Abstract
Programmed death-ligand 1 (PD-L1) down-modulates various immune responses by engaging the co-inhibitory receptor programmed death-1. Expression of PD-L1 and programmed death-1 is elevated in the salivary glands of patients with Sjögren’s syndrome (SS). The objective of this study is to define the role of endogenous PD-L1 in SS pathogenesis in non-obese diabetic (NOD) mouse model of this disease. We inhibited endogenous PD-L1 function by intraperitoneal administration of a blocking antibody to 6 week-old female NOD/ShiLtJ mice repeatedly during a 9-day period. PD-L1 blockade accelerated leukocyte infiltration and caspase-3 activation in the submandibular gland (SMG), production of antinuclear and anti-M3 muscarinic acetylcholine receptor (M3R) autoantibodies and impairment of saliva secretion, indicative of accelerated development and onset of SS. The effect of PD-L1 blockade was associated with increased T- and B cells and T helper 1 cytokine IFN-γ in the SMG. Local administration of exogenous IFN-γ to the SMG led to impaired salivary secretion accompanied by down-regulation of aquaporin 5 and an increase in anti-M3R autoantibodies. Conversely, neutralization of IFN-γ markedly improved salivary secretion and aquaporin 5 expression in anti-PD-L1-treated NOD/ShiLtJ mice. Hence, endogenous PD-L1 hinders the development and onset of SS in NOD mice, in part by suppressing IFN-γ production.
Sjӧgren’s syndrome (SS) is a systemic autoimmune disease affecting an estimated 2–4 million Americans1. It is characterized by lymphocytic infiltration of exocrine glands, particularly salivary and lacrimal glands, production of autoantibodies, exocrine gland destruction and secretory dysfunction2,3,4. The hallmark symptoms of SS are dry mouth and dry eyes2,4. It also frequently affects many other organs and causes an array of symptoms and health complications, including B cell lymphoma2,4,5. SS can occur alone as primary SS or in conjunction with other inflammatory connective tissue diseases as secondary SS6. T and B cells are the main immune cell populations that infiltrate exocrine glands and are essentially required for the development and onset of SS7,8,9,10. T cell-derived cytokines, including IFN-γ, IL-4, and IL-17, signature cytokines for the major T helper (Th) cell subsets, play indispensable roles in the pathogenesis of SS by promoting tissue inflammation and destruction and facilitating B cell activation and autoantibody production1,11. SS patients exhibit elevated Th1 cytokine IFN-γ levels and enhanced Th1 response in salivary glands and saliva compared to non-SS sicca patients12,13,14,15. Moreover, the degree of local Th1 response strongly correlates with the pathologies of the salivary glands16. Importantly, studies with IFN-γ-deficient mice demonstrate an indispensable role of this cytokine in the development and onset of SS17. IFN-γ contributes to the pathogenesis of SS by multiple mechanisms. It can induce tissue apoptosis, especially in cooperation with TNF-α18,19,20. It induces expression of chemoattractants CXCL9 and -10 in salivary gland tissues, thereby promoting the tissue recruitment of CXCR3-expressing T cells, which are predominantly Th1 and T cytotoxic (Tc) 1 cells21. IFN-γ also enhances the antigen presenting function of the salivary gland cells to facilitate immune activation7,22. Therefore, endogenous immunoregulatory pathways or exogenous immune-suppressive approaches that can attenuate Th1/Tc1 responses and IFN-γ production may have preventive or therapeutic potentials for SS disease.
A multitude of soluble factors and cell surface molecules are up-regulated during SS development, including both positive and negative regulators of the autoimmune responses and pathologies. The costimulatory pathway formed by programmed death-ligand 1 (PD-L1) and its receptor PD-1 plays a critical role in maintaining immune tolerance and limiting immune activation and tissue damage, predominantly by suppressing the differentiation, activation and IFN-γ production of Th1 and Tc1 cells23,24,25,26,27,28, as well as enhancing the differentiation and function of regulatory T (Treg) cells29. PD-1 is expressed on the surface of activated lymphocytes and antigen presenting cells (APCs)30. PD-L1 is constitutively expressed on resting lymphocytes and APCs and its expression is upregulated upon activation of these cells by various stimuli, including IFN-γ and TNF-α31,32,33,34. Apart from immune cells, PD-L1 is induced in various types of non-hematopoietic cells by pro-inflammatory cytokines including IFN-γ35,36, allowing it to exert immunoregulatory function in target organs of various autoimmune and inflammatory diseases. Hence, PD-L1-PD-1 pathway is activated as a result of immune activation and serves as a negative feedback mechanism that down-modulates T cell immune responses. Indeed, PD-L1 expression is elevated in the inflamed tissues of a number of autoimmune diseases, including type 1 diabetes, autoimmune encephalomyelitis (EAE), Crohn’s syndrome, and rheumatoid arthritis29,37,38, and the tissue-infiltrating T cells in these autoimmune disorders express surface PD-138,39,40,41. Loss-of-function studies in mouse models demonstrate that the endogenous PD-L1-PD1 activities restrain the development and reduce the severity of lupus-like glomerulonephritis and arthritis, EAE, autoimmune diabetes and collagen-induced arthritis29,42,43. Moreover, enforced activation of PD-L1-PD-1 pathway impedes the development and reduces the severity of these diseases44,45. The disease-inhibiting effect of PD-L1-PD-1 is predominantly associated with reduced Th1 and Tc1 responses and impaired IFN-γ production25,46,47, and in some cases, a dampened Th17 responses48,49.
PD-L1 and PD-1 expression are elevated in salivary gland epithelial cells and salivary gland-infiltrating lymphocytes, respectively, in SS patients35, suggesting a potential immune-suppressive and disease-inhibiting role of PD-L1-PD-1 pathway in this disease. In the present study, we investigated the role of endogenous PD-L1 in SS by inhibiting its function in non-obese diabetic (NOD) mice, a widely used model of SS, and demonstrated an inhibitory effect of endogenous PD-L1 that hinders the development and onset of this disease.
Results
Expression of PD-L1 and PD-1 in the submandibular glands (SMG) of NOD/ShiLtJ mice increases during the development of SS-like disease
Increased PD-L1 and PD-1 expressions are shown in patients with SS35. To examine whether PD-L1 and PD-1 expression is elevated during the development of SS-like disease in NOD/ShiLtJ mice, we determined their mRNA levels in the SMG from these mice at various ages. We first determined the time course of SS development in the female NOD/ShiLtJ mice by examining mice aged 4-, 7-, 10- and 12 weeks. The results showed that the disease onset started around 10 weeks of age in the great majority of these mice, based on the presence of leukocyte foci in the SMG and antinuclear antibodies (ANA) in the serum, as well as an impaired stimulated salivary flow rate compared to mice aged 4 weeks and control Balb/c mice (data not shown). We thus assessed PD-L1 and PD-1 gene expression at 4-, 7- and 10 weeks of age by real-time PCR analysis, which showed that the amounts of both PD-L1 and PD-1 mRNA in the SMG were significantly increased between age 4 and 7 weeks, and PD-1 mRNA levels were further increased between age 7 and 10 weeks (Fig. 1). Hence, PD-L1 and PD-1 expression in the SMG increases during the early phase of SS development in NOD/ShiLtJ mice, suggesting that PD-L1-PD-1 pathway may act as a negative feedback mechanism to suppress autoimmune responses and hinder the further development of this disease.
Figure 1. Expression level of PD-L1 and its receptor PD-1 in the SMG of NOD/ShiLtJ mice.
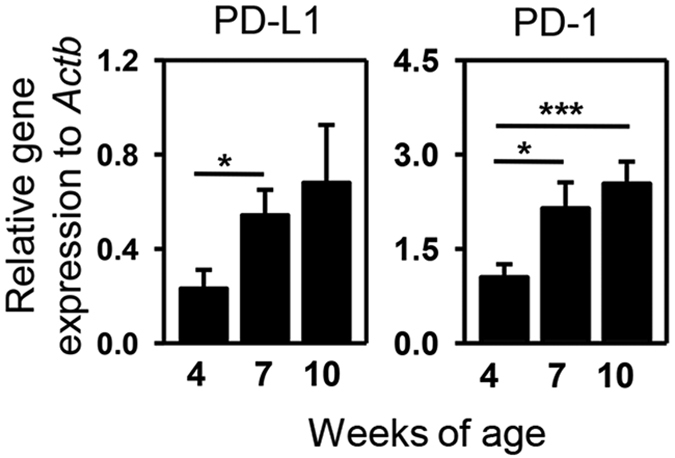
Real-time PCR analysis of PD-L1 and PD-1 mRNA levels in the SMG of NOD/ShiLtJ mice aged 4, 7 and 10 weeks, presented relative to that of β-actin. Data are the average of the analyses of 4–7 mice for each group. Error bars represent the standard error of mean (SEM). *P < 0.05, **P < 0.01; ***P < 0.001.
