GOT1B regulates COPII-mediated protein export from the endoplasmic reticulum exit sites in developing rice endosperm cells.
Abstract
Coat protein complex II (COPII) mediates the first step of anterograde transport of newly synthesized proteins from the endoplasmic reticulum (ER) to other endomembrane compartments in eukaryotes. A group of evolutionarily conserved proteins (Sar1, Sec23, Sec24, Sec13, and Sec31) constitutes the basic COPII coat machinery; however, the details of how the COPII coat assembly is regulated remain unclear. Here, we report a protein transport mutant of rice (Oryza sativa), named glutelin precursor accumulation4 (gpa4), which accumulates 57-kD glutelin precursors and forms two types of ER-derived abnormal structures. GPA4 encodes the evolutionarily conserved membrane protein GOT1B (also known as GLUP2), homologous to the Saccharomyces cerevisiae GOT1p. The rice GOT1B protein colocalizes with Arabidopsis thaliana Sar1b at Golgi-associated ER exit sites (ERESs) when they are coexpressed in Nicotiana benthamiana. Moreover, GOT1B physically interacts with rice Sec23, and both proteins are present in the same complex(es) with rice Sar1b. The distribution of rice Sar1 in the endomembrane system, its association with rice Sec23c, and the ERES organization pattern are significantly altered in the gpa4 mutant. Taken together, our results suggest that GOT1B plays an important role in mediating COPII vesicle formation at ERESs, thus facilitating anterograde transport of secretory proteins in plant cells.
INTRODUCTION
Coat protein complex II (COPII)-mediated anterograde transport of newly synthesized proteins from the endoplasmic reticulum (ER) to the Golgi apparatus is a vital cellular process in all eukaryotes so far analyzed (D’Arcangelo et al., 2013; Venditti et al., 2014). Numerous studies of yeast and mammalian cells have suggested a model in which five conserved proteins (Sar1, Sec23, Sec24, Sec13, and Sec31) constitute the basic COPII coat machinery that can fulfill the essential function of vesicle formation (Miller and Barlowe, 2010). The assembly of COPII coat occurs on the ER membrane in a step-wise fashion and is initiated by the small GTPase Sar1 (Secretion-associated and ras-superfamily related1), which is activated by the guanine nucleotide exchange factor Sec12, an ER-localized integral membrane protein (Barlowe and Schekman, 1993). The GTP binding of Sar1 causes a conformational change that exposes its N-terminal amphipathic α-helix, which inserts into the ER membrane to initiate vesicle formation. Membrane-bound activated Sar1 then recruits the heterodimeric cargo adaptor platform Sec23/Sec24 through direct interaction with Sec23, forming the prebudding complexes. Sec24 discriminates cargo molecules for incorporation into COPII vesicles by recognizing specific ER export signals on diverse proteins (Miller et al., 2002, 2003). The membrane-bound inner coat complex Sar1-Sec23-Sec24 in turn recruits the Sec13-Sec31 heterotetramer, which forms the cage-like outer layer of the COPII coat to drive ER membrane curvature and release of the vesicles (Aridor et al., 1998; Giraudo and Maccioni, 2003; Stagg et al., 2006). Downstream events, including hydrolysis of Sar1 in the completed coat, catalyzed by Sec23 and the outer coat, lead to uncoating of the transport vesicles and recycling of the COPII components (Bi et al., 2002, 2007).
In addition to the above five COPII proteins that constitute the minimal COPII coat machinery, several accessory factors that are responsible for modulating coat protein recruitment and COPII vesicle formation at ER exit sites (ERESs) have been identified, including Sec16, Sec12, Sed4, phosphatidylinositol 4-phosphate, p125A, and ALG-2 (D’Arcangelo et al., 2013). Another potential regulator of COPII vesicle formation in yeast is GOT1p (Golgi transport1), which is not essential for yeast growth, but its deletion significantly affects the transport efficiency between the ER and the Golgi compartments in vitro (Conchon et al., 1999). GOT1p is efficiently packaged into in vitro-generated COPII vesicles; however, efforts to demonstrate physical interaction between GOT1p and COPII coat components have failed (Lorente-Rodríguez et al., 2009). Thus, the exact role of GOT1p in the regulation of COPII vesicle-mediated transport remains elusive.
Increasing evidence has shown that COPII vesicles also mediate protein export from the ER in plants (Marti et al., 2010). Many of the major molecular players involved in COPII-mediated ER-Golgi trafficking have homologs in plants and seem to play similar roles as their yeast and mammalian counterparts. For example, transient expression of a dominant-negative Sar1 (Arabidopsis thaliana Sar1 H74L) mutant in tobacco (Nicotiana benthamiana) and Arabidopsis cultured cells leads to retention of two Golgi membrane proteins and a vacuolar storage protein in the ER, indicating a role for Sar1 in protein exit from the ER (Takeuchi et al. 2000). A partial loss-of-function mutation in Arabidopsis Sec24A affects the recruitment of Sec24A to ERESs, resulting in the formation of aberrant tubular clusters of ER and Golgi membranes, suggesting that COPII coat proteins are important for maintaining ER and Golgi membrane integrity in relation to ER protein export in plants (Faso et al., 2009). In addition, analysis of an Arabidopsis Sec16A loss-of-function mutant demonstrated that Sec16A is involved in the dynamic association of COPII coat components on the ER as Sec24 and Sec13 were found to cycle on and off the ERES at a much faster rate than in wild-type cells (Takagi et al., 2013). Through live-cell imaging analyses, the Arabidopsis COPII components (Sec13, Sec23, Sec24, and Sec31) have been found in punctate structures that are associated with the ER and move with the Golgi stacks when expressed in highly vacuolated leaf epidermal cells (Stefano et al., 2006; Hanton et al., 2007, 2009; Sieben et al., 2008; Wei and Wang, 2008; Faso et al., 2009; Takagi et al., 2013; Tanaka et al., 2013). These COPII coat protein-labeled punctate structures are commonly indicated as ERESs. Notably, Arabidopsis Sar1 has been found at the ERESs but also over the ER network to a variable degree that may depend on the specific Sar1 isoform (Hanton et al., 2008). In rice (Oryza sativa), simultaneous knockdown of three Sar1 isoforms prevents vacuolar storage proteins from exiting the ER in developing endosperm, suggesting an involvement of COPII vesicles in the early secretory pathway in monocotyledonous plants (Tian et al., 2013). Despite great efforts and advances, our knowledge of the highly regulated process of COPII vesicle formation and its regulation is still limited in plants.
Plants generally accumulate large amounts of storage proteins in the seeds, which provide nutrition for seed germination and seedling development. In rice, three types of major storage proteins accumulate in the endosperm, including glutelins, prolamins, and α-globulin. The prolamins are retained in the ER lumen after synthesis and are pinched off to form spherical protein bodies I (PBI) (Bechtel and Juliano, 1980; Tanaka et al., 1980; Yamagata and Tanaka, 1986). Glutelins are initially synthesized on the rough endoplasmic reticulum (RER) as 57-kD precursors and then transported to the protein storage vacuoles (PSVs; also called protein body II [PBII]) through the Golgi apparatus and the dense vesicle-mediated post-Golgi trafficking pathway (Krishnan et al., 1986; Takemoto et al., 2002; Liu et al., 2013, Ren et al., 2014). The α-globulin is deposited together with glutelins in PBIIs. Only those proglutelins arriving at the PBII/PSV can be cleaved into the mature 40-kD acidic and 20-kD basic subunits (Wang et al., 2009; Kumamaru et al., 2010). Any defects in the proglutelin trafficking process before reaching the PBII can lead to overaccumulation of the 57-kD precursor proteins (referred to as the 57H phenotype) in the seeds. Through the studies of 57H mutants, several key factors involved in post-Golgi trafficking of storage proteins have been characterized (Wang et al., 2010; Fukuda et al., 2011, 2013; Liu et al., 2013; Ren et al., 2014). However, how these storage proteins are first exported from the ER remains largely unknown.
In this study, we report the functional characterization of the rice gpa4 mutant, which overaccumulates proglutelins in the mature seeds. We show that GPA4 encodes an evolutionarily conserved membrane protein GOT1B, homologous to the Saccharomyces cerevisiae GOT1p, a protein known to be involved in vesicular trafficking. When expressed in N. benthamiana, rice GOT1B colocalizes with Arabidopsis Sar1b at the ERESs. Yeast two-hybrid hunting identified rice Sec23, a key component of the minimal COPII coat machinery, as an interacting partner of GOT1B. Through a series of biochemical and cellular studies, we concluded that GOT1B is associated with Sec23c and Sar1b in protein complexes in vivo and plays an important role in the proper assembly of the COPII prebudding complex at the ERES sites, thus affecting anterograde transport of secretory proteins (from ER to Golgi) in plant cells. Furthermore, we present evidence that this mechanism is likely to be conserved across the eukaryotic kingdom.
RESULTS
The gpa4-1 Mutant Has a Defect in Vacuolar Protein Trafficking
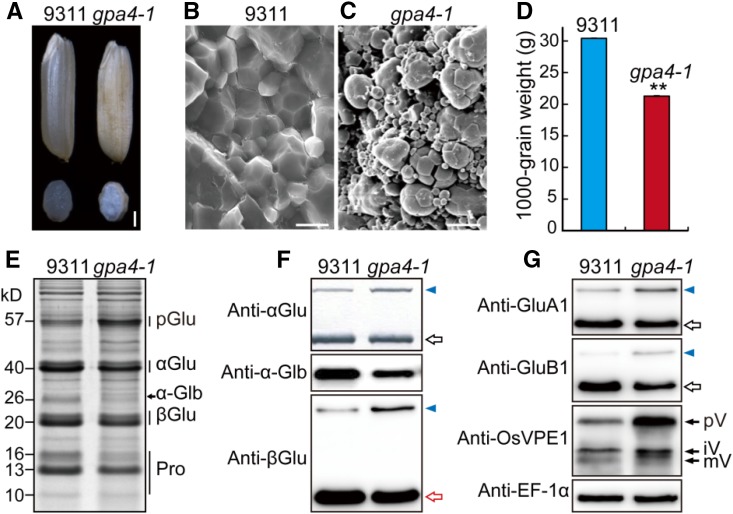
As part of our continuing efforts to dissect the vacuolar sorting mechanisms of seed storage proteins in rice, we isolated two allelic 57H mutants named gpa4-1 (in the indica variety 9311 background) and gpa4-2 (in the japonica variety Kinmaze background). Phenotypic characterization was performed with gpa4-1 (which carries a loss-of-function mutation; see below). In contrast to the transparent wild-type seeds, gpa4-1 seeds appeared floury (Figure 1A). Scanning electron microscopy analysis revealed that the gpa4-1 endosperm comprised round and loosely packaged compound starch granules instead of the tightly packaged, crystal-like structures found in the wild type (Figures 1B and 1C). Meanwhile, the 1000-grain weight was significantly reduced in the gpa4-1 mutant (Figure 1D; Supplemental Table 1). Interestingly, compared with the wild type, gpa4-1 accumulated a higher amount of 57-kD proglutelins, accompanied by a significant reduction of the 40-kD acidic and 20-kD basic subunits of the mature glutelins as well as the 26-kD α-globulin. Prolamins of 16 and 13 kD were both notably decreased as well (Figures 1E and 1F). Immunoblots with isoform-specific antibodies revealed increased accumulation of proglutelins for the major glutelin subfamilies (GluA and GluB). Moreover, the precursor of vacuole-localized VPE1 was also greatly elevated in gpa4-1 (Figure 1G; Wang et al., 2009). These results suggest that gpa4-1 is defective in the vacuolar trafficking pathway.
