Abstract
We have previously demonstrated in human subjects who under euglycemic clamp conditions GLP-1(9–36)amide infusions inhibit endogenous glucose production without substantial insulinotropic effects. An earlier report indicates that GLP-1(9–36)amide is cleaved to a nonapeptide, GLP-1(28–36)amide and a pentapeptide GLP-1(32–36)amide (LVKGR amide). Here we study the effects of the pentapeptide on whole body glucose disposal during hyperglycemic clamp studies. Five dogs underwent indwelling catheterizations. Following recovery, the dogs underwent a 180 min hyperglycemic clamp (basal glucose +98 mg/dl) in a cross-over design. Saline or pentapeptide (30 pmol kg−1 min−1) was infused during the last 120 min after commencement of the hyperglycemic clamp in a primed continuous manner. During the last 30 min of the pentapeptide infusion, glucose utilization (M) significantly increased to 21.4 ± 2.9 mg kg−1 min−1 compared to M of 14.3 ± 1.1 mg kg−1 min−1 during the saline infusion (P = 0.026, paired t-test; P = 0.062, Mann–Whitney U test). During this interval, no significant differences in insulin (26.6 ± 3.2 vs. 23.7 ± 2.5 µU/ml, P = NS) or glucagon secretion (34.0 ± 2.1 vs. 31.7 ± 1.8 pg/ml, P = NS) were observed. These findings demonstrate that under hyperglycemic clamp studies the pentapeptide modulates glucose metabolism by a stimulation of whole-body glucose disposal. Further, the findings suggest that the metabolic benefits previously observed during GLP-1(9–36)amide infusions in humans might be due, at least in part, to the metabolic effects of the pentapeptide that is cleaved from the pro-peptide, GLP-1(9–36)amide in the circulation.
Keywords: Glucagon-like peptide-1, Diabetes, Obesity, Insulin resistance, Glucose utilization
Introduction
The most potent naturally occurring glucoincretin hormone is GLP-1(7–36)amide. The insulinotropic action of GLP-1(7–36)amide is operative not only in states of glucose tolerance, but also in states of glucose intolerance [13]. This circumstance has led to the development of several GLP-1 mimetic drugs, which have been approved by the FDA and are currently in use for the treatment of type 2 diabetes [7]. A major action of native GLP-1 peptides is the stimulation of glucose-dependent insulin secretion [13]. However, in addition, GLP-1(7–36)amide, or its metabolites, has extrapancreatic effects on many organ systems, including cardiovascular, central nervous, and gastrointestinal. GLP-1(7–36)amide exerts cytoprotective, anti-oxidative, and hemodynamic actions, inhibits gastric emptying, and promotes satiety [1,3,9,15].
GLP-1(7–36)amide is rapidly cleaved by the ubiquitous enzyme diaminopeptidyl peptidase-4 (DPP-4) resulting in the formation of GLP-1(9–36)amide [13,14]. GLP-1(9–36)amide is generally regarded as an inactive metabolite with little or no insulinotropic properties. However, more recently it has been demonstrated that GLP-1(9–36)amide has insulinomimetic actions in many of the systems referred to above [1,4,5,8,19,25,27,28]. These insulin-like insulinomimetic actions occur without significant changes in plasma insulin levels. More important, these effects are more potent in states of “intolerance” such as obesity, insulin resistance, and cardiovascular and cerebrovascular disease [3,8,22]. Thus, the insulinomimetic action(s) appear to improve glucose homeostasis and glucose utilization in glucose intolerant states in which “normal” function is impaired.
GLP-1(7–36)amide is not only cleaved by DPP-4, but also by another ubiquitous endopeptidase, the neutral endopeptidase NEP 24.11 (neprilysin) forming a nonapeptide GLP-1(28–36)amide (FIAWLVKGRamide(and a pentapeptide GLP-1(32–36)amide (LVKGRamide) [11,26,31]. The nonapeptide has several biologic properties such as suppression of glucose production [12,29] and oxidative stress [29] in isolated mouse hepatocytes, curtailment of weight gain in diet-induced obese mice [30], and increased glucose disposal in streptozotocin-induced diabetic mice. The nonapeptide also protects beta cells against glucolipotoxic oxidative stress [17] as well as increases pancreatic beta cell mass and proliferation in mice [24]. Our aim in the present study was to examine the acute insulinomimetic properties of the pentapeptide LVKGRamide, in conscious unstrained dogs. We performed 3-h hyperglycemic clamps with the infusion of GLP-1(32–36)amide during the last 2 h of the clamp. We report that during the last 30 min of the clamp, glucose utilization was significantly increased when GLP-1(32–36)amide was infused in comparison to cross-over clamps when only the vehicle was infused. The increase in glucose utilization occurred without significant changes in plasma insulin, C-peptide or glucagon levels. These findings raise the possibility that by increasing glucose disposal and lowering plasma glucose levels GLP-1(32–36)amide might be useful for the treatment of type 2 diabetes.
