Abstract
Long noncoding RNAs (lncRNAs) play crucial roles in carcinogenesis. However, the function and mechanism of lncRNAs in human non–small-cell lung cancer (NSCLC) are still remaining largely unknown. Long intergenic noncoding RNA 00511 (LINC00511) has been found to be upregulated and acts as an oncogene in breast cancer, but little is known about its expression pattern, biological function and underlying mechanism in NSCLC. Herein, we identified LINC00511 as an oncogenic lncRNA by driving tumorigenesis in NSCLC. We found LINC00511 was upregulated and associated with oncogenesis, tumor size, metastasis, and poor prognosis in NSCLC. Moreover, LINC00511 affected cell proliferation, invasiveness, metastasis, and apoptosis in multiple NSCLC cell lines. Mechanistically, LINC00511 bound histone methyltransferase enhancer of zeste homolog 2 ((EZH2, the catalytic subunit of the polycomb repressive complex 2 (PRC2), a highly conserved protein complex that regulates gene expression by methylating lysine 27 on histone H3), and acted as a modular scaffold of EZH2/PRC2 complexes, coordinated their localization, and specified the histone modification pattern on the target genes, including p57, and consequently altered NSCLC cell biology. Thus, LINC00511 is mechanistically, functionally, and clinically oncogenic in NSCLC. Targeting LINC00511 and its pathway may be meaningful for treating patients with NSCLC.
Keywords: enhancer of zeste homolog 2, long noncoding RNA LINC00511, p57, non–small-cell lung cancer, polycomb repressive complex 2, tumorigenesis
Introduction
Non–small-cell lung cancer (NSCLC) is the major reason for cancer-relevant death around the world.1,2 Clarification of the mechanisms that underlie NSCLC tumorigenesis and progression is urgently needed.3,4 Though alterations in multiple oncogenes and tumor-suppressive genes have been reported in NSCLC,5,6,7 the precise molecular mechanisms underlying NSCLC pathogenesis are still remaining to be further elaborated. Hence, better understanding of the carcinogenesis is critical for the advance of diagnostic markers and aid novel effective therapies for NSCLC patients.
Long noncoding RNAs (lncRNAs), a class of noncoding RNAs more than 200 nucleotides (nt) in length,8,9 have been reported to participate in pathological and physiological process in numerous types of human diseases, including cancers.9,10,11 A large amount of studies12,13,14,15,16,17,18,19,20,21,22,23,24,25 have reported that lncRNAs could be crucial players in cancer biology, particularly resulting in dysregulation of gene products that contributed in the advance human tumors.26,27,28 Further, lncRNAs could also be considered as diagnostic or prognostic markers on account of their clinical significance with tumor outcomes.19,22,25,29
Recently, several lncRNAs have been found to involve in NSCLC tumorigenesis, such as NEAT1,30 TATDN1,31 LINC00473,32 AGAP2-AS1,33 BC087858,34 and LINC01133.35 Sun et al. reported NEAT1 functioned as an oncogene in NSCLC by acting as a competing endogenous RNA (ceRNA) for miR-377-3p, and then leading to de-suppression of E2F3, an endogenous target of miR-377-3p, which was a central oncogene in facilitating NSCLC progression.30 LINC00473 is a nuclear lncRNA which expresses highly in NSCLC, elevated LINC00473 expression correlated with poor prognosis.32 But up to date, the detail molecular mechanisms of lncRNAs in NSCLC are still needed to be further clarified.
Long intergenic noncoding RNA 00511 (LINC00511, NR 033876), is a newly identified lncRNA that is upregulated in human breast cancer,36 which may serve as an oncogene. Nevertheless, biological roles and underlying molecular mechanisms of LINC00511 in NSCLC tumorigenesis are still remaining unclearly defined. In our study, we are committed to investigate the potential molecular mechanisms of LINC00511 on NSCLC progression. Interestingly, we found that LINC00511 is highly upregulated in both NSCLC tissues and cell lines. Our data also show that LINC00511 is capable to facilitate cell growth, migration and invasion, and repress cell apoptosis through epigenetically suppressing p57 (an inhibitor for cyclin-dependent kinase, and is deemed as a candidate of tumor-suppressive gene that has been embroiled in numerous of cancers37,38,39,40,41) expression in NSCLC cells.
Results
LINC00511 is upregulated in NSCLC tissues and cell lines and indicates a poor prognosis
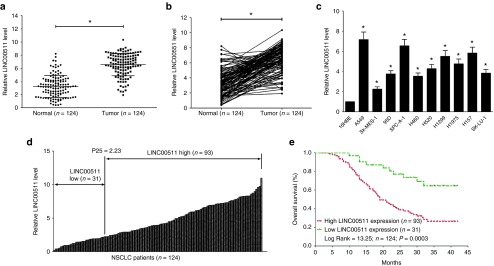
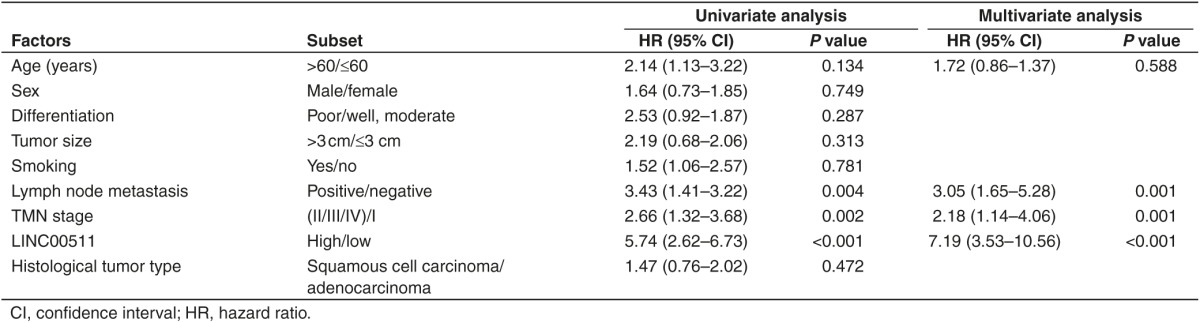
We first examined the LINC00511 expression in NSCLC by qRT-PCR, and found that LINC00511 levels in 124 NSCLC tissues were markedly higher than that of in their counterparts (P < 0.05) (Figure 1a,b). Next, we tested LINC00511 expression in NSCLC cell lines, and found that LINC00511 was higher expressed in NSCLC cell lines, including A549, SK-MES-1, H1299, 95D, H460, H520, H1975, H157, SK-LU-1, and SPC-A-1 cell lines, than that of in normal lung epithelial cells, 16HBE (Figure 1c). Among the ten NSCLC cell lines, LINC00511 are relative higher expressed in A549 and SPC-A-1 cells, thus, we chose A549 and SPC-A-1 cells to perform the following experiments. Subsequently, NSCLC patients were divided into a high group (≥2.23-fold, n = 93) and a low group (<2.23-fold, n = 31) on the basis of the P25 value of LINC00511 expression (Figure 1d). Moreover, to assess the clinical significance of LINC00511, we evaluated the correction between its level and clinic-pathological parameters. Results revealed that LINC00511 levels were remarkably corrected with tumor size (P < 0.0001), TNM stage (P < 0.0001), smoking history (P = 0.0005), and lymph node metastasis (P = <0.0001) in NSCLC. Nevertheless, LINC00511 levels were not associated with other clinical characteristics, including gender (P = 0.2988), differentiation (P = 0.9685), histological tumor type (P = 0.9169), or age (P = 0.2015) in NSCLC (Table 1). Additionally, multivariate Cox regression analysis revealed that high LINC00511 expression (≥2.23-fold, n = 93), positive lymph node metastasis, and advanced stage are independent predictors of OS in NSCLC patients (Table 2). Kaplan-Meier analysis indicated that high LINC00511 expression was related to a poorer OS (log-rank test, P =0.0003, Figure 1e). These results confirmed that high LINC00511 expression was related to poor prognosis, and upregulated expression of LINC00511 might be crucial in NSCLC tumorigenesis and progression.