Figure 1.
Phenotypic Analysis of the gpa4-1 Mutant.
(A) Transverse sections of representative wild-type (Indica variety 9311) and gpa4-1 mutant dry seeds. Bar = 1 mm.
(B) and (C) Scanning electron microscopy images of transverse sections of wild-type (B) and gpa4-1 mutant (C) seeds. Bars = 10 μm.
(D) The 1000-grain weight of the wild type and gpa4-1. Values are mean ± sd. **P < 0.01 (n = 3, Student’s t test).
(E) SDS-PAGE profiles of total proteins of dry seeds from the wild type and the gpa4-1 mutant. pGlu, 57-kD proglutelins; αGlu, 40-kD glutelin acidic subunits; α-Glb, 26-kD α-globulin; βGlu, 20-kD glutelin basic subunits; Pro, prolamins.
(F) Immunoblot analysis of seed storage proteins using antiglutelin and anti-α-globulin antibodies. Blue triangles indicate the 57-kD proglutelins. Hollow arrows denote the glutelin acidic subunits (black) and basic subunits (red) in (F) and (G).
(G) Immunoblot analysis of the major glutelin subfamily proteins (i.e., GluA and GluB) and VPE1. Arrows indicate different forms of VPE1. pV, VPE1 precursor; iV, intermediate VPE1; mV, mature VPE1. EF-1α was used as a loading control in (F) and (G).
In the wild-type seeds, most of the storage proteins started to accumulate from ∼6 d after flowering (DAF), but the storage proteins became detectable in gpa4-1 until ∼9 DAF (Supplemental Figure 1). The expression levels of the representative genes encoding major storage proteins were all lower in the 12 DAF endosperm of gpa4-1 compared with those of the wild type (Supplemental Figure 2), which is consistent with a lower protein content in the gpa4-1 mature seeds (Supplemental Table 1). These results suggest that the gpa4-1 mutant is also defective in storage protein biosynthesis.
The gpa4-1 Mutant Shows Defects in Protein Export from the ER in Developing Endosperm Cells
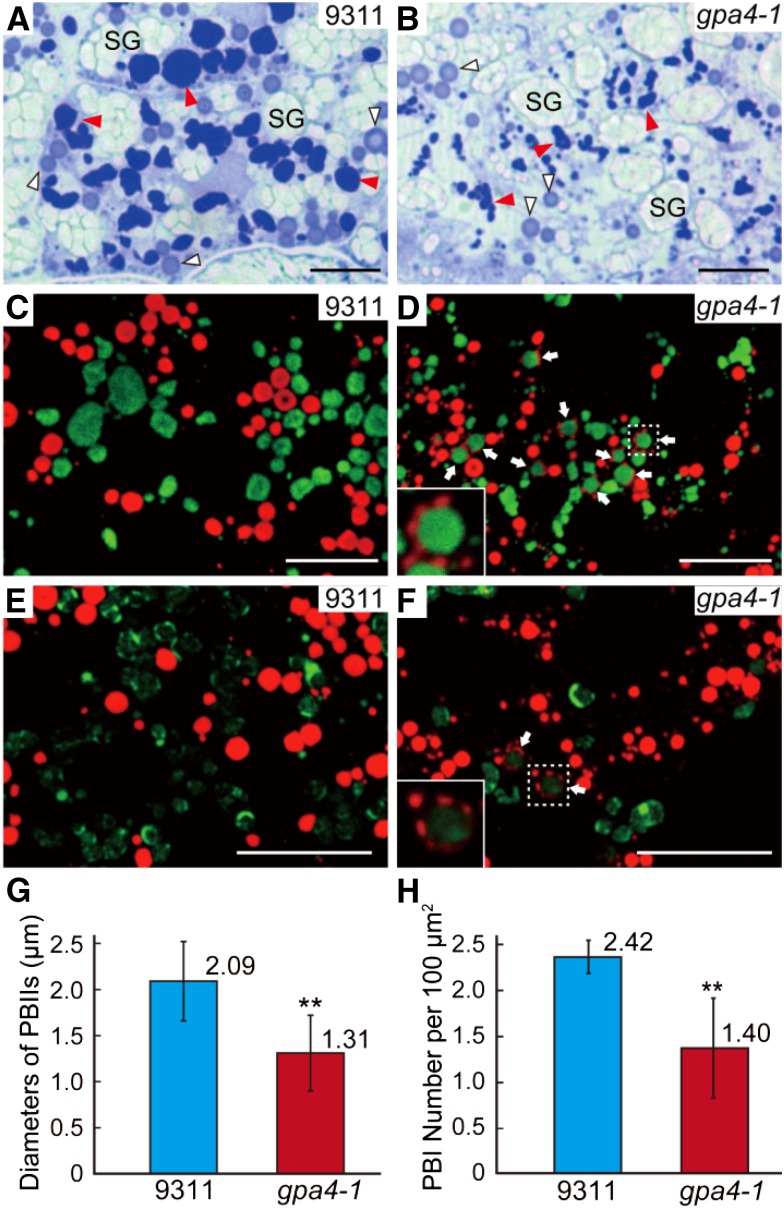
As live-cell imaging is not feasible in developing seeds, we compared storage protein trafficking in the subaleurone layers of the gpa4-1 mutant to that of the wild type. Semithin sections (0.5 μm) of 12 DAF endosperm were subjected to Coomassie blue staining. Two types of protein bodies were readily observed in the wild type: dark-stained irregular-shaped PBIIs (contain glutelins and α-globulin) and light-stained round-shaped PBIs (contain prolamins) (Figure 2A). In gpa4-1, the section area of the corresponding dark-stained structures was much smaller than that in 9311, and the mutant had much fewer light-stained spherical structures of normal size (∼1 to 2 µm in the wild type) (Figures 2A and 2B). This observation was further supported by immunofluorescence assays with specific antibodies against prolamins and glutelin acidic subunits (Figures 2G and 2H), suggesting that the gpa4-1 mutation affects the formation of both types of protein bodies.
Figure 2.
Light and Immunofluorescence Microscopy of Protein Bodies in the Subaleurone Cells of the Wild Type and the gpa4-1 Mutant.
(A) and (B) Sections of 12 DAF endosperm of the wild type (A) and gpa4-1 (B) stained with Coomassie blue. Red and white triangles indicate the dark-stained glutelin-containing structures (PBIIs in the wild type) and the light-stained prolamin-containing structures (PBIs in the wild type), respectively. SG, starch grains. Bars = 10 μm.
(C) to (F) Immunofluorescence microscopy images of storage proteins in wild-type ([C] and [E]) and gpa4-1 ([D] and [F]) seeds. Secondary antibodies conjugated with Alexa fluor 488 (green) and Alexa fluor 555 (red) were used to trace the antigens recognized by the antiglutelin and antiprolamin antibodies, respectively, in (C) and (D). Similar reactions were performed with anti-α-globulin antibodies instead of antiglutelin antibodies in (E) and (F). White arrows in (D) and (F) indicate the novel structures. The insets in (D) and (F) are the enlarged images of the corresponding boxed areas. Bars = 10 μm.
(G) and (H) Measurement of the diameters of PBIIs (G) and the number of PBIs with normal size per 100 µm2 (H). Values are mean ± sd. **P < 0.01 (n = 84 for the wild type and 56 for gpa4-1 in [G]; n = 4 [total 470 PBIs] for the wild type and 5 [total 334 PBIs] for gpa4-1 in [H]; Student’s t test).
Notably, in addition to the typical PBIs and PBIIs, novel structures with a glutelin core and a prolamin periphery were observed in the developing mutant endosperm (Figures 2C and 2D). Moreover, α-globulin was also detected in the cores (Figures 2E and 2F). As prolamins, glutelins, and α-globulin are all cotranslationally transported into their only colocalization site, the RER lumen, we deduced that the abnormal structures might reflect a defect in ER export of storage proteins in rice endosperm.
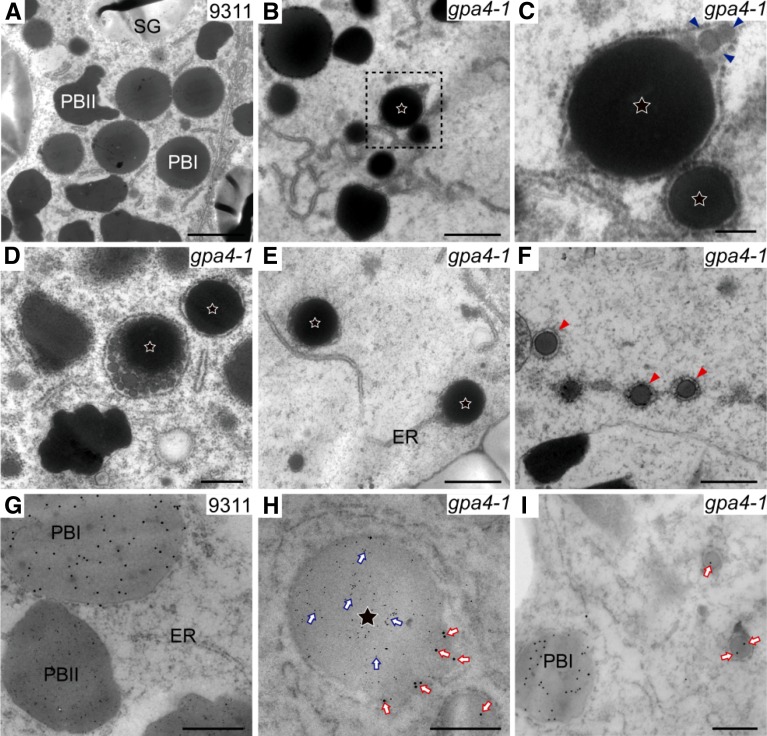
To trace the origin and the formation of these novel structures, transmission electron microscopy and immunogold-labeling assays were adopted for subcellular observation. In 12 DAF wild-type endosperm, individual irregular-shaped PBIIs and spherical PBIs were readily observed (Figure 3A), whereas novel structures were found only in gpa4-1 (Figure 3B). These structures were bounded by and attached to the RER network, indicating that they were ER-derived (Figures 3B to 3E). In 9 DAF endosperm, most of these structures had just a small electron-dense core (Figure 3B). Only a few larger ones had protein aggregates in the peripheral regions (Figure 3C). The structures continued to be filled with storage proteins as seed development progressed and they finally grew into the typical structures of 1 to 2 µm in diameter with an electron-dense core surrounded by large amounts of protein aggregates with low electron density (Figure 3D). In 12 DAF endosperm of the gpa4-1 mutant, numerous PBI-like structures with an average diameter of 336.8 nm (±54.2; n = 20 cells) were observed. These structures were bounded by and linked to the RER membranes as well, indicating an ER origin (Figure 3F).
Figure 3.
Ultrastructure of Subaleurone Cells of Developing Endosperm of the Wild Type and gpa4-1 Mutant.
(A) Two types of protein bodies were observed in wild-type endosperm. Bar = 2 μm.
(B) The first type of novel structure was observed from 9 DAF in endosperm of the gpa4-1 mutant. Stars represent this type of novel structure in (B) to (E). Bar = 1 μm.