Blockade of endogenous PD-L1 accelerates the development of characteristic SS-like pathologies in NOD/ShiLtJ mice
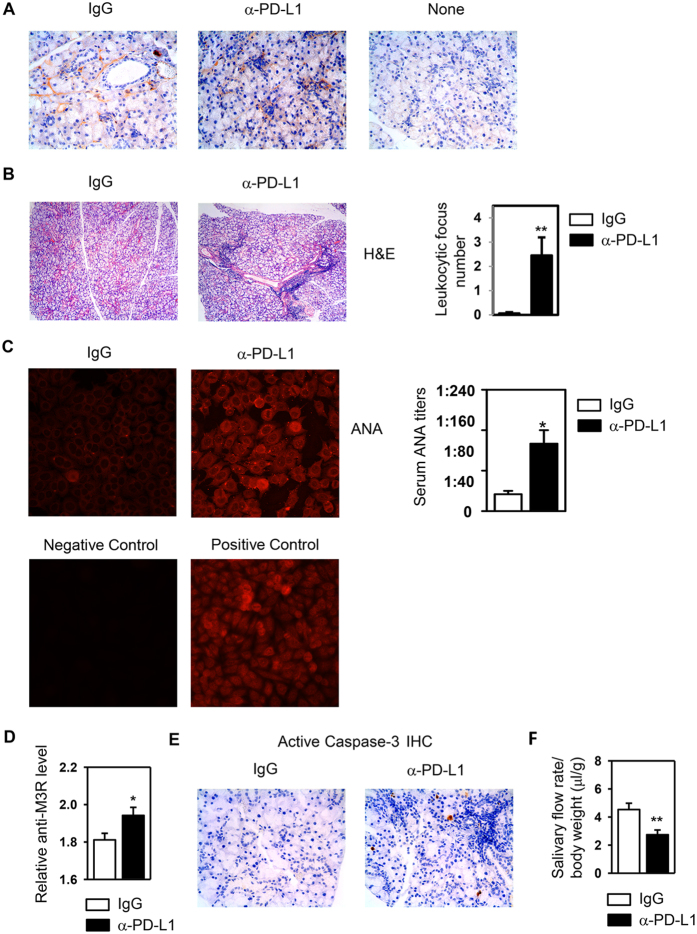
To investigate whether endogenous PD-L1 expressed in the SMG affects the pathogenesis of SS, we intraperitoneally (i.p.)-administered 200 μg of an anti-PD-L1 antibody, which blocks the interaction between PD-L1 and its receptors, or the isotype control, rat IgG2b, into 6 week-old female NOD/ShiLtJ mice every other day for a total of 4 times and analyzed the disease profiles in these mice. To determine whether systemically injected anti-PD-L1 antibody was delivered into the target organ SMG, the presence of rat IgG2b in this organ was assessed by immunohistochemical staining. The positive staining of rat IgG2b was detected in the SMG of both anti-PD-L1- and rat IgG2b-treated mice, whereas no staining was observed in that of untreated control mice (Fig. 2A), indicating that the injected antibody was present in the SMG and could inhibit the function of PD-L1 expressed locally as intended. We next analyzed a series of hallmark pathologies of SS to determine the role of endogenous PD-L1 in the development of this disease. Histological analysis by H&E staining demonstrated that at the time of the analysis, only 5.5% of IgG-treated NOD/ShiLtJ mice had leukocytic foci in the SMG tissues, whereas 55% of anti-PD-L1-treated mice had foci, with the average focus number higher than 2 (Fig. 2B, Supplementary Figure S1 and Table S1). To assess whether PD-L1 blockade alters the production of ANA, we determined the presence of ANA in the serum by indirect immunofluorescence staining for mouse IgG that recognizes human epithelial (HEp-2) cell substrates. The results showed that 47% of IgG-treated mice were positive for serum ANA, and this percentage was increased to 81% in anti-PD-L1-treated mice. Moreover, among the ANA-positive mice, PD-L1 blockade led to an increase in the levels of ANA as indicated by higher fluorescence intensity of the staining (Fig. 2C). Autoantibodies against M3 muscarinic acetylcholine receptor (M3R) have been shown to interfere with normal salivary secretion in response to neurotransmitters50,51, and ELISA assay demonstrated that PD-L1 blockade led to increased serum anti-M3R levels in the NOD/ShiLtJ mice (Fig. 2D). Immunohistochemical staining of SMG sections showed that at the time of the analysis, IgG-treated NOD/ShiLtJ mice had very low levels of active caspase-3 in the SMG tissues (Fig. 2E). In contrast, most of the anti-PD-L1-injected mice exhibited caspase-3 activation in the SMG (Fig. 2E), suggestive of tissue apoptosis and damage that can contribute to the development of SS pathologies and the secretory dysfunction of the SMG. Finally, anti-PD-L1-treatment significantly decreased the stimulated saliva flow rate, indicating an impaired secretory function and clinical onset of SS (Fig. 2F). Hence, PD-L1 blockade accelerates the development and clinical onset of SS.
Figure 2. Blockade of PD-L1 accelerates the appearance of SS-like pathologies.
NOD/ShiLtJ mice received injections of rat anti-mouse PD-L1 antibody and control rat IgG2b every other day for a total of 4 times. (A) The presence of the injected antibody and control IgG in the SMG was examined by horseradish peroxidase (HRP) based immunohistochemical staining with an anti-rat IgG antibody. SMG sections from mice not receiving any injections were used as negative controls. Original magnification: ×400. Data are representative of 6–7 mice for each group. (B) H&E staining of SMG sections with original magnification of ×200 (left), and mean leukocyte focus number in the SMG (right). (C) Detection of serum ANA (original magnification: ×400) and determination of its titers (right panel). (D) Serum anti-M3R autoantibody level was determined by ELISA. (E) Immunohistochemical staining of cleaved, active caspase-3 in SMG sections. Original magnification: ×400. (F) Stimulated saliva flow rate normalized to body weight. Data are representative or the average of the analyses of 18–20 mice for each group. Error bars represent the SEM. *P < 0.05, **P < 0.01; ***P < 0.001.