Figure 1.
LINC00511 is upregulated in primary human NSCLC and NSCLC cell lines, and benefits for prognosis. (a,b) LINC00511 is significantly decreased in primary human NSCLC tissues in comparison to adjacent-normal NSCLC tissues. n = 124 for each group. (c) The expression level of LINC00511 in ten NSCLC cell lines and normal 16HBE cells. Assays were performed in triplicate. (d) Kaplan-Meier survival analysis revealed that high-expressed LINC00511 is associated with poor prognosis in patients with non–small-cell lung cancer. *P < 0.05, Means ± SD was shown. Statistical analysis was conducted using student t-test and Log Rank test. NSCLC, non–small-cell lung cancer.
Table 1. Correlation between LINC00511 expression and clinicopathological parameters of non–small-cell lung cancer patients (n = 124).
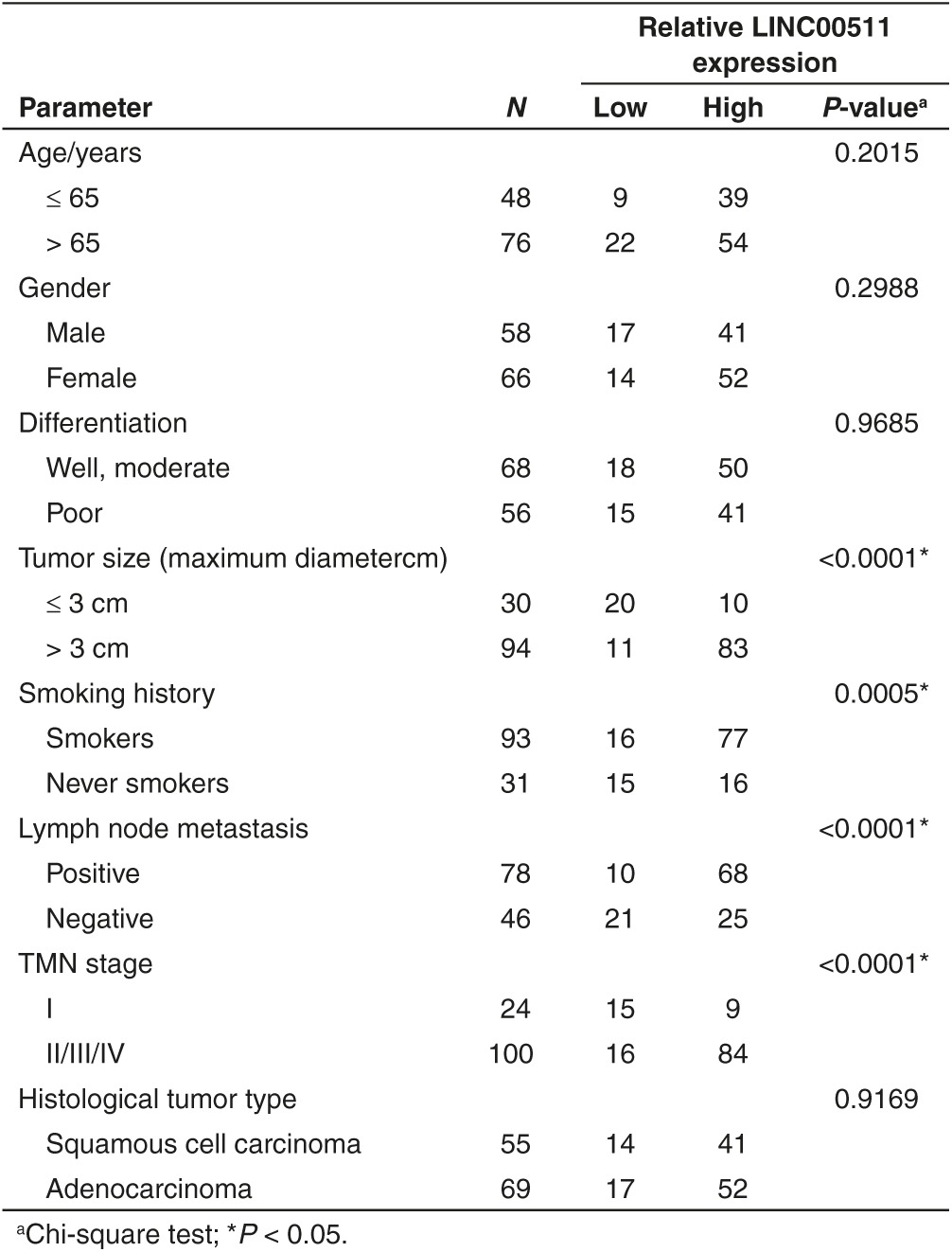
Table 2. Influence of LINC00511 expression and clinical characteristics on overall survival in non–small-cell lung cancer patients.
Knockdown of LINC00511 represses NSCLC cell growth in vitro
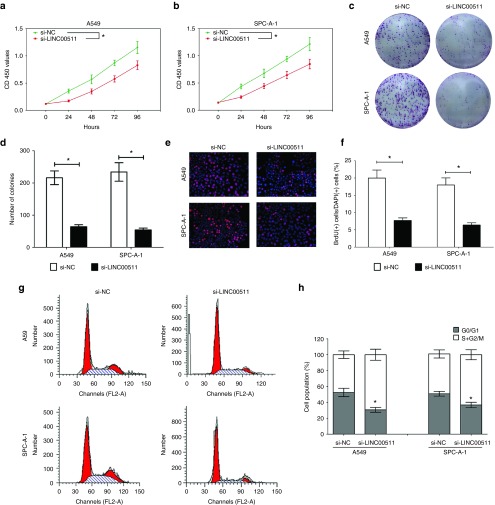
To validate if LINC00511 represses cell proliferation in A549 and SPC-A-1 cells, CCK8 as well as assays colony formation were performed (Figure 2a–d). Knockdown of LINC00511 remarkably inhibited cell proliferation at 48 hours treatment. These results were confirmed by BrdU colony formation assays (Figure 2e,f), which indicated that silence of LINC00511 retarded cell growth. Further, flow cytometry was conducted to analyze the role of LINC00511 on cell cycle in NSCLC cells. And results indicated knockdown of LINC00511 retarded the G1/S transition, namely, they promoted the number of cells in the G1 phase but reduced the number of cells in the S and G2/M phases, in comparison to si-NC group (Figure 2g,h). These results indicate knockdown of LINC00511 may repress cell growth in A549 and SPC-A-1 cells.
Figure 2.
Knockdown of LINC00511 represses NSCLC cell proliferation in vitro. (a,b). CCK8 assay indicate knockdown of LINC00511 represses NSCLC cell proliferation. (c,d). Colony formation assay indicate knockdown of LINC00511 represses NSCLC cell proliferation. (e,f) BrdU staining assay indicate knockdown of LINC00511 represses NSCLC cell proliferation. Bar = 100 μm. (g,h) Representative images and quantification of flow cytometry analysis of A549 and SPC-A-1 cells after transfection. Cell cycle analysis revealed that LINC00511 has influence the proliferation of A549 and SPC-A-1 cells by regulating its cell cycle. *P < 0.05, Means ± SD was shown. Statistical analysis was conducted using student t-test analysis. NSCLC, non–small-cell lung cancer.
