Summary
SNT adaptor proteins transduce activation of fibroblast growth factor receptors (FGFRs) and neurotrophin receptors (TRKs) to common signaling targets. The SNT-1 phosphotyrosine binding (PTB) domain recognizes activated TRKs at a canonical NPXpY motif and, atypically, binds to nonphosphorylated FGFRs in a region lacking tyrosine or asparagine. Here, using NMR and mutational analyses, we show that the PTB domain utilizes distinct sets of amino acid residues to interact with FGFRs or TRKs in a mutually exclusive manner. The FGFR1 peptide wraps around the β sandwich structure of the PTB domain, and its binding is possibly regulated by conformational change of a unique C-terminal β strand in the protein. Our results suggest mechanisms by which SNTs serve as molecular switches to mediate the essential interplay between FGFR and TRK signaling during neuronal differentiation.
Introduction
Growing evidence from studies of developmental biology, cell differentiation, and apoptosis suggests that proteins have evolved to undertake multifunctional tasks in response to external cues. Endowing proteins with such versatility is essential for economy and efficiency in biological systems. This emerging concept is well illustrated in protein modular domains (Pawson and Scott, 1997).
Phosphotyrosine binding (PTB) domains represent a large family of protein interaction modules that share a conserved structural fold similar to that of pleckstrin homology (PH) domains and display diverse functions in mediating various signaling pathways (Forman-Kay and Pawson, 1999). The discovery of PTB domains is attributed largely to the fact that these protein modules serve as alternatives to Src homology 2 (SH2) domains for binding to tyrosine-phosphorylated proteins through recognition of amino acid residues N-terminal (rather than C-terminal) to the phosphotyrosine (Blaikie et al., 1994; Kavanaugh and Williams, 1994). Particularly, PTB domains of the signaling proteins Shc and insulin receptor substrate 1 (IRS-1) preferentially bind to phosphorylated proteins containing an NPXpY motif and hydrophobic amino acids N-terminal to this sequence (Gustafson et al., 1995; Kavanaugh et al., 1995). Recent studies show that PTB domain-like protein modules can bind to proteins independently of tyrosine phosphorylation or can even bind to those proteins lacking the canonical NPXY motif. For example, the PTB domains of X11 and Fe65 bind to a β-amyloid precursor protein at an NPTY sequence (Borg et al., 1996; Zambrano et al., 1997). The Drosophila Numb PTB domain recognizes non-NPXY sequences, including GFSNMSFEDFP in a Ser/Thr protein kinase, Nak (Chien et al., 1998; Zwahlen et al., 2000), and a GPY motif, identified through screening of a tyrosine-oriented synthetic peptide library (Li et al., 1997, 1998). In addition, the PTB domains of Shc (Ravichandran et al., 1997) and mammalian protein Dab (Disabled) (Howell et al., 1999) can also interact with phospholipids.
A recently identified PTB domain at the N terminus of membrane-anchored adaptor proteins, SNT-1/2 (suc1-associated neurotrophic factor target, also known as FRS2α/β), serves as one of the most dramatic examples of functional diversity and versatility of the PTB domain family. SNTs function to transduce fibroblast growth factor receptor (FGFR) or neurotrophin receptor (TRK) activation to Shp2 tyrosine phosphatase and to the Ras/ MAPK signaling pathway (Wang et al., 1996; Kouhara et al., 1997; Hadari et al., 1998). Strikingly, this single PTB domain is capable of interacting with two seemingly unrelated receptor sequences, i.e., with a tyrosine-phosphorylated NPXpY motif in TRKs (Peng et al., 1995; Meakin et al., 1999) and with a nonphosphorylated region in FGFRs that contains no tyrosine or asparagine residues (Xu et al., 1998; Ong et al., 2000). This function of the SNT PTB domain could be partly due to a unique 12 residue insert seen in the sequence homology alignment with IRS proteins (Figure 1A). The importance of the PTB domain is further underscored by the possible role of SNTs in regulating differentiating neurons to undergo a well-known developmental switch from FGF to neurotrophin dependence (Birren and Anderson, 1990; Ip et al., 1994; Stemple et al., 1988). Here we report the solution structure of the SNT-1 PTB domain in complex with a human FGFR1 (hFGFR1) peptide, and we examine SNT-1 interactions with FGFRs and TRKs by using structure-based mutagenesis.