PD-L1 blockade increases the number of T and B cells in the SMG
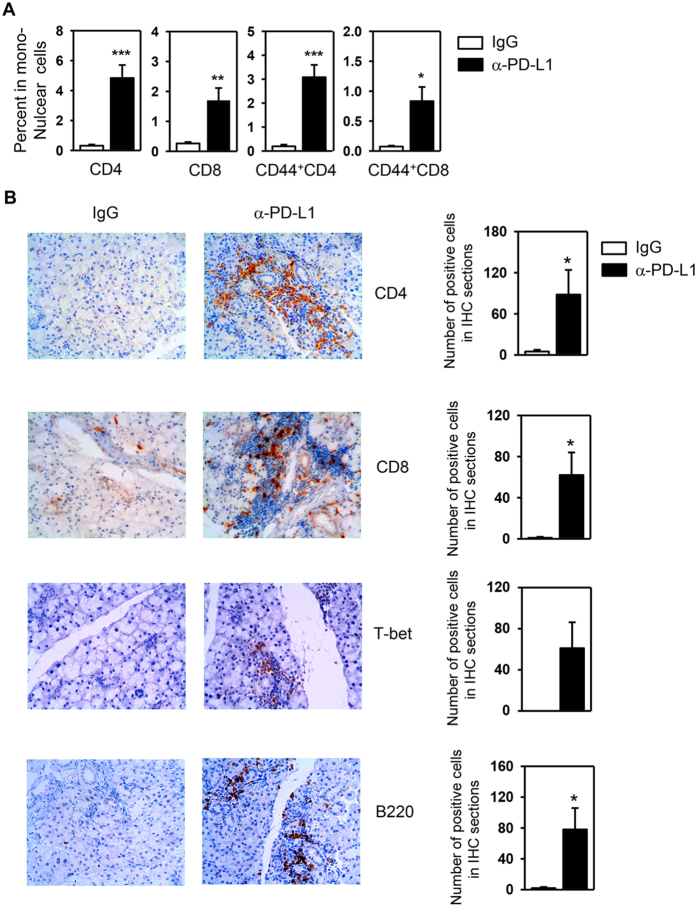
We subsequently examined the effect of PD-L1 blockade on lymphocyte numbers in the SMG. Flow cytometric analysis showed that the percentages of CD4+ and CD8+ T cells among the SMG-infiltrating mononuclear cells were markedly higher in anti-PD-L1-treated NOD/ShiLtJ mice than IgG2b-treated controls (Fig. 3A and Supplementary Figure S2). Moreover, the percentages of CD44+CD4 and CD44+CD8 T cells, which represented the effector or memory T cells, were also significantly increased by anti-PD-L1 treatment (Fig. 3A). Immunohistochemical staining of the SMG sections showed that a significant amount of CD4+, CD8+ and B220+ cells were present in the SMG of anti-PD-L1-treated mice, especially in the leukocytic foci (Fig. 3B). In contrast, very few of these cells were detected in the SMG from IgG-treated control mice, consistent with a lack of foci (Fig. 3B). In addition, the immunohistochemical staining provided evidence that anti-PD-L1 treatment increased the number of Th1 and Tc1 cells in the SMG, as indicated by increased amount of cells expressing T-bet, the signature transcription factor for Th1 and Tc1 subsets (Fig. 3B). In contrast, virtually no cells in the SMG expressed GATA3, the master regulator of Th2 cells (Supplementary Figure S3). Interestingly, the amount of cells that expressed Foxp3, the master regulator of Tregs, were significantly increased upon PD-L1 blockade, indicating that the promoting effect of anti-PD-L1 on SS development did not result from a decrease in Tregs (Supplementary Figure S3). In summary, the disease-accelerating effect of PD-L1 blockade on SS development is accompanied by increased T and B cells and elevated T-bet levels in the SMG.
Figure 3. PD-L1 blockade increases the number of T and B cells and in the SMG.
Anti-PD-L1 or control rat IgG was injected into 6 week-old NOD/ShiLtJ mice, every other day for a total of 4 times. (A) Flow cytometry of lymphocyte populations in SMG-infiltrating mononuclear cells. (B) Immunohistochemical staining of CD4, CD8, T-bet and B220 in SMG sections and the quantification of the number of positively-stained cells (right panels). Original magnification: ×400. Data are representative or the average of the analyses of 6–7 mice for each group. *P < 0.05, **P < 0.01; ***P < 0.001.
PD-L1 blockade increases the expression of Th1 signature molecules and lymphocyte chemoattractants in the SMG
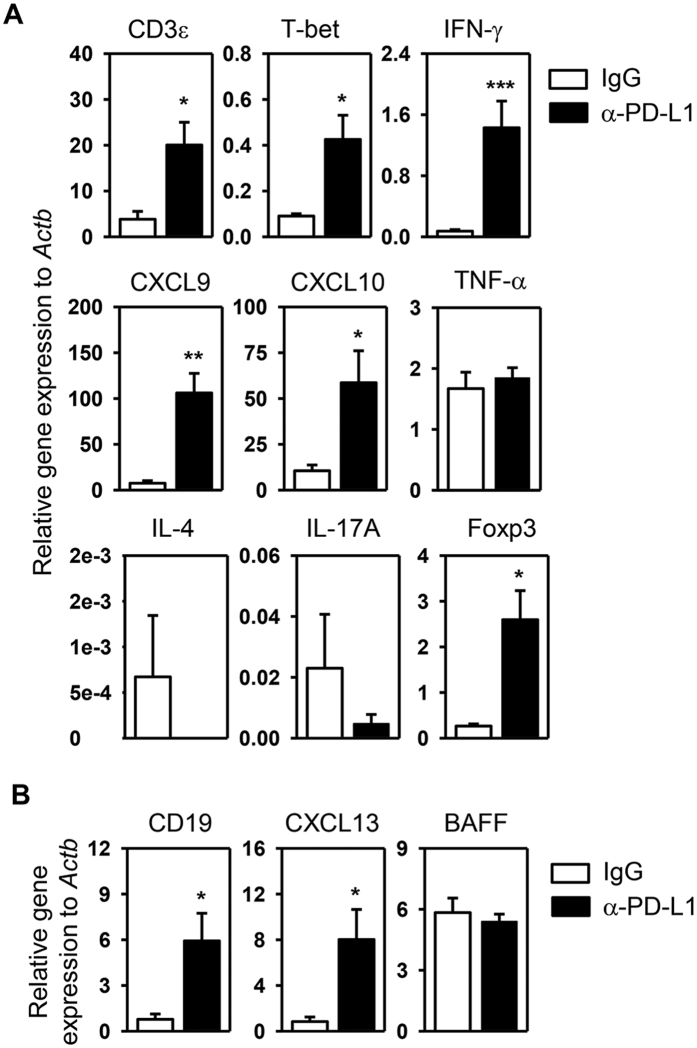
To further understand the effect of PD-L1 blockade on the autoimmune responses in the SMG, we assessed the expression of hallmark cytokines for various effector T cell subsets. In accordance with increased T cell numbers and T-bet levels in the SMG, mRNA levels of CD3ε, IFN-γ and T-bet were all elevated upon PD-L1 blockade (Fig. 4A). In contrast, PD-L1 blockade did not increase the amount of IL-4 and IL-17A mRNA, suggesting that endogenous PD-L1 plays a protective role in SS by suppressing Th1 and Tc1 type responses but not Th2 or Th17 responses (Fig. 4A). Consistent with the increase in Foxp3-expressing cells in the SMG as determined by immunohistochemical staining, the transcription level of Foxp3 was also increased by PD-L1 blockade (Fig. 4A). We next assessed if PD-L1 blockade affects the expression of CXCR3 ligands CXCL9 and -10, which are chemokines that can facilitate the recruitment of CXCR3-expressing Th1 and Tc1 cells to the inflamed organs. Real time PCR analysis indicated that PD-L1 blockade substantially up-regulated CXCL9 and -10 gene expression in the SMG of NOD/ShiLtJ mice (Fig. 4A), which may at least in part contribute to the increase in T cells in the SMG by promoting their tissue-infiltration. Hence, the disease-accelerating effect of PD-L1 blockade is accompanied by increased Th1/Tc1 chemoattractants. Furthermore, consistent with an increase in B cells in the SMG shown by flow cytometric analysis, mRNA levels of B cell marker CD19 and B cell chemoattractant CXCL13 in the SMG were significantly increased by PD-L1 blockade (Fig. 4B). Assessment of several cytokines that can promote B cell function and antibody production showed that the mRNA levels of B cell activating factor, IL-21 and IL-13 were not affected by PD-L1 blockade (Fig. 4B, and data not shown). Hence, the disease-accelerating effect of anti-PD-L1 is associated with increased amounts of B cells and CXCL13 in the SMG. In summary, PD-L1 blockade increases the expression of Th1/Tc1 signature molecules and T- and B-cell chemoattractants in the SMG.
Figure 4. PD-L1 blockade increases expression of Th1 signature molecules and lymphocyte chemoattractants in the SMG.
Anti-PD-L1 or control rat IgG was injected into NOD/ShiLtJ mice as described earlier. Real-time PCR analysis of (A) T cell-associated gene expression and (B) B cell-associated gene expression in the SMG, presented relative to that of β-actin. Data are the average of the analyses of 6–7 mice for each group. *P < 0.05, **P < 0.01; ***P < 0.001.
