and phylogenetic microarrays. In addition to the intrinsic differences that exist between platforms, how samples are prepared for analysis, including extraction, primers used for PCR amplification steps, and the depth of sequencing, all can influence conclusions about the community members present.
Finally, most attention so far has been directed to bacterial populations in the respiratory tract, but similar approaches can be applied to fungi using the 18S rRNA gene or internal transcribed spacer sequences. Although fungal microbiome methods and databases are considerably less developed than those for bacteria, studies have recently begun to apply fungal microbiome analysis to the upper respiratory tract and to the lungs (23, 47).
Key Questions and Challenges
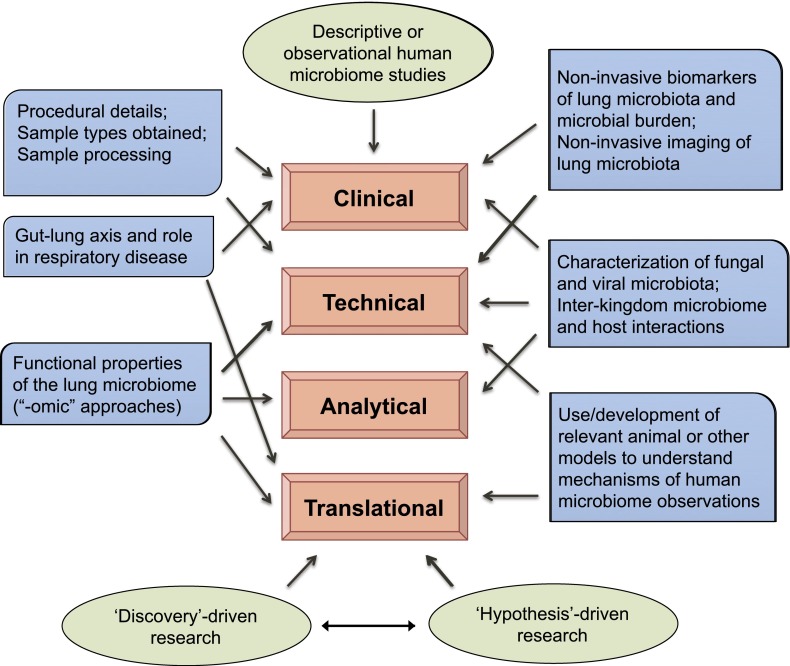
Given the nascency of lung microbiome research, there are many questions and challenges. Some of these overlap with ones encountered and already recognized in other areas of human microbiome research; others are unique to studying the lung microbiome. These issues may be organized into the following three areas: clinical, technological and analytical, and translational (Figure 1).
Figure 1.
Summary of workshop recommendations for future lung microbiome research. Recommendations, as discussed in the article, are shown in blue boxes. Areas into which the recommendations may be categorized are indicated (arrows). In addition, conceptual frameworks viewed as important considerations in approaching lung microbiome research are shown (green).
Clinical
Several fundamental questions about the lung microbiome remain unanswered. These include whether a core lung microbiome exists, either across all individuals or among individuals defined by the presence or absence of specific diseases. Because bacteria can be detected in the lungs by 16S rRNA-based approaches, even in healthy individuals, whether the microbial community is stable, transient, or subject to frequent turnover at some level is unclear. Moreover, sampling the lower airways and lung tissue in living research subjects presents considerations unique to lung microbiome research, because the upper airways must be traversed to obtain samples. Concerns about contamination by microbiota traditionally associated with the upper respiratory tract are important. Yet finding such microbiota in lower lung specimens alternatively could imply a lower burden of true colonization.
Many additional, clinically driven questions of interest encompass the nature and role of the lung microbiome in disease. Prior evidence of pathogenic roles for specific microbial infections is strongest in diseases such as CF, COPD, and asthma. However, how the microbial milieu potentially modulates pathogenicity of specific species is an active area of investigation (48, 49). Furthermore, how described heterogeneity in microbial community composition in the lungs relates to clinical manifestations of disease is unclear. Conversely, there is heterogeneity in where the lung tends to be affected by disease, and how this interfaces with the local microbiome present is unknown. Lung transplantation is another clinical setting in which microbial factors are believed to play a central role in long-term outcome, and studies to address microbiome community effects on transplant outcome have only just begun (47).