Knockdown of LINC00511 represses NSCLC cell migration and invasion in vitro
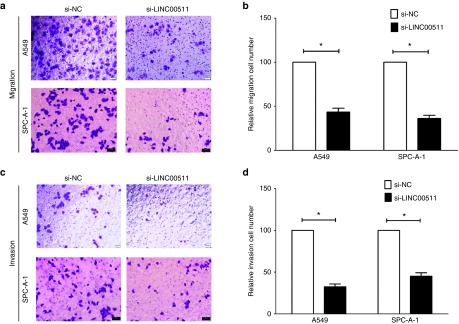
Next, we explored the efficiency of LINC00511 on migration and invasion in A549 and SPC-A-1 cells. Results showed that silence of LINC00511 repressed cell motility, migration, and invasion in comparison to si-NC group (Figure 3a–d). In detail, knockdown of LINC00511 nearly suppressed 55–60% of the cells' migratory activity in A549 and SPC-A-1 cells, and inhibited 53–62% of the cells' inhibitory activity in A549 and SPC-A-1 cells. These results, taken together, clearly indicated that knockdown of LINC00511 expression markedly retarded cell migration and invasion motility in NSCLC.
Figure 3.
Knockdown of LINC00511 represses NSCLC cell migration and invasion in vitro. (a–d) Transwell migration/invasion assays reveal knockdown of LINC00511 represses NSCLC cell migration and invasion. Bar = 100 μm. *P < 0.05, Means ± SD was shown. Statistical analysis was conducted using student t-test analysis. NSCLC, non–small-cell lung cancer.
Knockdown of LINC00511 facilitates cell apoptosis in NSCLC
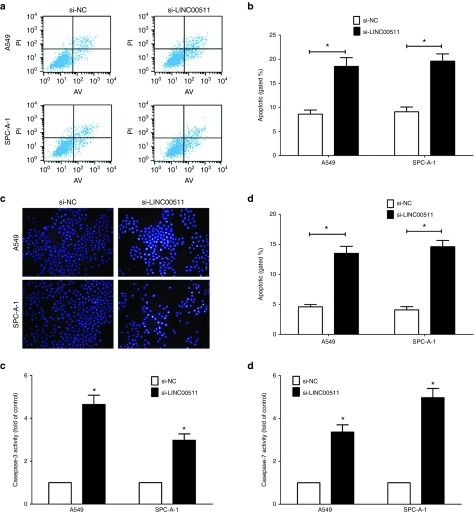
We also explored the efficiency of LINC00511 on cell apoptosis in vitro. And knockdown of LINC00511 led to a ~2.2-folds and ~2.4-folds of increase in apoptotic cell death of A549 and SPC-A-1 cells, as analyzed by flow cytometric analysis (Figure 4a,b), separately. Moreover, Hochest33342 staining confirmed that knockdown of LINC00511 led to a ~2.8-folds and ~3.2-folds of increase in apoptotic cell death of A549 and SPC-A-1 cells (Figure 4c,d), separately. Furthermore, we also examined the caspase-3/7 activity after transfection, and results demonstrated that knockdown of LINC00511 markedly augmented the caspase-3/7 activity in A549 and SPC-A-1 cell lysate, by nearly 4.6- and 2.9-folds increase (caspase-3 activity), 3.3- and 4.4-folds increase (caspase-7 activity), in comparison to that of in each si-NC group (Figure 4e,f), respectively. These data revealed that knockdown of LINC00511 actually facilitated cell apoptosis in A549 and SPC-A-1 cells.
Figure 4.
Knockdown of LINC00511 facilitates non–small-cell lung cancer cell apoptosis in vitro. (a,b) Representative images and quantification of flow cytometry analysis of A549 and SPC-A-1 cells after transfection. Cell apoptosis analysis revealed that LINC00511 has influenced the apoptosis of A549 and SPC-A-1 cells. (c,d) Representative images and quantification of Hochest33342 staining of A549 and SPC-A-1 cells after transfection. Bar = 100 μm. (e,f) Quantitative representation of caspase-3 and caspase-7 activity in A549 and SPC-A-1 cells after transfection for forty eight hours. Assays were performed in triplicate. *P < 0.05, Means ± SD was shown. Statistical analysis was conducted using student t-test analysis.
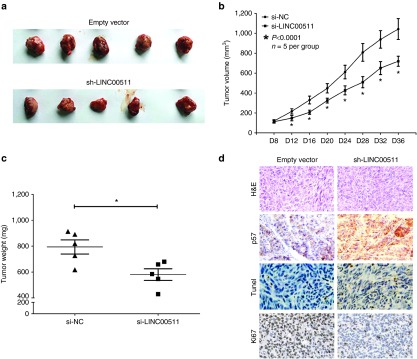
Knockdown of LINC00511 represses tumor growth in vivo
To validate the oncogenic efficiency of LINC00511 in vivo, we constructed a BALB/c nude mouse xenograft model by A549 cells. Results demonstrated the tumor volume and weight of tumors in nude mice treated with sh-LINC00511 were markedly suppressed (46% of decrease in tumor weight) relative to that of treated with empty vector (Figure 5a–c). These data indicated that knockdown of LINC00511 markedly inhibited the tumorigenicity of A549 cells in the nude mouse xenograft model. Moreover, in microscopic observation of tumors, lesser number of Ki-67 and more number of p57-positive cells and TUNEL-positive cells were observed in sh-LINC00511-treated group compared with empty vector-treated group (Figure 5d). The results revealed that knockdown of LINC00511 may result in NSCLC growth delay via repressing cell proliferation and medicating cell apoptosis. These in vivo data suggested that knockdown of LINC00511 could reduce tumorigenic capacity and increase survival in mouse models of human NSCLC.
Figure 5.
Knockdown of LINC00511 represses tumor growth in vivo. (a) Representative images tumors isolated from nude mice. (b) Tumor volume in nude mice. (c) Tumor weight in nude mice. Each group contained six mice (n = 5); the data are presented as the mean ± SD; *P < 0.05, compared with the empty vector group. (d) Representative images of H&E, TUNEL, p57, and Ki-67 staining. Bar = 50 μm. Assays were performed in triplicate. *P < 0.05, Means ± SD are shown. Statistical analysis was conducted using student t-test.
