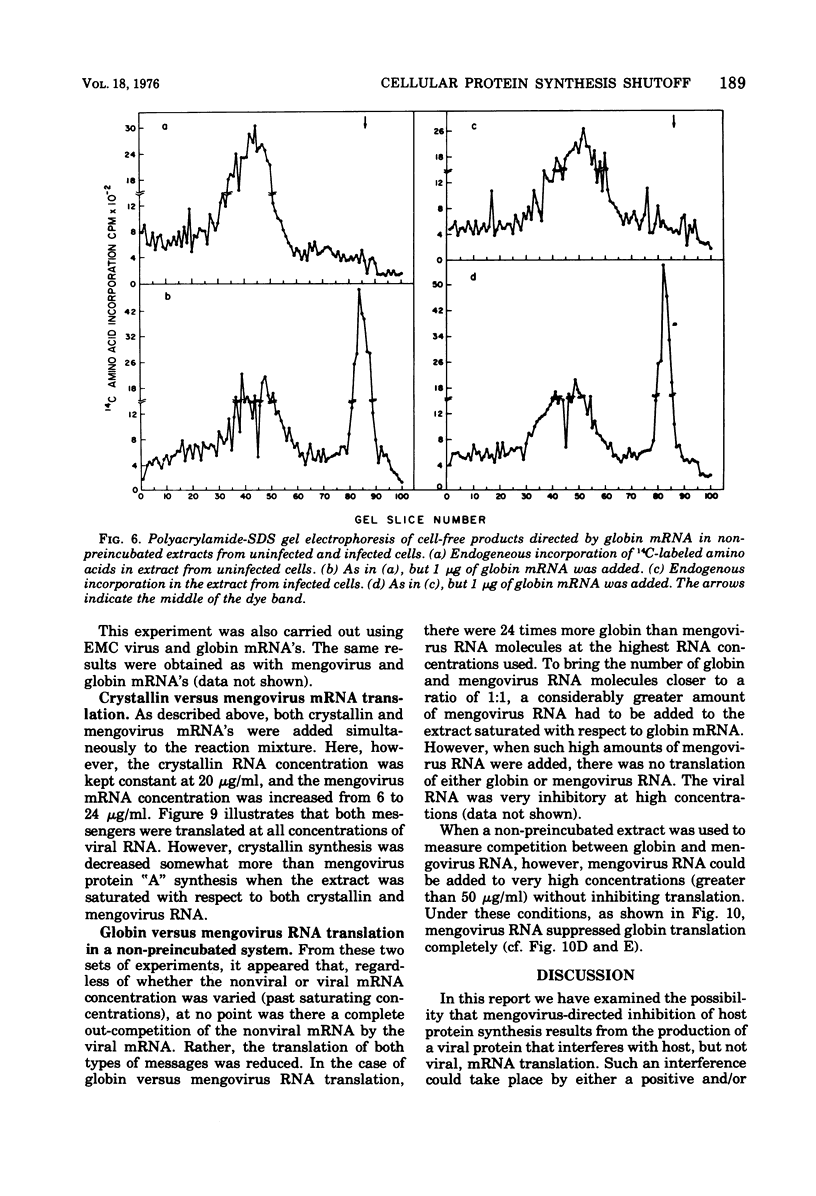
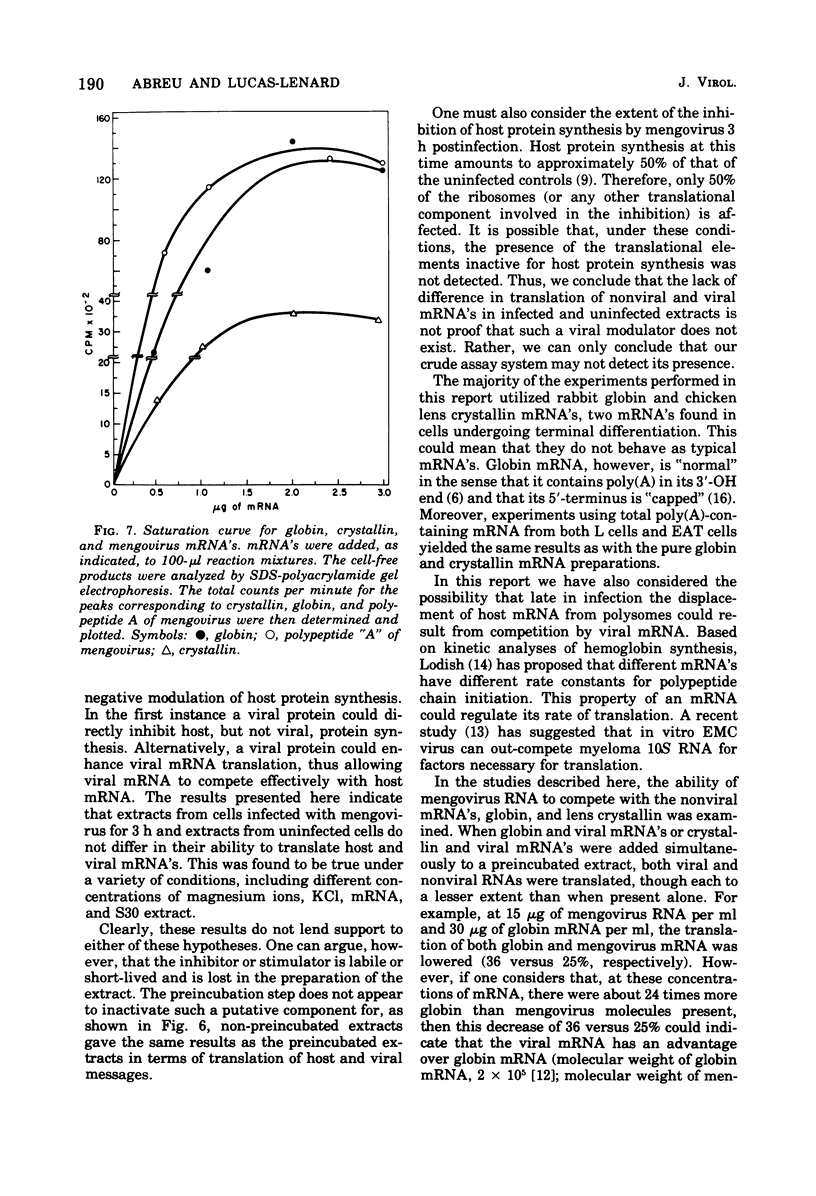
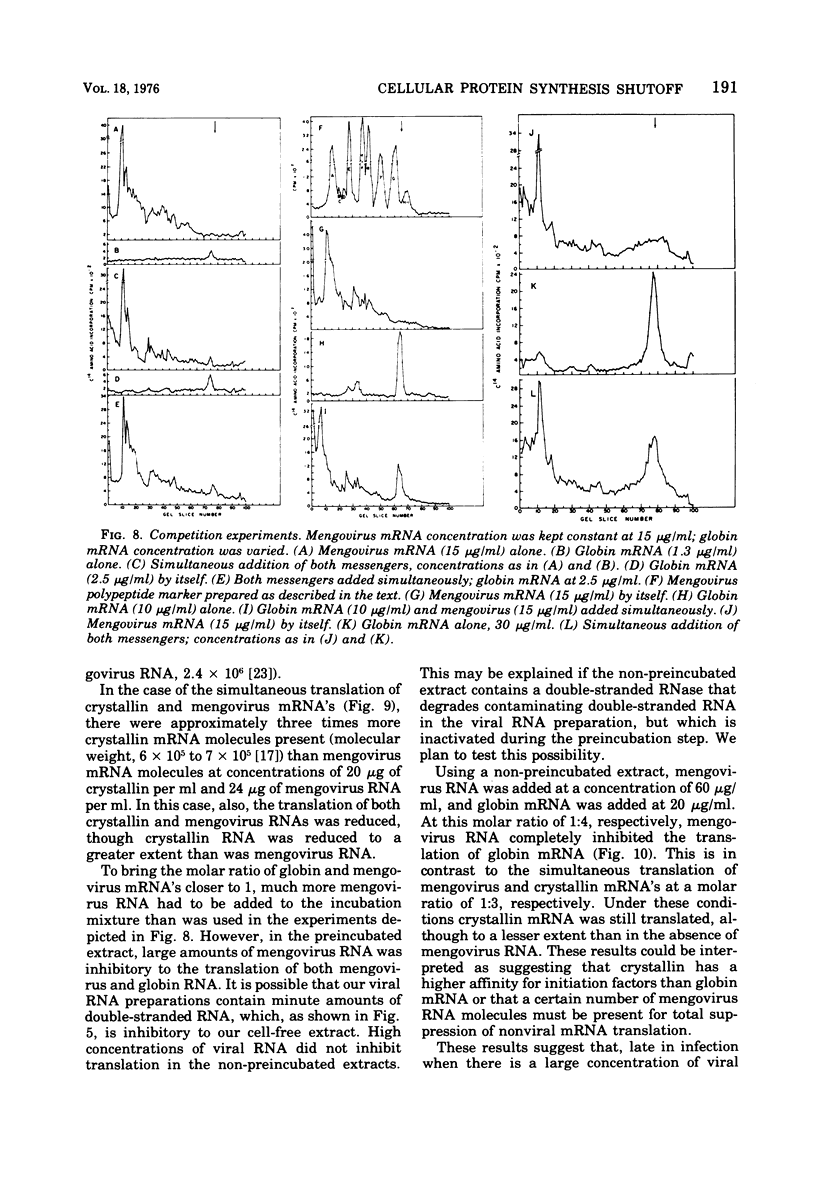
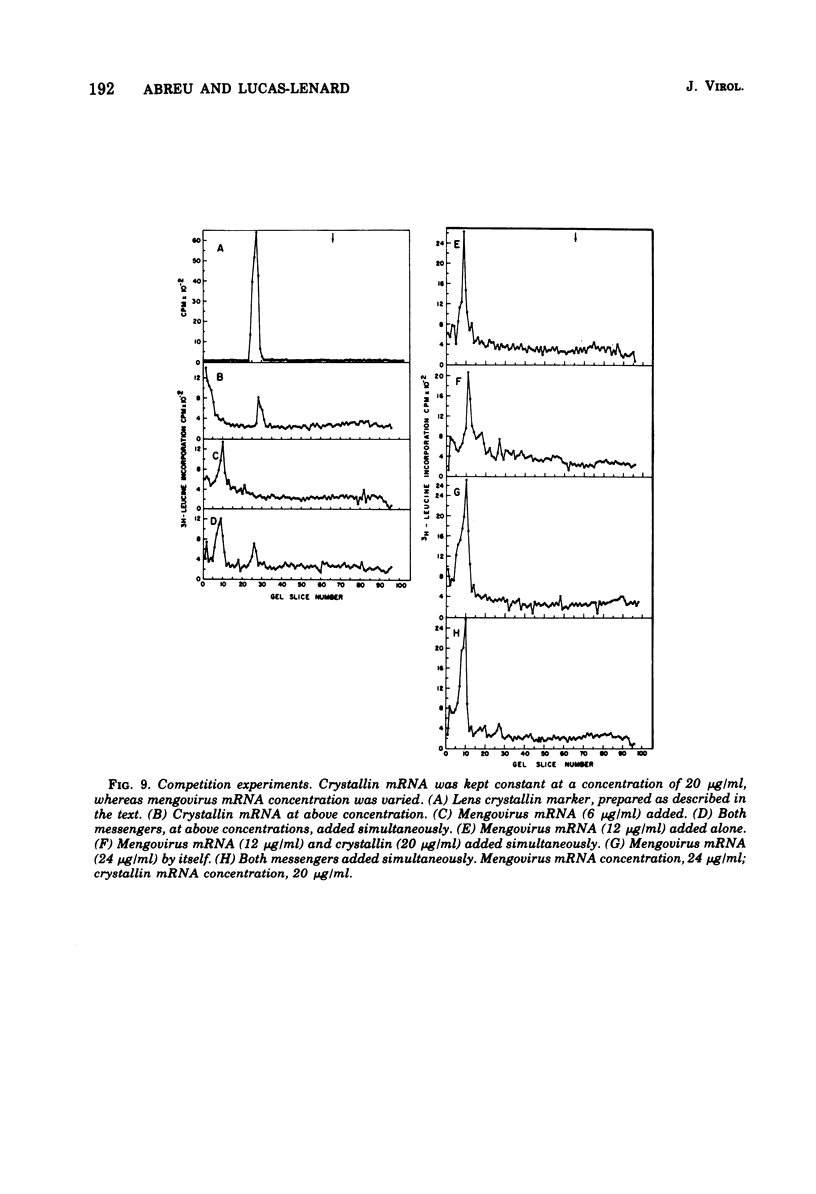
Abstract
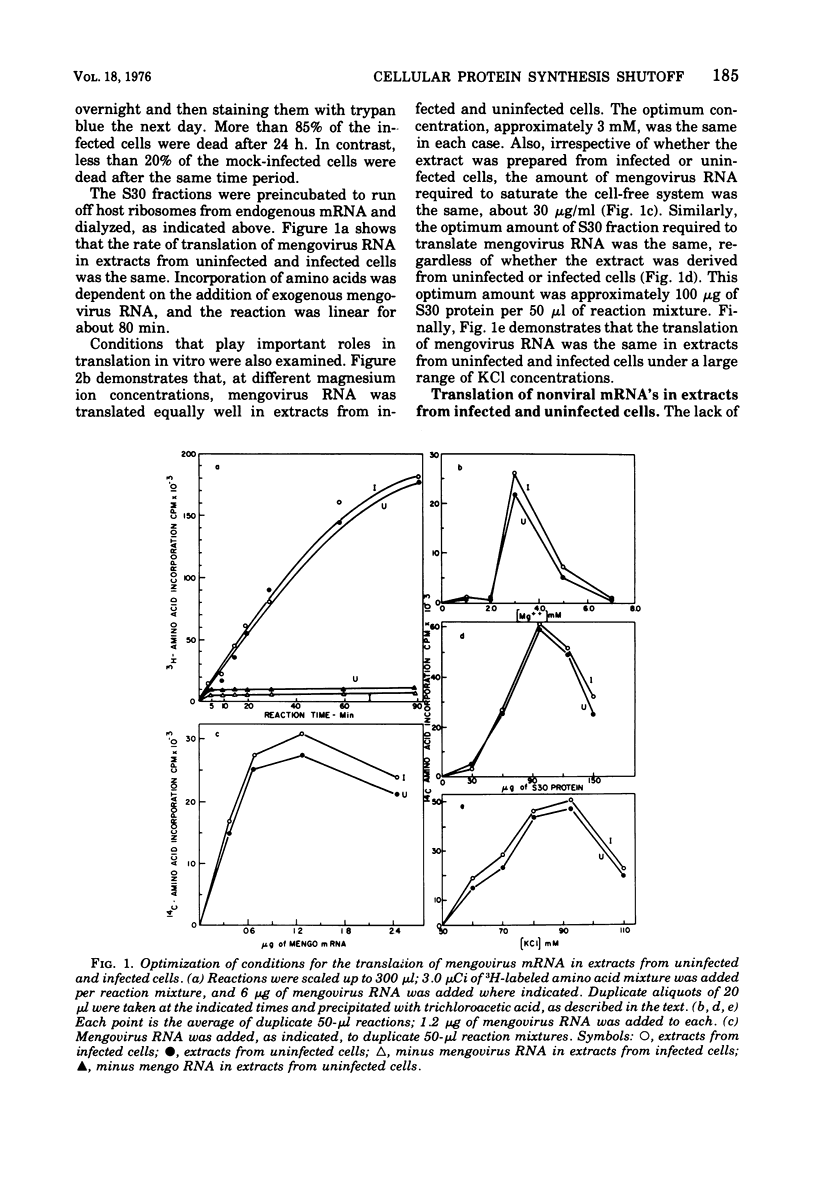
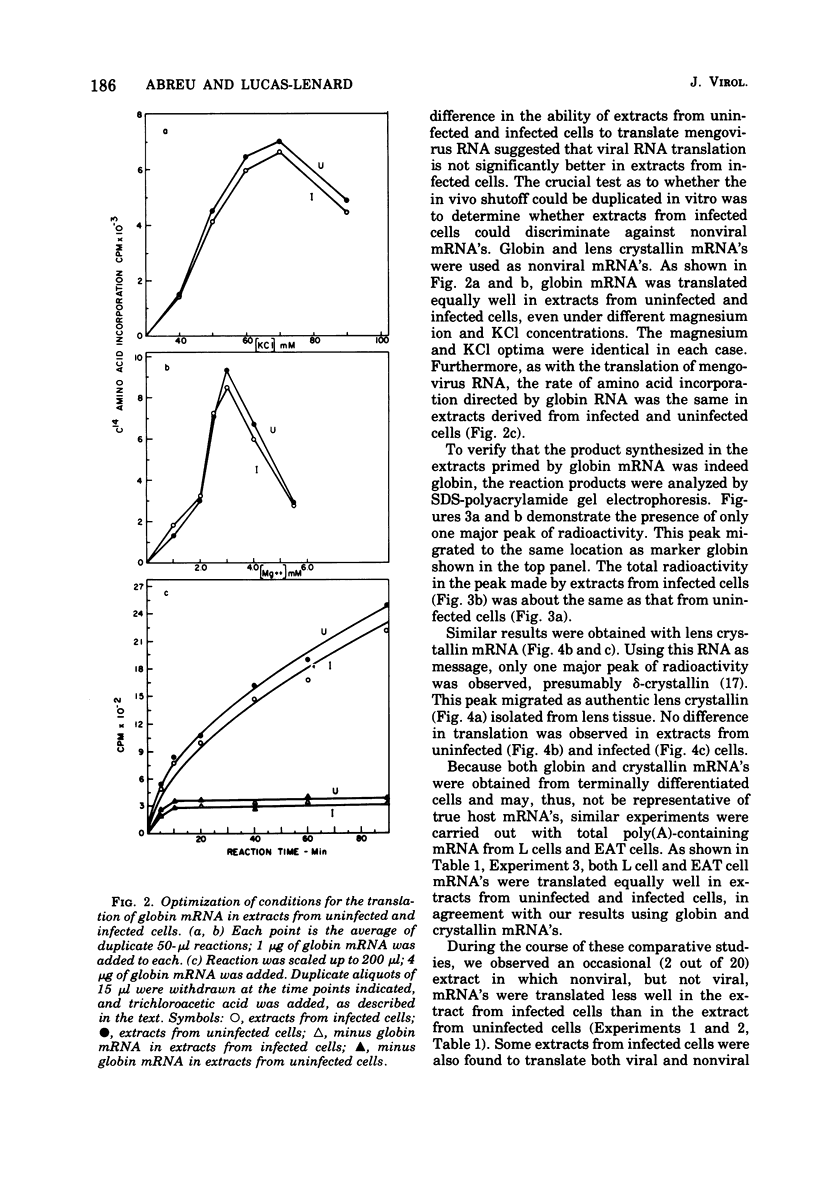
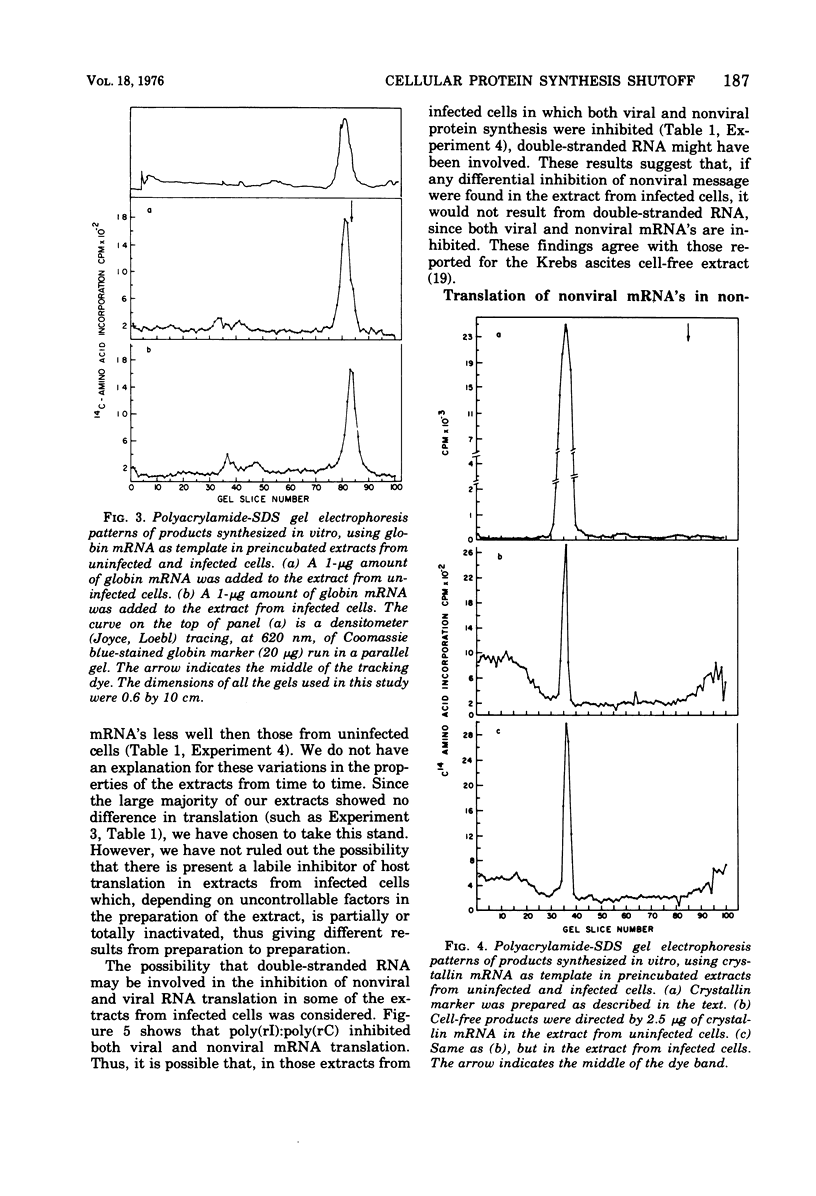
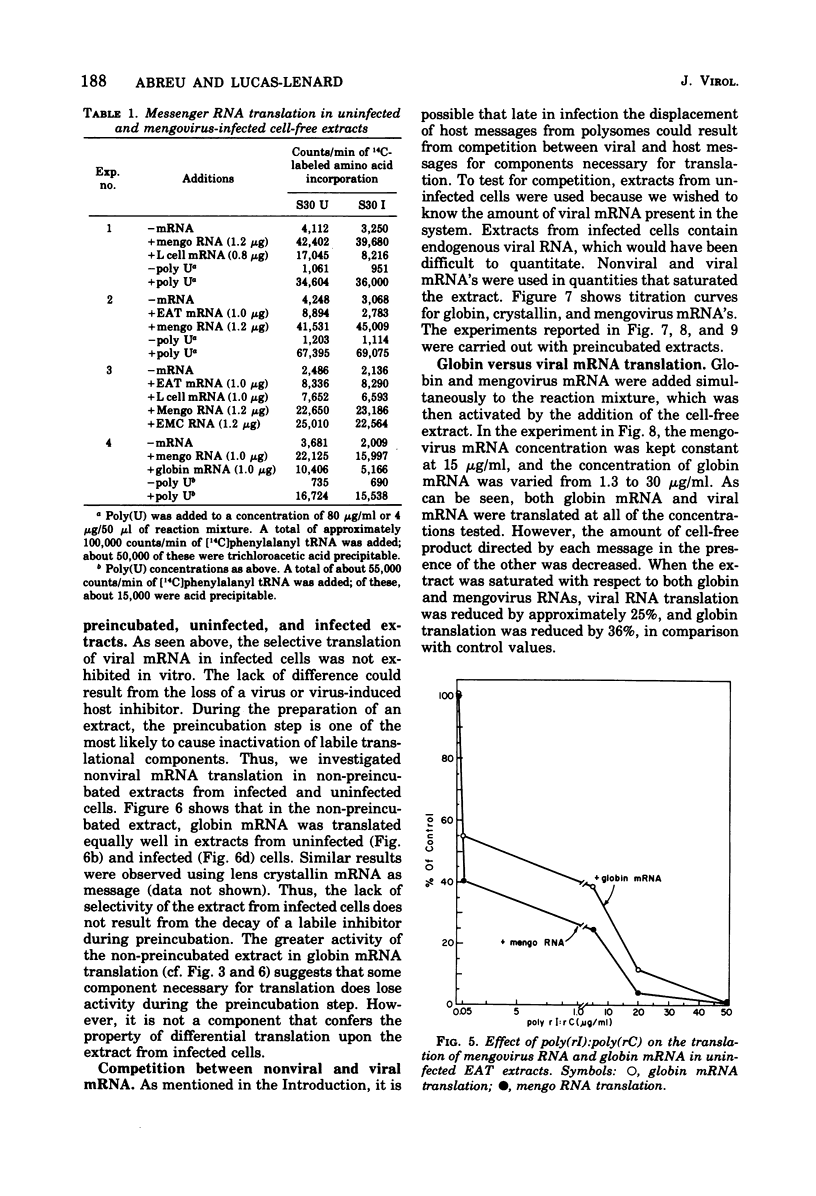
The mechanism whereby picornaviruses inhibit host protein synthesis while their own synthetic processes proceed unabated has remained elusive. One of our approaches to this problem was to study the ability of cell-free extracts derived from uninfected and mengovirus-infected Ehrlich ascites tumor cells to translate viral and nonviral mRNA's under various conditions of incubation. Our results indicate that viral messengers (from mengovirus and encephalomyocarditis virus) and