(C) Enlarged image of the boxed area in (B). Triangles indicate protein aggregates in the periphery of the first type of novel structure. Bar = 200 nm.
(D) The first type of novel structure in 12 DAF endosperm cells. Bar = 1 μm.
(E) The first type of novel structure is directly linked with the ER. Bar = 1 μm.
(F) The second type of novel structure (red triangles) in 12 DAF endosperm cells. Bar = 500 nm.
(G) to (I) Immunoelectron microscopy localization of glutelins and prolamins in subaleurone cells of developing wild-type (G) and the gpa4-1 mutant ([H] and [I]) endosperm. Bars = 500 nm in (G) and (H) and 200 nm in (I).
(G) Glutelins and prolamins are accumulated separately in the wild type.
(H) The first type of novel structure contains a glutelin (6-nm gold, blue arrows) core and a prolamin (15-nm gold, red arrows) periphery.
(I) The second type of novel structure contains prolamins (15-nm gold, red arrows).
Immunogold labeling analysis showed that in the first novel structure, the core contained glutelins whereas its periphery contained prolamins (Figures 3G and 3H), consistent with the immunofluorescence observations (Figure 2D). The second novel structure contained prolamins like PBI (Figure 3I). Together, these observations suggest that the export of all three types of storage proteins from the ER was compromised, resulting in protein retention in the ER lumen and subsequent formation of two types of abnormal structures. Consistent with the above results, centrifugation-based fractionation assay showed that gpa4-1 accumulated more storage proteins and VPE1 precursor in the P13 fraction (enriched in ER membranes) compared with the wild type (Supplemental Figure 3). Moreover, chaperone proteins like Bip1, PDIL1-1, and Hsp90 accumulated and the expression of all the ER stress-related genes analyzed was significantly higher in 12 DAF endosperm of the gpa4-1 mutant.
GPA4 Encodes a Conserved Membrane Protein Homologous to Yeast Got1p
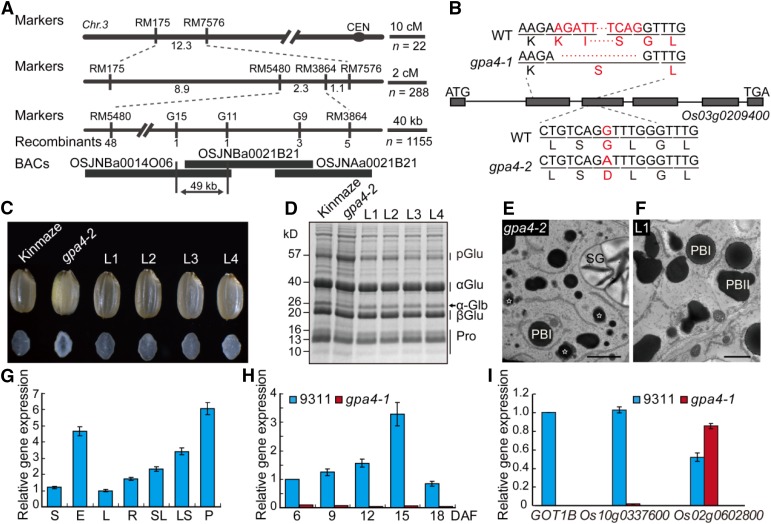
The gpa4-1 mutant was isolated from a 60Co-irradiated population of indica cultivar 9311. Genetic analysis revealed that the mutant phenotype was inherited as a single nuclear recessive mutation (Supplemental Table 2). Through map-based cloning, the GPA4 locus was delineated to a 49-kb genomic region (Figure 4A). Sequence analysis revealed that a 108-bp fragment was deleted in the coding region of Os03g0209400. Only the first 10 amino acids might be correctly translated in gpa4-1. In gpa4-2, a single nucleotide substitution in the 3rd exon of Os03g0209400 caused the replacement of the highly conserved Gly-47 with Asp. Thus, gpa4-1 most likely represents a null mutant of Os03g0209400, while gpa4-2 is likely a partial loss-of-function mutant (Figure 4B). Complementation tests with the entire coding region of Os03g0209400 driven by the Ubiquitin promoter showed complete rescue of the mutant phenotype, including the floury endosperm appearance, storage protein composition, and storage protein deposit pattern in the subaleurone cells of developing endosperm (Figures 4C to 4F). Therefore, we concluded that Os03g0209400 is the underlying gene for GPA4. Notably, a recent study reported that Os03g0209400, which encodes GOT1B, is also the underlying gene for the glup2 mutation (Fukuda et al., 2016). For simplicity, we term this gene GOT1B hereafter.
Figure 4.
Map-Based Cloning and Expression Analysis of GOT1B.
(A) Fine mapping of the GPA4 locus. The molecular markers and the number of recombinants are shown.
(B) Gene structure and the mutation site in Os03g0209400. Os03g0209400 comprises six exons (closed boxes) and five introns (lines). ATG and TGA represent the start and stop codon, respectively. A 108-bp fragment deletion and a nucleotide substitution occurred in the coding region of Os03g0209400 in gpa4-1 and gpa4-2, respectively.
(C) to (F) The Os03g0209400 cDNA under the control of Ubiquitin promoter rescues the grain appearance (C), the storage protein composition pattern (D), and the storage protein trafficking defects ([E] and [F]) of the gpa4-2 mutant. L1 to L4 denote the grains from four independent T1 transgenic lines. Stars represent the abnormal structure with a glutelin core and a prolamin periphery in (E). Bars = 2 μm in (E) and (F).
(G) RT-qPCR assay showing that GOT1B is expressed in various wild-type tissues examined, with the highest expression in panicles before heading. S, shoot; E, endosperm; L, leaf; R, root; SL, seedling; LS, leaf sheath; P, panicle before heading. EF-1α was used as an internal control in (G) to (I). For each RNA sample, three technical replicates were performed. Values are mean ± sd.
(H) RT-qPCR assay showing that GOT1B is expressed throughout endosperm development. The expression of GOT1B in gpa4-1 was much lower compared with that in the wild type. For each RNA sample, three technical replicates were performed. Values are mean ± sd.
(I) The expression of GOT1B and its homologs in 12 DAF endosperm cells. For each RNA sample, three technical replicates were performed. Values are mean ± sd.
RT-qPCR analysis revealed that the endogenous GOT1B was expressed in all tissues examined, with relatively higher expression in the endosperm and young panicles (Figure 4G). During endosperm development, the expression of GOT1B was lower at the early stage, peaked at ∼15 DAF, and then decreased at 18 DAF, which was correlated with the accumulation of storage proteins (Figure 4H). The rice genome has two other genes (Os10g0337600 and Os02g0602800) homologous to GOT1B (Figure 5A). The Os10g0337600 protein shares 91% sequence similarity with GOT1B. Unexpectedly, instead of compensating for the loss of GOT1B, the expression of Os10g0337600 was greatly downregulated in gpa4-1 developing endosperm (Figure 4I).
Figure 5.
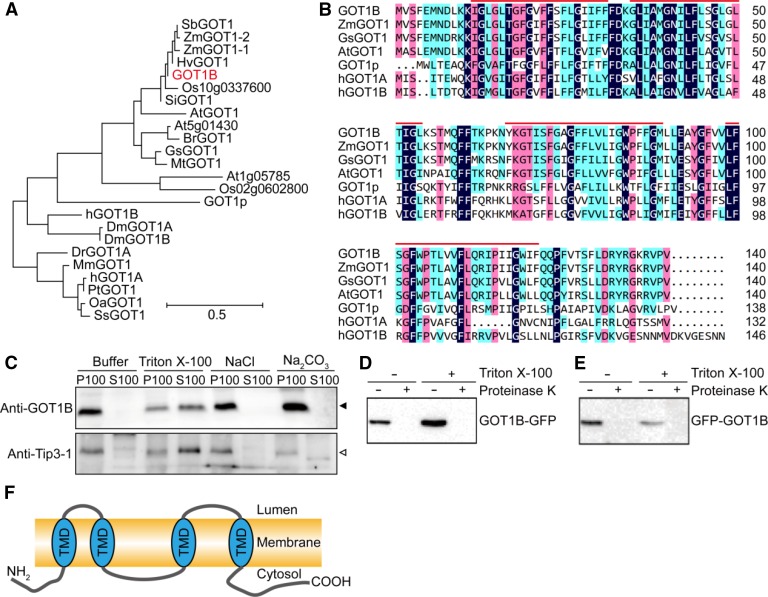
Phylogenic Tree and Topology Analysis of the GOT1B Protein.
(A) Phylogenic tree of the GOT1B protein and its homologs in eukaryotes. The phylogenic tree was constructed using MEGA version 5.0. Sb, Sorghum bicolor; Zm, Zea mays; Hv, Hordeum vulgare; Os, Oryza sativa; Si, Setaria italica; At, Arabidopsis thaliana; Br, Brassica rapa; Gs, Glycine soja; Mt, Medicago truncatula; H, Homo sapiens; Dm, Drosophila melanogaster; Mm, Mus musculus; Pt, Pan troglodytes; Oa, Ovis aries; Ss, Sus scrofa. The sequence alignment used for this analysis is available as Supplemental Data Set 1.
(B) Sequence alignment of GOT1B and its homologous proteins. Red lines indicate the four transmembrane domains predicted by TMHMM Server v. 2.0 (http://www.cbs.dtu.dk/services/TMHMM).
(C) Membrane association of GOT1B protein expressed in N. benthamiana. GOT1B was expressed in tobacco leaf epidermal cells. The 100,000g membrane pellets were prepared and extracted with indicated buffers before centrifugation and analysis of the pellets (P100) and supernatants (S100) by immunoblot. The closed arrowhead indicates the GOT1B protein band. The open arrowhead indicates the Tip3-1 protein band.
(D) and (E) Protease protection assays. Membrane preparations containing the GOT1B protein tagged at either the C (D) or N terminus (E), as indicated, were digested with proteinase K in the presence or absence of detergent and then analyzed by immunoblot.
(F) The proposed topology of GOT1B protein. TMD, transmembrane domain.
GOT1B was predicted to encode a polypeptide of 140-amino acid residues with a calculated molecular mass of 15.8 kD and four transmembrane domains. Phylogenic analysis showed that GOT1B is conserved in eukaryotes and homologous to yeast GOT1p and human GOT1 (hGOT1A and hGOT1B) (Figures 5A and 5B). To analyze the membrane association of GOT1B, cDNA sequence of GOT1B without any tags was subcloned into a binary vector and transiently expressed in N. benthamiana leaf epidermal cells. We used tonoplast-localized rice Tip3-1 as a positive control (Takahashi et al., 2004). After centrifuging cell homogenates at 100,000g, both GOT1B and Tip3-1 could be efficiently pelleted and could not be extracted with sodium chloride or sodium carbonate but could be solubilized by Triton X-100, confirming their membrane-inserted nature (Figure 5C). To determine the topology of GOT1B, a protease digestion assay was performed using the microsomal pellets prepared from N. benthamiana leaves transiently expressing GFP-GOT1B and GOT1B-GFP. Regardless of the presence or absence of a detergent, the N- and C-terminal GFP tags were completely digested (Figures 5D and 5E), suggesting that both termini of GOT1B are cytoplasmically exposed (Figure 5F).
