ABSTRACT
The transposase-derived transcription factor genes FAR-RED ELONGATED HYPOCOTYL3 (FHY3) and FAR-RED IMPAIRED RESPONSE1 (FAR1) have redundant and multifaceted roles in plant growth and development during the vegetative stage, including phytochrome A-mediated far-red light (FR) signaling and circadian clock entrainment. Little is known about their functions in the reproductive stage. We recently demonstrated that FHY3 plays important roles in shoot apical meristem (SAM) maintenance and floral meristem (FM) determinacy through its target genes CLAVATA3 (CLV3), SEPALLATA1 (SEP1) and SEP2. Here we present data that FHY3 but not its homolog, FAR1, has a distinct role in FM determinacy in a manner independent of its light signaling and circadian pathway functions. Moreover, genome-wide gene expression profiling showed that the homeostasis of the FM is critical for the regulation of FM activity.
KEY WORDS: Arabidopsis; FAR1; FHY3, floral meristem determinacy
Abbreviations
- AG
AGAMOUS
- CCA1
Circadian Clock Associated1
- CLV3
CLAVATA3
- DEGs
differentially expressed genes
- ELF4
Early Flowering4
- FAR1
FAR-RED IMPAIRED RESPONSE1
- FHY3
FAR-RED ELONGATED HYPOCOTYL3
- FM
Floral Meristem
- phyA
phytochrome A
- SAM
Shoot Apical Meristem
- SEP2
SEPALLATA2
- TOC1
TIMING OF CAB1
Light is one of the most important environmental signals for plant growth and development. In Arabidopsis, 5 distinct photoreceptors perceive and respond to different wavelengths of light. While phytochrome B (phyB) is the primary photoreceptor of the red-light (R) signal, phytochrome A (phyA) is responsible for the transduction of the far-red light (FR) signal and the early R response.1,2 Upon FR and R irradiation, phyA is light activated and translocates to the nucleus to regulate the FR/R response. These events are mediated by FAR-RED ELONGATED HYPOCOTYL1 (FHY1) and its homolog FHY1-LIKE (FHL), as evidenced by the cytosolic localization of phyA in the fhy1 fhl double mutant.3,4
FAR-RED ELONGATED HYPOCOTYL3 (FHY3) and its homolog FAR-RED IMPAIRED RESPONSE1 (FAR1) were isolated as positive regulators of the phyA signaling pathway in Arabidopsis and were found to encode Mutator-like transposase-derived transcription factors.5-8 In this response, FHY3 functions redundantly with but dominantly over FAR1.9 Further molecular analysis revealed that FHY3/FAR1 induce FHY1/FHL expression by directly binding to FHY3/FAR1-binding sites (FBS, CACGCGC) in their promoters and facilitating the nuclear accumulation of light-activated phyA.8,10 FHY3 is also involved in the gating of light signals to the circadian clock.11 The complex network of interconnected feedback loops and endogenous key oscillators, including CIRCADIAN CLOCK ASSOCIATED1 (CCA1), EARLY FLOWERING4 (ELF4) and TIMING OF CAB1 (TOC1), ensures that daily processes occur at the optimum time of day and that metabolism proceeds in an efficient sequence.12,13 In fhy3 mutants, CCA1 and ELF4 are dramatically reduced, and the expression of TOC1 becomes arrhythmic.14 Besides phytochrome and circadian signaling, FHY3/FAR1 have been found to function in diverse plant developmental and physiological processes such as UV-B signaling, programmed cell death, chloroplast biogenesis, chlorophyll biosynthesis, ABA signaling, branching and plant architecture.15 However, these findings focus on FHY3/FAR1 functions at the plant vegetative stage. The roles of FHY3/FAR1 in flower development remain enigmatic.