Local administration of exogenous IFN-γ into the SMG causes impaired salivary secretion
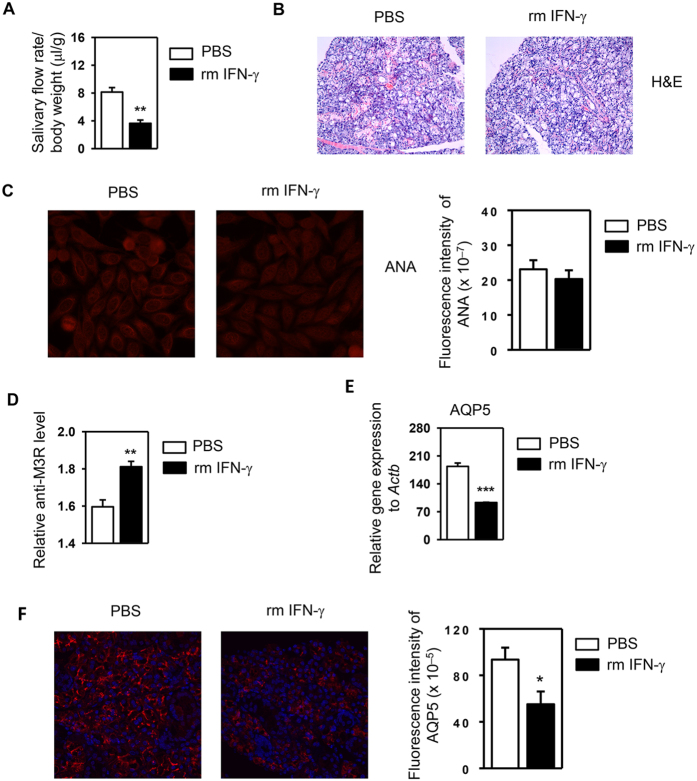
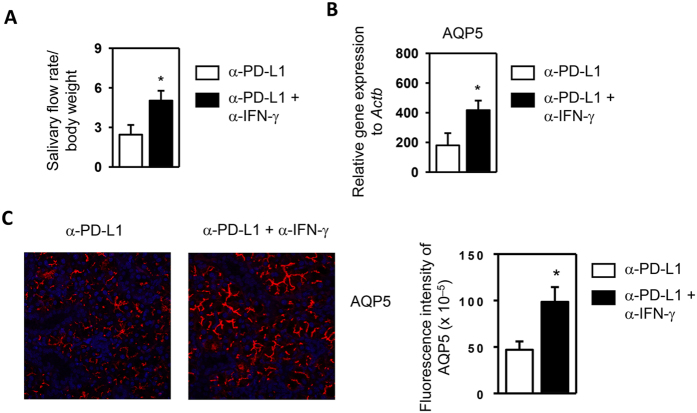
Genetic deficiency of IFN-γ in NOD/ShiLtJ mice prevents the development of SS-like pathologies including SMG tissue inflammation and apoptosis, ANA production, and hyposalivation, indicating a crucial requirement for IFN-γ in the development of SS. Since PD-L1 blockade led to enhanced Th1 and Tc1 responses and increased IFN-γ production, we reasoned that the excess IFN-γ may account for the disease-acceleration induced by PD-L1 blockade. To test this hypothesis, we injected 3 μg of recombinant murine (rm) IFN-γ or control PBS directly into the SMG of 6 week-old female NOD/ShiLtJ mice every 3 days for a total of 3 times, and analyzed the SS disease profile. Treatment with rm IFN-γ significantly reduced the stimulated saliva flow rate in NOD/ShiLtJ mice (Fig. 5A). IFN-γ treatment did not promote leukocyte infiltration or caspase-3 activation in SMG tissues (Fig. 5B, and data not shown). Interestingly, IFN-γ treatment did not affect serum ANA levels (Fig. 5C), but increased the levels of anti-M3R autoantibodies, which have the ability to impair normal salivary secretion (Fig. 5D)50,51. To further define additional mechanisms of IFN-γ effect on salivary secretion, we examined the expression of aquaporin 5 (AQP5), a water channel protein critical for normal salivary secretion52,53,54. IFN-γ treatment reduced AQP5 mRNA amounts in the SMG (Fig. 5E) and decreased AQP5 protein levels as determined by immunofluorescence staining (Fig. 5F). Hence, intra-SMG administration of IFN-γ leads to impaired saliva secretion in NOD/ShiLtJ mice, which is accompanied by reduced AQP5 expression and augmented anti-M3R production. These results indicate that the increased IFN-γ expression caused by PD-L1 blockade may be responsible for the exacerbated secretory dysfunction but not for other pathological changes induced by this treatment.
Figure 5. Local administration of exogenous IFN-γ into the SMG causes impaired salivary secretion.
Recombinant mouse IFN-γ was directly injected into the SMG of NOD/ShiLtJ mice every 3 days for a total of 3 times. (A) Stimulated saliva flow rate normalized to body weight. (B) H&E staining of SMG sections. Original magnification ×200. (C) Detection of serum ANA and the quantification of the fluorescence intensity of ANA staining (right panel). Original magnification: ×400. (D) Serum anti- M3R autoantibody level was determined by ELISA. (E) Real-time PCR analysis of relative AQP5 mRNA levels in the SMG. (F) Immunofluorescence staining of AQP5 protein in SMG sections and the quantification of the fluorescence intensity of AQP5 staining (right panel). Data are representative or the average of the analyses of 4 mice for each group. *P < 0.05, **P < 0.01; ***P < 0.001.
Neutralization of IFN-γ improves salivary secretion and AQP5 expression in anti-PD-L1-treated NOD/ShiLtJ mice
Having shown that exogenous IFN-γ administration impairs salivary secretion, we next determined whether IFN-γ blockade can improve salivary secretion in anti-PD-L1-treated NOD/ShiLtJ mice. We i.p.-injected the anti-PD-L1 antibody to NOD/ShiLtJ mice in conjunction with a neutralizing anti-IFN-γ antibody or the isotype control IgG. Our results demonstrated that anti-IFN-γ markedly improved the salivary flow rate (Fig. 6A). Real-time PCR analysis and immunofluorescence staining demonstrated that anti-IFN-γ treatment markedly increased AQP5 mRNA and protein levels in the SMG (Fig. 6B and C). IFN-γ neutralization did not reduce the inflammation of SMG or the levels of serum autoantibodies (Data not shown). Hence, increased amount of IFN-γ upon PD-L1 blockade down-regulates AQP5 production and contributes to the salivary gland secretory dysfunction.
Figure 6. Neutralization of IFN-γ improves salivary secretion in anti-PD-L1-treated NOD/ShiLtJ mice.
6 week-old female NOD/ShiLtJ mice were i.p.-injected with 200 μg of rat IgG1 or anti-IFN-γ together with 200 μg of anti-PD-L1 every other day for a total of 4 times. (A) Stimulated saliva flow rate normalized to body weight. (B) Real-time PCR analysis of relative AQP5 mRNA levels in the SMG. (C) Immunofluorescence staining of AQP5 protein in SMG sections, and the quantification of the fluorescence intensity of AQP5 staining (right panel). Original magnification: ×400. Data are representative or the average of the analyses of 8 mice for each group.
In summary, we propose a model in which the interaction between PD-L1 and PD-1 impedes the recruitment of Th1 and Tc1 to the salivary glands in part by down-regulating CXCL9. Reduced IFN-γ production resulting from impaired local Th1/Tc1 responses in turn increases AQP5 expression and diminishes anti-M3R autoantibody production, thereby hindering the development of salivary gland hypofunction. In addition, PD-L1-PD-1 interaction also impedes B cell recruitment to the salivary glands in part by down-regulating CXCL13, and thereby curtails B cell activation and autoantibody production. Hence, endogenous PD-L1, which is up-regulated during early phase of SS development, impedes the development and delays the onset of SS in a negative feedback fashion (Supplementary Figure S4).
Discussion
In the present study, we demonstrate a protective role of endogenous PD-L1 in the development and onset of SS-like disease in NOD mice and elucidate the cellular mechanisms underlying its immune-regulatory function in this disease.