GOT1B Is Localized to the Golgi-Associated ERESs
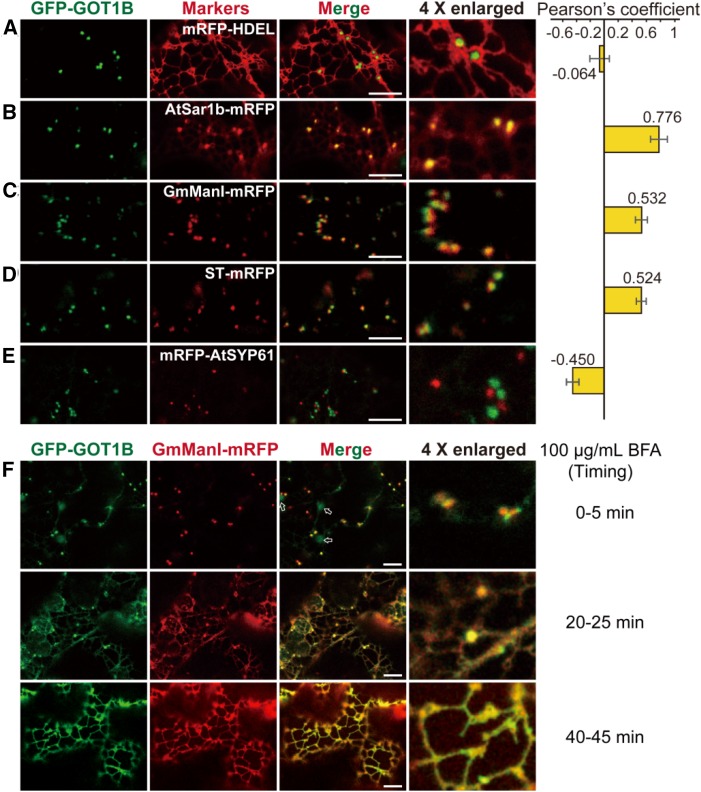
To determine the subcellular localization of GOT1B, two fusion constructs (GFP-GOT1B and GOT1B-GFP) under the control of GOT1B native promoter were transformed into the gpa4-2 mutant. Strikingly, only the GFP-GOT1B construct showed rescue of the mutant phenotype (Supplemental Figure 4). From these results, we concluded that the GFP-GOT1B fusion protein is biologically functional in vivo and thus should be present at its correct subcellular localization. Then we observed the fluorescence of GFP-GOT1B in the roots of the complemented rice lines. The GFP signal showed a punctate pattern rather than the ER-like tubular structures (Supplemental Figure 5A). When expressed in N. benthamiana leaf epidermal cells, GFP-GOT1B was localized in dynamically moving punctate structures (Supplemental Movie 1), but faint signals in the ER networks were observed in cells with higher expression levels (Supplemental Figures 5B and 5C). To determine the nature of these punctate structures, GFP-GOT1B was coexpressed with several fluorescent markers characteristic for the ER (mRFP-HDEL), ERES (AtSar1b-mRFP; Hanton et al., 2008), cis-Golgi (GmMan1-mRFP; Ren et al., 2014), trans-Golgi (ST-mRFP; Saint-Jore et al., 2002), and the trans-Golgi networks (mRFP-SYP61; Ren et al., 2014) in N. benthamiana leaf epidermal cells (Figures 6A to 6E). Confocal microscopy analysis revealed strong correlation between GFP-GOT1B and the ERES marker signals (rs = 0.776) (Figure 6B; French et al., 2008) and relative weaker correlation with the Golgi markers (rs = 0.532 and 0.524 for cis-Golgi and trans-Golgi, respectively) (Figures 6C and 6D). Therefore, GFP-GOT1B is mainly localized at the ERESs and is associated with Golgi stacks.
Figure 6.
Subcellular Localization of GOT1B in the Leaf Epidermal Cells of N. benthamiana.
(A) to (E) Confocal microscopy images showing that GFP-GOT1B generates punctate signals in the cytosol and its distribution is obviously distinct from that of marker proteins characteristic for the ER (mRFP-HDEL [A]) and the trans-Golgi networks (mRFP-SYP61 [E]) but is partially localized with the marker proteins characteristic for ER exit sites (AtSar1b-mRFP [B]), cis-Golgi (GmMan1-mRFP [C]), and trans-Golgi apparatus (ST-mRFP [D]). PSC coefficients (rs) between GFP-GOT1B and each marker are shown in the right panel. Values are mean ± sd. n = 3. Bars = 10 μm.
(F) BFA treatment (100 μg/mL) of leaf epidermal cells coexpressing GFP-GOT1B and GmMan1-mRFP. The localization pattern of GFP-GOT1B and GmMan1-mRFP gradually changed from punctate patterns (0 to 5 min) to typical ER patterns (40 to 45 min). Bars = 10 μm.
This localization pattern suggests the presence of sorting signals in GOT1B terminal regions. To identify the possible signals, we generated a series of N- and C-terminal deletion and site-directed mutagenesis constructs and examined their localizations in N. benthamiana leaf epidermal cells (Supplemental Figure 6A). GFP fusions of three N-terminal mutant constructs (ΔN2-10, ΔN5-10, and V2A F4A) were mainly localized to the ER tubule networks except the construct ΔN5-10 (Supplemental Figures 6B to 6D). Similarly, GFP fusions of three C-terminal mutant constructs (ΔC125-140, ΔC125-133, and V138G V140G) were fully retained in the ER except ΔC125-133 (Supplemental Figures 6E to 6G). Thus, GOT1B protein possesses sorting signals at both termini. The N-terminal VSF and the C-terminal RGKRVPV residues are essential for its proper localization.
Previous studies have demonstrated that Golgi markers and COPII components are sensitive to Brefeldin A (BFA) treatment, which inhibits COPI-mediated retrograde transport and ultimately disassembles the Golgi apparatus. Upon BFA treatment, the distribution of COPII components is significantly changed; Nt-Sar1 is redirected to the ER, while At-Sec13 and At-Sec24 are released into the cytosol (Brandizzi et al., 2002; daSilva et al., 2004; Yang et al., 2005; Hanton et al., 2009). We observed that upon BFA treatment, the fluorescence of GmMan1-mRFP (a cis-Golgi marker) was completely redirected to the ER as expected. Interestingly, the punctate-localized GFP-GOT1B gradually changed to a typical ER pattern within 40 to 45 min (Figure 6F), indicating that GFP-GOT1B is also sensitive to BFA treatment, like COPII components and Golgi markers. This observation lent further support to the notion that GOT1B might play a role in COPII-mediated ER-Golgi protein transport.
GOT1B Directly Interacts with the COPII Component Sec23
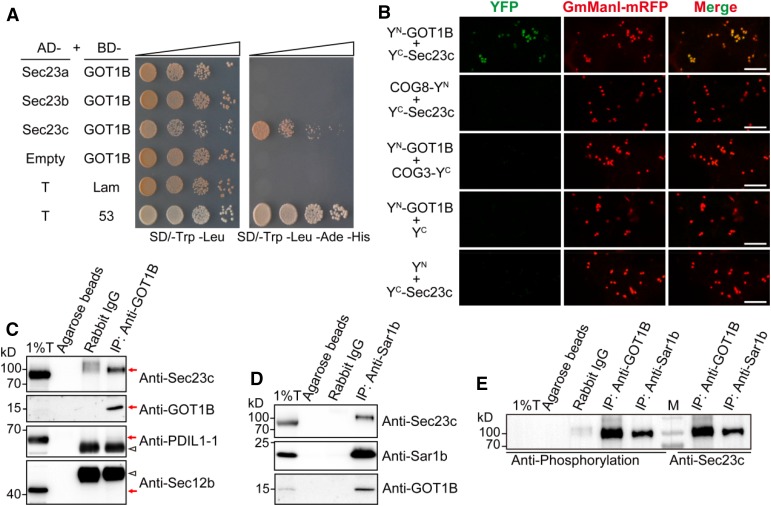
COPII-mediated vesicle trafficking is essential for ER-Golgi anterograde protein transport in all eukaryotes so far analyzed (Marti et al., 2010; Miller and Barlowe, 2010; D’Arcangelo et al., 2013; Venditti et al., 2014). The phenotypic characterization and the specific subcellular localization suggest a possible relationship between GOT1B and the COPII system. Thus, we performed a yeast two-hybrid (Y2H) study between GOT1B and the five basic components of COPII coat as well as Sec12 and Sec16. We found that GOT1B could interact with Sec23c, but not other COPII components (Figure 7A; Supplemental Figure 7A). As GOT1B encodes an integral membrane protein, we next used a split-ubiquitin based yeast two-hybrid system to verify this interaction (Johnsson and Varshavsky, 1994). In this assay, GOT1B clearly interacted with both Sec23b and Sec23c. The interaction between GOT1B and Sec23a could not be clearly determined because of the self-activation activity of pBT3-N-GOT1B (Supplemental Figure 7B). In the subsequent in vivo bimolecular fluorescence complementation (BiFC) assay in N. benthamiana leaf epidermal cells, GOT1B was found to interact with all three Sec23 isoforms (Figure 7B; Supplemental Figure 7C).
Figure 7.
GOT1B Physically Interacts with Sec23 Proteins.
(A) Y2H assay showing that GOT1B interacts with Sec23c. The bait plasmid (pGBKT7-Lam or pGBKT7-53) was cotransformed into the AH109 yeast strain with the prey plasmid (pGADT7-T) to serve as negative and positive controls, respectively.
(B) BiFC assay showing that GOT1B can interact with three Sec23 isoforms in leaf epidermal cells of N. benthamiana. The Golgi-localized membrane proteins COG3 and COG8 (a pair of interacting proteins) were used as the negative control (Tan et al., 2016). Bars = 10 μm.
(C) Co-IP assay showing that Sec23c can be immunoprecipitated by anti-GOT1B polyclonal antibodies in total extract of developing endosperm. Immunoblots with anti-PDIL1-1 and anti-Sec12b antibodies showing no ER lumenal and membrane protein contamination in the IP samples. Red arrows indicate the corresponding protein bands. Triangles indicate the heavy chain of rabbit IgG protein. T, total extract; IP, immunoprecipitation; IB, immunoblot.
(D) Co-IP assay showing that both Sec23c and GOT1B can be immunoprecipitated by anti-Sar1b antibodies in total extract of developing endosperm.
(E) Immunoblot analysis of the IP samples with antiphosphorylation antibodies (left) and anti-Sec23c antibodies (right), respectively. M, protein markers.
As Sec23c had the highest expression level in endosperm (Supplemental Figure 7E), we focused on verifying the GOT1B -Sec23c interaction in vivo. We raised specific antibodies against these proteins (Supplemental Figure 8) and used them in a coimmunoprecipitation (co-IP) assay with total extract of developing endosperm (Figure 7C). In the wild type, Sec23c protein was immunoprecipitated by the anti-GOT1B antibodies, while in the gpa4-1 mutant, no Sec23c protein was pulled down by the same antibodies (Supplemental Figure 7D). The yeast GOT1p and human GOT1 could also interact with their corresponding Sec23 partner in the Y2H assay. Furthermore, the GOT1 proteins from all three species (human, rice, and yeast) could interact with rice Sec23c (Supplemental Figure 7F). In addition, yeast GOT1 could rescue the mutant phenotypes of gpa4-2 (Supplemental Figure 9). These observations suggest that GOT1B performs a highly conserved role in eukaryotes.