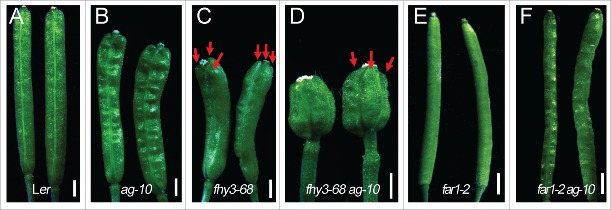
In a genetic screen, we found that mutations in the FHY3 gene could enhance the FM determinacy defect of ag-10, a weak agamous allele, which is characterized by increased carpel numbers and a bulged gynoecium with additional tissue growing inside (Fig. 1A–D).16,17 While far1-2 produced normal siliques with 2 carpels, the siliques of fhy3-68 had 2.59 ± 0 .7 carpels (n = 46), indicative of a weak FM determinacy defect of fhy3-68 (Fig. 1C and E).16 We therefore crossed ag-10 with far1-2, a null mutant,7 to investigate the role of FAR1 in FM determinacy. The FM determinacy phenotype of far1-2 ag-10 resembled that of far1-2 and ag-10 with normal carpel numbers (Fig. 1B and F). This finding indicated that FAR1 does not play a discernible role in FM determinacy in Arabidopsis as FHY3 does.
Figure 1.
Siliques of Ler (A), ag-10 (B), fhy3-68 (C), fhy3-68 ag-10 (D), far1-2 (E) and far1-2 ag-10 (F). Carpels are marked by red arrows in C and D. Scale bars, 1 mm.
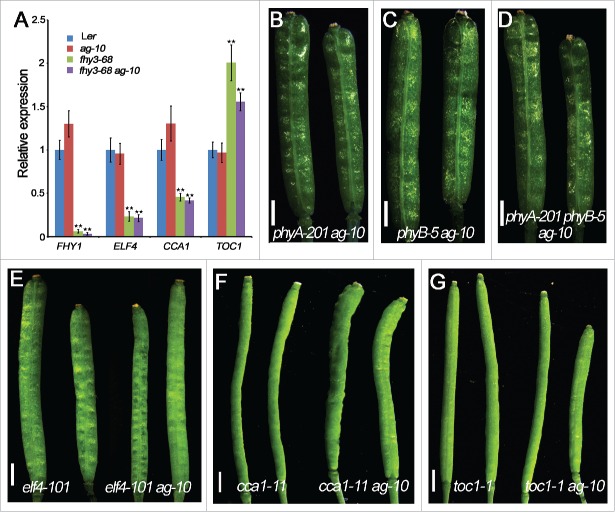
FHY3 is a key positive regulator of phyA signaling and functions in the transduction of light signals to the circadian clock.15 To investigate whether the light signaling or circadian clock functions mediated by FHY3 were involved in FM determinacy regulation, we measured the transcript levels of key FHY3 target genes in wild-type and mutant flowers by quantitative PCR (qPCR) and also performed genetic analyses. FHY1 transcript levels were dramatically down-regulated in fhy3-68 and fhy3-68 ag-10 floral tissue compared to Ler and ag-10, respectively (Fig. 2A), indicating that phyA signaling was impaired in the mutant floral organs. For the genetic analysis, ag-10 was crossed to phyA-201 phyB-5, the strong phyA and phyB double mutant. phyA-201 phyB-5 siliques resembled those of Ler, and similarly, the siliques of phyA-201 ag-10, phyB-5 ag-10 and phyA-201 phyB-5 ag-10 were indistinguishable from those of ag-10 in terms of FM determinacy defects (compare Fig. 2B–D to Fig. 1B). Thus, neither phyA signaling nor phyB signaling was involved in FM determinacy in the ag-10 background.
Figure 2.
Light signaling and circadian clock genes are not involved in FM determinacy. (A) The transcript levels of light signaling and clock genes in the floral tissue of various genotypes measured by real-time RT-PCR. Three biological replicates were performed. Error bars represent SD from 3 biological repeats. **P < 0 .01. (B-G) Siliques of the indicated double and triple mutants exhibited no obvious FM determinacy defects compared to Ler or the corresponding parental lines. Scale bars, 1 mm in (B-G).