Salivary gland-infiltrating lymphocytes in SS patients express higher levels of PD-1 than those from control subjects and PD-L1 is expressed in salivary gland epithelial cells in most of the patients35. Consistent with these findings, we showed that PD-L1 and PD-1 expression in the salivary glands of NOD/ShiLtJ mice increases considerably, accompanying the development of SS. Hence, up-regulation of this inhibitory pathway, possibly as a result of production of the inflammatory mediators at this stage, acts as a negative feedback mechanism to impede the development and onset of this disease. The cellular sources of PD-1 and PD-L1 in this study may include activated T and B cells, Tregs, salivary gland epithelial cells and APCs. The most well-characterized interaction between PD-L1 and PD-1 in autoimmune and inflammatory conditions is the one between PD-L1 expressed on tissue cells or APCs and PD-1 expressed on T cells25,35,38,43. Additionally, PD-L1 expressed by activated T cells24 as well as by Tregs29,55,56 can also engage PD-1 to suppress the activation and function of T cells. The precise cellular sources of PD-L1 critical for the inhibition of SS development in the NOD/ShiLtJ mice require further determination by specifically abolishing PD-L1 expression in these cell populations.
SS have a strong female propensity, with 90% of the patients being women50,57. It has been well-documented that female NOD mice mostly develop spontaneous autoimmune sialadenitis, whereas male NOD mice mostly develop autoimmune dacryoadenitis (dry eyes)58,59. Our recent analysis of the disease profiles in both female and male NOD/ShiLtJ mice also confirmed these findings. In this study, we only used female mice to study the effect of PD-L1 blockade on SS-like sialadenitis, which has the same gender propensity in both human patients and NOD mice. However, it will be important to assess whether PD-L1 blockade can similarly accelerate the development and onset of SS-like lacrimal gland inflammation and dysfunction using male NOD mice. Furthermore, our future investigations will also assess whether enhancing PD-L1-PD-1 activity with exogenous PD-L1-Fc can prevent the development and onset of SS, and reverse or attenuate the established SS in both male and female NOD mice.
In this study, we administered anti-PD-L1 antibody repeatedly into NOD/ShiLtJ mice between 6 to 8 weeks of age. Previous reports have shown that most of NOD/ShiLtJ mice become diabetic between 12 and 16 weeks of age, and the development and onset of the type-1 diabetes is accelerated upon PD-L1 blockade37,60. We therefore monitored the urine glucose levels in mice during the course of anti-PD-L1 treatment and found that they remained normal in all the mice throughout the experiments. Therefore, the acceleration of SS development induced by PD-L1 blockade in the present study is not a secondary consequence of clinical diabetes.
Consistent with the findings in a number of other autoimmune disorders24,25,46,47, here we showed that PD-L1 blockade and preferentially enhances Th1/Tc1 responses and IFN-γ production, without affecting Th2 and Th17 responses, in the SS disease setting. It has been reported that IFN-γ-gene-deficiency prevents the development of pathologies of SS and the secretory dysfunction in NOD/ShiLtJ mice17. Here we show that indeed, administration of IFN-γ replicates, whereas neutralization of IFN-γ attenuates the detrimental effect of PD-L1 blockade on salivary gland secretory function. The effect of IFN-γ correlates with the alteration in the level of AQP5, a key water channel protein for normal salivary secretion52,53,54. AQP5 deficiency leads to significantly reduced salivary volume due to defective water export from the salivary epithelial cells52,53, and local gene therapy that overexpresses AQP1 to compensates for defective AQP5 expression restores salivary secretion in a mouse model of SS54. It is interesting that IFN-γ administration leads to increased production of anti-M3R antibody, which has a reported effect of impairing the salivary gland cell secretory function in response to parasympathetic neurotransmitters50,51. It is not clear to us how it affects this specific autoantibody without affecting the ANA production and future investigations will be needed to elucidate the underlying mechanisms. We also find that excess IFN-γ in the SMG does not replicate, and neutralization of IFN-γ does not mitigate the accelerating effect of PD-L1 blockade on leukocyte infiltration, ANA production and tissue apoptosis. Hence, the excessive IFN-γ production is likely a consequence of enhanced Th1/Tc1 response in the SMG resulting from PD-L1 blockade, which in turn promotes the salivary gland secretory dysfunction possibly by inhibiting AQP5 expression and enhancing anti-M3R production. It is not essentially required for the exacerbation of other pathological events induced by PD-L1 blockade.
Another intriguing finding in this study is that anti-PD-L1 treatment increases the expression level of Foxp3, the key transcription factor of Treg cells. PD-L1 promotes the development and sustains the suppressive function of Treg cells by regulating Foxp361, and PD-L1 expressed by Tregs constitutes as one of the pivotal mechanisms by which Tregs exert their suppressive effect29,55,56. However, a recent report demonstrates that PD-L1 can also negatively regulate the expansion of Treg cells in the context of chronic viral inflammation62, supporting the notion that the effect of PD-L1 on Tregs is disease context-dependent. Hence, the specific effect of PD-L1 on Treg expansion and function in the SS setting needs to be characterized in greater depth in future. Furthermore, the specific characteristics of Tregs and their functional importance in the SS disease setting also await investigation by using mouse models.
In addition to autoreactive T cell responses, B cell infiltration of exocrine glands and their activation and autoantibody production are also characteristic events in SS that are crucial to the full onset of salivary gland secretory dysfunction9,63,64. We found that the number of B cells in the salivary gland of NOD/ShiLtJ mice and the production of ANA and anti-M3R antibodies are significantly enhanced by PD-L1 blockade. Although several reports have shown that PD-L1 can directly affect B cell activation and proliferation65,66, we did not observe a direct effect of PD-L1 on B cell expansion and antibody production in in vitro cultures (Data not shown). We therefore hypothesize that PD-L1 indirectly suppresses B cell function and autoantibody production by inhibiting T cell responses, in particular, Th1/Tc1 response and IFN-γ production. Indeed, in both SS patients and mouse SS models, T cell infiltration of target organs precedes B cell infiltration4,64. Moreover, deletion or inhibition of T cell cytokines IFN-γ and IL-17 prevents the development of all major pathologies of SS including B cell infiltration and ANA production17,67. These findings highlight the crucial role of T cell response for the induction of subsequent autoreactive B cell response. Hence, it is plausible that by inhibiting T cell activation and cytokine production, PD-L1 can suppress the subsequent B cell activation and antibody production. One specific event induced by anti-PD-L1 treatment is the up-regulation of B cell chemoattractant CXCL13, which is elevated in a number of autoimmune diseases including SS68,69. Importantly, studies in both human patients and mouse models strongly suggest CXCL13 as a biomarker and an essential pathogenic player in SS69. Here, we showed that PD-L1 blockade leads to elevated CXCL13 expression in the salivary glands, which may promote B cell recruitment and the consequent production of autoantibodies. Future studies will investigate the main cellular sources of CXCL13 and the mechanisms of enhanced CXCL13 production as a result of PD-L1 blockade in the SS disease context.
Conclusions
The present study demonstrated that PD-L1 expression is up-regulated in the salivary glands of female NOD mice during the developmental phase of SS, which in turn hinders the development and onset of this disease in a negative feedback fashion. Thus, enhancing the activities PD-L1-dependent pathways may be an effective therapeutic strategy to combat this disease for which no effective treatment is currently available.
Methods
Mice
Female non-obese diabetic (NOD/ShiLtJ) mice were purchased from the Jackson Laboratory and were kept under specific pathogen-free conditions. All experimental protocols were approved by the Institutional Animal Care and Use Committee of the Forsyth Institute. All methods were carried out in accordance with the National Institutes of Health guidelines for the care and use of laboratory animals.
Antibodies, peptides and cytokines
Purified monoclonal anti-mouse PD-L1 (10F.9G2), anti-mouse IFN-γ (XMG1.2), and isotype control rat IgG2b and rat IgG1 were purchased from BioXCell. Recombinant mouse (rm) IFN-γ was purchased from Peprotech. The muscarinic acetycholine receptor (M3R) peptide was synthesized by Biomatik Corporation. For Flow cytometry, anti-CD4, anti-CD8 and anti-CD44 antibodies were purchased from BioLegend. For immunohistological chemistry, biotin conjugated anti-CD4 antibody was obtained from eBioscience, anti-T-bet and biotin conjugated anti-B220 antibodies were from BioLegend, and biotin conjugated anti-rat IgG2bwere purchased from Vector Laboratories. For immunofluorescence staining, anti-aquaporin 5 (AQP5) and Alexa Fluor647-conjugated anti-rabbit IgG antibodies were purchased from Abcam.