cellular messengers [L cell and Ehrlich ascites tumor poly(A)-containing mRNA's, rabbit globin mRNA, and chicken embryo lens crystallin mRNA] are translated equally well in both extracts. We also examined the simultaneous translation of viral and nonviral mRNA's in extracts from uninfected Ehrlich ascites tumor cells. Our results indicate that under certain conditions mengovirus RNA can suppress completely the translation of globin mRNA. The significance of these results in terms of the shutoff of host protein synthesis is discussed.
Full text
PDF



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Aviv H., Leder P. Purification of biologically active globin messenger RNA by chromatography on oligothymidylic acid-cellulose. Proc Natl Acad Sci U S A. 1972 Jun;69(6):1408–1412. doi: 10.1073/pnas.69.6.1408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- BALTIMORE D., FRANKLIN R. M., CALLENDER J. MENGOVIRUS-INDUCED INHIBITION OF HOST RIBONUCLEIC ACID AND PROTEIN SYNTHESIS. Biochim Biophys Acta. 1963 Nov 22;76:425–430. [PubMed] [Google Scholar]
- Bablanian R. Depression of macromolecular synthesis in cells infected with guanidine-dependent poliovirus under restrictive conditions. Virology. 1972 Jan;47(1):255–259. doi: 10.1016/0042-6822(72)90260-7. [DOI] [PubMed] [Google Scholar]
- Burr H., Lingrel J. B. Poly A sequences at the 3' termini of rabbit globin mRNAs. Nat New Biol. 1971 Sep 8;233(36):41–43. doi: 10.1038/newbio233041a0. [DOI] [PubMed] [Google Scholar]
- Butterworth B. E., Hall L., Stoltzfus C. M., Rueckert R. R. Virus-specific proteins synthesized in encephalomyocarditis virus-infected HeLa