Earlier studies showed that the expression of 3 key components of the Arabidopsis central clock, ELF4, CCA1 and TOC1, was altered in the fhy3 mutant.14,18 To investigate the involvement of the circadian clock in FM determinacy, we measured ELF4, CCA1 and TOC1 transcript levels in the floral tissues of fhy3-68 mutants. Consistent with previous findings in seedling tissues,14 ELF4 and CCA1 levels decreased significantly and TOC1 levels increased in the floral organs of fhy3-68 and fhy3-68 ag-10 relative to Ler and ag-10, respectively (Fig. 2A). Thus, circadian clock function was compromised in the floral organs of fhy3-68 and fhy3-68 ag-10. For further analysis, ag-10 was crossed with elf4-101, cca1-11 and toc1-1. The FM determinacy defects of elf4-101 ag-10, cca1-11 ag-10 and toc1-1 ag-10 were indistinguishable from that of ag-10 (compare Fig. 2E–G to Fig. 1B), indicating that the circadian clock was unlikely to be involved in FM determinacy in the ag-10 background.
Using RNA-seq, we analyzed the gene expression profiles from the inflorescences of Ler, ag-10, fhy3-68 and fhy3-68 ag-10.16 A very small number of DEGs (29 up-regulated and 3 downregulated genes) were observed in the ag-10 sample, in line with its point mutation and weak FM determinacy defects. The greater number of upregulated genes than down-regulated genes in fhy3 compared to Ler indicated that FHY3 plays a repressive role in flower development (Fig. 3). This finding was corroborated by the analysis of fhy3-68 ag-10 vs. ag-10 and fhy3-68 ag-10 vs. Ler.16 Decreased numbers of both up- and downregulated genes between fhy3-68 vs. Ler and fhy3-68 vs. ag-10 (1442 to 1138 up-regulated genes and 399 to 211 down-regulated genes) indicated that FHY3 may co-regulate a group of genes with AG (Fig. 3). In contrast with the dramatically different FM determinacy phenotype between fhy3-68 and fhy3-68 ag-10 (Fig. 1C and D), only 2 genes showed a significant difference in expression between fhy3-68 and fhy3-68 ag-10 (Fig. 3). This surprising finding indicates that although the ag-10 point mutation results in only minor changes in terms of genome-wide gene expression (Fig. 3), the homeostasis of the FM is destroyed in the ag-10 plant. Thus, even small perturbations in the FM determinacy regulatory network may be amplified and lead to severe FM determinacy defects in the ag-10 genetic background. Using an EMS genetic screening system in ag-10, we have identified several FM determinacy genes, including FHY3 and SEP2 along with others reported previously.16,19 Thus, the present study, along with our recent findings,16 highlights how proper FM determinacy hinges upon FM homeostasis and clearly distinguishes the role of FHY3 in the flower from its functions during the vegetative stage.
Figure 3.
