Abstract
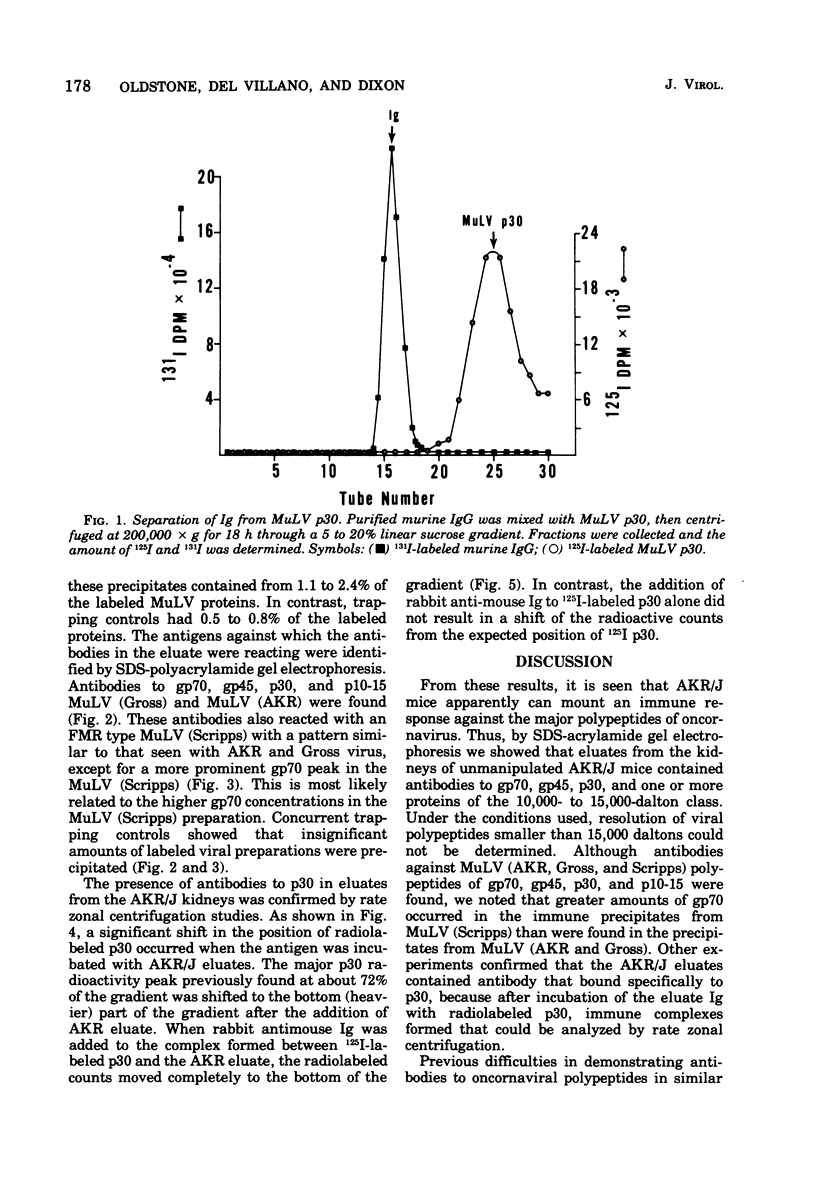
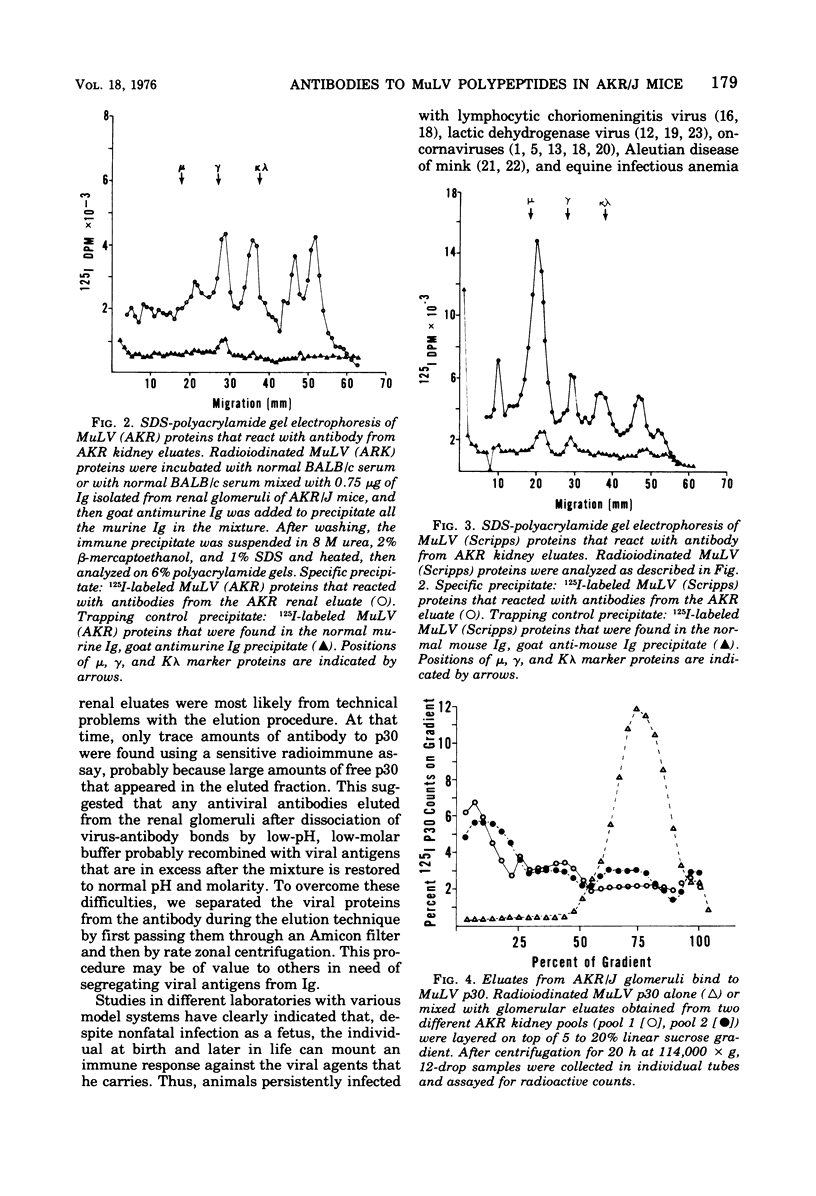
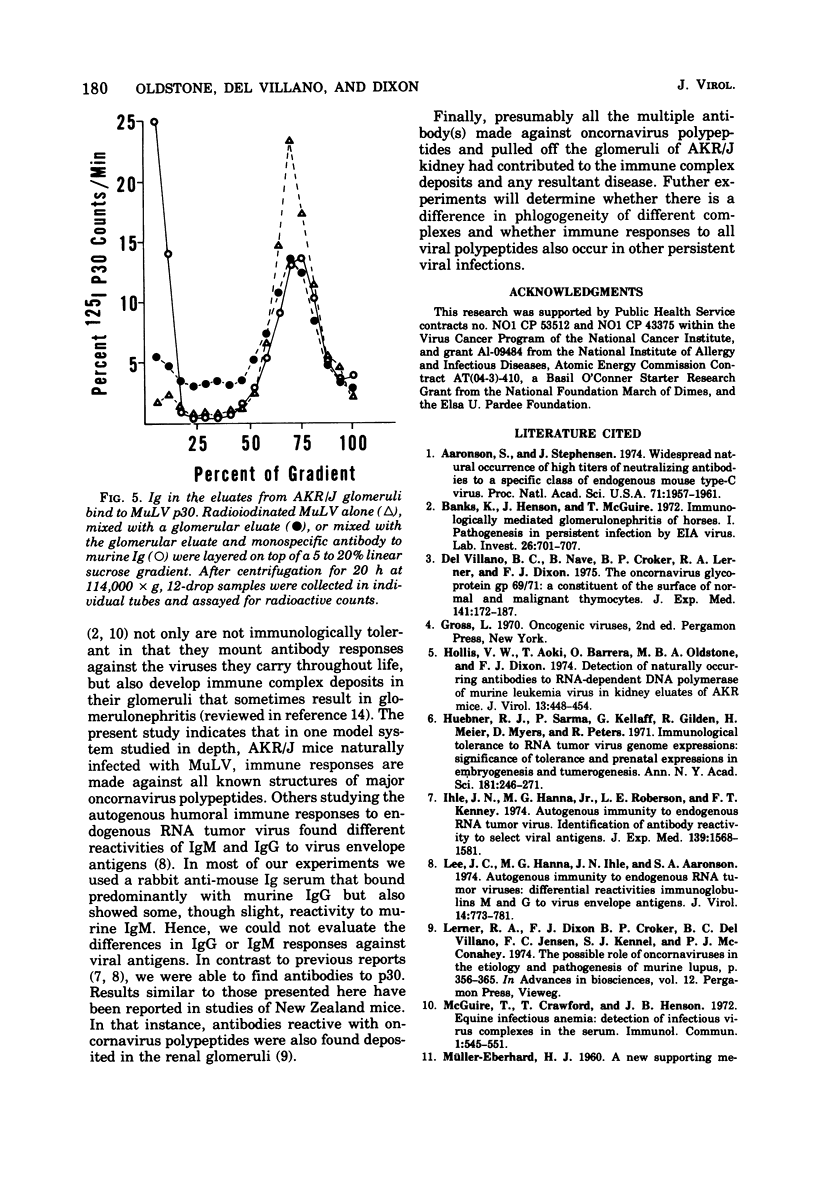
The autologous immune response of AKR/J mice to the structural proteins of murine leukemia virus (MuLV) was examined. Immunoglobulins from the renal glomeruli were chemically eluted, separated from antigens, recovered, and tested for immunological reactivity against MuLV structural proteins. Analyzing immune precipitates obtained after mixing radiolabeled Tween-disrupted MuLV preparations with eluates from AKR/J mice on sodium dodecyl sulfategel electrophoresis, we found evidence of antibodies to the major classes of MuLV structural components: gp70, gp45, p30, and one or more proteins in the 10,000- to 15,000-dalton class. Using rate zonal centrifugation we confirmed that the eluates from AKR/J glomeruli contained antibody(s) that bound specifically to p30. These results indicate that AKR/J mice spontaneously mount immune responses against the major oncornavirus polypeptide antigens.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Aaronson S. A., Stephenson J. R. Widespread natural occurrence of high titers of neutralizing antibodies to a specific class of endogenous mouse type-C virus. Proc Natl Acad Sci U S A. 1974 May;71(5):1957–1961. doi: 10.1073/pnas.71.5.1957. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Banks K. L., Henson J. B., McGuire T. C. Immunologically mediated glomerulitis of horses. I. Pathogenesis in persistent infection by equine infectious anemia virus. Lab Invest. 1972 Jun;26(6):701–707. [PubMed] [Google Scholar]