In vivo administration of anti-PD-L1 antibody, IFN-γ and anti-IFN-γ antibody
Female NOD/ShiLtJ mice were i.p.-injected with 200 μg of IgG or anti-PD-L1 every other day for a total of 4 times, starting from 6 weeks of age. For IFN-γ administration, 6 week-old female NOD/ShiLtJ mice were anesthetized with ketamine hydrochloride/xylazine hydrochloride and 3 μg rm IFN-γ was injected directly into the submandibular gland (SMG) every 3 days for a total of 3 times. For anti-IFN-γ antibody administration, 6 week-old female NOD/ShiLtJ mice were i.p.-injected with 200 μg anti-PD-L1, together with 200 μg rat IgG1 or anti-IFN-γ, every other day for a total of 4 times. All the analyses were performed 2 days after the last injection.
Histological analysis
SMG tissue samples were fixed in 4% paraformaldehyde, embedded in paraffin and sectioned to 5 μm thickness. Sections were then stained with hematoxylin and eosin (H&E) and examined for leukocytic infiltration. The number of leukocytic foci in each of the three non-consecutive sections of each SMG sample was counted, and the highest number among the three was used for further calculation and statistical analysis.
Immunofluorescence staining
Rehydrated SMG sections were subjected to antigen unmasking process and then incubated with rabbit anti-mouse AQP5 antibody at 4 °C overnight, followed by Alexa Fluor647-conjugated anti-rabbit IgG for 1 h at room temperature. The stained samples were examined and imaged (magnification: ×400) under a Leica laser scanning confocal microscope (Zeiss, Oberkochen, Germany). Quantification of the fluorescence intensity was performed using ImageJ 1.50i software. Briefly, images were converted into grayscale (8-bit) and the grey color was segmented using thresholding. Integrated density measurement was performed to determine the fluorescence intensity of the staining.
Immunohistochemical staining
SMG sections were subjected to de-paraffinization and stained with anti-mouse CD4, anti-mouse T-bet, or anti-mouse B220 antibody at 4 °C overnight using VECTASTAIN Elite ABC Kit (Vector Laboratories) following the manufacturer’s instructions. Active caspase-3 was detected by SignalStain® Apoptosis (Cleaved Caspase-3) IHC Detection Kit (Cell Signaling Technology), according to the manufacturer’s manual. The stained samples were examined and imaged (magnification ×400) under a light microscope. Quantification of the positively-stained cells in the samples was performed using ImageJ 1.50i software. Briefly, images were saved as RGB Tiff files and only the brown-stained cells were segmented using appropriate color thresholding. The total number of the thresholded cells, which were positively-stained cells, in each sample was measured and calculated.
Detection of serum antinuclear antibodies (ANA)
ANA in mouse sera were detected using Alexa Fluor568-conjugated anti-mouse IgG (ThermoFisher Scientific) and HEp-2 human epithelial cell substrate slides (INOVA Diagnostics) following the manufacturer’s instructions. The stained samples were examined with inverted wide-field fluorescence microscope (Zeiss) at a magnification of 400X. Images presented were processed using Zeiss software (ZEN blue edition). Quantification of the fluorescence intensity was performed using ImageJ 1.50i software as described. Integrated density measurement was performed to determine the fluorescence intensity of the staining.
Measurement of salivary flow rate
Non-anesthetized mice were weighed and given an i.p.- injection of 100 μl PBS-based secretagogue solution containing isoproterenol (0.02 mg/ml) and pilocarpine (0.05 mg/ml). One min after secretagogue injection, saliva was collected continuously for 5 min from the oral cavity of mice with a micropipette. The volume of saliva was measured and normalized to the body weight.
RNA isolation and Real-time RT-PCR
SMG lobes were cut into small fragments and grinded into single cells using frosted glass slides. The total SMG cells were resuspended in RNA lysis buffer and subjected to RNA isolation using RNeasy Micro kit (Qiagen) followed by reverse transcription with M-MLV reverse transcriptase (Promega). The resulting cDNA was subjected to SYBR Green-based real-time PCR amplification (Qiagen) for 40 cycles with annealing and extension temperature at 60 °C, on a LightCycler 480 Real-Time PCR System (Roche). Primer sequences are: mouse PD-L1 forward, 5′-GGTGCGGACTACAAGCGAAT-3′, reverse, 5′-TTCATGCTCAGAAGTGGCTGG-3′; PD-1 forward, 5′-AAATCGAGGAGAGCCCTGGA-3′, reverse, 5′-CATGCCTTGAAACCGGCCTT-3′; T-bet forward, 5′-CCAACAACCCCTTTGCCAAAG-3′, reverse, 5′-TCCCCCAAGCAGTTGACAGT-3′; IFN-γ forward, 5′-GGATGCATTCATGAGTATTGC-3′, reverse, 5′-CTTTTCCGCTTCCTGAGG-3′; TNF-α forward, 5′-CCTTTCACTCACTGGCCCAA-3′, reverse, 5′-AGTGCCTCTTCTGCCAGTTC-3′. Other sequences will be provided upon request. The relative mRNA level of each gene relative to that of β-actin was analyzed using the LightCycler@480 software.
ELISA
The M3R peptide solution, at 2 μg/ml in the ELISA coating buffer (Biolegend), was adsorbed onto a Nunc™ MaxiSorp™ flat-bottom 96 well plate overnight at 4 °C. Non-specific binding sites on the plate were then blocked by incubating with ELISA assay diluent buffer (Biolegend) for 1 h at room temperature. Mouse sera (1:6 diluted in the blocking buffer) were added to the plate and incubated overnight at 4 °C. The plate was washed and incubated with biotinlated goat anti-mouse IgG antibody (Vector Labratories) at 1:300 dilution for 1 h at room temperature. After washing, the bound antibodies were detected by incubation with Avidin-HRP solution for 30 min, followed by incubation with TMB substrate solution. Finally, the absorbance was read on a microplate reader (BioTek) at 450 nm.
Statistical analysis
All statistical significance was determined by Student’s t-test (two-tailed, two sample equal variance). P values smaller than 0.05 were considered as statistically significant.
Additional Information
How to cite this article: Zhou, J. et al. Endogenous programmed death ligand-1 restrains the development of Sjögren’s syndrome in non-obese diabetic mice. Sci. Rep. 6, 39105; doi: 10.1038/srep39105 (2016).
Publisher's note: Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Material
Acknowledgments
We thank Juan Du, Dr. Ekta S Patel and Dr. Atsushi Ikeda for technical assistance in this study. This study was supported by grants from the National Institutes of Health to QY (R01 DE023838, P30 DE020751).
Footnotes
Author Contributions Q.Y. conceived the project, designed the experiments, analyzed data and wrote the manuscript. J.Z. carried out most of the experiments, analyzed data and participated in writing of the manuscript. J.O.J. and T.K. contributed to the design/analysis/reagents of some experiments.