Figure 1. Protein Sequence Homology Alignment.
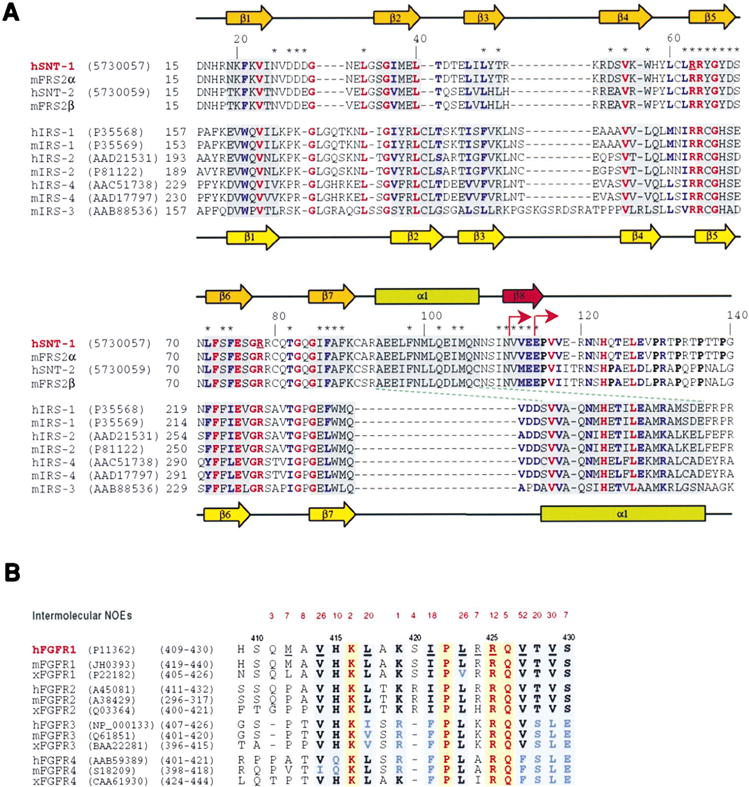
(A) Sequence alignment of PTB domains of the SNT and IRS proteins. Amino acid sequence and accession numbers of the proteins are indicated along the protein sequences. Protein sequences of FRS2α and FRS2β were reported previously (Ong et al., 2000). The experimentally determined secondary-structural elements are displayed above or below the sequences of the PTB domains of SNTs or IRSs (Zhou et al., 1996), respectively. Asterisks highlight residues in the SNT-1 PTB domain that show intermolecular NOEs to the hFGFR1 peptide. Absolutely or highly conserved residues among the SNT and IRS PTB domains are shown in red and blue, respectively. Two underlined arginine residues of SNT-1 were both changed by site-directed mutagenesis to glutamine. Arrows indicate constructs used in truncation analysis of the binding of the SNT-1 PTB domain to hFGFR1 or TRK. Proline residues located C-terminal to the SNT-1 PTB domain are shown in bold.
(B) Sequence alignment of the juxtamembrane region of the FGFR family. For each FGFR group (FGFR1–4), protein sequences from three representative species (i.e., human, mouse, and Xenopus) are selected. The number of observed intermolecular NOEs identified for a particular amino acid residue of the hFGFR1 peptide is shown in red above the sequence. Absolutely or highly conserved residues are highlighted in yellow and blue backgrounds, respectively.