- Del Vellano B. C., Nave B., Croker B. P., Lerner R. A., Dixon F. J. The oncornavirus glycoprotein gp69/71: a constituent of the surface of normal and malignant thymocytes. J Exp Med. 1975 Jan 1;141(1):172–187. doi: 10.1084/jem.141.1.172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hollis V. W., Jr, Aoki T., Barrera O., Oldstone M. B., Dixon F. J. Detection of naturally occurring antibodies to RNA-dependent DNA polymerase of murine leukemia virus in kidney eluates of AKR mice. J Virol. 1974 Feb;13(2):448–454. doi: 10.1128/jvi.13.2.448-454.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ihle J. N., Hanna M. G., Jr, Roberson L. E., Kenney F. T. Autogenous immunity to endogenous RNA tumor virus. Identification of antibody reactivity to select viral antigens. J Exp Med. 1974 Jun 1;139(6):1568–1581. doi: 10.1084/jem.139.6.1568. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee J. C., Hanna M. G., Jr, Ihle J. N., Aaronson S. A. Autogenous immunity to endogenous RNA tumor virus: differential reactivities of immunoglobulins M and G to virus envelope antigens. J Virol. 1974 Oct;14(4):773–781. doi: 10.1128/jvi.14.4.773-781.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MULLER-EBERHARD H. J. A new supporting medium for preparative electrophoresis. Scand J Clin Lab Invest. 1960;12:33–37. [PubMed] [Google Scholar]