To further confirm that GOT1B is involved in the COPII system, we performed co-IP in the 12 DAF endosperm extract of the wild type using anti-Sar1b antibodies. Interestingly, both Sec23c and GOT1B were coimmunoprecipitated (Figure 7D), indicating that these three proteins were present in the same complex(es) in vivo. Notably, the migration of the Sec23c immunoprecipitated by anti-GOT1B and anti-Sar1b antibodies was a little slower than that in the total extracts (Figures 7C and 7D), suggesting that Sec23c might be subject to posttranslational modification and that the modified Sec23c might be the preferred binding partner for GOT1B and Sar1b. Immunoblot analysis with antiphosphorylation antibodies showed that the larger band was a phosphorylated form of Sec23c in both IP samples (Figure 7E). This result is consistent with the earlier finding that yeast Sec23p is a phosphoprotein (Lord et al., 2011).
The Distribution Patterns of Sar1 and ERESs Are Affected in the gpa4-1 Mutant
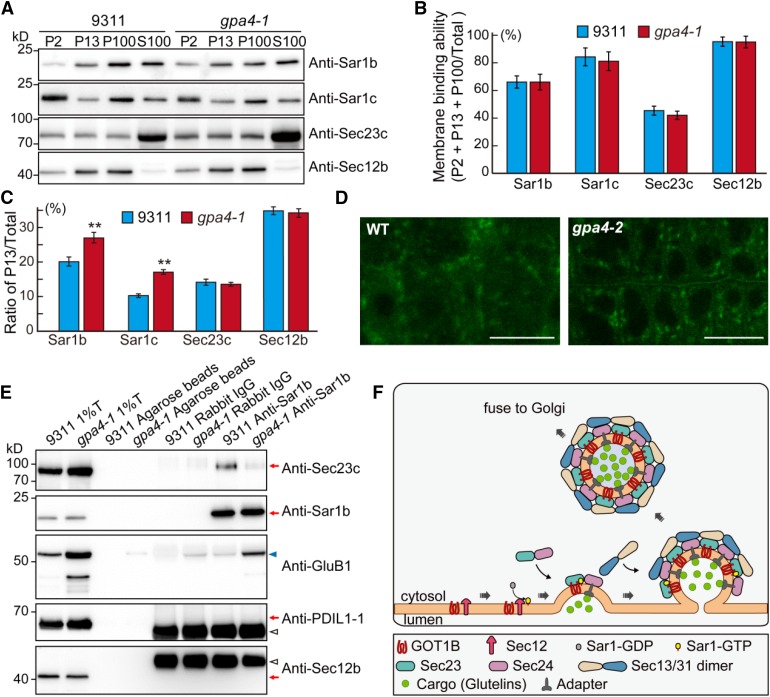
Previous studies have suggested that yeast GOT1p likely participates in the budding step of COPII vesicles (Lorente-Rodríguez et al., 2009). To examine the possible defects of COPII vesicle formation in the gpa4 mutant, we first prepared the microsomal and soluble fractions from endosperm of the wild type and gpa4-1. Although the amounts of membrane-associated fractions of endogenous Sec12b, Sar1b, Sar1c, and Sec23c showed no significant difference between the wild type and gpa4-1 (Figures 8A and 8B), the distribution of the Sar1 proteins in the endomembrane system was affected; more Sar1b and Sar1c accumulated in the P13 fraction of the gpa4-1 mutant compared with the wild type (Figure 8C; Supplemental Table 3), while the distribution of Sec23c showed no obvious change. Previous studies have shown that Sar1 is mainly localized to the ERESs in plant cells (Hanton et al., 2008). The altered distribution of Sar1 proteins in endomembrane system might affect the formation or distribution of ERESs. To directly test this, the ERES maker AtSar1b-GFP was transformed into the wild type and the gpa4-2 mutant. Expression of AtSar1b-GFP in rice did not notably affect rice growth and development (Supplemental Figure 10 and Supplemental Table 4). In wild-type root cells, most of the fluorescence of AtSar1b-GFP was dispersed throughout the cytoplasm, with only some sporadic punctate ERES structures. In the gpa4-1 mutant, AtSar1b-GFP showed a typical, more concentrated punctate pattern (Figure 8D). These results suggest that more rice Sar1 proteins might be associated with the ERESs, which is consistent with the above fractionation assay result. Furthermore, co-IP assays showed that less Sec23c but more proglutelin protein was pulled down by anti-Sar1b antibodies in the gpa4-1 mutant than that in the wild type (Figure 8E). These observations together support the notion that GOT1B plays an important role in the formation of COPII vesicles at the ERESs and proper sorting of proglutelins from the ER to the Golgi.
Figure 8.
Mutation of GOT1B Affects the Distribution of COPII Coat Components and ERESs.
(A) Immunoblot analysis showing the distribution of COPII coat components in the various membrane fractions in the wild type and gpa4-1. P2, pellet obtained following the centrifugation at 2000g; P13, pellet obtained following the centrifugation at 13,000g; P100, pellet obtained following centrifugation at 100,000g; S100, supernatant obtained following centrifugation at 100,000g.
(B) Quantitative analysis of the ratio of the signal intensity of membrane fractions compared with total proteins (P2 + P13 + P100/total, total = P2 + P13 + P100 + S100) in the immunoblot shown in (A). Three independent experiments were performed. Values are mean ± sd.
(C) Quantitative analysis of the ratio of signal intensity of each protein in P13 compared with total protein in the immunoblot shown in (A). **P < 0.01 (n = 3 independent experiments, Student’s t test).
(D) Fluorescence observation of the ERES status (marked by AtSar1b-GFP) in root cells of the wild type and the gpa4-2 mutant. Bars = 10 µm.
(E) In vivo co-IP assay showing that reduced amounts of Sec23c were precipitated in gpa4-1 mutant compared with the wild type. Immunoblots with anti-PDIL1-1 and anti-Sec12b antibodies showing no ER lumenal and membrane protein contamination in the immunoprecipitation samples.
(F) A working model depicting the function of GOT1B in the formation of COPII vesicles. GOT1B participates in COPII coat formation at the ERESs via interaction with Sec23c. The heterodimer of Sec23/Sec24 is recruited by GOT1B and Sar1 cooperatively to form the prebudding complexes in which proglutelin cargos are loaded. Then, the heterotetrimer of Sec13/Sec31 is recruited to form the outer coat of the COPII vesicles before vesicle budding and subsequent fusion with the cis-Golgi apparatus.
DISCUSSION
gpa4 Is Defective in Storage Protein Exit from the ER
In this study, we isolated a rice glutelin precursor accumulation mutant named gpa4-1, which is allelic with the recently reported glup2/got1b mutant (Fukuda et al., 2016). Our cytological, immunocytochemical, and biochemical studies showed that export of storage proteins from the ER is significantly repressed, resulting in the retention of these proteins in the ER lumen in gpa4-1 endosperm cells and formation of two novel types of ER-derived abnormal structures. These structures are quite different to those observed in previously reported 57H mutants in which the 57-kD proglutelins are overaccumulated. The esp2 mutant, resulting from the knockout of PDIL1-1, develops large amounts of ER-derived small PBI-like vesicles containing cross-linked glutelins and prolamins in developing endosperm (Takemoto et al., 2002). The w379/glup3 mutants, both resulting from mutation of VPE1, develop round-shaped PBII (Wang et al., 2009; Kumamaru et al., 2010). The other three mutants defective in post-Golgi trafficking, gpa1/glup4/rab5a, gpa2/glup6/vps9a, and gpa3, all develop large amounts of protein granules and paramural bodies in the apoplast (Wang et al., 2010; Fukuda et al., 2011, 2013; Liu et al., 2013; Ren et al., 2014). Moreover, PDIL1-1 functions in the ER lumen, and VPE1 plays a role in PSV/PBII, while GPA1, GPA2, and GPA3 form a complex that is present in the trans-Golgi networks and prevacuolar compartments. The localizations of these proteins are quite different from that of GOT1B. Thus, gpa4 represents a novel type of 57H mutant in rice.
Notably, the first type of abnormal structure in gpa4-1 was very similar to the MAG bodies observed in the Arabidopsis mag2, mag4, and mag5 mutants (Li et al., 2006; Takahashi et al., 2010; Takagi et al., 2013). Each of these MAG bodies contains an electron-dense core (composed of 2S albumin) and a peripheral matrix region (composed of 12S globulin). In Arabidopsis, MAG2 interacts with two ER-localized t-SNAREs (target-soluble NSF attachment protein receptor), Sec20 and Ufe1 (Li et al., 2006). MAG4 is a Golgi-localized tethering factor that has domains homologous to those found in bovine vesicular transport factor p115 (Takahashi et al., 2010). MAG5 is the ortholog of the S. cerevisiae and Homo sapiens Sec16, which is localized to the cup-shaped ERESs and regulates COPII turnover (Takagi et al., 2013). These three factors all function in protein export from ER and are related to the COPII vesicles. The high phenotypic similarity between gpa4-1 and the mag mutants suggests that the protein trafficking defect in gpa4-1 is likely associated with the COPII system. Consistent with this notion, simultaneous knockdown of rice Sar1a/b/c results in almost the same phenotype as observed in the gpa4-1 mutant (Tian et al., 2013). In addition, the blocking of large amounts of storage proteins in the ER lumen may affect the normal function of ER, which probably in turn affects the biogenesis of PBIs, resulting in the formation of the second type of small PBI-like structures (immature PBIs) in the gpa4-1 mutant.
GOT1B Encodes a Golgi-Associated ERES-Localized Membrane Protein
Cloning and characterization of GPA4 revealed that it encodes GOT1B, which is homologous to the yeast and human GOT1 with four transmembrane domains. Both termini face the cytosol. The structure and topology of GOT1B is highly conserved relative to the yeast GOT1p (Conchon et al., 1999; Lorente-Rodríguez et al., 2009). However, earlier studies have reported conflicting subcellular localization of yeast GOT1p. Conchon et al. (1999) demonstrated that both GOT1p and human GOT1A (hGOT1A) show a punctate pattern overlapping with the cis-Golgi apparatus. Further subcellular fractionation assays confirmed their cis-Golgi localization. Notably, a faint ER-like staining was also observed for hGOT1A, in addition to the punctate pattern. However, Huh et al. (2003) showed that a C-terminally GFP-tagged GOT1p (GOT1p-GFP) is localized to the ER. In a recent study, it was proposed that GOT1p may cycle between the ER and the Golgi compartments (Lorente-Rodríguez et al., 2009). In this study, we showed that the biologically functional GFP-GOT1B fusion protein indeed exhibited a typical punctate localization pattern in the root cells of the complemented transgenic lines. This fusion protein is predominantly localized at the AtSar1b-mRFP-labeled ERESs with faint signals in the ER when transiently expressed in N. benthamiana leaf epidermal cells (Figure 6; Supplemental Figure 5) and still shows relatively high correspondence (rs > 0.5) with Golgi markers. Thus, our result seems to support the ER-Golgi secretory unit model, in which ERESs are associated with mobile Golgi stacks (daSilva et al., 2004). The interaction between GOT1B and Sec23c and their coexistence with Sar1b further support this localization pattern. Based on these results, we conclude that GOT1B is predominantly localized at the Golgi-associated ERESs and likely plays a role in COPII-mediated anterograde protein transport from the ER to the Golgi.