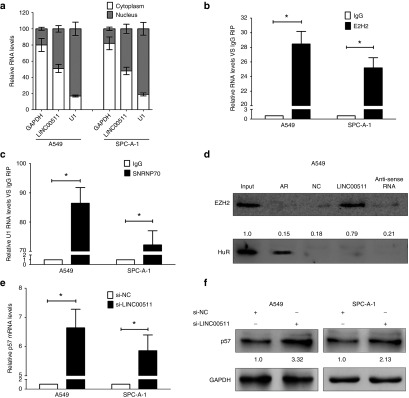
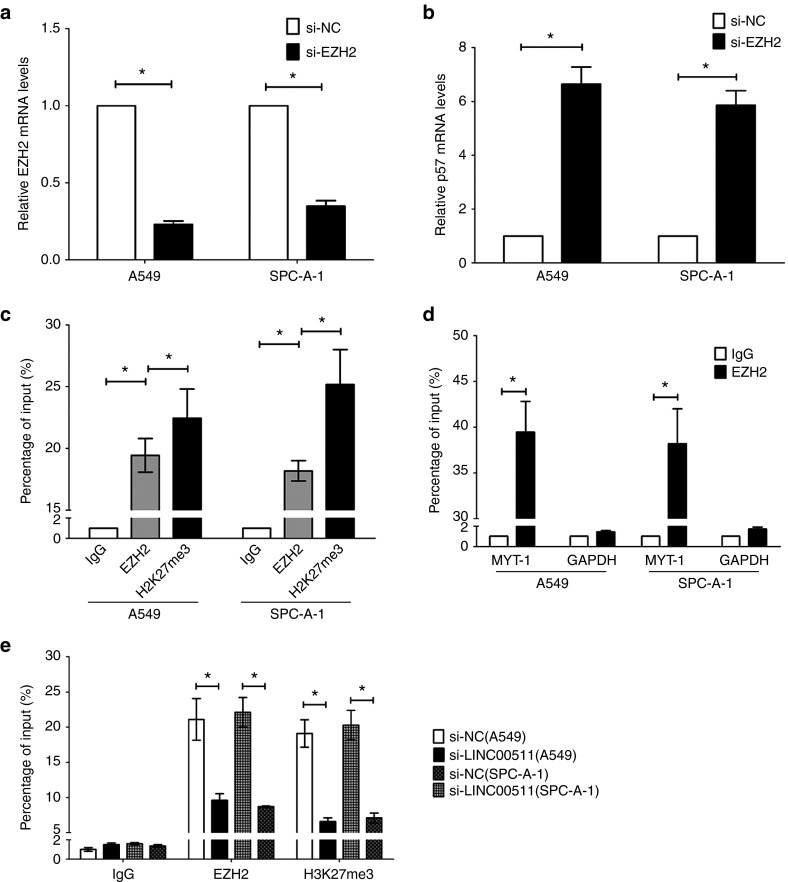
LINC00511 represses p57 expression via directly binding with EZH2 in NSCLC cells
LncRNAs could regulate their target genes expression through functioning as competing endogenous RNAs (ceRNA) for miRNAs or interacting with RNA binding proteins such as polycomb repressive complex 2 (PRC2, leading to methylating lysine 27 in histone H3 (3meH3K27)).42 To explore the molecular mechanisms of LINC00511 involved in NSCLC cells, we firstly analyzed the distribution of LINC00511 in NSCLC cells. And we found that LINC00511 is distributed in both cytoplasm and nucleus, but the ratio of LINC00511 in nucleus is higher than that of in cytoplasm (Figure 6a). Furthermore, we performed RIP assays and the results showed that LINC00511 could directly binds with enhancer of zeste homolog 2 (EZH2, the catalytic subunit of the PRC2) in A549 and SPC-A-1 cells (Figure 6b), while U1 binding with SNRNP70 was used as positive control (Figure 6c). In addition, RNA-pulldown assays confirmed that LINC00511 indeed binds with EZH2 in A549 cells (Figure 6d). These data suggest that LINC00511 could epigenetically repress underlying targets expression at transcriptional level. p57 is an inhibitor for cyclin-dependent kinase, and is deemed as a candidate of tumor-suppressive gene that has been embroiled in numerous of cancers.37,38,39,40,41 In addition, p57 is also a direct target of EZH2 and repressed by serveral epigenetic mechanisms in ovarian cancer43 and breast cancer.44 We suppose LINC00511 repressed p57 expression via interacting with EZH2 in NSCLC cells. To test this hypothesis, we evaluated their expression after knockdown of EZH2 in NSCLC cells. Interestingly, knockdown of EZH2 upregulated p57 expression (Figure 7a,b). To confirm whether EZH2 could directly bind the promoter region p57, we designed four pairs of primers across 2,000 bp of the promoter region. ChIP assays demonstrated that EZH2 could directly bind to the p57 promoter region (Figure 7c,d). Moreover, knockdown of LINC00511 reduced their binding to p57 promoter regions (Figure 7e). We next explore the role of LINC00511 on expression of p57. The qPCR results revealed that LINC00511 knockdown increased the expression of p57 (Figure 6e,f).
Figure 6.
LINC00511 represses p57 expression via directly binding with EZH2 in non–small-cell lung cancer cells. (a) Relative LINC00511 levels in A549 and SPC-A-1 cell cytoplasm or Nucleus were detected by qPCR. GAPDH was used as cytoplasm control and U1 was used as nuclear control. (b) LINC00511 RNA levels in immunoprecipitates with EZH2 antibodies are determined by qPCR. The expression levels of LINC00511 RNA were presented as fold enrichment relative to IgG. (c) SNRNP70 RNA levels in immunoprecipitates with U1 antibodies were used as positive control. (d) EZH2 protein level in immunoprecipitates with LINC00511 RNA is determined by western blot. HuR protein immunoprecipitates with AR RNA was used as positive control. (e) The level of p57 mRNA is determined by qPCR when knockdown of LINC00511 in A549 and SPC-A-1 cells. (f) The level of p57 protein is determined by western-blot when knockdown of LINC00511 in A549 and SPC-A-1 cells. Assays were performed in triplicate. *P < 0.05, Means ± SD was shown. Statistical analysis was conducted using student t-test.
Figure 7.
LINC00511 recruits EZH2 to p57 promoter and represses their transcription. (a) The EZH2 expression levels were detected by qPCR when knockdown of EZH2 in A549 and SPC-A-1 cells. (b) The p57 expression levels were detected by qPCR when knockdown of EZH2 in A549 and SPC-A-1 cells. (c) ChIP–qPCR analysis of EZH2 occupancy and H3K27me3 binding in the p57 promoter in A549 and SPC-A-1 cells, and IgG as a negative control. (d) ChIP–qPCR analysis of EZH2 occupancy in the MYT-1 promoter in A549 and SPC-A-1 cells, which was used as positive control. (e) ChIP–qPCR analysis of and EZH2 occupancy H3K27me3 binding in the p57 promoter after knockdown of LINC00511. Assays were performed in triplicate. *P < 0.05, Means ± SD was shown. Statistical analysis was conducted using student t-test.
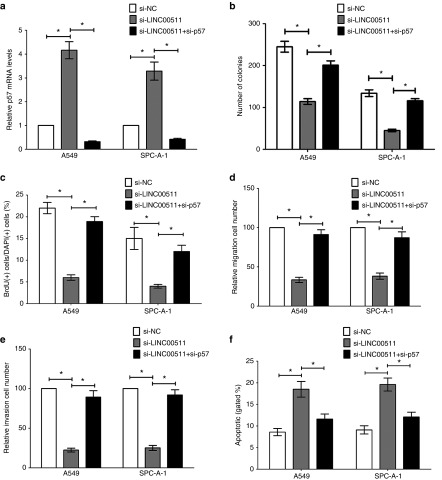
Silencing of p57 is partly mediated in the oncogenic efficiency of LINC00511
We conducted rescue assays to identify whether p57 is mediated in the LINC00511-induced NSCLC cell growth, metastasis, and apoptosis. Actually, A549 and SPC-A-1 cells were cotransfected with si-NC, si-LINC00511 or si-LINC00511+si-p57. qPCR demonstrated that knockdown of LINC00511 increased mRNA levels of p57, and knockdown of p57 decreased p57 expression (Figure 8a). First, colony formation and BrdU assays demonstrated that the inhibitory role of si-LINC00511 on cell growth was reversed by si-p57 treatment (Figure 8b,c). Next, transwell migration/invasion assay revealed that the inhibitory role of si-LINC00511 on cell migration and invasion was also reversed by si-p57 treatment (Figure 8d,e). Moreover, flow cytometric analysis demonstrated that the favorable role of si-LINC00511 on cell apoptosis was also reversed by si-p57 treatment (Figure 8f). These findings indicate that LINC00511 exerting oncogenic effects in NSCLC cells may partly through repressing p57 expression.
Figure 8.
Silencing of p57 is partly involved in the oncogenic function of LINC00511. A549 and SPC-A-1 cells were transfected with si-NC, si-LINC00511, or cotransfected with si-LINC00511 and si-p57. (a) Relative p57 mRNA expression was tested by qRT-PCR. (b,c) Colony-forming anf BrdU staining assays were performed to determine the cell viability. (d,e) Transwell migration/invasion assays were performed to determine the cell migration and invasion. (f) Quantification of flow cytometry analysis of A549 and SPC-A-1 cells after transfection. Assays were performed in triplicate. *P < 0.05, Means ± SD was shown. Statistical analysis was conducted using student t-test.