Results and Discussion
Structure of the SNT PTB Domain
Nuclear magnetic resonance (NMR) studies were conducted using a 1:1 complex of SNT-1 PTB domain (residues 11–140) and a 22 residue peptide derived from the juxtamembrane region of hFGFR1 (residues 409–430) (Figures 1A and 1B). The dissociation constant (KD) of the protein/peptide complex was estimated to be ~10 μM using the isothermal titration calorimetry (ITC) technique. This result is consistent with the interaction being in slow-to-intermediate exchange on the NMR timescale in NMR titration experiments. The well-defined structure of this complex was determined from a total of 2844 NMR-derived restraints (Figure 2A). The protein structure consists of a β sandwich containing two nearly orthogonal, antiparallel β sheets capped at one end by an amphipathic α helix (Figures 2B and 2C), as expected from a classical PTB domain. The SNT-1 structure, however, possesses several unforeseen features that are unique for the conserved PTB domain scaffold. First, unlike all other known PTB domain structures that end with a C-terminal α helix (Forman-Kay and Pawson, 1999), the SNT-1 PTB domain has an additional β strand (β8) extending from its C-terminal α helix (α1) that molds the hFGFR1 peptide into the second antiparallel β sheet (see below). Second, boundaries of secondary-structural elements between SNT and IRS PTB domains do not necessarily coincide with their amino acid conservation, such as the conserved VEE motif of β8 (residues 113–115). This observation suggests that tertiary interactions are very important in defining structural elements. Third, a C-terminal portion of the SNT-1 construct (residues 116–136) (Figure 1A), which is highly homologous (~45% identity) to α1 in the IRS-1 PTB domain, is largely structurally disordered. The loss of helical conformation is perhaps due to the presence of proline residues and the change of the amphipathic nature of the sequence, which could disrupt helical propensity and alter interactions with other parts of the protein, respectively. While reasons for the conformational discrepancy between these homologous sequences are evident from structural analysis, the functional implications of their evolutionary relationship are not clear. Finally, the sequence comprising residues 94–107 in SNT-1, predicted to be a large insert from sequence homology alignment with IRS proteins, actually forms an α helix (α1) that blocks one side of the β sandwich. Together, these unique structural features of the SNT-1 PTB domain may confer its distinct function.
Figure 2. Structure of the SNT-1 PTB Domain/hFGFR1 Complex.

(A) Stereoview of the backbone atom superposition of the final 20 NMR-derived structures of the complex. The figure shows the SNT-1 PTB domain residues 18–116 and the hFGFR1 peptide residues 411–430. The terminal residues, which are structurally disordered, are omitted for clarity. For the final 20 structures, the root-mean-square deviations (rmsd) of the backbone and all heavy atoms for protein residues 18–116 are 0.74 ± 0.16 Å and 1.46 ± 0.16 Å, respectively. The corresponding rmsd for the protein secondary- structural regions (protein residues 19–24, 35–40, 45–49, 52–57, 63–68, 71–76, 85–90, 94–107, and 111–115) are 0.40 ± 0.05 A and 0.88 ± 0.05 A, respectively. The rmsd of the backbone and all heavy atoms for the hFGFR1 peptide (residues 412–430) are 0.56 ± 0.10 Å and 1.25 ± 0.15 Å, respectively.
(B) Ribbons (Carson, 1991) depiction of the averaged minimized NMR structure of the SNT-1 PTB domain/hFGFR1 complex. The orientation of (B) is as shown in (A).
(C) Ribbon diagram of the SNT-1 PTB domain structure from the top of the protein, which is rotated ~90° from the orientation in (B).
(D) Molecular surface representation of the SNT-1 PTB domain structure calculated in GRASP (Nicholls et al., 1993). The protein is color coded by surface curvature, and the color gradient from green to dark gray reflects decreasing solvent exposure. The hFGFR1 peptide molecule is shown as a ball-and-stick representation color coded by atom type.
Interactions between hFGFR1 Peptide and the SNT PTB Domain
The hFGFR1 peptide wraps around the protein molecule with an unusual backbone conformation containing two nearly 90° turns that are oriented orthogonal to each other (Figures 2B and 2C). The peptide interacts extensively with the protein by clasping both sides of the β sandwich (Figure 2D). The estimated surface area of SNT-1 that the bound peptide buries is ~2025 Å2, with 18 of the 22 peptide residues displaying intermolecular nuclear Overhauser effects (NOEs) to many protein residues (Figure 1B). The C-terminal QVTVS segment of the peptide (residues 426–430) adopts an antiparallel β strand (β′) sandwiched between β5 and β8. Two intermolecular hydrogen bonds bridging β′ and β5 and a large number of NOEs characteristic of the antiparallel β sheet are observed between backbone atoms of the complex (Figure 3A). Side chains of Val-427 and Val-429 interact extensively with Leu-62, Tyr-65, Phe-98, Met- 105, Ile-110, and Val-112 in a hydrophobic core formed between β5 and α1 (Figure 3B). The peptide fastens onto the other side of the β sandwich by embedding its N-terminal MAVH segment (residues 412–415) into a large hydrophobic cavity bounded by the three loops connecting β1/β2, β3/β4, and β6/β7. In particular, methyl groups of Val-414 are completely immersed in an aromatic pool of Trp-57, Phe-74, Phe-87, and Phe-89. The methyl groups also contact Leu-47, Val-55, and Thr-82 (Figure 3C). Moreover, Leu-417, Ile-421, and Leu-423, located in the center of the peptide, bind to otherwise solvent-exposed hydrophobic residues Leu-71, Ile-86, and Ala-88 on the surface of the second β sheet (Figure 3D). In addition to hydrophobic interactions, complementary electrostatic interactions are observed largely localized at the two turns in the peptide. At one turn, Arg-424 pairs with Asp-68, while Arg-425 interacts with Glu-114 and Glu-119. At the other turn, Lys-416 and Lys-419 show interactions with a contiguous patch of three solvent-accessible residues, Asp-27, Asp-28, and Asp-29 (Figure 3E). These results suggest that both hydrophobic and electrostatic interactions are important for SNT-1 and hFGFR1 recognition.