A distinct but also clinically relevant question is whether microbiome techniques, by their minimally biased, comprehensive, and quantitative nature, might enhance understanding and diagnosing specific infections in the lower respiratory tract. Similarly, studies are just beginning to explore the usefulness of these approaches to investigations into the etiology or progression of lung diseases such as sarcoidosis and α1 antitrypsin deficiency (www.gradslung.org [50]).
Technological and Analytical
In addition to technical considerations in obtaining human specimens for study that accurately represent the intended source, such as the lung, additional issues arise in specimen processing and in the generation and analysis of complex datasets. Our capacity now to detect bacteria by culture-independent approaches is unprecedented in scale. However, given the sensitivity of tools such as next-generation sequencing platforms and microarrays, attention to how data are obtained is warranted. At the proximal end of the process, unintentional introduction of microorganisms from environmental sources can lead to their identification by these technologies. As inhalation of organisms in the air is an obvious route of entry, distinguishing contamination from what is truly resident in the respiratory tract is potentially challenging. Thus, controlling for potential sources, such as reagents used to obtain and process samples, is an important consideration.
Biases that could be introduced in the process of attempting to characterize microbiota composition are also important to note. For example, some types of organisms are more difficult to lyse and extract their nucleic acid, such as gram-positive bacteria and fungi, so extraction protocols should be sound. Subsequent to this, amplification by PCR is a common step in current techniques to profile bacterial communities. In sequencing experiments, primers targeting one of the variable regions of the 16S rRNA gene are used. Depending on the variable region targeted, certain bacterial groups or species may not be well captured (51). Detection of fungal microbiota can be done using 18S rRNA gene and internal transcribed spacer sequencing, whereas identification of viral members of the microbiome requires approaches other than marker-gene–directed PCR analysis.
The amount of data generated from high-throughput sequencing methods presents formidable challenges in analysis. Datasets from microarray-based platforms are generally more manageable. Normalization of data and approaches for handling data noise, artifacts, and errors in reads are all important issues that require robust bioinformatics to ensure the most accurate conclusions. Depth of sequencing also can influence conclusions about the composition of the microbial community in a sample. The lower the number of reads on a per-sample basis, the less resolution achieved in identifying members of the microbiota, especially if communities are dominated by one or a few types of organisms.
The necessary bioinformatics and analytic support required for performing microbiome studies are a major challenge, coupled with attendant risks of obtaining potentially biased information for the reasons discussed. Moreover, there currently are no robust ways of estimating power and significance calculation for purposes of study planning, given the high-dimensional nature of data generated. Collectively, all of the above issues factor additionally into deciding how many subjects or samples can feasibly be studied well in a given study.
Translational
Ultimately, translating findings about the composition of lung microbiota into knowledge about their behavior and role in health or disease will require additional approaches. Determining how to cultivate as yet uncultured organisms would facilitate further characterization about the genetics and function of specific bacterial species of interest. This also would enable evaluation in vivo of whether an individual species or a collection of species in the microbiota community are most relevant. Metagenomics enables obtaining information on all the genomes present such that gene functions can be predicted. However, this requires adequate reference databases, which for some organisms are sparse. Metatranscriptomics aims to characterize the collective functional gene expression profiles present, including microbial and host. However, intrinsic challenges include the preponderance of rRNA that composes total RNA and the lack of distinguishing features of mature mRNA in prokaryotes to facilitate their isolation for analysis (in contrast to the polyadenylation present on the 3′ end of eukaryotic mRNA). These are active areas of methodological research. Metabolomics represents another potentially useful approach to examine phenotypic and organism–host environment relationships. For example, specific small-molecule metabolites, which require gut microbiota for their generation, have been mechanistically linked to cardiovascular disease (52).