cells. Proc Natl Acad Sci U S A. 1971 Dec;68(12):3083–3087. doi: 10.1073/pnas.68.12.3083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cassel W. A., McCaskill K. M. Ehrlich ascites tumor preservation for fifteen years--a simple method. Appl Microbiol. 1974 Oct;28(4):726–726. doi: 10.1128/am.28.4.726-726.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Colby D. S., Finnerty V., Lucas-Lenard J. Fate of mRNA of L-cells infected with mengovirus. J Virol. 1974 Apr;13(4):858–869. doi: 10.1128/jvi.13.4.858-869.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Collins F. D., Roberts W. K. Mechanism of Mengo virus-induced cell injury in L cells: use of inhibitors of protein synthesis to dissociate virus-specific events. J Virol. 1972 Nov;10(5):969–978. doi: 10.1128/jvi.10.5.969-978.1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- FRANKLIN R. M., BALTIMORE D. Patterns of macromolecular synthesis in normal and virus-infected mammalian cells. Cold Spring Harb Symp Quant Biol. 1962;27:175–198. doi: 10.1101/sqb.1962.027.001.019. [DOI] [PubMed] [Google Scholar]
- Gould H. J., Hamlyn P. H. The molecular weight of rabbit globin messenger RNA's. FEBS Lett. 1973 Mar 15;30(3):301–304. doi: 10.1016/0014-5793(73)80674-x. [DOI] [PubMed] [Google Scholar]
- Lawrence C., Thach R. E. Encephalomyocarditis virus infection of mouse plasmacytoma cells. I. Inhibition of cellular protein synthesis. J Virol. 1974 Sep;14(3):598–610. doi: 10.1128/jvi.14.3.598-610.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lodish H. F. Model for the regulation of mRNA translation applied to haemoglobin synthesis. Nature. 1974 Oct 4;251(5474):385–388. doi: 10.1038/251385a0. [DOI] [PubMed] [Google Scholar]