Figure 3. Intermolecular Interactions in the SNT-1 PTB Domain/hFGFR1 Complex.
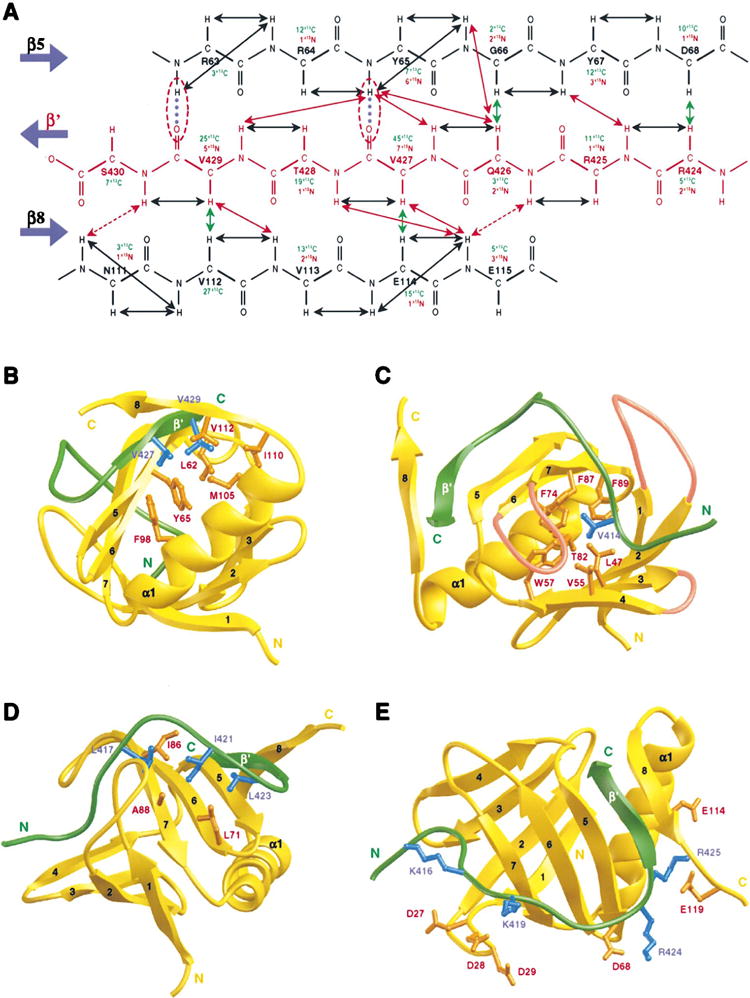
(A) Secondary structure of the intermolecular antiparallel β sheet of the complex. The number of intermolecular NOEs observed in 13C- or 15N-edited (F1), 13C/15N-filtered (F3) 3D NOESY spectra is summarized for individual amino acid residues. NOEs that define the structure of the β sheet are indicated by arrows. Arrows for intramolecular, 13C- and 15N-based intermolecular NOEs are color coded in black, green, and red, respectively. Broken lines (blue) highlight two intermolecular hydrogen bonds that are supported by amide exchange data. The intermolecular interactions are depicted for three regions of the hFGFR1 peptide (green).
(B) The C-terminal region of the hFGFR1 peptide (residues 424–430). The side chains of the protein and the peptide residues are displayed in orange and blue, respectively.