GOT1B Likely Participates in Regulating the Assembly of COPII Prebudding Complexes in Vivo
Yeast GOT1p was initially identified in a synthetic lethal screen with sft2Δ and was proposed to be involved in the fusion of uncoated COPII vesicles to the Golgi (Conchon et al., 1999). However, later studies found the trafficking defects of the original got1p strain could be due to additional mutation(s) in the mutant background (Lorente-Rodríguez et al., 2009). GOT1 was also isolated as a strong suppressor of thermosensitive allele of yip1-2 (Lorente-Rodríguez et al., 2009). Yip1p is an evolutionarily conserved, essential 35.5-kD integral Golgi membrane protein functioning in COPII budding in the membrane scission stage (Matern et al., 2000; Heidtman et al., 2005). Meanwhile, multicopy GOT1 is a suppressor of genes involved in vesicle formation rather than those participating in vesicle tethering, fusing, and retrograde transport. Moreover, in vitro assays showed that GOT1p can be packaged into COPII vesicles (Lorente-Rodríguez et al., 2009). These results together suggest that GOT1p likely participates in the assembly or budding stage of COPII vesicles.
In this study, using a combination of Y2H, BiFC, and co-IP assays, we showed that GOT1B specifically interacts with rice Sec23 isoforms (Figures 7A to 7C). We also showed that the yeast and human counterparts of GOT1B can physically interact with their corresponding Sec23 proteins (Supplemental Figure 7F). Our co-IP assays further showed that Sar1b, GOT1B, and Sec23c are present in the same complex(es) in vivo (Figure 7D). These observations together suggest that GOT1B likely functions before the budding of COPII vesicles, as Sar1 hydrolyzes its bound GTP and dissociates from the membrane once the COPII vesicle is released. The altered fractionation pattern of the prebudding complex components (Sar1b and Sar1c) and the lower amount of modified Sec23c protein immunoprecipitated by anti-Sar1b antibodies in the gpa4 mutant (Figures 8A to 8C and 8E) also support the notion that GOT1B functions in COPII vesicle formation, probably participating in the regulation of the formation or stability control of the prebudding complex of the COPII coat. In further support of this, we observed distinct patterns of ERESs in the wild type and gpa4 (Figure 8D). The dispersed distribution of ERESs in the wild type might be due to the rapid recycling of COPII vesicles between the ER and the Golgi apparatus, while in the mutant, the recycling of COPII vesicles is likely disrupted, causing blockage of COPII components as well as proglutelins (cargos) in the ERESs.
Based on the above results, we propose a speculative model for GOT1B in COPII coat assembly. As GOT1B itself is localized to the ERESs, it may work together with Sar1 to facilitate the recruitment of the Sec23/Sec24 heterodimer to form the prebudding complexes in which cargos have been preloaded. Then the Sec13/Sec31 heterotetramer is recruited to form the outer coat of the COPII vesicles before vesicle budding and subsequent fusion with the cis-Golgi apparatus (Figure 8F). In the gpa4-1 mutant, the absence of GOT1B protein may reduce the binding strength or efficiency between Sar1 and the Sec23/Sec24 heterodimer, resulting in the instability of the prebudding complexes and defective COPII vesicle formation. The assembly deficiency of COPII vesicles may in turn affect the recycling of COPII coat components, leading to blockage of COPII coat components in the ERESs.
The COPII system is highly conserved in eukaryotes for anterograde protein transport, and defects in this system cause many types of diseases in human (Miller and Schekman, 2013). Our results reveal the in vivo function of GOT1B in regulating COPII-mediated secretory protein trafficking and provide evidence that GOT1B might function in regulating the formation or stability of the COPII prebudding complexes. However, earlier studies have shown that COPII vesicle budding can be reconstituted in vitro in the absence of GOT1p (Matsuoka et al., 1998); thus, it is possible that GOT1B may function as a modulator to facilitate COPII coat formation rather than being a stoichiometric subunit of the COPII coat. Notably, a recent study reported that prolamin mRNA sorting is defective in the glup2/got1b mutant and that GOT1B is required for localization of prolamin and α-globulin RNAs to the protein body-ER and for efficient export of proglutelin and α-globulin proteins from the ER to the Golgi apparatus (Fukuda et al., 2016). The identification and functional studies of GOT1B now pave the way for further investigating the detailed biophysical mechanisms of COPII vesicle formation and its regulation in eukaryotes.
METHODS
Plant Materials and Growth Conditions
Two allelic gpa4 mutants, named gpa4-1 and gpa4-2, were isolated in this study. gpa4-1 was isolated from a 60Co-irradiated M2 population of the indica rice (Oryza sativa) cultivar 9311. gpa4-2 was isolated from an MNU-treated M2 population of the japonica rice cultivar Kinmaze. Both mutants were backcrossed at least three times with their corresponding wild type to remove other mutation sites. All plants were grown in paddy fields during normal growing seasons or in a greenhouse.
Protein Extraction from Rice Seeds and Immunoblot Analysis
Total protein extraction and immunoblot assays were performed as described previously (Wang et al., 2010; Liu et al., 2013; Ren et al., 2014).
Map-Based Cloning
To map the GPA4 locus, an F2 population derived from the cross between the gpa4-1 mutant (indica) and a japonica variety Nipponbare was developed. In this population, total proteins were extracted from one half of an individual F2 seed and resolved by SDS-PAGE gel to monitor the accumulation of proglutelins. Meanwhile, the other half of the identified mutant seed with embryo was grown for DNA isolation. In total, 1155 recessive individuals were used for fine mapping of GPA4. The primers used in fine mapping are listed in Supplemental Table 5.
Microscopy Observation
The immunofluorescence analyses, scanning electron microscopy, transmission electron microscopy, and subsequent immunogold labeling experiments were performed as described previously (Wang et al., 2010; Liu et al., 2013; Ren et al., 2014).
Phylogenetic Analysis
Homologs of rice GOT1B were identified using the BLASTP search program of the National Center for Biotechnology Information (http://www.ncbi.nlm.nih.gov). The phylogenetic tree was constructed using MEGA 5.0 (http://www.megasoftware.net), based on the neighbor-joining method with the following parameters: p-distance model, pairwise deletion, and bootstrap (1000 replicates; random seed).
Antibodies
Synthetic peptides of GOT1B (Os03g0209400, TSFLDRYRGKRVPV), GluA1 (Os01g0762500, RRGSPRECRFDR), GluB1 (Os02g0249800, SQSQKFRDEHQK), α-globulin (Os05g0499100, CEGSSSEQGYYGEGS), 10-kD prolamin (Os03g0766100, Pro10, TLAMGTMDPCRQ), 13-kD prolamin-a (Os07g0206400, Pro13a, RFDPLSQSYRQY), 13-kD prolamin-b (Os07g0219300, Pro13b, QLRNNQVLQQLR), 16-kD prolamin (Os06g0507200, Pro16, EQSRRLQLSSCQ), PDIL1-1 (Os11g0199200, CKAESAPAEPLKDEL), Sar1c (Os06g0225000, PTQHPTSEELSIGRC), and Tip3-1 (Os10g0492600, RPGRRFTVGRSEDAC) were synthesized. Recombinant proteins of Sec12b (Os11g0610700, 1 to 227 amino acids), Sar1b (Os01g0338000, 1 to 193 amino acids), Sec23c (Os11g0433500, 1 to 366 amino acids), Bip1 (Os02g0115900, 461 to 665 amino acids), and VPE1 (Os04g0537900, 24 to 497 amino acids) were bacterially produced in pET-32a and purified. The synthetic peptides or recombinant proteins were injected to rabbits or mice to generate corresponding polyclonal antibodies or monoclonal antibody at Yingji Biotech Co. The anti-EF-1α antibodies were purchased from Agrisera (As111633, lot 1209). The anti-Hsp90 antibodies were purchased from BGI (AbM51099-31-PU, lot 2012022301). Antibodies of phosphorylation were purchased from Abcam (ab17464, lot GR276547-1). The anti-GFP antibodies were purchased from Roche (11814460001, lot 14717400). All the antibodies were used in 1:1000 dilutions in immunoblot analyses.
Yeast Two-Hybrid Assay
The cDNA of GOT1B was cloned into pGBT9, while the coding regions of the COPII components were cloned into pGADT7 by Clontech In-Fusion HD cloning kit (639650). Various combinations of plasmids were cotransformed into the yeast strain AH109 for interaction study following the manufacturer’s instructions.
The split ubiquitin based DUAL hunter starter kit was purchased from Dualsystems Biotech (P01601-P01609). The cDNA of GOT1B was cloned into pBT3-N, while the coding regions of the rice Sec23 isoforms were cloned into pPR3-N. Corresponding plasmids were cotransformed into the yeast strain NMY51 following the manufacturer’s instructions. Primers used in this assay are listed in Supplemental Table 5.
RT-qPCR Analysis
Total RNA was isolated using the RNA prep pure plant kit (Tiangen). The first-strand cDNA was synthesized using oligo(dT)18 as the primer and PrimeScript reverse transcriptase (TaKaRa) for reverse transcription. Rice Ubiquitin (UBQ) was used as an internal control. Real-time PCR analysis was performed using an ABI 7500 real-time PCR system with the SYBR Green Mix (Bio-Rad) and three biological repeats (three plants grown separately). Primers used in this assay are listed in Supplemental Table 5.
Subcellular Fractionation
The wild-type and the gpa4-1 developing endosperm were used for fractionation as described previously with some modifications (Tamura et al., 2005). Developing 12 DAF endosperm were homogenized in a mortar on ice in a triple volume of buffer A (100 mM HEPES-KOH, pH 7.5, 0.3 M sucrose, 5 mM EGTA, 5 mM MgCl2, and protease inhibitor [complete cocktail tablets; Roche]). The homogenate was filtered through cheesecloth and centrifuged at 50g for 20 min to remove starch. The supernatant was further centrifuged at 2000g for 20 min at 4°C to obtain the supernatant (S2) and pellet fractions (P2). Then the S2 fraction was further centrifuged at 13,000g and 100,000g to obtain the P13, P100, and S100 fractions for immunoblot analysis.
To perform subcellular fractionation, Nicotiana benthamiana leaves (∼3 g) transiently expressing GOT1B were homogenized in 10 mL of the above buffer. The homogenate was filtered and centrifuged at 2000g for 20 min at 4°C to remove the cell debris. The supernatant was ultracentrifuged at 100,000g for 1 h at 4°C to obtain the microsomal pellet. Then the microsomal pellets were resuspended in 150 μL of each solution of buffer A, high-salt buffer (1 M NaCl, 100 mM HEPES-KOH, pH 7.5, 0.3 M sucrose, 5 mM EGTA, and 5 mM EDTA), alkaline buffer (0.1 M Na2CO3, pH 11, 0.3 M sucrose, 5 mM EGTA, and 5 mM EDTA), and Triton X-100 buffer (1% [v/v] Triton X-100, 100 mM HEPES-KOH, pH 7.5, 0.3 M sucrose, 5 mM EGTA, and 5 mM EDTA). After incubation for 20 min on ice, these supernatants were ultracentrifuged at 100,000g for 1 h at 4°C to obtain the supernatant and pellet fractions for immunoblot analysis.
To determine the membrane topology of GOT1B, the microsomal pellets were resuspended in buffer A in the presence or absence of 1% (v/v) Triton X-100 and then were incubated with 10 ng/μL proteinase K (Roche) for 15 min on ice. The reactions were terminated by adding equal volume of 2× SDS-PAGE loading buffer and then were subjected to immunoblot analysis.