Lower p57 expression is positively correlated with the outcome of NSCLC patients
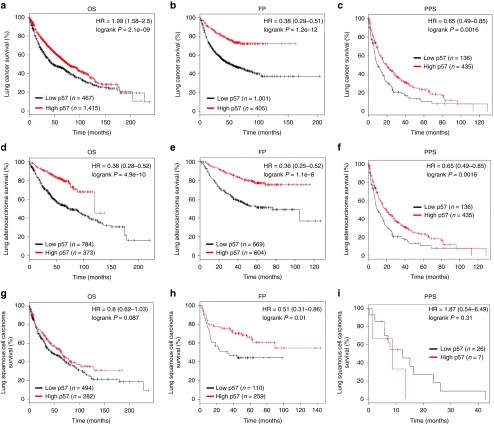
To further explore the critical efficiency of p57 in the survival of NSCLC patients, Kaplan Meier Plotter tools were used to analyze the correlation between the p57 mRNA levels and the survival of NSCLC patients from 2437 lung tumors using publicly available datasets (2015 version) (http://kmplot.com/analysis/index.php?p=service&cancer=lung). Results demonstrated that lower p57 mRNA levels in NSCLC patients are markedly correlated with an improvement of the overall survival (OS), progression-free survival (FP), and post progression survival (PPS) of patients (Figure 9a–i). These analyses further verified the tumor-suppressive effect of p57 in NSCLC.
Figure 9.
Prognostic significance of p57 in lung cancer. (a–c) The effect of p57 mRNA expression level on the overall survival (OS), progression-free survival (FP), and post progression survival (PPS) in lung cancer patients was analyzed and the Kaplan-Meier plots were generated by Kaplan-Meier Plotter (http://www.kmplot.com). (d–f) The effect of p57 mRNA expression level on the overall survival (OS), progression-free survival (FP), and post progression survival (PPS) in lung adenocarcinoma patients was analyzed and the Kaplan-Meier plots were generated by Kaplan-Meier Plotter (http://www.kmplot.com). (g–i) The effect of p57 mRNA expression level on the overall survival (OS), progression-free survival (FP), and post progression survival (PPS) in lung squamous cell carcinoma patients was analyzed and the Kaplan-Meier plots were generated by Kaplan-Meier Plotter (http://www.kmplot.com).
Discussion
LncRNAs are implicated in pathologic and physiologic processes in numerous of human diseases. The levels of certain lncRNAs are associated with the metastasis, development, and prognosis of cancers.44,45,46,47,48 Given that LINC00511 is upregulated in breast cancer,45,46 and silence of LINC00511 showed tumor-suppressive roles via inhibition of cell proliferation in breast cancer.45 We speculated that LINC00511 might involve in the NSCLC progression. Here, we identified and characterized LINC00511 as an oncogenic lncRNA in NSCLC by analyzing a cohort of 124 pairs NSCLC tumorous and normal tissues, and results revealed that LINC00511 was upregulated in NSCLC tumorous tissues and markedly correlated with poor prognosis and shorter survival. Moreover, silencing LINC00511 also impaired cell proliferation, migration and invasion, and facilitated cell apoptosis in vitro, and inhibited tumorigenesis of NSCLC cells in vivo.
Generally, lncRNA involved in regulation of cancer cells phenotypes by regulating target gene expression by different mechanisms, including chromatin modification, genomic imprinting, RNA decay and sponging miRNAs. p57 is an inhibitor for cyclin-dependent kinase, and is deemed as a candidate of tumor-suppressive gene that has been embroiled in numerous of cancers.37,38,39,40,41 In this study, we analyzed the correction between the p57 mRNA levels and the survival of NSCLC patients from 2437 lung tumors using publicly available datasets (2015 version) (http://kmplot.com/analysis/index.php?p=service&cancer=lung). And Kaplan-Meier analysis demonstrated lower p57 mRNA levels were markedly correlated with the outcome of NSCLC patients. In addition, we also found that knockdown of LINC00511 increased the expression of p57 in NSCLC cells. We also found knockdown of LINC00511 and repressed cell proliferation, migration and invasion, and facilitated cell apoptosis, while knockdown of p57 reversed the negative role of cell proliferation, migration and invasion, and favorable role of cell apoptosis in LINC00511-defected A549 and SPC-A-1 cells, which indicated p57 was a novel LINC00511 target, and LINC00511 could function as oncogene through suppressing p57 expression in NSCLC cells.
We next explore the potential mechanism of LINC00511's suppressive role on p57 expression. p57 is also a direct target of EZH2 and repressed by serveral epigenetic mechanisms in ovarian cancer43 and breast cancer.44 Here, we discovered that LINC00511 directly binding with EZH2 in NSCLC cells, which suggesting that LINC00511 might also could regulate underlying targets at transcriptional levels. Further experiments revealed that LINC00511 simultaneously recruits EZH2 to p57 promoter region and represses their transcription via mediating trimethylation of histone H3 at lysine 27 (H3K27me3). Hypothesis of the mechanism of LINC00511 on NSCLC is shown in Figure 10. These findings indicated that LINC00511 play crucial roles in EZH2 mediated repression of tumor suppressors in NSCLC cancer cells.
In conclusion, our data clarify that LINC00511 is upregulated in NSCLC tumorous tissues and cell lines, and corrected with poor prognosis in NSCLC patients. LINC00511 knockdown repressed cell proliferation, migration and invasion, and facilitated cell apoptosis in vitro, and inhibited tumorigenesis in vivo. Furthermore, LINC00511-mediated oncogenic effects are partially through its epigenetically silencing of the p57 expression via directly binding with EZH2 (a part of PRC2). Our findings elucidate a potential mechanism underlying the tumor-oncogenic role of LINC00511 in NSCLC, and indicate that LINC00511 could be a useful marker and potential therapeutic target in NSCLC. However, whether LINC00511 could regulate other possible targets and the mechanisms that underlie regulatory behaviors were not investigated in this study, which needs to be further investigated.
Materials and methods
Tissue collection. Fresh and formalin-fixed, paraffin-embedded, NSCLC tumor tissue samples were obtained from patients who were diagnosed with NSCLC. Elective surgery was carried out on these patients at ZhongNan Hospital of Wuhan University (Wuhan, China). In total, 124 pairs of fresh NSCLC and adjacent non-tumor tissues (more than 5 cm away from the tumor) were freshly frozen in liquid nitrogen and stored at −80 °C until further use. 124 cases of archived, formalin-fixed, paraffin-embedded NSCLC tissue samples were collected and used in clinicopathological and prognostic investigation of LINC00511. The use of tissues for this study has been approved by the ethics committee of ZhongNan Hospital of Wuhan University. Before using these clinical materials for research purposes, all the patients have signed the informed consent. None of these patients received any preoperative chemotherapy or radiotherapy.
Cell culture and transfection. Ten NSCLC cell lines (A549, SK-MES-1, H1299, 95D, H460, H520, H1975, H157, SK-LU-1, and SPC-A-1) and the 16HBE cell lines were purchased from the Institute of Biochemistry and Cell Biology of the Chinese Academy of Sciences (Shanghai, China). Cells were cultured in RPMI 1640 (Gibco, Grand Island, NY) medium supplemented with 10% fetal bovine serum, 100 U/ml penicillin, and 100 mg/ml streptomycin (Gibco) in humidified air at 37 °C with 5% CO2. si-LINC00511, si-p57, were purchased from GenePharma (Shanghai, China). Complete medium without antibiotics was used to culture the cells at least 24 hours prior to transfection. The cells were washed with 1× phosphate buffer saline (PBS) (pH7.4) and then transiently transfected with 50 nmol/l si-NC, si-LINC00511, or si-p57, using Lipofectamine 2000 (Invitrogen, Carlsbad, CA) according to the manufacturer's instructions.