Finally, linking knowledge gained about the lung microbiota to downstream outcomes on host disease or health requires a potential shift in the usual view of approaching translational research. Traditionally, this has been a linear view from basic science, hypothesis-driven research to translational experiments and subsequently clinical investigations. In the current era of microbiome studies, these links are more bidirectional between observational findings from surveying the microbiome and hypothesis-driven basic science. For instance, hypotheses may be generated from findings about the microbiome in clinical research studies and then tested in animal models.
Recommendations
From the workshop, a number of issues were identified as most important to address for the future of lung microbiome research (Figure 1). These are summarized as follows:
-
1.
Challenges in lung sample collection, and recognition of the potential for upper respiratory tract carryover as well as environmental sources of admixture. Given the great sensitivity of newer molecular tools for microbiota profiling, findings are subject to potential confounding from representation of organisms not necessarily derived from the lung or lower airways. These issues are important to consider when designing and performing lung microbiome studies.
-
2.
Observational studies are necessary to continue to build knowledge and reference about the types, abundance, and distributions of microbial populations throughout the human respiratory tract. Such studies are important for hypothesis generation and for subsequent controlled experiments or clinical intervention studies. The resultant data also can be used to perform mechanistic experiments to determine why observed clinical associations exist. Focus on a few targeted disease populations in which viral, bacterial, and/or fungal organisms are likely to play a role may initially be most useful. In this context, high-quality molecular datasets of the microbiome could be obtained from leveraging existing disease study cohorts rather than development of new study cohorts.
-
3.
Investigation of viral and fungal microbial communities in the lungs, including their characterization and potential interactions with other members of the microbiome and host. Most of the focus in studies to date has been on bacterial microbiota. Because specific viral and fungal species are known to cause respiratory disease, further knowledge of the viral and fungal microbiota is needed and may yield important insights.
-
4.
Development of noninvasive biomarkers of lung microbial populations. Useful methodologies would include ways to study lung microbial burden and measure relevant biomarkers of the microbiome in airway samples collected in a minimally invasive manner.
-
5.
Development of noninvasive imaging techniques, new or adapted from existing ones that might provide reliable spatial maps of the lung microbiome. In addition, techniques that could characterize the metabolic state of existing microorganisms in the lung microbiome would be useful.
-
6.
Investigations of functional properties of the lung microbiome to better define consequences of perturbation of microbial communities and understand what constitutes homeostasis or lack thereof. This might include transcriptomic, metabolomic, or proteomic analysis of microbial populations, in addition to comprehensive identification, and could be accomplished through integration with genomic and other “-omic” datasets in healthy and disease states.
-
7.
Research on the gut–lung axis and the potential role that gut microbial populations might play in the development of respiratory disease. This might include metabolic disturbances or autoimmune mechanisms that affect airway health.
Effective use of animal studies and other models to further develop mechanistic understanding of respiratory microbiome–host interactions suggested by descriptive human studies.
Acknowledgments
Acknowledgment
Participants: James Beck, M.D. (University of Michigan); Homer A. Boushey, M.D. (University of California San Francisco); Frederic Bushman, Ph.D. (University of Pennsylvania); Emily Charlson, M.D., Ph.D candidate (University of Pennsylvania); Ronald Collman, M.D. (University of Pennsylvania); Rima Kaddurah-Daouk, Ph.D. (Duke University Medical Center); Garth D. Ehrlich, Ph.D. (Allegheny-Singer Research Institute); Patricia Finn, M.D. (University of California San Diego); Sonia Castro Flores, M.D. (University of Colorado Denver); Andrew Fontenot, M.D. (University of Colorado); Mary Foulkes, Ph.D. (George Washington University); Elodie Ghedin, Ph.D. (University of Pittsburgh); Danial Haft, Ph.D. (J. Craig Venter Institute); Laurence Huang, M.D. (University of California San Francisco); Yvonne J. Huang, M.D. (University of California San Francisco); Gary Huffnagle, Ph.D. (University of Michigan); Kathleen Jablonski, Ph.D. (George Washington University); John Lipuma, M.D. (University of Michigan); Susan Lynch, Ph.D. (University of California San Francisco); Fernando D. Martinez, M.D. (University of Arizona); Fernando J. Martinez, M.D. (University of Michigan); Alison Morris, M.D., M.S. (University of Pittsburgh); Rubin Tuder, M.D. (University of Colorado Denver); Robert Senior, M.D. (Washington University); Homer Twigg III, M.D. (Indiana University); George Weinstock, Ph.D. (Washington University); Scott Weiss, M.D., M.S. (Brigham and Women’s Hospital); Vincent Young, M.D. (University of Michigan); Stanley Hazen, Ph.D. (Cleveland Clinic).