- Loening U. E. The determination of the molecular weight of ribonucleic acid by polyacrylamide-gel electrophresis. The effects of changes in conformation. Biochem J. 1969 Jun;113(1):131–138. doi: 10.1042/bj1130131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Muthukrishnan S., Both G. W., Furuichi Y., Shatkin A. J. 5'-Terminal 7-methylguanosine in eukaryotic mRNA is required for translation. Nature. 1975 May 1;255(5503):33–37. doi: 10.1038/255033a0. [DOI] [PubMed] [Google Scholar]
- Piatigorsky J., Zelenka P., Simpson R. T. Molecular weight and subunit structure of delta-crystallin from embryonic chick lens fibers. Exp Eye Res. 1974 May;18(5):435–446. doi: 10.1016/0014-4835(74)90080-3. [DOI] [PubMed] [Google Scholar]
- Puckett L., Chambers S., Darnell J. E. Short-lived messenger RNA in HeLa cells and its impace on the kinetics of accumulation of cytoplasmic polyadenylate. Proc Natl Acad Sci U S A. 1975 Jan;72(1):389–393. doi: 10.1073/pnas.72.1.389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robertson H. D., Mathews M. B. Double-stranded RNA as an inhibitor of protein synthesis and as a substrate for a nuclease in extracts of Krebs II ascites cells. Proc Natl Acad Sci U S A. 1973 Jan;70(1):225–229. doi: 10.1073/pnas.70.1.225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scraba D. G., Hostvedt P., Colter J. S. Physical and chemical studies of Mengo virus variants. 3. Absorbance--temperature profiles, sedimentation in dextran sulfate gradients, and total-infectious particle ratios. Can J Biochem. 1970 Apr;48(4):412–417. doi: 10.1139/o70-067. [DOI] [PubMed] [Google Scholar]
- Steiner-Pryor A., Cooper P. D. Temperature-sensitive poliovirus mutants defective in repression of host protein synthesis are also defective in structural protein. J Gen Virol. 1973 Nov;21(2):215–225. doi: 10.1099/0022-1317-21-2-215. [DOI] [PubMed] [Google Scholar]
- Zelenka P., Piatigorsky J. Isolation and in vitro translation of delta-crystallin mRNA from embryonic chick lens fibers. Proc Natl Acad Sci U S A. 1974 May;71(5):1896–1900. doi: 10.1073/pnas.71.5.1896. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ziola B. R., Scraba D. G. Structure of the Mengo virion. I. Polypeptide and ribonucleate components of the virus particle. Virology. 1974 Feb;57(2):531–542. doi: 10.1016/0042-6822(74)90192-5. [DOI] [PubMed] [Google Scholar]