Materials and methods
Animals
Ten hyperglycemic clamps were conducted in five conscious adult mongrel dogs (4F; 12.8 ± 1.1 kg; (X ± SE); range: 10.1–15.9 kg) in a randomized crossover design. Their age ranged from 2 to 6 years (3.2 ± 0.7 years). The animals were fed Purina Dog Chow (300 g/day) along with 450 g/day of canned meal. All procedures were performed in accordance with NIH protocols for humane use of experimental animals and approved by the institutional animal care and use committee of the University of Pennsylvania.
Hyperglycemic clamp studies
The dogs had an indwelling polyethylene catheter in the aorta and right atria, inserted at least two weeks before conducting the first study. The dogs were trained to stand quietly in a sling, which supported their weight. The two hyperglycemic clamps, one with infusion of the peptide and the other with infusion of vehicle, were conducted at least one week apart. The dogs were fasted for 10–12 h prior to the start of the clamp studies. With each clamp, the dogs were placed in the harness, and approximately 30 min later four basal blood samples were obtained at 10-min intervals. Then, the hyperglycemic clamp was started, which rapidly created a square wave of hyperglycemia, 98 mg/dl above the basal glucose level with a falling prime infusion of 20% glucose as previously described by Andres [2]. Body surface area was calculated as SA = Ht(m) × Wt(kg)0.367 × 0.282 [20]. The stable hyperglycemic level was maintained for 180 min. At 60 min a falling primed dose (10 min, with change every 2 min; 118, 51, 47, 44, 40, 30 pmol kg−1 min−1) constant (30 pmol kg−1 min−1) of the pentapeptide, LVKGRamide, or saline was started and continued for 120 min (until 180 min). This falling primed-continuous infusion rate of peptide has been used previously with the administration of GLP-1(7–36)amide and GLP-1(9–36)amide; a square wave of GLP-1 is created with this mode of infusion [8]. At 180 min both 20% glucose and GLP-1(32–36)amide or saline were discontinued and the fall in plasma glucose was followed for the next 30 min. GLP-1(32–36)amide was synthesized by the Protein/Peptide Core Facility at Massachusetts General Hospital. The peptide was 99% pure, displayed a single peak on high performance liquid chromatography and mass spectrometry analysis, with a peptide content of 85%.
During each GLP-1(32–36)amide clamp, the peptide was diluted with normal saline to a total volume of 50 ml, which contained 2 ml of the dogs’blood. During the “control” clamp a 50 ml saline solution containing 2 ml of the dogs’blood was prepared (without any peptide) and infused in the same primed continuous manner as when the infusate contained GLP-1(32–36)amide. During each clamp blood samples were obtained every 5 min for determination of plasma glucose and every 2 min for the first 10 min and then every 10 min thereafter until 210 min for determination of plasma insulin, and C-peptide and every 10 min for the determination of glucagon concentrations.
Assays of blood samples
Blood samples were collected using heparinized syringes. Plasma glucose was immediately assayed using the glucose oxidase method (Stat Strip, Nova Biomedical, Waltham, MA) as previously described [21]. The remaining blood samples were placed in pre-chilled test tubes containing peptide inhibitors, centrifuged at 4 degrees, aliquoted in small tubes, and stored at −70 degrees for subsequent determination of plasma insulin, C-peptide, and glucagon levels [16].
Statistical analyses
Standard methods were used to compute means and SEM. Glucose infusion rates were calculated for each 2-min interval from 0 to 10 min and at 5-min intervals thereafter until 180 min. Glucose utilization rates, M, were calculated from the glucose infusion rate, corrected for the volume component and metabolic component (but not urine spillage), and hormone responses were calculated for each 30-min interval by area under the curve (AUC) from 0 to 210 min using the trapezoidal rule. The integrated rates and hormone levels were divided by the time interval, which resulted in mean rate or concentration. The Mann–Whitney U test, Paired t-test, and ANOVA with Tukey’spost hoc test was used to evaluate changes between the two clamps. All statistical tests were two tailed and when a statistical P value as observed with the paired-t test a U test P value is also provided.