Footnotes
Originally Published in Press as DOI: 10.1164/rccm.201303-0488WS on April 24, 2013
Author disclosures are available with the text of this article at www.atsjournals.org.
References
- 1.Brotman RM. Vaginal microbiome and sexually transmitted infections: an epidemiologic perspective. J Clin Invest. 2011;121:4610–4617. doi: 10.1172/JCI57172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Diaz PI. Microbial diversity and interactions in subgingival biofilm communities. Front Oral Biol. 2012;15:17–40. doi: 10.1159/000329669. [DOI] [PubMed] [Google Scholar]
- 3.Eckburg PB, Bik EM, Bernstein CN, Purdom E, Dethlefsen L, Sargent M, Gill SR, Nelson KE, Relman DA. Diversity of the human intestinal microbial flora. Science. 2005;308:1635–1638. doi: 10.1126/science.1110591. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Grice EA, Kong HH, Conlan S, Deming CB, Davis J, Young AC, Bouffard GG, Blakesley RW, Murray PR, Green ED, et al. NISC Comparative Sequencing Program. Topographical and temporal diversity of the human skin microbiome. Science. 2009;324:1190–1192. doi: 10.1126/science.1171700. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Wade WG. Has the use of molecular methods for the characterization of the human oral microbiome changed our understanding of the role of bacteria in the pathogenesis of periodontal disease? J Clin Periodontol. 2011;38:7–16. doi: 10.1111/j.1600-051X.2010.01679.x. [DOI] [PubMed] [Google Scholar]
- 6.Nelson KE, Weinstock GM, Highlander SK, Worley KC, Creasy HH, Wortman JR, Rusch DB, Mitreva M, Sodergren E, Chinwalla AT, et al. Human Microbiome Jumpstart Reference Strains Consortium. A catalog of reference genomes from the human microbiome. Science. 2010;328:994–999. doi: 10.1126/science.1183605. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Telle O, Winogradsky Y.editors. MetaHIT. INRA-MICA Division [accessed 2013 Jan]. Available from: http://www.metahit.eu/
- 8.Proctor LM. The Human Microbiome Project in 2011 and beyond. Cell Host Microbe. 2011;10:287–291. doi: 10.1016/j.chom.2011.10.001. [DOI] [PubMed] [Google Scholar]
- 9.National Institutes of HealthHuman Microbiome Project. The NIH Common Fund. Division of Program Coordination, Planning, and Strategic Initiatives. Available from: http://commonfund.nih.gov/hmp
- 10.Savage DC. Microbial ecology of the gastrointestinal tract. Annu Rev Microbiol. 1977;31:107–133. doi: 10.1146/annurev.mi.31.100177.000543. [DOI] [PubMed] [Google Scholar]
- 11.Qin J, Li R, Raes J, Arumugam M, Burgdorf KS, Manichanh C, Nielsen T, Pons N, Levenez F, Yamada T, et al. MetaHIT Consortium. A human gut microbial gene catalogue established by metagenomic sequencing. Nature. 2010;464:59–65. doi: 10.1038/nature08821. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.NIHHuman Microbiome Project. Data Analysis and Coordination Center [accessed 2013 Jan]. Available from: http://www.hmpdacc.org/
- 13.Lung HIV Microbiome ProjectGeorge Washington University Biostatistics Center [accessed 2013 Jan]. Available from: https://lhmp.bsc.gwu.edu/