(C) The middle region of the hFGFR1 peptide (residues 417–423). The three loops that connect β1 to β2, β3 to β4, and β6 to β7 and that form a hydrophobic binding pocket for binding to the peptide residue Val-414 are colored in pink.
(D) The N-terminal region of the hFGFR1 peptide (residues 409–416).
(E) Complementary electrostatic interactions between the SNT-1 PTB domain and hFGFR1 peptide residues.
To determine the relative contributions of specific hFGFR1 residues to SNT-1 recognition, we used site- directed mutagenesis to alter the peptide residues that show intermolecular NOEs to the protein (Figure 1B). We used yeast two-hybrid binding assays to analyze the resulting hFGFR1 mutants for SNT-1 interaction. Substitution of Ala for either Leu-423 or Val-429 completely eliminated peptide binding to the SNT-1 PTB domain, while mutation of Val-414, Leu-417, Arg-425, or Val-427 to alanine significantly reduced binding (Figure 4A). The reduced protein-protein interactions of the individual FGFR1 mutants were not likely to be due to variations of protein expression in the yeast cells (Figure 4A). Particularly, expression levels of the Leu-423-Ala and Val-429-Ala mutants were at least as high as that of the wild type. These mutagenesis data agree with (1) the observed intermolecular NOEs (Figure 1B), (2) the calculated solvent-accessible surface area (particularly for the peptide hydrophobic residues) (data not shown), and (3) amino acid conservation of the juxtamembrane region of the FGFR family (Figure 1B). In addition, these data are consistent with recently reported results of mutational analysis of FGFR1 and SNT-1 interactions (Ong et al., 2000). Collectively, these results demonstrate that the nature of the protein and peptide binding is highly specific and extensive, with multiple types of interactions stabilizing the complex. It is interesting to note that utilization of the hydrophobic side of the β sandwich opposite to α1 for protein interactions in SNT-1 is not seen in other PTB domains (Forman-Kay and Pawson, 1999). Amino acid residues in the corresponding loops, however, have been shown to interact with phospholipids in the PTB domain of Shc (Ravichandran et al., 1997) and in the structurally homologous PH domains of several signaling molecules (Lemmon et al., 1996). Remarkably, SNT-1 is also capable of interacting with tyrosine-phosphorylated TRKs, which possess no sequence homology to hFGFR1 (see below). This ability illustrates the unique functional diversity of this conserved PTB domain fold.
Figure 4. Mutational Analysis of the SNT-1 PTB Domain Interactions with FGFRs or TRKs.

(A) Effects of hFGFR1 point mutations on interactions with the SNT-1 PTB domain as determined by yeast two-hybrid binding assays. Data for peptide mutants are calculated from an average of five independent experiments. Western blot shows BD fusion protein expression of wild-type and mutant hFGFR1 in the yeast cells.
(B) Structure of the SNT-1 PTB domain/hFGFR1 complex shows locations of Arg-63 and Arg-78 (blue) that are essential for binding to the phosphotyrosine in the NPXpY motif. The backbone of the hFGFR1 peptide is shown in green. The distinct β8 strand of the SNT-1 PTB domain is displayed in red.
(C) Structure of the IRS-1 PTB domain in complex with a tyrosine-phosphorylated peptide derived from interleukin-4 receptor (LVIAGNPApYRS, residues 489–499) as determined by NMR (Zhou et al., 1996). The peptide residues are shown in green, and the two key arginine residues (Arg-212 and Arg-227) of the PTB domain that are essential for phosphotyrosine binding are displayed in blue.
(D) Yeast two-hybrid binding studies of the effect of truncation of SNT-1 β8 on its interactions with hFGFR1 and tyrosine-phosphorylated TRKB. The panel framed in red shows the loss of interaction between hFGFR1 and the SNT-1 PTB domain protein lacking the β8 strand. Colony formation on the synthetic complete medium lacking leucine and tryptophan (Leu−, Trp−) illustrates the efficiency of cotransformation with the two plasmids, while growth on the corresponding medium lacking histidine, leucine, and tryptophan (His−, Leu−, Trp−) shows the level of protein-protein interaction.