Results
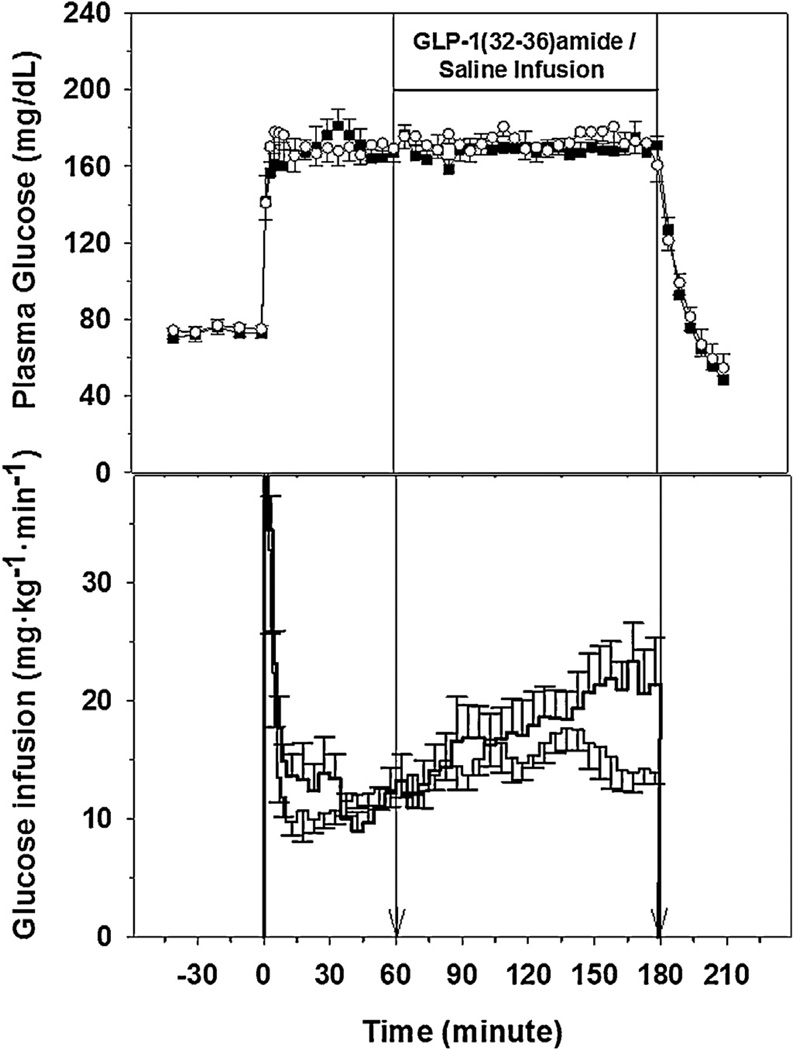
Basal plasma glucose levels for the five dogs were similar during the GLP-1(32–36)amide and saline infusion studies (72.8 ± 4.2 vs. 74.9 ± 4.6 mg/dl). Plasma glucose levels were rapidly raised by 98 mg/dl in both studies, held stable, and the plasma glucose profiles during the 180 min hyperglycemic clamp studies were nearly identical (Fig. 1). The 0–180 min average plasma glucose levels for the GLP-1(32–36)amide and saline studies were 167.8 ± 4.1 and 171.1 ± 4.7 mg/dl, which represented 98.9 ± 0.2 and 99.3 ± 0.4 of the desired goal. The corresponding coefficient of variances (CVs) for the two studies were 4.1 ± 0.7 and 4.5 ± 1.0%. At 180 min both the 20% glucose and GLP-1(32–36)amide or saline infusions were terminated and plasma glucose levels fell rapidly, reaching a level of 48.5 ± 13.1 and 54.6 ± 4.1 mg/dl at 210 min. The slope of the decay or t1/2 was nearly identical 5.8 ± 0.9 vs. 4.5 ± 0.6 per cent per min (p = 0.25) and 13.8 ± 3.0 and 16.2 ± 1.8 min (p = 0.48).
Fig. 1.
Plasma glucose levels (top panel) in five dogs receiving GLP-1(32–36)amide (■) or saline (○) vehicle and glucose infusion rates (lower panel, thick line = GLP infusion). The vertical line at 60 and 180 min indicate the interval of GLP-1(32–36)amide or saline infusions.
The 2-min priming rates of 20% glucose infusion for the first 10 min, and the 5-min glucose infusion rates necessary to maintain the stable plasma glucose levels of the hyperglycemic clamp, are presented in Fig. 1. Of note, the glucose infusion requirements during the GLP-1(32–36)amide infusion studies were higher than the glucose infusion requirements during the saline studies between 150 and 180 min of the clamps as demonstrated by the glucose utilization rates, M, during this period GLP-1(31–36)amide, 21.4 ± 2.9 vs. Control, 14.3 ± 1.1 mg kg−1 min−1 (P = 0.026, paired t-test; P = 0.062, Mann–Whitney U test).