Western blot analysis. Western blot was performed using the protocol described previously.47,48,49 The following primary antibodies were used: rabbit anti-EZH2 (Santa Cruz), rabbit anti-p57 (Santa Cruz Biotechnology, Santa Cruz, CA), rabbit anti-GAPDH (Santa Cruz).
siRNA knockdown experiments. Stealth siRNA oligonucleotides were synthesized by Invitrogen. The following siRNA sequences were used for knockdown of LINC00511 (NR 033876): LINC00511 siRNA 1 (5′-CCCAUGUCUGCUGUGCCUUUGUACU-3′), LINC00511 siRNA 2 (5′-CCAGUGUGUGCUGAUGACACAUACA-3′) or a control (a scrambled matched %GC oligonucleotide synthesized by Invitrogen). And we used siRNA 1 as a representative siRNA for LINC00511 on account of its strong knockout efficiency (Supplementary Figure S1). The following siRNA sequences were used for knockdown of p57 (NM 000076): p57 siRNA 1 (5′-CAGCACATCCACGATGGAGCGTCTT-3′), p57 siRNA 2 (5′-TCGCCCGTGGGACCTTCCCAGTACT-3′) or a control (a scrambled matched %GC oligonucleotide synthesized by Invitrogen). And we used siRNA 1 as a representative siRNA for p57 on account of its strong knockout efficiency (Supplementary Figure S2). Cells were transfected with 50 pmol of siRNA and the scrambled control oligo with RNAimax Lipofecatmine (Invitrogen) following manufacturer's instructions. Knockdown efficiency was determined by quantitative PCR at time of plating for assay.
RNA isolation and qRT-PCR. RNA isolation and qRT-PCR was carried out using the protocol described previously.50,51 Glyceraldehyde 3-phosphate dehydrogenase (GADPH) and U1 were used as endogenous controls. The relative expression level was calculated using the 2-ΔΔCt method. The primer sequences used in this study are as follows: human LINC00511: sense: 5′-CGCAAGGACCCTCTGTTAGG-3′, antisense: 5′-GAAGGCGGATCGTCTCTCAG-3′; human p57: sense: 5′-AGACCATGTGGACCTGTCACTG3-3′, antisense: 5′-GTTTGGAGTGGTAGAAATCTGTC-3′; human EZH2: sense: 5′-TGCACATCCTGACTTCTGTG-3′, antisense: 5′-AAGGGCATTCACCAACTCC-3′; human U1: sense: 5′-CAGGGCGAGGCTTATCCA-3′, antisense: 5′-GCAGGGGTCAGCACATCC-3′; human GAPDH: sense: 5′-CTCTGCTCCTCCTGTTCGAC-3′, antisense: 5′-ACCAAATCCGTTGACTCCGA-3′. The formula and its derivations were obtained from the ABI Prism 7300 sequence detection system user guide. Statistical analysis was performed on the fold change.
Colony formation assay. Colony formation assay was carried out using the protocol described previously.52,53,54,55
Tumor formation in BALB/c nude mice. BALB/c athymic nude mice (male, 4–6 weeks old and 16–20 g) were purchased from Hubei Research Center of Laboratory Animal (Wuhan, China). All animal experiments were carried out in accordance with the Guide for the Care and Use of Laboratory Animals of Wuhan University. To establish lung cancer xenograft model, 5 × 105 empty vector or sh-LINC00511-A549 cells were suspended in 100 μl phosphate-buffered saline and inoculated subcutaneously into the flanks of 10 nude mice (n = 5 each). The following shRNA sequences were used for knockdown of LINC00511 (NR 033876): LINC00511 shRNA 1 (5′-CACCGCCCATGTCTGCTGTGCCTTTGTACTCTCGAGAGTACAAAGGCACACAGACATGGG-3′), LINC00511 shRNA 2 (5′-CACCGCCAGTGTGTGCTGATGACACATACACTCGAGTGTATGTGTCATCAGCACACACTGG-3′). And we used shRNA 1 as a representative shRNA for LINC00511 on account of its strong knockout efficiency (Supplementary Figure S1). The tumor size was monitored by measuring the length (L) and width (W) with calipers every 4 day, and the volumes were calculated using the formula: (L × W2)/2. Mice were killed by cervical dislocation in day 36, and the tumors were excised and snap-frozen for protein and RNA extraction.
RNA pull-down assays. LINC00511 transcripts were transcribed using T7 RNA polymerase (Ambio life) in vitro, then by using the RNeasy Plus Mini Kit (Qiagen) and treated with DNase I (Qiagen). Purified RNAs were biotin-labeled with the Biotin RNA Labeling Mix (Ambio life). Positive control, negative control and Biotinylated RNAs were mixed and incubated with A549 cell lysates. Then, magnetic beads were added to each binding reaction, and incubated at room temperature. Finally, the beads were washed, and the eluted proteins were detected by western blot analysis.
Flow cytometry
Apoptosis analysis. A549 and SPC-A-1 cells transfected with si-NC or si-LINC00511 were trypsinized and resuspended in 1× binding buffer at 1 × 106 cells/ml. 100 μl of this cell suspension was incubated with 5 μl of FITC-Annexin V and 5 μl propridium iodide (PI) for 15 minutes in the dark. The reaction was terminated with the addition of 400 μl 1× binding buffer and analyzed with FACSCalibur using the CellQuest software (Becton Dickinson). FITC-Annexin V-positive and PI-negative cells were considered as apoptotic and the experiments were carried out in triplicates.
Cell-cycle analysis. Transfected cells were harvested 48 hours after transfection. The cells were fixed in 70% ethanol, washed once with PBS, and then labeled with propidium iodide (Sigma-Aldrich) in the presence of RNase A (Sigma-Aldrich) for 30 minutes in the dark (50 g/ml). Samples were run on a FACSalibur flow cytometer (Becton-Dickinson, FL, NJ), and the percentages of cells within each phase of the cell cycle were analyzed using Cell Quest software.
Caspase-3/7 activity assay. A caspase-3/7 ELISA kit (BD Pharmingen, San Diego, CA) was used for in vitro determination of caspase-3/7 activity in cell lysates according to manufacturer's instruction.
Chromatin immunoprecipitation. A549 and SPC-A-1 cells were treated with formaldehyde and incubated for 10 minutes to generate DNA-protein cross-links. Cell lysates were then sonicated to generate chromatin fragments of 200–300 bp and immunoprecipitated with EZH2-specific antibody (Millipore) or IgG as control. Precipitated chromatin DNA was recovered and analyzed by qRT-PCR. ChIP qPCR primers for p57 are as follows: sense: 5′-GGTGTCTAGGTGCTCCAGGT-3′, antisense: 5′-GCACTCTCCAGGAGGACACA-3′.
Immunohistochemistry. Immunohistochemistry of the tumor tissues was performed as described previously.35,36,37 3-μm tumor sections were incubated with commercial rabbit polyclonal antibodies against Ki67 (Santa Cruz), p57 (Santa Cruz) at 1/100 dilution overnight at 4 °C. Then, the sections were conjugated with horseradish peroxidase antibody (1:500 dilution; Santa Cruz Biotechnology, Santa Cruz, CA) at room temperature for 2 hours, then covered by diaminobenzidine (DAB) (Vector Laboratories, Burlingame, CA), and slides were mounted with Vectashield mounting medium (Vector Laboratories). Subsequently, all fields were observed under light microscopy (Olympus 600 auto-biochemical analyzer, Tokyo, Japan). Control experiments without primary antibody demonstrated that the signals observed were specific.
Statistical analysis. The Students t-test (two tailed), one-way analysis of variance, and Mann-Whitney U-test were conducted to analyze the in vitro and in vivo data by SPSS 19.0 software. P < 0.05 was considered significant.
SUPPLEMENTARY MATERIAL Figure S1. Relative LINC00511 expression in A549 and SPC-A-1 cells after transfecting with si-LINC00511, namely, siRNA1 and siRNA2 for forty-eight hours. Figure S2. Relative p57 expression in A549 and SPC-A-1 cells after transfecting with si-p57, namely, siRNA1 and siRNA2 for forty-eight hours.
Author contributions
Participated in research design: C-C.S., S-J.L., and D-J.L. Conducted experiments: C-C.S., S-J.L., and D-J.L. Contributed new reagents or analytic tools: C-C.S., S-J.L., and D-J.L. Performed data analysis: C-C.S., S-J.L., G.L., R-X.H., X-H.Z., and D-J.L. Wrote or contributed to the writing of the manuscript: C-C.S., S-J.L., and D-J.L.
Figure 10.
Hypothesis of the mechanism of LINC00511 on non–small-cell lung cancer.