- McGuire T. C., Crawford T. B., Henson J. B. Equine infectious anemia: detection of infections virus-antibody complexes in the serum. Immunol Commun. 1972;1(6):545–551. doi: 10.3109/08820137209022963. [DOI] [PubMed] [Google Scholar]
- Notkins A. L., Mahar S., Scheele C., Goffman J. Infectious virus-antibody complex in the blood of chronically infected mice. J Exp Med. 1966 Jul 1;124(1):81–97. doi: 10.1084/jem.124.1.81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nowinski R. C., Kaehler S. L. Antibody to leukemia virus: widespread occurrence in inbred mice. Science. 1974 Sep 6;185(4154):869–871. doi: 10.1126/science.185.4154.869. [DOI] [PubMed] [Google Scholar]
- Oldstone M. B., Aoki T., Dixon F. J. The antibody response of mice to murine leukemia virus in spontaneous infection: absence of classical immunologic tolerance (AKR mice-complement-fixing antibodies-lymphocytic choriomeningitis virus-immunofluorescence-glomerular deposits of antigen-antibody complexes). Proc Natl Acad Sci U S A. 1972 Jan;69(1):134–138. doi: 10.1073/pnas.69.1.134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oldstone M. B., Dixon F. J. Early events in allergic encephalomyelitis. Trans Am Neurol Assoc. 1968;93:257–259. [PubMed] [Google Scholar]
- Oldstone M. B., Dixon F. J. Lymphocytic choriomeningitis: production of antibody by "tolerant" infected mice. Science. 1967 Dec 1;158(3805):1193–1195. doi: 10.1126/science.158.3805.1193. [DOI] [PubMed] [Google Scholar]