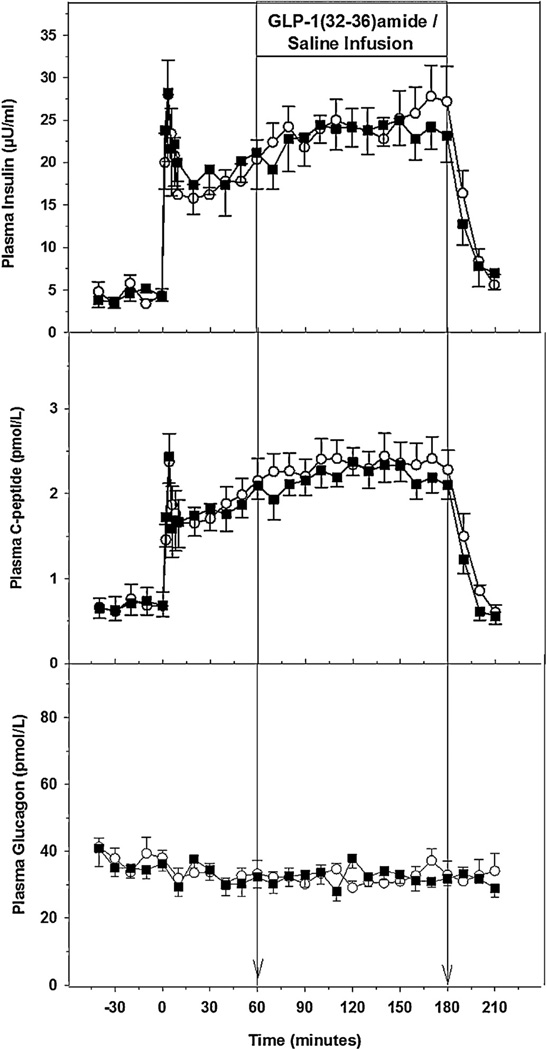
Basal plasma insulin levels for the GLP-1(32–36)amide and saline studies were 4.3 ± 0.8 and 4.3 ± 0.6 µU/ml. With the establishment of the square wave of hyperglycemia, a first and a second phase insulin response occurred and their profiles were nearly identical (Fig. 2). The first phase response (0–10 min) for the GLP-1(32–36)amide and saline were similar with a peak response on 28.0 ± 4.1 and 28.2 ± 8.0 µU/ml. After the nadir of the first phase response at 10 min, the second phase insulin response to glucose alone, (10–60 min prior to beginning the peptide or saline infusions), started to increase and was similar in the two studies. The 60-min plasma insulin levels for the GLP-1(32–36)amide and saline were 18.0 ± 1.6 and 19.5 ± 3.1 µU/ml. With the start of, and during the continuous infusion of the peptide or saline, the second phase insulin response continued to increase until the infusions were terminated at 180 min. However, no change in plasma insulin levels occurred in response to the infusion of GLP-1(32–36)amide. The 150–180 min AUC’swere 26.6 ± 3.2 and 23.7 ± 2.5 µU/ml for the GLP-1 and saline infusion (p = 0.43). With the termination of the infusions at 180 min and in parallel to the fall of plasma glucose, plasma insulin levels also fell, nearly identically, and the 210 min values were 5.6 ± 1.2 and 7.0 ± 1.9 µU/ml for the GLP-1(32–36)amide and saline studies.
Fig. 2.
Plasma levels of insulin (top panel), plasma C-peptide (middle panel), plasma glucagon (lower panel) in the five dogs receiving GLP-1(32–36)amide (■) or vehicle (○). The vertical line at 60 and 180 min indicate the interval of GLP-1(32–36)amide or saline infusions.
Basal plasma C-peptide levels for the GLP-1(32–36)amide and saline studies were 0.68 ± 0.15 and 0.68 ± 0.16 pmol/L. The C-peptide responses during the square wave of hyperglycemia with infusion of either glucose alone or glucose plus the peptide or the vehicle were similar to the insulin responses during the entire study (Fig. 2). No significant augmentation of C-peptide response during the infusion of the peptide occurred. The 150–180 min AUV’s were 2.4 ± 0.25 and 2.2 ± 0.18 pmol/L for the GLP-1 and saline infusion (p = 0.55). Basal plasma glucagon levels for the GLP-1(32–36)amide and saline infusion studies were 38.1 ± 1.9 and 36.4 ± 2.3 pmol/L. With the establishment of the hyperglycemic plateau and the response of the beta cell, plasma glucagon levels fell slightly in both studies (Fig. 2). However, infusion of GLP-1(32–36)amide did not have any further suppressive effect on the α cell (Fig. 2). The 60-min glucagon levels for the GLP-1(32–36)amide and saline studies were 33.4 ± 4.0 and 32.3 ± 3.2 pmol/L. The 150–180 min AUC were 34.0 ± 2.1 and 31.7 ± 1.8 pmol/L for the GLP-1 and saline infusion (p = 0.08), indicating that there was no further suppression of glucagon secretion during the GLP-1(32–36)amide infusion.