Structural Insights into SNT Binding to Tyrosine-Phosphorylated TRKs
The new SNT-1 PTB domain structure yields insights into how the protein might interact with tyrosine-phosphorylated TRKs. Conserved Arg-63 and Arg-78 residues in SNT-1 are structurally analogous to Arg-212 and Arg-227 of IRS-1 PTB domain, respectively (Figures 4B and 4C). The latter pair of arginine residues are essential for IRS-1 binding to phosphotyrosine in the canonical NPXpY motif (Zhou et al., 1996). Our mutagenesis and yeast two-hybrid binding studies showed that mutation of Arg-63 and Arg-78 to glutamine resulted in complete elimination of SNT-1 interaction with TRKB but the mutation had no effect on binding to hFGFR1. Additionally, Ala substitution of Ile(pY-5) in a TRKA peptide (HIIENPQpYFSDA, which is highly homologous to TRKB) caused marked reduction in binding to the PTB domain (data not shown). Based on these results, we postulate that the mechanism by which SNT-1 binds to TRKs is similar to the way in which the IRS-1 PTB domain binds to NPXpY-containing proteins (Figure 4C). Specifically, the phosphotyrosine of the NPXpY motif in TRKs would coordinate with Arg-63 and Arg-78 in the SNT-1 PTB domain, and residues N-terminal to the phosphotyrosine would adopt an extended conformation with hydrophobic side chains intercalating into the hydrophobic pocket between β5 and α1, which is also a site for interactions with hFGFR1. Furthermore, NMR titration experiments showed that hFGFR1 and TRK peptides compete for binding to the PTB domain (data not shown), which agrees with the results of the peptide competition experiments in an SNT GST pull-down assay (Ong et al., 2000). Together, these results strongly argue that binding of SNT to either nonphosphorylated FGFRs or tyrosine- phosphorylated TRKs is mutually exclusive.
Regulation of SNT and FGFR Association by a Possible Local Conformational Change
The β8 strand is structurally unique for the PTB domain fold, but is it functionally important for SNT-1 binding to hFGFR1 or phosphorylated TRKs? We performed truncation studies of SNT-1 to address this question. Yeast two-hybrid assays showed that a truncated SNT-1 PTB domain lacking the β8 region (residues 2–111) almost abolished its ability to interact with hFGFR1 without decreasing its TRKB binding (Figure 4C). Another SNT-1 truncation mutant (residues 11–114), which ends with β8 shortened by one residue, showed markedly reduced binding to hFGFR1 peptide in NMR binding studies (supported by significant line broadening of the protein NMR signals) but did not impair the protein’s interactions with the tyrosine-phosphorylated TRKB peptide. The effects of the β8 truncation were further confirmed in ITC measurements of the binding of the SNT PTB domain to hFGFR1 or TRK peptides (data not shown). These results assert that both the presence and structural integrity of the β8 region are necessary for SNT-1 binding to FGFRs but not for its interaction with TRKs. While the overall structural fold of the SNT-1 PTB domain may be similar in its free, TRK-, or hFGFR1- bound forms, the structural requirement of β8 is unique for its hFGFR1 association. This observation is consistent with the fact that the antiparallel β′ strand of the hFGFR1 peptide (7 residues, Figure 3A) is much longer than the NPXpY or related sequences that are recognized by the PTB domains of Shc (Zhou et al., 1995), IRS-1 (Eck et al., 1996; Zhou et al., 1996), or Numb (Zwahlen et al., 2000). These findings imply that conformational perturbation of β8 would compromise SNT-1 binding to FGFRs, and they suggest that this β8 strand could act as an on/off switch for SNT-1/FGFR association.
Possible Role of SNTs as Molecular Switches in FGFR and TRK Signaling
Our structural and mutational analyses of the SNT-1 PTB domain suggest mechanisms by which SNTs might coordinate FGF and neurotrophin signaling during neuronal differentiation. The ability of SNTs to interact with nonphosphorylated FGFR raises the possibility that SNTs are sequestered by FGFRs in unstimulated cells and are only available for activation by FGFs. Events that trigger release of SNTs from constitutive FGFR association would allow for SNT interaction with TRKs and lead to a neurotrophin-responsive state in differentiating neurons. Such events may include FGFR downregulation or conformational perturbation to the β8 region of the SNT PTB domain by posttranslational modifications or interactions with other protein(s). Alternatively, even in the absence of SNT/FGFR complexes, the conformational flexibility of the SNT PTB domain may be constrained during neurogenesis to regulate its availability for interaction with and activation by neurotrophin receptors. These models may provide new clues to explain how differentiating neuronal precursors undergo the well-documented switch from FGF to neurotrophin dependence (Birren and Anderson, 1990; Ip et al., 1994; Stemple et al., 1988), which is not simply due to changes in TRK expression (Ip et al., 1994).