Transient Expression Analysis in N. benthamiana
For transient expression analysis in N. benthamiana leaf epidermal cells, the coding region of GOT1B was amplified and inserted into the binary vector pCAMBIA1305GFP to produce the GFP-GOT1B (BglII site) or GOT1B-GFP (XbaI-BamHI sites) fusion constructs. For BiFC assay, the coding sequences of GOT1B and rice Sec23 isoforms were cloned into pYN1 and pYC1 vectors (kind gift of John A. Lindbo, OARDC, The Ohio State University, Wooster, OH) to produce YN-GOT1B and YC-Sec23a/b/c, respectively. All the constructs were introduced into the Agrobacterium tumefaciens strain EHA105 and then used to infiltrate N. benthamiana leaves, as described previously (Waadt and Kudla, 2008). Confocal imaging was performed using a Zeiss LSM780 laser scanning confocal microscope. Image analysis was performed using Image J software.
Coimmunoprecipitation
Developing endosperm was homogenized and solubilized in triple volumes of NB1 buffer (50 mM Tris-MES, pH 7.5, 0.5 M sucrose, 1 mM MgCl2, 10 mM EDTA, 5 mM DTT, 0.2% [v/v] Nonidet P-40, 10 mM NaF, and complete proteinase inhibitors) and then centrifuged at 20,000g for 20 min at 4°C to remove the cell debris and fatty acids. One milliliter of total extract was preincubated with 20 μL of Protein-A-agarose beads (Roche; 11134515001). The extracts were incubated with the corresponding antibodies or the same amount of IgG from rabbit serum (Sigma-Aldrich; I5006) for 3 h and then incubated with Protein-A-agarose beads for additional 2 h. After extensive washing, the agarose beads were centrifuged at 500g and boiled in the 5× SDS-PAGE sample buffer for subsequent immunoblot analysis. For phosphorylation assay, the transblotted PVDF membrane was cut into two halves along the marker lane. Then the left half was incubated with antiphosphorylation antibodies, while the right half was incubated with anti-Sec23c antibodies.
Accession Numbers
Sequence data from this article can be found in the GenBank/EMBL databases under the following accession numbers: GOT1B (Os03g0209400), Sec12b (Os11g0610700), Sar1a (Os01g0254000), Sar1b (Os01g0338000), Sar1c (Os06g0225000), Sar1d (Os12g0560300), Sec23a (Os01g0321700), Sec23b (Os08g0474700), Sec23c (Os11g0433500), Sec24 (Os11g0482100), Sec13a (Os02g0135800), Sec13b (Os03g0831800), Sec13c (Os07g0246300), and Sec31 (Os07g0657200). The accession numbers for proteins in the phylogenetic analysis are as follows: SbGOT1, XP_003617250.1; ZmGOT1-2, NP_001150565.1; ZmGOT1-1, NP_001141744.1; HvGOT1, BAJ99406.1; GOT1B, NP_001049334.1; Os10g0337600, BAH00874.1; SiGOT1, XP_004985256.1; AtGOT1, NP_186968.2; At5g01430, NP_190511.1; BrGOT1, XP_009130652.1; GsGOT1, KHN46642.1; MtGOT1, XP_003617250.1; At1g05785, NP_683279.1; Os02g0602800, NP_001047360.1; GOT1p, NP_014020.1; hGOT1B, NP_057156; DmGOT1A, NP_727945.1; DmGOT1B, NP_001014746.1; DrGOT1A, XP_001336385.1; MmGOT1, NP_080956.1; hGOT1A, NP_940849; PtGOT1, XP_009439600.1; OaGOT1, XP_012042315.1; and SsGOT1, XP_003130159.1.
Supplemental Data
Supplemental Figure 1. Time-Course Analysis of Storage Proteins during Endosperm Development of the Wild-Type 9311 and the gpa4-1 Mutant.
Supplemental Figure 2. RT-qPCR Assay of the Expression of Representative Genes Coding for Storage Proteins in 12 DAF Endosperm.
Supplemental Figure 3. Immunoblot Analyses Showing Defective Export of Storage Proteins from the ER in gpa4-1.
Supplemental Figure 4. Rescue of the gpa4-2 Mutant Phenotype by the GFP-GOT1B Fusion Construct.
Supplemental Figure 5. Localization Pattern of GFP-GOT1B Fusion Protein.
Supplemental Figure 6. Both the N and C Termini Are Essential for the Proper Localization of GOT1B Protein.
Supplemental Figure 7. Interaction between GOT1B and Sec23.
Supplemental Figure 8. Immunoblot Analyses Showing the Specificity of Antibodies.
Supplemental Figure 9. Rescue of the gpa4-2 Mutant Phenotypes by Yeast GOT1.
Supplemental Figure 10. The Seeds of Transgenic Rice Lines Expressing AtSar1b-GFP Show No Difference from Their Corresponding Recipient Plants.
Supplemental Table 1. Comparison of Important Agronomic Traits between the Wild Type and gpa4 Mutants.
Supplemental Table 2. Segregation of Mutant Phenotype in Reciprocal Crosses between the Wild Type and the gpa4-1 Mutant.
Supplemental Table 3. The Distribution of COPII-Related Proteins in the Endomembrane System.
Supplemental Table 4. Comparison of Important Agronomic Traits between AtSar1b-GFP Transgenic Lines and Their Corresponding Recipient Plants.
Supplemental Table 5. Primers Used in This Study.
Supplemental Data Set 1. Text File of the Alignment Used for the Phylogenetic Analysis in Figure 5A.
Supplemental Movie 1. Time-Lapse Microscopy of N. benthamiana Leaf Epidermal Cells Expressing GFP-GOT1B.
Supplementary Material
Acknowledgments
This work was supported by grants from the National Natural Science Foundation of China (Grants 31330054, 31371598, and 31401360), a project from the ministry of Agriculture of China for Transgenic Research (Grants 2014ZX0800925B, 2014ZX08009-003, and 2014ZX08001006), and the Fundamental Research Funds for Excellent Young Scientists of ICS-CAAS (Grants 2014JB04-009 and 1610092015003-08 to Y.R.). This work was also supported by the Key Laboratory of Biology, Genetics, and Breeding of Japonica Rice in Mid-lower Yangtze River, Ministry of Agriculture, P.R. China, and the Jiangsu Collaborative Innovation Center for Modern Crop Production. We thank Jinxing Lin and Xiaojuan Li (Beijing Forestry University), Xiaohua Wang, and Jingjing Xing (Institute of Botany, The Chinese Academy of Sciences) for kind help with fluorescence observation. We also thank Shuhua Yang (China Agricultural University) for kindly providing the split ubiquitin-based Y2H hybrid vectors.
AUTHOR CONTRIBUTIONS
J.W., H.W., Y.B., and Y.H.W. designed the research. X.L., W.L., and D.W. screened the mutant materials. F.L., Y.R, and Y.L.W. performed immunofluorescence and immunogold labeling experiments. Y.H.W., F.L., and J.Z. performed the co-IP assays. X.Z. and R.J. performed vector constructions. Y.H.W., F.L., M.W., L.J., and C.W. performed other experiments. J.W., H.W., Y.H.W., and F.L. analyzed the data and wrote the article. Y.H.W., F.L., and Y.R. contributed equally to this work.
Glossary
- ER
endoplasmic reticulum
- ERES
ER exit site
- PSV
protein storage vacuole
- DAF
days after flowering
- BFA
Brefeldin A
- Y2H
yeast two-hybrid
- BiFC
bimolecular fluorescence complementation
- co-IP
coimmunoprecipitation
Footnotes
Articles can be viewed without a subscription.
References
- Aridor M., Weissman J., Bannykh S., Nuoffer C., Balch W.E. (1998). Cargo selection by the COPII budding machinery during export from the ER. J. Cell Biol. 141: 61–70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barlowe C., Schekman R. (1993). SEC12 encodes a guanine-nucleotide-exchange factor essential for transport vesicle budding from the ER. Nature 365: 347–349. [DOI] [PubMed] [Google Scholar]
- Bechtel D.B., Juliano B.O. (1980). Formation of protein bodies in the starchy endosperm of rice (Oryza sativa L.): a re-investigation. Ann. Bot. (Lond.) 45: 503–509. [Google Scholar]
- Bi X., Corpina R.A., Goldberg J. (2002). Structure of the Sec23/24-Sar1 pre-budding complex of the COPII vesicle coat. Nature 419: 271–277. [DOI] [PubMed] [Google Scholar]
- Bi X., Mancias J.D., Goldberg J. (2007). Insights into COPII coat nucleation from the structure of Sec23.Sar1 complexed with the active fragment of Sec31. Dev. Cell 13: 635–645. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brandizzi F., Snapp E., Roberts A., Lippincott-Schwartz J., Hawe C. (2002). Membrane protein transport between the ER and Golgi in tobacco leaves is energy dependent but cytoskeleton independent: evidence from selective photobleaching. Plant Cell 14: 1293–1309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Conchon S., Cao X., Barlowe C., Ṕelham H.R. (1999). Got1p and Sft2p: membrane proteins involved in traffic to the Golgi complex. EMBO J. 18: 3934–3946. [DOI] [PMC free article] [PubMed] [Google Scholar]
- D’Arcangelo J.G., Stahmer K.R., Miller E.A. (2013). Vesicle-mediated export from the ER: COPII coat function and regulation. Biochim. Biophys. Acta 1833: 2464–2472. [DOI] [PMC free article] [PubMed] [Google Scholar]
- daSilva L.L., Snapp E.L., Denecke J., Lippincott-Schwartz J., Hawes C., Brandizzi F. (2004). Endoplasmic reticulum export sites and Golgi bodies behave as single mobile secretory units in plant cells. Plant Cell 16: 1753–1771. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Faso C., Chen Y.N., Tamura K., Held M., Zemelis S., Marti L., Saravanan R., Hummel E., Kung L., Miller E., Hawes C., Brandizzi F. (2009). A missense mutation in the Arabidopsis COPII coat protein Sec24A induces the formation of clusters of the endoplasmic reticulum and Golgi apparatus. Plant Cell 21: 3655–3671. [DOI] [PMC free article] [PubMed] [Google Scholar]
- French A.P., Mills S., Swarup R., Bennett M.J., Pridmore T.P. (2008). Colocalization of fluorescent markers in confocal microscope images of plant cells. Nat. Protoc. 3: 619–628. [DOI] [PubMed] [Google Scholar]
- Fukuda M., Kawagoe Y., Murakami T., Washida H., Sugino A., Nagamine A., Okita T.W., Ogawa M., Kumamaru T. (2016). The dual roles of the Golgi Transport 1 (GOT1B): RNA localization to the cortical endoplasmic reticulum and the export of proglutelin and α-globulin from the cortical-ER to the Golgi. Plant Cell Physiol. 57: 2380–2391. [DOI] [PubMed] [Google Scholar]
- Fukuda M., Satoh-Cruz M., Wen L., Crofts A.J., Sugino A., Washida H., Okita T.W., Ogawa M., Kawagoe Y., Maeshima M., Kumamaru T. (2011). The small GTPase Rab5a is essential for intracellular transport of proglutelin from the Golgi apparatus to the protein storage vacuole and endosomal membrane organization in developing rice endosperm. Plant Physiol. 157: 632–644. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fukuda M., et al. (2013). A guanine nucleotide exchange factor for Rab5 proteins is essential for intracellular transport of the proglutelin from the Golgi apparatus to the protein storage vacuole in rice endosperm. Plant Physiol. 162: 663–674. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Giraudo C.G., Maccioni H.J. (2003). Endoplasmic reticulum export of glycosyltransferases depends on interaction of a cytoplasmic dibasic motif with Sar1. Mol. Biol. Cell 14: 3753–3766. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hanton S.L., Chatre L., Matheson L.A., Rossi M., Held M.A., Brandizzi F. (2008). Plant Sar1 isoforms with near-identical protein sequences exhibit different localisations and effects on secretion. Plant Mol. Biol. 67: 283–294. [DOI] [PubMed] [Google Scholar]