References
- Jin J. O. & Yu Q. T Cell-Associated Cytokines in the Pathogenesis of Sjogren’s Syndrome. Journal of clinical & cellular immunology S!, doi: 10.4172/2155-9899.S1-009 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fox P. C. Autoimmune diseases and Sjogren’s syndrome: an autoimmune exocrinopathy. Annals of the New York Academy of Sciences 1098, 15–21, doi: 10.1196/annals.1384.003 (2007). [DOI] [PubMed] [Google Scholar]
- Lee B. H., Tudares M. A. & Nguyen C. Q. Sjogren’s syndrome: an old tale with a new twist. Archivum immunologiae et therapiae experimentalis 57, 57–66, doi: 10.1007/s00005-009-0002-4 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Voulgarelis M. & Tzioufas A. G. Pathogenetic mechanisms in the initiation and perpetuation of Sjogren’s syndrome. Nat Rev Rheumatol 6, 529–537, doi: 10.1038/nrrheum.2010.118 (2010). [DOI] [PubMed] [Google Scholar]
- Theander E., Manthorpe R. & Jacobsson L. T. Mortality and causes of death in primary Sjogren’s syndrome: a prospective cohort study. Arthritis and rheumatism 50, 1262–1269, doi: 10.1002/art.20176 (2004). [DOI] [PubMed] [Google Scholar]
- Feltsan T., Stanko P. & Mracna J. Sjogren s syndrome in present. Bratislavske lekarske listy 113, 514–516 (2012). [DOI] [PubMed] [Google Scholar]
- Katsifis G. E., Moutsopoulos N. M. & Wahl S. M. T lymphocytes in Sjogren’s syndrome: contributors to and regulators of pathophysiology. Clinical reviews in allergy & immunology 32, 252–264, doi: 10.1007/s12016-007-8011-8 (2007). [DOI] [PubMed] [Google Scholar]
- Singh N. & Cohen P. L. The T cell in Sjogren’s syndrome: force majeure, not spectateur. Journal of autoimmunity 39, 229–233, doi: 10.1016/j.jaut.2012.05.019 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hayakawa I., Tedder T. F. & Zhuang Y. B-lymphocyte depletion ameliorates Sjogren’s syndrome in Id3 knockout mice. Immunology 122, 73–79, doi: 10.1111/j.1365-2567.2007.02614.x (2007). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li H., Dai M. & Zhuang Y. A. T cell intrinsic role of Id3 in a mouse model for primary Sjogren’s syndrome. Immunity 21, 551–560, doi: 10.1016/j.immuni.2004.08.013 (2004). [DOI] [PubMed] [Google Scholar]
- Roescher N., Tak P. P. & Illei G. G. Cytokines in Sjogren’s syndrome: potential therapeutic targets. Annals of the rheumatic diseases 69, 945–948, doi: 10.1136/ard.2009.115378 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fox R. I., Kang H. I., Ando D., Abrams J. & Pisa E. Cytokine mRNA expression in salivary gland biopsies of Sjogren’s syndrome. Journal of immunology 152, 5532–5539 (1994). [PubMed] [Google Scholar]
- Boumba D., Skopouli F. N. & Moutsopoulos H. M. Cytokine mRNA expression in the labial salivary gland tissues from patients with primary Sjogren’s syndrome. British journal of rheumatology 34, 326–333 (1995). [DOI] [PubMed] [Google Scholar]
- van Woerkom J. M. et al. Salivary gland and peripheral blood T helper 1 and 2 cell activity in Sjogren’s syndrome compared with non-Sjogren’s sicca syndrome. Annals of the rheumatic diseases 64, 1474–1479, doi: 10.1136/ard.2004.031781 (2005). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kang E. H., Lee Y. J., Hyon J. Y., Yun P. Y. & Song Y. W. Salivary cytokine profiles in primary Sjogren’s syndrome differ from those in non-Sjogren sicca in terms of TNF-alpha levels and Th-1/Th-2 ratios. Clinical and experimental rheumatology 29, 970–976 (2011). [PubMed] [Google Scholar]
- Mitsias D. I. et al. The Th1/Th2 cytokine balance changes with the progress of the immunopathological lesion of Sjogren’s syndrome. Clinical and experimental immunology 128, 562–568 (2002). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cha S. et al. A dual role for interferon-gamma in the pathogenesis of Sjogren’s syndrome-like autoimmune exocrinopathy in the nonobese diabetic mouse. Scandinavian journal of immunology 60, 552–565, doi: 10.1111/j.0300-9475.2004.01508.x (2004). [DOI] [PubMed] [Google Scholar]
- Kulkarni K., Selesniemi K. & Brown T. L. Interferon-gamma sensitizes the human salivary gland cell line, HSG, to tumor necrosis factor-alpha induced activation of dual apoptotic pathways. Apoptosis: an international journal on programmed cell death 11, 2205–2215, doi: 10.1007/s10495-006-0281-8 (2006). [DOI] [PubMed] [Google Scholar]
- Baker O. J. et al. Proinflammatory cytokines tumor necrosis factor-alpha and interferon-gamma alter tight junction structure and function in the rat parotid gland Par-C10 cell line. American journal of physiology. Cell physiology 295, C1191–1201, doi: 10.1152/ajpcell.00144.2008 (2008). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu A. J. et al. Interferon-gamma induced cell death in a cultured human salivary gland cell line. Journal of cellular physiology 167, 297–304, doi: (1996 ). [DOI] [PubMed] [Google Scholar]
- Ogawa N., Ping L., Zhenjun L., Takada Y. & Sugai S. Involvement of the interferon-gamma-induced T cell-attracting chemokines, interferon-gamma-inducible 10-kd protein (CXCL10) and monokine induced by interferon-gamma (CXCL9), in the salivary gland lesions of patients with Sjogren’s syndrome. Arthritis and rheumatism 46, 2730–2741, doi: 10.1002/art.10577 (2002). [DOI] [PubMed] [Google Scholar]
- Arellano-Garcia M. E., Misuno K., Tran S. D. & Hu S. Interferon-gamma induces immunoproteasomes and the presentation of MHC I-associated peptides on human salivary gland cells. PloS one 9, e102878, doi: 10.1371/journal.pone.0102878 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Khoury S. J. & Sayegh M. H. The roles of the new negative T cell costimulatory pathways in regulating autoimmunity. Immunity 20, 529–538 (2004). [DOI] [PubMed] [Google Scholar]
- Latchman Y. E. et al. PD-L1-deficient mice show that PD-L1 on T cells, antigen-presenting cells, and host tissues negatively regulates T cells. Proceedings of the National Academy of Sciences of the United States of America 101, 10691–10696, doi: 10.1073/pnas.0307252101 (2004). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Keir M. E. et al. Tissue expression of PD-L1 mediates peripheral T cell tolerance. The Journal of experimental medicine 203, 883–895, doi: 10.1084/jem.20051776 (2006). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sandner S. E. et al. Role of the programmed death-1 pathway in regulation of alloimmune responses in vivo. Journal of immunology 174, 3408–3415 (2005). [DOI] [PubMed] [Google Scholar]
- Sugita S. et al. Human corneal endothelial cells expressing programmed death-ligand 1 (PD-L1) suppress PD-1+ T helper 1 cells by a contact-dependent mechanism. Investigative ophthalmology & visual science 50, 263–272, doi: 10.1167/iovs.08-2536 (2009). [DOI] [PubMed] [Google Scholar]
- Valero-Pacheco N. et al. PD-L1 expression induced by the 2009 pandemic influenza A(H1N1) virus impairs the human T cell response. Clinical & developmental immunology 2013, 989673, doi: 10.1155/2013/989673 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Francisco L. M., Sage P. T. & Sharpe A. H. The PD-1 pathway in tolerance and autoimmunity. Immunological reviews 236, 219–242, doi: 10.1111/j.1600-065X.2010.00923.x (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yao S. & Chen L. PD-1 as an immune modulatory receptor. Cancer journal 20, 262–264, doi: 10.1097/PPO.0000000000000060 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamazaki T. et al. Expression of programmed death 1 ligands by murine T cells and APC. Journal of immunology 169, 5538–5545 (2002). [DOI] [PubMed] [Google Scholar]
- Liang S. C. et al. Regulation of PD-1, PD-L1, and PD-L2 expression during normal and autoimmune responses. European journal of immunology 33, 2706–2716, doi: 10.1002/eji.200324228 (2003). [DOI] [PubMed] [Google Scholar]
- Spranger S. et al. Up-regulation of PD-L1, IDO, and T(regs) in the melanoma tumor microenvironment is driven by CD8(+) T cells. Science translational medicine 5, 200ra116, doi: 10.1126/scitranslmed.3006504 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ou J. N., Wiedeman A. E. & Stevens A. M. TNF-alpha and TGF-beta counter-regulate PD-L1 expression on monocytes in systemic lupus erythematosus. Scientific reports 2, 295, doi: 10.1038/srep00295 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kobayashi M. et al. Enhanced expression of programmed death-1 (PD-1)/PD-L1 in salivary glands of patients with Sjogren’s syndrome. The Journal of rheumatology 32, 2156–2163 (2005). [PubMed] [Google Scholar]