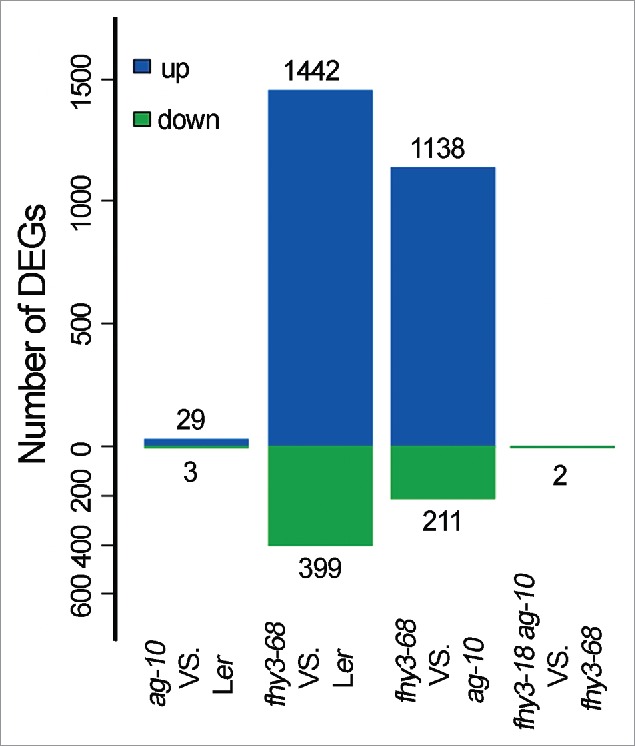
Number of differentially expressed genes (DEGs) identified in pairwise comparisons of RNA-seq data. The numbers of altered genes are indicated above and below the bars. The higher number of upregulated genes than downregulated genes in fhy3 indicated that FHY3 plays a repressive role in flower development.
Disclosure of potential conflicts of interest
No potential conflicts of interest were disclosed.
Funding
This work was funded by National Basic Research Program of China Grant 2014CB138100, National Science Foundation of China (NSFC) Project 31471168 and an award from the CAS Pioneer Hundred Talents Program to X.L.
ORCID
Xigang Liu http://orcid.org/0000-0003-4473-2900
References
- 1.Li J, Li G, Wang H, Wang Deng X. Phytochrome signaling mechanisms. Arabidopsis Book 2011; 9:e0148; PMID:22303272; http://dx.doi.org/ 10.1199/tab.0148 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Chen M, Chory J. Phytochrome signaling mechanisms and the control of plant development. Trends Cell Biol 2011; 21:664-71; PMID:21852137; http://dx.doi.org/ 10.1016/j.tcb.2011.07.002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Hiltbrunner A, Viczian A, Bury E, Tscheuschler A, Kircher S, Toth R, Honsberger A, Nagy F, Fankhauser C, Schafer E. Nuclear accumulation of the phytochrome A photoreceptor requires FHY1. Curr Biol 2005; 15:2125-30; PMID:16332538; http://dx.doi.org/ 10.1016/j.cub.2005.10.042 [DOI] [PubMed] [Google Scholar]
- 4.Hiltbrunner A, Tscheuschler A, Viczian A, Kunkel T, Kircher S, Schafer E. FHY1 and FHL act together to mediate nuclear accumulation of the phytochrome A photoreceptor. Plant Cell Physiol 2006; 47:1023-34; PMID:16861711; http://dx.doi.org/ 10.1093/pcp/pcj087 [DOI] [PubMed] [Google Scholar]
- 5.Wang H, Deng XW. Arabidopsis FHY3 defines a key phytochrome A signaling component directly interacting with its homologous partner FAR1. EMBO J 2002; 21:1339-49; PMID:11889039; http://dx.doi.org/ 10.1093/emboj/21.6.1339 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Hudson ME, Lisch DR, Quail PH. The FHY3 and FAR1 genes encode transposase-related proteins involved in regulation of gene expression by the phytochrome A-signaling pathway. Plant J 2003; 34:453-71; PMID:12753585; http://dx.doi.org/ 10.1046/j.1365-313X.2003.01741.x [DOI] [PubMed] [Google Scholar]
- 7.Hudson M, Ringli C, Boylan MT, Quail PH. The FAR1 locus encodes a novel nuclear protein specific to phytochrome A signaling. Genes Dev 1999; 13:2017-27; PMID:10444599; http://dx.doi.org/ 10.1101/gad.13.15.2017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Lin R, Ding L, Casola C, Ripoll DR, Feschotte C, Wang H. Transposase-derived transcription factors regulate light signaling in Arabidopsis. Science 2007; 318:1302-5; http://dx.doi.org/ 10.1126/science.1146281 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Lin R, Teng Y, Park HJ, Ding L, Black C, Fang P, Wang H. Discrete and essential roles of the multiple domains of Arabidopsis FHY3 in mediating phytochrome