Discussion
Herein we have shown that the pentapeptide, GLP-1(32–36)amide, a peptide derived from the C-terminus of GLP-1 (7–36)amide or its metabolites [11], increases whole body glucose uptake in conscious dogs when administered acutely during a stable hyperglycemic state. This increase in glucose uptake in response to the pentapeptide is not attributable to changes in plasma insulin, C-peptide, or glucagon levels. We note that the AUC insulin levels during the last 30 min of the GLP-1 (32–36)amide infusion studies are 3 µU/ml higher than during the same period of the saline infusion studies, with no change in C-peptide and a small increase in glucagon (2 pmol/L). Although the small increase in insulin could account, in part, for the increased GIR, we do not believe that the small increase in insulin is sufficient to account for the larger increase in GIR (7.1 mg kg−1 min−1). The acute infusions of the pentapeptide take at least 60 min before significant changes in glucose utilization were apparent. This finding suggests that the actions of the pentapeptide are probably not mediated through a cell surface receptor, but rather the pentapeptide might be a cell penetrating peptide that is transported through the plasma membrane into cells, as has been proposed for the GLP-1-derived nonapeptide [29]. Transport into cells and targeting to organelles such as mitochondria might account for the delay in the onset of the biologic actions of the pentapeptide. This finding of increased whole body glucose uptake mediated by the pentapeptide is of importance because it might also have similar effects in insulin resistant, glucose intolerance states such as obesity, diabetes, and related metabolic disorders.
There are several limitations to our study. The number of dogs experimented upon was small. We did not use tracer methodology to evaluate changes in hepatic glucose production (HGP). However, the magnitude of the insulin response to hyperglycemia is such that HGP was probably fully suppressed in both protocols and thus the action of the pentapeptide is on peripheral glucose uptake by skeletal muscle. We were unable to measure plasma levels of the pentapeptide as specific assays for its detection are not available. However, using mass spectroscopy we have preliminary data suggesting that GLP-1(9–36)amide is converted to GLP-1(32–36)amide and is present at approximately 100-fold lower concentrations than GLP-1(28–36)amide [31].
The observation that the decay value (K) or t1/2 for plasma glucose of ≈15 min are similar in the pentapeptide and vehicle-alone treated groups following termination of the infusions of the 20% glucose and pentapeptide/vehicle support a short half-life of the pentapeptide. It should be noted that similar to other agents that increase insulin-sensitivity with chronic administration (e.g. Metformin), the chronic administration of the pentapeptide might also have a prolonged beneficial effect.
Earlier we reported that GLP-1(9–36)amide, the major form of circulating GLP-1, inhibits hepatic glucose production in lean glucose-tolerant human subject volunteers [8]. In that study, we found that the suppression of hepatic glucose production by GLP-1(9–36)amide is even more pronounced in the obese state [8]. It is important to note that in the studies of infusions of GLP-1(9–36)amide in human subjects the glucose infusion rates required to maintain stable hyperglycemia in the obese group of subjects was greater than was accounted for by the suppression of hepatic glucose production. This circumstance indicates that GLP-1(9–36)amide increased glucose uptake in skeletal muscle in addition to a suppression of glucose production by the liver. Furthermore, this effect on glucose uptake was not mediated through the known GLP-1 receptor as the co-infusion of GLP-1(9–36)amide and exendin(9–39), an antagonist of the known GLP-1(7–36)amide receptor, did not reduce the suppressive effects of GLP-1(9–36)amide on hepatic glucose production (HGP). In fact, HGP was further suppressed in both the lean and the obese groups of subjects and glucose infusion rates were greater than could be accounted for by the suppression of HGP, again implying increased glucose uptake by skeletal muscle. In agreement with our findings of the actions of GLP-1(9–36)amide in human subjects are the findings that GLP-1(9–36)amide reduces postprandial glycemia independently of gastric emptying and insulin secretion in humans [18] and reduces blood glucose in pigs by a mechanism that does not involve insulin secretion [6]. In contrast to our earlier study with GLP-1(9–36)amide, a recent study from Sathananthan et al. demonstrated that the GLP-1(9–36) amide has no effects on insulin action, beta-cell function, and whole body glucose metabolism in nondiabetic subjects [23]. These apparent disparate findings might be related to different doses, timing of administration, and methodology used, highlighting the need for further investigations in clinical settings.