- 14.Charlson ES, Bittinger K, Haas AR, Fitzgerald AS, Frank I, Yadav A, Bushman FD, Collman RG. Topographical continuity of bacterial populations in the healthy human respiratory tract. Am J Respir Crit Care Med. 2011;184:957–963. doi: 10.1164/rccm.201104-0655OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Erb-Downward JR, Thompson DL, Han MK, Freeman CM, McCloskey L, Schmidt LA, Young VB, Toews GB, Curtis JL, Sundaram B, et al. Analysis of the lung microbiome in the “healthy” smoker and in COPD. PLoS ONE. 2011;6:e16384. doi: 10.1371/journal.pone.0016384. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Harris JK, De Groote MA, Sagel SD, Zemanick ET, Kapsner R, Penvari C, Kaess H, Deterding RR, Accurso FJ, Pace NR. Molecular identification of bacteria in bronchoalveolar lavage fluid from children with cystic fibrosis. Proc Natl Acad Sci USA. 2007;104:20529–20533. doi: 10.1073/pnas.0709804104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Hilty M, Burke C, Pedro H, Cardenas P, Bush A, Bossley C, Davies J, Ervine A, Poulter L, Pachter L, et al. Disordered microbial communities in asthmatic airways. PLoS ONE. 2010;5:e8578. doi: 10.1371/journal.pone.0008578. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Huang YJ, Kim E, Cox MJ, Brodie EL, Brown R, Wiener-Kronish JP, Lynch SV. A persistent and diverse airway microbiota present during chronic obstructive pulmonary disease exacerbations. OMICS. 2010;14:9–59. doi: 10.1089/omi.2009.0100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Huang YJ, Nelson CE, Brodie EL, Desantis TZ, Baek MS, Liu J, Woyke T, Allgaier M, Bristow J, Wiener-Kronish JP, et al. National Heart, Lung, and Blood Institute’s Asthma Clinical Research Network Airway microbiota and bronchial hyperresponsiveness in patients with suboptimally controlled asthma J Allergy Clin Immunol 2011127372–381.e1–e3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Zhao J, Schloss PD, Kalikin LM, Carmody LA, Foster BK, Petrosino JF, Cavalcoli JD, VanDevanter DR, Murray S, Li JZ, et al. Decade-long bacterial community dynamics in cystic fibrosis airways. Proc Natl Acad Sci USA. 2012;109:5809–5814. doi: 10.1073/pnas.1120577109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Sze MA, Dimitriu PA, Hayashi S, Elliott WM, McDonough JE, Gosselink JV, Cooper J, Sin DD, Mohn WW, Hogg JC. The lung tissue microbiome in chronic obstructive pulmonary disease. Am J Respir Crit Care Med. 2012;185:1073–1080. doi: 10.1164/rccm.201111-2075OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Cox MJ, Allgaier M, Taylor B, Baek MS, Huang YJ, Daly RA, Karaoz U, Andersen GL, Brown R, Fujimura KE, et al. Airway microbiota and pathogen abundance in age-stratified cystic fibrosis patients. PLoS ONE. 2010;5:e11044. doi: 10.1371/journal.pone.0011044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Ghannoum MA, Jurevic RJ, Mukherjee PK, Cui F, Sikaroodi M, Naqvi A, Gillevet PM. Characterization of the oral fungal microbiome (mycobiome) in healthy individuals. PLoS Pathog. 2010;6:e1000713. doi: 10.1371/journal.ppat.1000713. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Kistler A, Avila PC, Rouskin S, Wang D, Ward T, Yagi S, Schnurr D, Ganem D, DeRisi JL, Boushey HA. Pan-viral screening of respiratory tract infections in adults with and without asthma reveals unexpected human coronavirus and human rhinovirus diversity. J Infect Dis. 2007;196:817–825. doi: 10.1086/520816. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Reyes A, Haynes M, Hanson N, Angly FE, Heath AC, Rohwer F, Gordon JI. Viruses in the faecal microbiota of monozygotic twins and their mothers. Nature. 2010;466:334–338. doi: 10.1038/nature09199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Holmes E, Li JV, Athanasiou T, Ashrafian H, Nicholson JK. Understanding the role of gut microbiome-host metabolic signal disruption in health and disease. Trends Microbiol. 2011;19:349–359. doi: 10.1016/j.tim.2011.05.006. [DOI] [PubMed] [Google Scholar]