Acknowledgments
This work was supported by National Natural Science Foundation of China (No. 81271943) to D-J.L. The plan for Scientific and Technological Innovation Team of High-tech Industries of Wuhan Municipal Science and Technology Bureau (No. 2015070504020219) to D-J.L. and the Fundamental Research Funds for the Central Universities (No. 2015305020202) to C-C.S. The authors declare that they have no competing interests.
Supplementary Material
References
- DeSantis, CE, Lin, CC, Mariotto, AB, Siegel, RL, Stein, KD, Kramer, JL et al. (2014). Cancer treatment and survivorship statistics, 2014. CA Cancer J Clin 64: 252–271. [DOI] [PubMed] [Google Scholar]
- Sanoff, HK, Sargent, DJ, Campbell, ME, Morton, RF, Fuchs, CS, Ramanathan, RK et al. (2008). Five-year data and prognostic factor analysis of oxaliplatin and irinotecan combinations for advanced colorectal cancer: N9741. J Clin Oncol 26: 5721–5727. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sung, JJ, Ng, SC, Chan, FK, Chiu, HM, Kim, HS, Matsuda, T et al.; Asia Pacific Working Group. (2015). An updated Asia Pacific Consensus Recommendations on colorectal cancer screening. Gut 64: 121–132. [DOI] [PubMed] [Google Scholar]
- Torre, LA, Bray, F, Siegel, RL, Ferlay, J, Lortet-Tieulent, J and Jemal, A (2015). Global cancer statistics, 2012. CA Cancer J Clin 65: 87–108. [DOI] [PubMed] [Google Scholar]
- De Rosa, M, Pace, U, Rega, D, Costabile, V, Duraturo, F, Izzo, P et al. (2015). Genetics, diagnosis and management of colorectal cancer (Review). Oncol Rep 34: 1087–1096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sung, JJ, Lau, JY, Young, GP, Sano, Y, Chiu, HM, Byeon, JS et al.; Asia Pacific Working Group on Colorectal Cancer. (2008). Asia Pacific consensus recommendations for colorectal cancer screening. Gut 57: 1166–1176. [DOI] [PubMed] [Google Scholar]
- Rerknimitr, R, Angsuwatcharakon, P, Ratanachu-ek, T, Khor, CJ, Ponnudurai, R, Moon, JH et al.; Asia-Pacific Working Group on Hepatobiliary Cancers. (2013). Asia-Pacific consensus recommendations for endoscopic and interventional management of hilar cholangiocarcinoma. J Gastroenterol Hepatol 28: 593–607. [DOI] [PubMed] [Google Scholar]
- Mercer, TR, Dinger, ME and Mattick, JS (2009). Long non-coding RNAs: insights into functions. Nat Rev Genet 10: 155–159. [DOI] [PubMed] [Google Scholar]
- Ørom, UA, Derrien, T, Beringer, M, Gumireddy, K, Gardini, A, Bussotti, G et al. (2010). Long noncoding RNAs with enhancer-like function in human cells. Cell 143: 46–58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huarte, M, Guttman, M, Feldser, D, Garber, M, Koziol, MJ, Kenzelmann-Broz, D et al. (2010). A large intergenic noncoding RNA induced by p53 mediates global gene repression in the p53 response. Cell 142: 409–419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Geisler, S, Lojek, L, Khalil, AM, Baker, KE and Coller, J (2012). Decapping of long noncoding RNAs regulates inducible genes. Mol Cell 45: 279–291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rinn, JL, Kertesz, M, Wang, JK, Squazzo, SL, Xu, X, Brugmann, SA et al. (2007). Functional demarcation of active and silent chromatin domains in human HOX loci by noncoding RNAs. Cell 129: 1311–1323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pandey, GK, Mitra, S, Subhash, S, Hertwig, F, Kanduri, M, Mishra, K et al. (2014). The risk-associated long noncoding RNA NBAT-1 controls neuroblastoma progression by regulating cell proliferation and neuronal differentiation. Cancer Cell 26: 722–737. [DOI] [PubMed] [Google Scholar]
- Garzon, R, Volinia, S, Papaioannou, D, Nicolet, D, Kohlschmidt, J, Yan, PS et al. (2014). Expression and prognostic impact of lncRNAs in acute myeloid leukemia. Proc Natl Acad Sci USA 111: 18679–18684. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang, F, Huo, XS, Yuan, SX, Zhang, L, Zhou, WP, Wang, F et al. (2013). Repression of the long noncoding RNA-LET by histone deacetylase 3 contributes to hypoxia-mediated metastasis. Mol Cell 49: 1083–1096. [DOI] [PubMed] [Google Scholar]
- Barnhill, LM, Williams, RT, Cohen, O, Kim, Y, Batova, A, Mielke, JA et al. (2014). High expression of CAI2, a 9p21-embedded long noncoding RNA, contributes to advanced-stage neuroblastoma. Cancer Res 74: 3753–3763. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang, F, Zhang, L, Huo, XS, Yuan, JH, Xu, D, Yuan, SX et al. (2011). Long noncoding RNA high expression in hepatocellular carcinoma facilitates tumor growth through enhancer of zeste homolog 2 in humans. Hepatology 54: 1679–1689. [DOI] [PubMed] [Google Scholar]
- Prensner, JR, Iyer, MK, Balbin, OA, Dhanasekaran, SM, Cao, Q, Brenner, JC et al. (2011). Transcriptome sequencing across a prostate cancer cohort identifies PCAT-1, an unannotated lincRNA implicated in disease progression. Nat Biotechnol 29: 742–749. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang, F, Zhang, L, Huo, XS, Yuan, JH, Xu, D, Yuan, SX et al. (2011). Long noncoding RNA high expression in hepatocellular carcinoma facilitates tumor growth through enhancer of zeste homolog 2 in humans. Hepatology 54: 1679–1689. [DOI] [PubMed] [Google Scholar]
- Wang, F, Yuan, JH, Wang, SB, Yang, F, Yuan, SX, Ye, C et al. (2014). Oncofetal long noncoding RNA PVT1 promotes proliferation and stem cell-like property of hepatocellular carcinoma cells by stabilizing NOP2. Hepatology 60: 1278–1290. [DOI] [PubMed] [Google Scholar]
- Yuan, SX, Wang, J, Yang, F, Tao, QF, Zhang, J, Wang, LL et al. (2016). Long noncoding RNA DANCR increases stemness features of hepatocellular carcinoma by derepression of CTNNB1. Hepatology 63: 499–511. [DOI] [PubMed] [Google Scholar]
- Yuan, SX, Yang, F, Yang, Y, Tao, QF, Zhang, J, Huang, G et al. (2012). Long noncoding RNA associated with microvascular invasion in hepatocellular carcinoma promotes angiogenesis and serves as a predictor for hepatocellular carcinoma patients' poor recurrence-free survival after hepatectomy. Hepatology 56: 2231–2241. [DOI] [PubMed] [Google Scholar]
- Yap, KL, Li, S, Muñoz-Cabello, AM, Raguz, S, Zeng, L, Mujtaba, S et al. (2010). Molecular interplay of the noncoding RNA ANRIL and methylated histone H3 lysine 27 by polycomb CBX7 in transcriptional silencing of INK4a. Mol Cell 38: 662–674. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pasmant, E, Laurendeau, I, Héron, D, Vidaud, M, Vidaud, D and Bièche, I (2007). Characterization of a germ-line deletion, including the entire INK4/ARF locus, in a melanoma-neural system tumor family: identification of ANRIL, an antisense noncoding RNA whose expression coclusters with ARF. Cancer Res 67: 3963–3969. [DOI] [PubMed] [Google Scholar]
- Gupta, RA, Shah, N, Wang, KC, Kim, J, Horlings, HM, Wong, DJ et al. (2010). Long non-coding RNA HOTAIR reprograms chromatin state to promote cancer metastasis. Nature 464: 1071–1076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang, KC and Chang, HY (2011). Molecular mechanisms of long noncoding RNAs. Mol Cell 43: 904–914. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsai, MC, Manor, O, Wan, Y, Mosammaparast, N, Wang, JK, Lan, F et al. (2010). Long noncoding RNA as modular scaffold of histone modification complexes. Science 329: 689–693. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yuan, JH, Yang, F, Wang, F, Ma, JZ, Guo, YJ, Tao, QF et al. (2014). A long noncoding RNA activated by TGF-β promotes the invasion-metastasis cascade in hepatocellular carcinoma. Cancer Cell 25: 666–681. [DOI] [PubMed] [Google Scholar]