- Oldstone M. B., Dixon F. J. Pathogenesis of chronic disease associated with persistent lymphocytic choriomeningitis viral infection. I. Relationship of antibody production to disease in neonatally infected mice. J Exp Med. 1969 Mar 1;129(3):483–505. doi: 10.1084/jem.129.3.483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oldstone M. B., Tishon A., Chiller J. M. Chronic virus infection and immune responsiveness. II. Lactic dehydrogenase virus infection and immune response to non-viral antigens. J Immunol. 1974 Jan;112(1):370–375. [PubMed] [Google Scholar]
- Oldstone M. B. Virus neutralization and virus-induced immune complex disease. Virus-antibody union resulting in immunoprotection or immunologic injury--two sides of the same coin. Prog Med Virol. 1975;19:84–119. [PubMed] [Google Scholar]
- Porter D. D., Larsen A. E., Porter H. G. The pathogenesis of Aleutian disease of mink. 3. Immune complex arteritis. Am J Pathol. 1973 May;71(2):331–344. [PMC free article] [PubMed] [Google Scholar]
- Porter D. D., Porter H. G. Deposition of immune complexes in the kidneys of mice infected with lactic dehydrogenase virus. J Immunol. 1971 May;106(5):1264–1266. [PubMed] [Google Scholar]
- Weber K., Osborn M. The reliability of molecular weight determinations by dodecyl sulfate-polyacrylamide gel electrophoresis. J Biol Chem. 1969 Aug 25;244(16):4406–4412. [PubMed] [Google Scholar]