- 27.Ly NP, Litonjua A, Gold DR, Celedón JC.Gut microbiota, probiotics, and vitamin D: interrelated exposures influencing allergy, asthma, and obesity? J Allergy Clin Immunol 20111271087–1094.quiz 1095–1096 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Nagalingam NA, Kao JY, Young VB. Microbial ecology of the murine gut associated with the development of dextran sodium sulfate-induced colitis. Inflamm Bowel Dis. 2011;17:917–926. doi: 10.1002/ibd.21462. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Round JL, Mazmanian SK. The gut microbiota shapes intestinal immune responses during health and disease. Nat Rev Immunol. 2009;9:313–323. doi: 10.1038/nri2515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Stecher B, Hardt WD. Mechanisms controlling pathogen colonization of the gut. Curr Opin Microbiol. 2011;14:82–91. doi: 10.1016/j.mib.2010.10.003. [DOI] [PubMed] [Google Scholar]
- 31.Tilg H, Kaser A. Gut microbiome, obesity, and metabolic dysfunction. J Clin Invest. 2011;121:2126–2132. doi: 10.1172/JCI58109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Wu GD, Chen J, Hoffmann C, Bittinger K, Chen YY, Keilbaugh SA, Bewtra M, Knights D, Walters WA, Knight R, et al. Linking long-term dietary patterns with gut microbial enterotypes. Science. 2011;334:105–108. doi: 10.1126/science.1208344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.McDonald D, Price MN, Goodrich J, Nawrocki EP, DeSantis TZ, Probst A, Andersen GL, Knight R, Hugenholtz P. An improved Greengenes taxonomy with explicit ranks for ecological and evolutionary analyses of bacteria and archaea. ISME J. 2012;6:610–618. doi: 10.1038/ismej.2011.139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Wooley JC, Godzik A, Friedberg I. A primer on metagenomics. PLOS Comput Biol. 2010;6:e1000667. doi: 10.1371/journal.pcbi.1000667. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Reeves AE, Theriot CM, Bergin IL, Huffnagle GB, Schloss PD, Young VB. The interplay between microbiome dynamics and pathogen dynamics in a murine model of Clostridium difficile Infection. Gut Microbes. 2011;2:145–158. doi: 10.4161/gmic.2.3.16333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Yi P, Li L. The germfree murine animal: an important animal model for research on the relationship between gut microbiota and the host. Vet Microbiol. 2012;157:1–7. doi: 10.1016/j.vetmic.2011.10.024. [DOI] [PubMed] [Google Scholar]
- 37.Gonzalez A, Stombaugh J, Lozupone C, Turnbaugh PJ, Gordon JI, Knight R. The mind-body-microbial continuum. Dialogues Clin Neurosci. 2011;13:55–62. doi: 10.31887/DCNS.2011.13.1/agonzalez. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Koren O, Spor A, Felin J, Fåk F, Stombaugh J, Tremaroli V, Behre CJ, Knight R, Fagerberg B, Ley RE, et al. Human oral, gut, and plaque microbiota in patients with atherosclerosis. Proc Natl Acad Sci USA. 2011;108:4592–4598. doi: 10.1073/pnas.1011383107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Kwon HK, Lee CG, So JS, Chae CS, Hwang JS, Sahoo A, Nam JH, Rhee JH, Hwang KC, Im SH. Generation of regulatory dendritic cells and CD4+Foxp3+ T cells by probiotics administration suppresses immune disorders. Proc Natl Acad Sci USA. 2010;107:2159–2164. doi: 10.1073/pnas.0904055107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Karimi K, Inman MD, Bienenstock J, Forsythe P. Lactobacillus reuteri-induced regulatory T cells protect against an allergic airway response in mice. Am J Respir Crit Care Med. 2009;179:186–193. doi: 10.1164/rccm.200806-951OC. [DOI] [PubMed] [Google Scholar]