Several studies report that the nonapeptide GLP-1(28–36)amide, consisting of the pentapeptide GLP-1 (32–36)amide extended at the N-terminus by four amino acids, to give the nonapeptide, GLP-1 (28–36)amide has glucoregulatory effects [12,17,24,29,30]. The nonapeptide appears to enter isolated mouse hepatocytes [29] and pancreatic islet cells [17] and to associate with mitochondria [17,29], resulting in the inhibition of gluconeogenesis [29] and oxidative stress [17,29]. It has been proposed that the transport of the peptide might be mediated by a yet unidentified receptor or transporter or that the peptide might be a cell penetrating peptide that is permeable to the cell membrane by energy-independent mechanisms due to its amphiphilic structure [29]. The administration of the peptide to diet-induced obese mice inhibited weight gain, the accumulation of liver triglycerides, and improved insulin sensitivity [30]. The nonapeptide also improved glucose disposal and attenuated glucose production during a pyruvate test [12], and improved glucose disposal in streptozotocin-induced diabetic mice, accompanied by an increased beta cell proliferation and increased beta cell mass [24]. The in vivo observations described above with the infusion of GLP-1(9–36)amide could be potentially attributable to the cleaved products of GLP-1, namely; GLP-1(9–36)amide, GLP-1(28–36)amide, or GLP-1(32–36)amide.
The mechanism(s) by which GLP-1(32–36)amide increases whole body glucose disposal remains unknown. However, based on the recent findings that the pentapeptide activates AMP kinase (AMPK) in skeletal muscle cells [32], we propose that a possible mechanism might involve AMPK-mediated translocation of glucose transporter-4 in skeletal muscle [10].
In summary, we report finding glucose-lowering actions of GLP-1(32–36)amide, a novel bioactive pentapeptide derived from the C-terminus of GLP-1. The pentapeptide enhances whole-body glucose disposal during hyperglycemic clamps in dogs. The metabolic benefits of GLP-1 observed in humans might be, at least in part, attributable to the pentapeptide. It seems plausible that this novel pentapeptide might have potential benefit in the regulation of glucose homeostasis in insulin resistant states such as obesity and diabetes.
Acknowledgments
We thank Drs. You-Tang Shen and Li Chen for performing the surgery in the dogs. This study was funded in part from the Department of Medicine at the Perelman School of Medicine, from the Intramural Research Program of the National Institutes on Aging, and from the Laboratory of Molecular Endocrinology at the Massachusetts General Hospital (Grant No. P30DK05751).
Footnotes
Author contributions
DE, FSA, AV, ODC and JME assisted in the conduct of the study. DE, JFH, and RPS designed the study. DE, FSA, ET, JME, JFH, and RPS assisted in the interpretation of the data. DE wrote the first draft of the manuscript. All authors contributed to the writing of the manuscript.
Conflict of interest
The authors report no conflict of interest.
References
- 1.Abu-Hamdah R, Rabiee A, Meneilly GS, Shannon RP, Andersen DK, Elahi D. Clinical review: the extrapancreatic effects of glucagon-like peptide-1 and related peptides. J Clin Endocrinol Metab. 2009;94:1843–1852. doi: 10.1210/jc.2008-1296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Andres R, Swerdloff R, Pozefsky T, Coleman D. Manual feedback technique for control of glucose concentration. In: Skeggs L Jr, editor. Automation in analytic chemistry. New York: Medaid, Inc.; 1966. pp. 486–501. [Google Scholar]
- 3.Angeli FS, Shannon RP. Incretin based therapies: can we achieve glycemic control and cardioprotection? J Endocrinol. 2013 doi: 10.1530/JOE-13-0195. http://dx.doi.org/10.1530/JOE-13-0195. [DOI] [PubMed] [Google Scholar]
- 4.Ban K, Kim KH, Cho CK, Sauve M, Diamandis EP, Backx PH, et al. Glucagon-like peptide (GLP)-1(9–36)amide-mediated cytoprotection is blocked by exendin(9–39) yet does not require the known GLP-1 receptor. Endocrinology. 2010;151:1520–1531. doi: 10.1210/en.2009-1197. [DOI] [PubMed] [Google Scholar]