- Hanton S.L., Chatre L., Renna L., Matheson L.A., Brandizzi F. (2007). De novo formation of plant endoplasmic reticulum export sites is membrane cargo induced and signal mediated. Plant Physiol. 143: 1640–1650. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hanton S.L., Matheson L.A., Chatre L., Brandizzi F. (2009). Dynamic organization of COPII coat proteins at endoplasmic reticulum export sites in plant cells. Plant J. 57: 963–974. [DOI] [PubMed] [Google Scholar]
- Heidtman M., Chen C.Z., Collins R.N., Barlowe C. (2005). Yos1p is a novel subunit of the Yip1p-Yif1p complex and is required for transport between the endoplasmic reticulum and the Golgi complex. Mol. Biol. Cell 16: 1673–1683. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huh W.K., Falvo J.V., Gerke L.C., Carroll A.S., Howson R.W., Weissman J.S., O’Shea E.K. (2003). Global analysis of protein localization in budding yeast. Nature 425: 686–691. [DOI] [PubMed] [Google Scholar]
- Johnsson N., Varshavsky A. (1994). Split ubiquitin as a sensor of protein interactions in vivo. Proc. Natl. Acad. Sci. USA 91: 10340–10344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krishnan H.B., Franceschi V.R., Okita T.W. (1986). Immunochemical studies on the role of the Golgi complex in protein-body formation in rice seeds. Planta 169: 471–480. [DOI] [PubMed] [Google Scholar]
- Kumamaru T., Uemura Y., Inoue Y., Takemoto Y., Siddiqui S.U., Ogawa M., Hara-Nishimura I., Satoh H. (2010). Vacuolar processing enzyme plays an essential role in the crystalline structure of glutelin in rice seed. Plant Cell Physiol. 51: 38–46. [DOI] [PubMed] [Google Scholar]
- Li L., Shimada T., Takahashi H., Ueda H., Fukao Y., Kondo M., Nishimura M., Hara-Nishimura I. (2006). MAIGO2 is involved in exit of seed storage proteins from the endoplasmic reticulum in Arabidopsis thaliana. Plant Cell 18: 3535–3547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu F., et al. (2013). OsVPS9A functions cooperatively with OsRAB5A to regulate post-Golgi dense vesicle-mediated storage protein trafficking to the protein storage vacuole in rice endosperm cells. Mol. Plant 6: 1918–1932. [DOI] [PubMed] [Google Scholar]
- Lord C., Bhandari D., Menon S., Ghassemian M., Nycz D., Hay J., Ghosh P., Ferro-Novick S. (2011). Sequential interactions with Sec23 control the direction of vesicle traffic. Nature 473: 181–186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lorente-Rodríguez A., Heidtman M., Barlowe C. (2009). Multicopy suppressor analysis of thermosensitive YIP1 alleles implicates GOT1 in transport from the ER. J. Cell Sci. 122: 1540–1550. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marti L., Fornaciari S., Renna L., Stefano G., Brandizzi F. (2010). COPII-mediated traffic in plants. Trends Plant Sci. 15: 522–528. [DOI] [PubMed] [Google Scholar]
- Matern H., Yang X., Andrulis E., Sternglanz R., Trepte H.H., Gallwitz D. (2000). A novel Golgi membrane protein is part of a GTPase-binding protein complex involved in vesicle targeting. EMBO J. 19: 4485–4492. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matsuoka K., Orci L., Amherdt M., Bednarek S.Y., Hamamoto S., Schekman R., Yeung T. (1998). COPII-coated vesicle formation reconstituted with purified coat proteins and chemically defined liposomes. Cell 93: 263–275. [DOI] [PubMed] [Google Scholar]
- Miller E., Antonny B., Hamamoto S., Schekman R. (2002). Cargo selection into COPII vesicles is driven by the Sec24p subunit. EMBO J. 21: 6105–6113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller E.A., Beilharz T.H., Malkus P.N., Lee M.C.S., Hamamoto S., Orci L., Schekman R. (2003). Multiple cargo binding sites on the COPII subunit Sec24p ensure capture of diverse membrane proteins into transport vesicles. Cell 114: 497–509. [DOI] [PubMed] [Google Scholar]
- Miller E.A., Schekman R. (2013). COPII - a flexible vesicle formation system. Curr. Opin. Cell Biol. 25: 420–427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller E.A., Barlowe C. (2010). Regulation of coat assembly--sorting things out at the ER. Curr. Opin. Cell Biol. 22: 447–453. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ren Y., et al. (2014). GLUTELIN PRECURSOR ACCUMULATION3 encodes a regulator of post-Golgi vesicular traffic essential for vacuolar protein sorting in rice endosperm. Plant Cell 26: 410–425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saint-Jore C.M., Evins J., Batoko H., Brandizzi F., Moore I., Hawes C. (2002). Redistribution of membrane proteins between the Golgi apparatus and endoplasmic reticulum in plants is reversible and not dependent on cytoskeletal networks. Plant J. 29: 661–678. [DOI] [PubMed] [Google Scholar]
- Sieben C., Mikosch M., Brandizzi F., Homann U. (2008). Interaction of the K(+)-channel KAT1 with the coat protein complex II coat component Sec24 depends on a di-acidic endoplasmic reticulum export motif. Plant J. 56: 997–1006. [DOI] [PubMed] [Google Scholar]
- Stagg S.M., Gürkan C., Fowler D.M., LaPointe P., Foss T.R., Potter C.S., Carragher B., Balch W.E. (2006). Structure of the Sec13/31 COPII coat cage. Nature 439: 234–238. [DOI] [PubMed] [Google Scholar]
- Stefano G., Renna L., Chatre L., Hanton S.L., Moreau P., Hawes C., Brandizzi F. (2006). In tobacco leaf epidermal cells, the integrity of protein export from the endoplasmic reticulum and of ER export sites depends on active COPI machinery. Plant J. 46: 95–110. [DOI] [PubMed] [Google Scholar]
- Takagi J., Renna L., Takahashi H., Koumoto Y., Tamura K., Stefano G., Fukao Y., Kondo M., Nishimura M., Shimada T., Brandizzi F., Hara-Nishimura I. (2013). MAIGO5 functions in protein export from Golgi-associated endoplasmic reticulum exit sites in Arabidopsis. Plant Cell 25: 4658–4675. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takahashi H., Rai M., Kitagawa T., Morita S., Masumura T., Tanaka K. (2004). Differential localization of tonoplast intrinsic proteins on the membrane of protein body Type II and aleurone grain in rice seeds. Biosci. Biotechnol. Biochem. 68: 1728–1736. [DOI] [PubMed] [Google Scholar]
- Takahashi H., Tamura K., Takagi J., Koumoto Y., Hara-Nishimura I., Shimada T. (2010). MAG4/Atp115 is a golgi-localized tethering factor that mediates efficient anterograde transport in Arabidopsis. Plant Cell Physiol. 51: 1777–1787. [DOI] [PubMed] [Google Scholar]
- Takemoto Y., Coughlan S.J., Okita T.W., Satoh H., Ogawa M., Kumamaru T. (2002). The rice mutant esp2 greatly accumulates the glutelin precursor and deletes the protein disulfide isomerase. Plant Physiol. 128: 1212–1222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takeuchi M., Ueda T., Sato K., Abe H., Nagata T., Nakano A. (2000). A dominant negative mutant of sar1 GTPase inhibits protein transport from the endoplasmic reticulum to the Golgi apparatus in tobacco and Arabidopsis cultured cells. Plant J. 23: 517–525. [DOI] [PubMed] [Google Scholar]
- Tamura K., Shimada T., Kondo M., Nishimura M., Hara-Nishimura I. (2005). KATAMARI1/MURUS3 Is a novel golgi membrane protein that is required for endomembrane organization in Arabidopsis. Plant Cell 17: 1764–1776. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tan X., et al. (2016). Arabidopsis COG complex subunits COG3 and COG8 modulate Golgi morphology, vesicle trafficking homeostasis and are essential for pollen tube growth. PLoS Genet. 12: e1006140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tanaka K., Sugimoto T., Ogawa M., Kasai Z. (1980). Isolation and characterization of 2-types of protein bodies in the rice endosperm. Agric. Biol. Chem. 44: 1633–1639. [Google Scholar]
- Tanaka Y., Nishimura K., Kawamukai M., Oshima A., Nakagawa T. (2013). Redundant function of two Arabidopsis COPII components, AtSec24B and AtSec24C, is essential for male and female gametogenesis. Planta 238: 561–575. [DOI] [PubMed] [Google Scholar]
- Tian L., Dai L.L., Yin Z.J., Fukuda M., Kumamaru T., Dong X.B., Xu X.P., Qu Q. (2013). Small GTPase Sar1 is crucial for proglutelin and α-globulin export from the endoplasmic reticulum in rice endosperm. J. Exp. Bot. 64: 2831–2845. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Venditti R., Wilson C., De Matteis M.A. (2014). Exiting the ER: what we know and what we don’t. Trends Cell Biol. 24: 9–18. [DOI] [PubMed] [Google Scholar]
- Waadt R., Kudla J. (2008). In planta visualization of protein interactions using bimolecular fluorescence complementation (BiFC). CSH Protoc. 2008: pdb.prot4995. [DOI] [PubMed] [Google Scholar]
- Wang Y., et al. (2010). OsRab5a regulates endomembrane organization and storage protein trafficking in rice endosperm cells. Plant J. 64: 812–824. [DOI] [PubMed] [Google Scholar]
- Wang Y., Zhu S., Liu S., Jiang L., Chen L., Ren Y., Han X., Liu F., Ji S., Liu X., Wan J. (2009). The vacuolar processing enzyme OsVPE1 is required for efficient glutelin processing in rice. Plant J. 58: 606–617. [DOI] [PubMed] [Google Scholar]
- Wei T., Wang A. (2008). Biogenesis of cytoplasmic membranous vesicles for plant potyvirus replication occurs at endoplasmic reticulum exit sites in a COPI- and COPII-dependent manner. J. Virol. 82: 12252–12264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamagata H., Tanaka K. (1986). The site of synthesis and accumulation of storage proteins. Plant Cell Physiol. 27: 135–145. [Google Scholar]
- Yang Y.D., Elamawi R., Bubeck J., Pepperkok R., Ritzenthaler C., Robinson D.G. (2005). Dynamics of COPII vesicles and the Golgi apparatus in cultured Nicotiana tabacum BY-2 cells provides evidence for transient association of Golgi stacks with endoplasmic reticulum exit sites. Plant Cell 17: 1513–1531. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.