- 41.Strickland DH, Thomas JA, Mok D, Blank F, McKenna KL, Larcombe AN, Sly PD, Holt PG. Defective aeroallergen surveillance by airway mucosal dendritic cells as a determinant of risk for persistent airways hyper-responsiveness in experimental asthma. Mucosal Immunol. 2012;5:332–341. doi: 10.1038/mi.2012.13. [DOI] [PubMed] [Google Scholar]
- 42.Huang YJ, Lynch SV. The emerging relationship between the airway microbiota and chronic respiratory disease: clinical implications. Expert Rev Respir Med. 2011;5:809–821. doi: 10.1586/ers.11.76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Han MK, Huang YJ, Lipuma JJ, Boushey HA, Boucher RC, Cookson WO, Curtis JL, Erb-Downward J, Lynch SV, Sethi S, et al. Significance of the microbiome in obstructive lung disease. Thorax. 2012;67:456–463. doi: 10.1136/thoraxjnl-2011-201183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Charlson ES, Chen J, Custers-Allen R, Bittinger K, Li H, Sinha R, Hwang J, Bushman FD, Collman RG. Disordered microbial communities in the upper respiratory tract of cigarette smokers. PLoS ONE. 2010;5:e15216. doi: 10.1371/journal.pone.0015216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Lemon KP, Klepac-Ceraj V, Shiffer HK, Brodie EL, Lynch SV, Kolter R. Comparative analyses of the bacterial microbiota of the human nostril and oropharynx. MBio. 2010;1:e00129–10. doi: 10.1128/mBio.00129-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Willner D, Haynes MR, Furlan M, Schmieder R, Lim YW, Rainey PB, Rohwer F, Conrad D. Spatial distribution of microbial communities in the cystic fibrosis lung. ISME J. 2012;6:471–474. doi: 10.1038/ismej.2011.104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Charlson ES, Diamond JM, Bittinger K, Fitzgerald AS, Yadav A, Haas AR, Bushman FD, Collman RG. Lung-enriched organisms and aberrant bacterial and fungal respiratory microbiota after lung transplant. Am J Respir Crit Care Med. 2012;186:536–545. doi: 10.1164/rccm.201204-0693OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Ichinohe T, Pang IK, Kumamoto Y, Peaper DR, Ho JH, Murray TS, Iwasaki A. Microbiota regulates immune defense against respiratory tract influenza A virus infection. Proc Natl Acad Sci USA. 2011;108:5354–5359. doi: 10.1073/pnas.1019378108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Sibley CD, Duan K, Fischer C, Parkins MD, Storey DG, Rabin HR, Surette MG. Discerning the complexity of community interactions using a Drosophila model of polymicrobial infections. PLoS Pathog. 2008;4:e1000184. doi: 10.1371/journal.ppat.1000184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.GRADSGenomic Research in Alpha-1 Antitrypsin Deficiency and Sarcoidosis. Epidemology Data Center [accessed 2013 Jan]. Available from: www.gradslung.org/
- 51.Chakravorty S, Helb D, Burday M, Connell N, Alland D. A detailed analysis of 16S ribosomal RNA gene segments for the diagnosis of pathogenic bacteria. J Microbiol Methods. 2007;69:330–339. doi: 10.1016/j.mimet.2007.02.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Wang Z, Klipfell E, Bennett BJ, Koeth R, Levison BS, Dugar B, Feldstein AE, Britt EB, Fu X, Chung YM, et al. Gut flora metabolism of phosphatidylcholine promotes cardiovascular disease. Nature. 2011;472:57–63. doi: 10.1038/nature09922. [DOI] [PMC free article] [PubMed] [Google Scholar]