A signal transduction. Plant Physiol 2008; 148:981-92; PMID:18715961; http://dx.doi.org/ 10.1104/pp.108.120436 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Ouyang X, Li J, Li G, Li B, Chen B, Shen H, Huang X, Mo X, Wan X, Lin R, et al.. Genome-wide binding site analysis of FAR-RED ELONGATED HYPOCOTYL3 reveals its novel function in Arabidopsis development. Plant Cell 2011; 23:2514-35; PMID:21803941; http://dx.doi.org/ 10.1105/tpc.111.085126 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Allen T, Koustenis A, Theodorou G, Somers DE, Kay SA, Whitelam GC, Devlin PF. Arabidopsis FHY3 specifically gates phytochrome signaling to the circadian clock. Plant Cell Online 2006; 18:2506-16; http://dx.doi.org/ 10.1105/tpc.105.037358 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Pruneda-Paz JL, Kay SA. An expanding universe of circadian networks in higher plants. Trends Plant Sci 2010; 15:259-65; PMID:20382065; http://dx.doi.org/ 10.1016/j.tplants.2010.03.003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Nagel DH, Kay SA. Complexity in the wiring and regulation of plant circadian networks. Curr Biol 2012; 22:R648-57; PMID:22917516; http://dx.doi.org/ 10.1016/j.cub.2012.07.025 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Li G, Siddiqui H, Teng Y, Lin R, Wan XY, Li J, Lau OS, Ouyang X, Dai M, Wan J, et al.. Coordinated transcriptional regulation underlying the circadian clock in Arabidopsis. Nat Cell Biol 2011; 13:616-22; PMID:21499259; http://dx.doi.org/ 10.1038/ncb2219 [DOI] [PubMed] [Google Scholar]
- 15.Wang H, Wang H. Multifaceted roles of FHY3 and FAR1 in light signaling and beyond. Trends Plant Sci 2015; 20:453-61; PMID:25956482; http://dx.doi.org/ 10.1016/j.tplants.2015.04.003 [DOI] [PubMed] [Google Scholar]
- 16.Li D, Fu X, Guo L, Huang Z, Li Y, Liu Y, He Z, Cao X, Ma X, Zhao M, et al.. FAR-RED ELONGATED HYPOCOTYL3 activates SEPALLATA2 but inhibits CLAVATA3 to regulate meristem determinacy and maintenance in Arabidopsis. Proc Natl Acad Sci U S A. 2016 Aug 16;113(33):9375-80; http://dx.doi.org/ 10.1073/pnas.1602960113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Ji LJ, Liu XG, Yan J, Wang WM, Yumul RE, Kim YJ, Dinh TT, Liu J, Cui X, Zheng BL, et al.. ARGONAUTE10 and ARGONAUTE1 regulate the termination of floral stem cells through two MicroRNAs in Arabidopsis. PLoS Genet 2011; 7; PMID:21483759; http://dx.doi.org/ 10.1371/journal.pgen.1001358 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Doyle MR, Davis SJ, Bastow RM, McWatters HG, Kozma-Bognar L, Nagy F, Millar AJ, Amasino RM. The ELF4 gene controls circadian rhythms and flowering time in Arabidopsis thaliana. Nature 2002; 419:74-7; PMID:12214234; http://dx.doi.org/ 10.1038/nature00954 [DOI] [PubMed] [Google Scholar]
- 19.Cao X, He Z, Guo L, Liu X. Epigenetic Mechanisms Are Critical for the Regulation of WUSCHEL Expression in Floral Meristems. Plant Physiol 2015; 168:1189-96; PMID:25829464; http://dx.doi.org/ 10.1104/pp.15.00230 [DOI] [PMC free article] [PubMed] [Google Scholar]