- 5.Ban K, Noyan-Ashraf MH, Hoefer J, Bolz SS, Drucker DJ, Husain M. Cardioprotective and vasodilatory actions of glucagon-like peptide 1 receptor are mediated through both glucagon-like peptide 1 receptor-dependent and -independent pathways. Circulation. 2008;117:2340–2350. doi: 10.1161/CIRCULATIONAHA.107.739938. [DOI] [PubMed] [Google Scholar]
- 6.Deacon CF, Plamboeck A, Moller S, Holst JJ. GLP-1(9–36)amide reduces blood glucose in anesthetized pigs by a mechanism that does not involve insulin secretion. Am J Physiol Endocrinol Metab. 2002 Apr 4;282:E873–E879. doi: 10.1152/ajpendo.00452.2001. [DOI] [PubMed] [Google Scholar]
- 7.Drucker DJ, Nauck MA. The incretin system: glucagon-like peptide-1 receptor agonists and dipeptidyl peptidase-4 inhibitors in type 2 diabetes. Lancet. 2006;368(9548):1696–1705. doi: 10.1016/S0140-6736(06)69705-5. [DOI] [PubMed] [Google Scholar]
- 8.Elahi D, Egan JM, Shannon RP, Meneilly GS, Khatri A, Habener JF, et al. GLP-1 (9–36) amide, cleavage product of GLP-1 (7–36) amide, is a glucoregulatory peptide. Obesity (Silver Spring, Md) 2008;16:1501–1509. doi: 10.1038/oby.2008.229. [DOI] [PubMed] [Google Scholar]
- 9.Flint A, Raben A, Astrup A, Holst JJ. Glucagon-like peptide 1 promotes satiety and suppresses energy intake in humans. J Clin Invest. 1998;101:515–520. doi: 10.1172/JCI990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Hardie DG. Energy sensing by the AMP-activated protein kinase and its effects on muscle metabolism. Proc Nutr Soc. 2011;70:92–99. doi: 10.1017/S0029665110003915. [DOI] [PubMed] [Google Scholar]
- 11.Hupe-Sodmann K, McGregor GP, Bridenbaugh R, Goke R, Goke B, Thole H, et al. Characterisation of the processing by human neutral endopeptidase 24.11 of GLP-1(7–36) amide and comparison of the substrate specificity of the enzyme for other glucagon-like peptides. Regul Pept. 1995;58:149–156. doi: 10.1016/0167-0115(95)00063-h. [DOI] [PubMed] [Google Scholar]
- 12.Ip W, Shao W, Chiang YT, Jin T. GLP-1-derived nonapeptide GLP-1(28–36)amide represses hepatic gluconeogenic gene expression and improves pyruvate tolerance in high-fat diet-fed mice. Am J Physiol Endocrinol Metab. 2013 Dec 11;305:E1348–E1358. doi: 10.1152/ajpendo.00376.2013. [DOI] [PubMed] [Google Scholar]
- 13.Kieffer TJ, Habener JF. The glucagon-like peptides. Endocr Rev. 1999;20:876–913. doi: 10.1210/edrv.20.6.0385. [DOI] [PubMed] [Google Scholar]
- 14.Kieffer TJ, McIntosh CH, Pederson RA. Degradation of glucose-dependent insulinotropic polypeptide and truncated glucagon-like peptide 1 in vitro and in vivo by dipeptidyl peptidase IV. Endocrinology. 1995;136:3585–3596. doi: 10.1210/endo.136.8.7628397. [DOI] [PubMed] [Google Scholar]
- 15.Larsen PJ, Fledelius C, Knudsen LB, Tang-Christensen M. Systemic administration of the long-acting GLP-1 derivative NN2211 induces lasting and reversible weight loss in both normal and obese rats. Diabetes. 2001;50:2530–2539. doi: 10.2337/diabetes.50.11.2530. [DOI] [PubMed] [Google Scholar]
- 16.Lee CJ, Brown T, Magnuson TH, Egan JM, Carlson O, Elahi D. Hormonal response to a mixed-meal challenge after reversal of gastric bypass for hypoglycemia. J Clin Endocrinol Metab. 2013;98:E1208–E1212. doi: 10.1210/jc.2013-1151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Liu Z, Stanojevic V, Brindamour LJ, Habener JF. GLP-1-derived nonapeptide GLP-1(28–36)amide protects pancreatic beta-cells from glucolipotoxicity. J Endocrinol. 2012;213:143–154. doi: 10.1530/JOE-11-0328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Meier JJ, Gethmann A, Nauck MA, Gotze O, Schimtz F, Deacon CF, et al. The glucagon-like peptide-1 metabolite GLP-1(9–36)amide reduces postprandial glycemia independently of gastric emptying and insulin secretion in humans. Am J Physiol Endocrinol Metab. 2006 Jun 6;290:E1118–E1123. doi: 10.1152/ajpendo.00576.2005. [DOI] [PubMed] [Google Scholar]