- de Kok, JB, Verhaegh, GW, Roelofs, RW, Hessels, D, Kiemeney, LA, Aalders, TW et al. (2002). DD3(PCA3), a very sensitive and specific marker to detect prostate tumors. Cancer Res 62: 2695–2698. [PubMed] [Google Scholar]
- Sun, C, Li, S,Zhang, F, Xi, Y, Wang, L, Bi, Y et al. (2016). Long non-coding RNA NEAT1 promotes non-small cell lung cancer progression through regulation of miR-377-3p-E2F3 pathway. Oncotarget 7: 51784–51814. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zequn, N, Xuemei, Z, Wei, L, Zongjuan, M, Yujie, Z, Yanli, H et al. (2016). The role and potential mechanisms of LncRNA-TATDN1 on metastasis and invasion of non-small cell lung cancer. Oncotarget 7: 18219–18228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen, Z, Li, JL, Lin, S, Cao, C, Gimbrone, NT, Yang, R et al. (2016). cAMP/CREB-regulated LINC00473 marks LKB1-inactivated lung cancer and mediates tumor growth. J Clin Invest 126: 2267–2279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li, W, Sun, M, Zang, C, Ma, P, He, J, Zhang, M et al. (2016). Upregulated long non-coding RNA AGAP2-AS1 represses LATS2 and KLF2 expression through interacting with EZH2 and LSD1 in non-small-cell lung cancer cells. Cell Death Dis 7: e2225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pan, H et al. (2016). Long non-coding RNA BC087858 induces non-T790M mutation acquired resistance to EGFR-TKIs by activating PI3K/AKT and MEK/ERK pathways and EMT in non-small-cell lung cancer. Oncotarget 7: 49948–49960. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zang, C, Nie, FQ, Wang, Q, Sun, M, Li, W, He, J et al. (2016). Long non-coding RNA LINC01133 represses KLF2, P21 and E-cadherin transcription through binding with EZH2, LSD1 in non small cell lung cancer. Oncotarget 7: 11696–11707. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang, YS, Chang, CC, Lee, SS, Jou, YS and Shih, HM (2016). Xist reduction in breast cancer upregulates AKT phosphorylation via HDAC3-mediated repression of PHLPP1 expression. Oncotarget 7: 43256–43266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zou, P, Yoshihara, H, Hosokawa, K, Tai, I, Shinmyozu, K, Tsukahara, F et al. (2011). p57(Kip2) and p27(Kip1) cooperate to maintain hematopoietic stem cell quiescence through interactions with Hsc70. Cell Stem Cell 9: 247–261. [DOI] [PubMed] [Google Scholar]
- Avrahami, D, Li, C, Yu, M, Jiao, Y, Zhang, J, Naji, A et al. (2014). Targeting the cell cycle inhibitor p57Kip2 promotes adult human β cell replication. J Clin Invest 124: 670–674. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Naito, M, Mori, M, Inagawa, M, Miyata, K, Hashimoto, N, Tanaka, S et al. (2016). Dnmt3a regulates proliferation of muscle satellite cells via p57Kip2. PLoS Genet 12: e1006167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sun, CC, Li, SJ and Li, DJ (2016). Hsa-miR-134 suppresses non-small cell lung cancer (NSCLC) development through down-regulation of CCND1. Oncotarget 7: 35960–35978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sun, C, Huang, C, Li, S, Yang, C, Xi, Y, Wang, L et al. (2016). Hsa-miR-326 targets CCND1 and inhibits non-small cell lung cancer development. Oncotarget 7: 8341–8359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nie, F, Yu, X, Huang, M, Wang, Y, Xie, M, Ma, H et al. (2016). Long noncoding RNA ZFAS1 promotes gastric cancer cells proliferation by epigenetically repressing KLF2 and NKD2 expression. Oncotarget (epub ahead of print). [DOI] [PMC free article] [PubMed]
- Guo, J, Cai, J, Yu, L, Tang, H, Chen, C and Wang, Z (2011). EZH2 regulates expression of p57 and contributes to progression of ovarian cancer in vitro and in vivo. Cancer Sci 102: 530–539. [DOI] [PubMed] [Google Scholar]
- Yang, X, Karuturi, RK, Sun, F, Aau, M, Yu, K, Shao, R et al. (2009). CDKN1C (p57) is a direct target of EZH2 and suppressed by multiple epigenetic mechanisms in breast cancer cells. PLoS One 4: e5011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oh, TG, Wang, SC, Acharya, BR, Goode, JM, Graham, JD, Clarke, CL et al. (2016). The nuclear receptor, RORγ, regulates pathways necessary for breast cancer metastasis. EBioMedicine 6: 59–72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang, F, Lyu, S, Dong, S, Liu, Y, Zhang, X and Wang, O (2016). Expression profile analysis of long noncoding RNA in HER-2-enriched subtype breast cancer by next-generation sequencing and bioinformatics. Onco Targets Ther 9: 761–772. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sun, C, Yang, C, Xue, R, Li, S, Zhang, T, Pan, L et al. (2015). Sulforaphane alleviates muscular dystrophy in mdx mice by activation of Nrf2. J Appl Physiol (1985) 118: 224–237. [DOI] [PubMed] [Google Scholar]
- Sun, CC, Li, SJ, Yang, CL, Xue, RL, Xi, YY, Wang, L et al. (2015). Sulforaphane attenuates muscle inflammation in dystrophin-deficient mdx mice via NF-E2-related factor 2 (Nrf2)-mediated inhibition of NF-κB signaling pathway. J Biol Chem 290: 17784–17795. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- Sun, C, Li, S and Li, D (2016). Sulforaphane mitigates muscle fibrosis in mdx mice via Nrf2-mediated inhibition of TGF-β/Smad signaling. J Appl Physiol (1985) 120: 377–390. [DOI] [PubMed] [Google Scholar]
- Sun, C, Liu, Z, Li, S, Yang, C, Xue, R, Xi, Y et al. (2015). Down-regulation of c-Met and Bcl2 by microRNA-206, activates apoptosis, and inhibits tumor cell proliferation, migration and colony formation. Oncotarget 6: 25533–25574. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sun, C, Sang, M, Li, S, Sun, X, Yang, C, Xi, Y et al. (2015). Hsa-miR-139-5p inhibits proliferation and causes apoptosis associated with down-regulation of c-Met. Oncotarget 6: 39756–39792. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- Sun, CC, Li, SJ and Li, DJ (2016). Hsa-miR-134 suppresses non-small cell lung cancer (NSCLC) development through down-regulation of CCND1. Oncotarget 7: 35960–35978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sun, C, Huang, C, Li, S, Yang, C, Xi, Y, Wang, L et al. (2016). Hsa-miR-326 targets CCND1 and inhibits non-small cell lung cancer development. Oncotarget 7: 8341–8359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sun, CC, Li, SJ, Zhang, F, Pan, JY, Wang, L, Yang, CL et al. (2016). Hsa-miR-329 exerts tumor suppressor function through down-regulation of MET in non-small cell lung cancer. Oncotarget 7: 21510–21526. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sun, C, Li, S, Yang, C, Xi, Y, Wang, L, Zhang, F et al. (2016). MicroRNA-187-3p mitigates non-small cell lung cancer (NSCLC) development through down-regulation of BCL6. Biochem Biophys Res Commun 471: 82–88. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.