- Schoop R. et al. Suppressed T-cell activation by IFN-gamma-induced expression of PD-L1 on renal tubular epithelial cells. Nephrology, dialysis, transplantation: official publication of the European Dialysis and Transplant Association - European Renal Association 19, 2713–2720, doi: 10.1093/ndt/gfh423 (2004). [DOI] [PubMed] [Google Scholar]
- Ansari M. J. et al. The programmed death-1 (PD-1) pathway regulates autoimmune diabetes in nonobese diabetic (NOD) mice. The Journal of experimental medicine 198, 63–69, doi: 10.1084/jem.20022125 (2003). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Salama A. D. et al. Critical role of the programmed death-1 (PD-1) pathway in regulation of experimental autoimmune encephalomyelitis. The Journal of experimental medicine 198, 71–78, doi: 10.1084/jem.20022119 (2003). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pauken K. E., Jenkins M. K., Azuma M. & Fife B. T. PD-1, but not PD-L1, expressed by islet-reactive CD4+ T cells suppresses infiltration of the pancreas during type 1 diabetes. Diabetes 62, 2859–2869, doi: 10.2337/db12-1475 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moret F. M., van der Wurff-Jacobs K. M., Bijlsma J. W., Lafeber F. P. & van Roon J. A. Synovial T cell hyporesponsiveness to myeloid dendritic cells is reversed by preventing PD-1/PD-L1 interactions. Arthritis research & therapy 16, 497, doi: 10.1186/s13075-014-0497-x (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kanai T. et al. Blockade of B7-H1 suppresses the development of chronic intestinal inflammation. Journal of immunology 171, 4156–4163 (2003). [DOI] [PubMed] [Google Scholar]
- Nishimura H., Nose M., Hiai H., Minato N. & Honjo T. Development of lupus-like autoimmune diseases by disruption of the PD-1 gene encoding an ITIM motif-carrying immunoreceptor. Immunity 11, 141–151 (1999). [DOI] [PubMed] [Google Scholar]
- Raptopoulou A. P. et al. The programmed death 1/programmed death ligand 1 inhibitory pathway is up-regulated in rheumatoid synovium and regulates peripheral T cell responses in human and murine arthritis. Arthritis and rheumatism 62, 1870–1880, doi: 10.1002/art.27500 (2010). [DOI] [PubMed] [Google Scholar]
- Wang C. J. et al. Protective role of programmed death 1 ligand 1 (PD-L1)in nonobese diabetic mice: the paradox in transgenic models. Diabetes 57, 1861–1869, doi: 10.2337/db07-1260 (2008). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sharpe A. H., Wherry E. J., Ahmed R. & Freeman G. J. The function of programmed cell death 1 and its ligands in regulating autoimmunity and infection. Nature immunology 8, 239–245, doi: 10.1038/ni1443 (2007). [DOI] [PubMed] [Google Scholar]
- Guleria I. et al. Mechanisms of PDL1-mediated regulation of autoimmune diabetes. Clinical immunology 125, 16–25, doi: 10.1016/j.clim.2007.05.013 (2007). [DOI] [PubMed] [Google Scholar]
- Dai S., Jia R., Zhang X., Fang Q. & Huang L. The PD-1/PD-Ls pathway and autoimmune diseases. Cellular immunology 290, 72–79, doi: 10.1016/j.cellimm.2014.05.006 (2014). [DOI] [PubMed] [Google Scholar]
- McAlees J. W. et al. Differential control of CD4(+) T-cell subsets by the PD-1/PD-L1 axis in a mouse model of allergic asthma. European journal of immunology 45, 1019–1029, doi: 10.1002/eji.201444778 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dulos J. et al. PD-1 blockade augments Th1 and Th17 and suppresses Th2 responses in peripheral blood from patients with prostate and advanced melanoma cancer. Journal of immunotherapy 35, 169–178, doi: 10.1097/CJI.0b013e318247a4e7 (2012). [DOI] [PubMed] [Google Scholar]
- Rhodus N. L. Sjogren’s syndrome. Quintessence international 30, 689–699 (1999). [PubMed] [Google Scholar]
- Sumida T. et al. The role of M3 muscarinic acetylcholine receptor reactive T cells in Sjogren’s syndrome: a critical review. Journal of autoimmunity 51, 44–50, doi: 10.1016/j.jaut.2013.12.012 (2014). [DOI] [PubMed] [Google Scholar]
- Ma T. et al. Defective secretion of saliva in transgenic mice lacking aquaporin-5 water channels. The Journal of biological chemistry 274, 20071–20074 (1999). [DOI] [PubMed] [Google Scholar]
- Culp D. J. et al. A mouse caries model and evaluation of aqp5-/- knockout mice. Caries research 39, 448–454, doi: 10.1159/000088179 (2005). [DOI] [PubMed] [Google Scholar]
- Lai Z. et al. Aquaporin gene therapy corrects Sjogren’s syndrome phenotype in mice. Proceedings of the National Academy of Sciences of the United States of America 113, 5694–5699, doi: 10.1073/pnas.1601992113 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Francisco L. M. et al. PD-L1 regulates the development, maintenance, and function of induced regulatory T cells. The Journal of experimental medicine 206, 3015–3029, doi: 10.1084/jem.20090847 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kasagi S., Kawano S. & Kumagai S. PD-1 and autoimmunity. Critical reviews in immunology 31, 265–295 (2011). [DOI] [PubMed] [Google Scholar]
- Patel R. & Shahane A. The epidemiology of Sjogren’s syndrome. Clinical epidemiology 6, 247–255, doi: 10.2147/CLEP.S47399 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Toda I. et al. Impact of gender on exocrine gland inflammation in mouse models of Sjogren’s syndrome. Experimental eye research 69, 355–366, doi: 10.1006/exer.1999.0715 (1999). [DOI] [PubMed] [Google Scholar]
- Lieberman S. M., Kreiger P. A. & Koretzky G. A. Reversible lacrimal gland-protective regulatory T-cell dysfunction underlies male-specific autoimmune dacryoadenitis in the non-obese diabetic mouse model of Sjogren syndrome. Immunology 145, 232–241, doi: 10.1111/imm.12439 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Giarratana N., Penna G. & Adorini L. Animal models of spontaneous autoimmune disease: type 1 diabetes in the nonobese diabetic mouse. Methods in molecular biology 380, 285–311, doi: 10.1007/978-1-59745-395-0_17 (2007). [DOI] [PubMed] [Google Scholar]
- Beswick E. J., Pinchuk I. V., Das S., Powell D. W. & Reyes V. E. Expression of the programmed death ligand 1, B7-H1, on gastric epithelial cells after Helicobacter pylori exposure promotes development of CD4+ CD25+ FoxP3+ regulatory T cells. Infection and immunity 75, 4334–4341, doi: 10.1128/IAI.00553-07 (2007). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Franceschini D. et al. PD-L1 negatively regulates CD4+ CD25+ Foxp3+ Tregs by limiting STAT-5 phosphorylation in patients chronically infected with HCV. The Journal of clinical investigation 119, 551–564, doi: 10.1172/JCI36604 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mackay F., Groom J. R. & Tangye S. G. An important role for B-cell activation factor and B cells in the pathogenesis of Sjogren’s syndrome. Current opinion in rheumatology 19, 406–413, doi: 10.1097/BOR.0b013e328277ef4c (2007). [DOI] [PubMed] [Google Scholar]
- Nguyen C. Q., Cha S. R. & Peck A. B. Sjogren’s syndrome (SjS)-like disease of mice: the importance of B lymphocytes and autoantibodies. Frontiers in bioscience: a journal and virtual library 12, 1767–1789 (2007). [DOI] [PubMed] [Google Scholar]
- Thibult M. L. et al. PD-1 is a novel regulator of human B-cell activation. International immunology 25, 129–137, doi: 10.1093/intimm/dxs098 (2013). [DOI] [PubMed] [Google Scholar]
- Bodhankar S., Galipeau D., Vandenbark A. A. & Offner H. PD-1 Interaction with PD-L1 but not PD-L2 on B-cells Mediates Protective Effects of Estrogen against EAE. Journal of clinical & cellular immunology 4, 143, doi: 10.4172/2155-9899.1000143 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nguyen C. Q., Yin H., Lee B. H., Chiorini J. A. & Peck A. B. IL17: potential therapeutic target in Sjogren’s syndrome using adenovirus-mediated gene transfer. Laboratory investigation; a journal of technical methods and pathology 91, 54–62, doi: 10.1038/labinvest.2010.164 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klimatcheva E. et al. CXCL13 antibody for the treatment of autoimmune disorders. BMC immunology 16, 6, doi: 10.1186/s12865-015-0068-1 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kramer J. M., Klimatcheva E. & Rothstein T. L. CXCL13 is elevated in Sjogren’s syndrome in mice and humans and is implicated in disease pathogenesis. Journal of leukocyte biology 94, 1079–1089, doi: 10.1189/jlb.0113036 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.