- 19.Nikolaidis LA, Elahi D, Shen YT, Shannon RP. Active metabolite of GLP-1 mediates myocardial glucose uptake and improves left ventricular performance in conscious dogs with dilated cardiomyopathy. Am J Physiol Heart Circ Physiol. 2005;289:H2401–H2408. doi: 10.1152/ajpheart.00347.2005. [DOI] [PubMed] [Google Scholar]
- 20.Price GS, Frazier DL. Use of body surface area (BSA)-based dosages to calculate chemotherapeutic drug dose in dogs: I. Potential problems with current BSA formulae. J Vet Intern Med/Am Coll Vet Intern Med. 1998;12:267–271. doi: 10.1111/j.1939-1676.1998.tb02121.x. [DOI] [PubMed] [Google Scholar]
- 21.Rabiee A, Magruder JT, Grant C, Salas-Carrillo R, Gillette A, DuBois J, et al. Accuracy and reliability of the Nova StatStrip(R) glucose meter for real-time blood glucose determinations during glucose clamp studies. J Diab Sci Technol. 2010;4:1195–1201. doi: 10.1177/193229681000400519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Salcedo I, Tweedie D, Li Y, Greig NH. Neuroprotective and neurotrophic actions of glucagon-like peptide-1: an emerging opportunity to treat neurodegenerative and cerebrovascular disorders. Br J Pharmacol. 2012;166:1586–1599. doi: 10.1111/j.1476-5381.2012.01971.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Sathananthan M, Farrugia LP, Miles JM, Piccinini F, Dalla Man C, Zinsmeister AR, et al. Direct effects of exendin-(9,39) and GLP-1-(9,36)amide on insulin action, beta-cell function, and glucose metabolism in nondiabetic subjects. Diabetes. 2013;62(8):2752–2756. doi: 10.2337/db13-0140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Shao W, Wang Z, Ip W, Chiang YT, Xiong X, Chai T, et al. GLP-1(28–36) improves beta-cell mass and glucose disposal in streptozotocin induced diabetes mice and activates PKA-beta-catenin signaling in beta-cells in vitro. Am J Physiol. 2013 doi: 10.1152/ajpendo.00600.2012. http://dx.doi.org/10.1152/ajpendo.00600.2012. [DOI] [PubMed] [Google Scholar]
- 25.Sonne DP, Engstrom T, Treiman M. Protective effects of GLP-1 analogues exendin-4 and GLP-1(9–36) amide against ischemia-reperfusion injury in rat heart. Regul Pept. 2008;146:243–249. doi: 10.1016/j.regpep.2007.10.001. [DOI] [PubMed] [Google Scholar]
- 26.Tomas E, Habener JF. Insulin-like actions of glucagon-like peptide-1: a dual receptor hypothesis. Trends Endocrinol Metab. 2010;21:59–67. doi: 10.1016/j.tem.2009.11.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Tomas E, Stanojevic V, Habener JF. GLP-1 (9–36) amide metabolite suppression of glucose production in isolated mouse hepatocytes. Horm Metab Res. 2010;42:657–662. doi: 10.1055/s-0030-1253421. [DOI] [PubMed] [Google Scholar]
- 28.Thomas E, Wood JA, Stanojevic V, Habener JF. Glucagon-like peptide-1(9–36)amide metabolite inhibits weight gain and attenuates diabetes and hepatic steatosis in diet-induced obese mice. Diab Obes Metab. 2011;13:26–33. doi: 10.1111/j.1463-1326.2010.01316.x. [DOI] [PubMed] [Google Scholar]
- 29.Tomas E, Stanojevic V, Habener JF. GLP-1-derived nonapeptide GLP-1(28–36)amide targets to mitochondria and suppresses glucose production and oxidative stress in isolated mouse hepatocytes. Regul Pept. 2011;167:177–184. doi: 10.1016/j.regpep.2011.01.003. [DOI] [PubMed] [Google Scholar]
- 30.Tomas E, Wood JA, Stanojevic V, Habener JF. GLP-1-derived nonapeptide GLP-1(28–36)amide inhibits weight gain and attenuates diabetes and hepatic steatosis in diet-induced obese mice. Regul Pept. 2011;169:43–48. doi: 10.1016/j.regpep.2011.04.006. [DOI] [PubMed] [Google Scholar]
- 31.Tomas E, Stanojevic V, Laudone R, Everill P, Bachovchin W, Habener JF. GLP-1-derived pentapeptide GLP-1(32–36)amide attenuates the development of obesity, diabetes, hepatic steatosis, and increases energy expenditure in diet-induced obese mice [abstract] Diabetes. 2012;61(Suppl):1915-P. [Google Scholar]
- 32.Tomas E, Habener JF. GLP-1(32–36)amide pentapeptide regulates oxidative phosphorylation by activating AMPK signaling and raising the NAD+/NADH ratio in C2C12 myotubes. Diabetes. 2014;63(Suppl):1857-P. [Google Scholar]