Conclusion
The three-dimensional structure of the SNT-1 PTB domain/hFGFR1 complex reveals the unique features that enable SNT-1 to recognize two radically different receptor sequences in a mutually exclusive manner. Our results demonstrate that both adaptive hydrophobic interactions and complementary electrostatic interactions are important factors that underlie specificity and versatility of molecular recognition by the conserved PTB domain structural fold (Forman-Kay and Pawson, 1999). Our findings further suggest that cellular events that cause a local conformational change in the PTB domain may govern SNT interactions with either FGFRs or TRKs. Thus, the intrinsic adaptability and flexibility of the SNT PTB domain may serve as a focal point for the essential interplay between FGF and neurotrophin receptor signaling that governs neuronal survival and differentiation.
Experimental Procedures
Sample Preparation
A cDNA fragment encoding the SNT-1 PTB domain was cloned into a modified pET28bvector (Novagen) that produced the recombinant protein with a cleavable hexa-histidine (His6) tag at the C terminus. We uniformly labeled proteins with 15N and 15N/13C by growing Escherichia coli BL21(DE3) cells in a minimal medium containing 15NH4Cl with or without 13C6-glucose. A uniformly 15N/13C-labeled and fractionally deuterated protein was prepared by using medium with 75% 2H2O. The protein was overexpressed in largely soluble form and purified by affinity chromatography on a nickel-IDA column (Invitrogen) followed by cleavage of the His6 tag upon thrombin treatment. The cleaved protein contained an additional LVPR sequence at the C terminus from the engineered thrombin site. The protein was unstable in its free form and quickly aggregated or became partially unfolded at room temperature. We ensured structural integrity by further subjecting the protein to a refolding procedure followed by ion-exchange chromatography. Synthetic peptides were prepared on a MilliGen 9050 peptide synthesizer (Perkin Elmer) with Fmoc/HBTU chemistry. NMR samples contained the SNT-1 PTB domain/hFGFR1 peptide complex (1:1) of ~0.5 mM in 100 mM phosphate buffer of pH 6.5, 5 mM DTT-d10, and 0.5 mM EDTA in H2O/2H2O (9/1) or 2H2O.
NMR Spectroscopy
NMR spectra were acquired at 30°C on a Bruker DRX600 or DRX500 spectrometer. The backbone and side chain 1H, 13C, and 15N resonances of the protein were assigned using deuterium-decoupled triple-resonance experiments of HNCA, HN(CO)CA, HNCACB, HN(CO)CACB, and (H)C(CO)NH-TOCSY (Yamazaki et al., 1994; Sattler et al., 1999) recorded by using uniformly 15N/13C-labeled and fractionally deuterated protein in complex with a nonisotopically labeled hFGFR1 peptide. The side chain assignments were completed using 3D HCCH-TOCSY (Clore and Gronenborn, 1994) data collected from a uniformly 15N/13C labeled-protein/nonlabeled-peptide complex. NOE-derived distance restraints were obtained from 15N- or 13C-edited 3D NOESY spectra (Clore and Gronenborn, 1994). ϕ-angle restraints were determined from 3JHN,Hα coupling constants measured in a 3D HNHA-J spectrum (Clore and Gronenborn, 1994). Slowly exchanging amide protons were identified from a series of 2D 15N-HSQC spectra recorded after the H2O buffer was changed to 2H2O buffer. The peptide resonances were assigned using 13C/15N- filtered 2D NOESY and TOCSY spectra (Sattler et al., 1999) collected from a 15N/13C labeled-protein/nonlabeled-peptide complex. The intermolecular NOEs used in defining the structure of the SNT-1 PTB domain/hFGFR1 complex were detected in 13C- or 15N-edited (F1), 13C/15N-filtered (F3) 3D NOESY spectra. All NMR spectra were processed with NMRPipe/NMRDraw (Delaglio et al., 1995) and analyzed using NMRView (Johnson and Blevins, 1994).
Structure Calculations
Structures of the SNT-1 PTB domain in complex with the hFGFR1 peptide were calculated with a distance geometry and simulated annealing protocol by using the X-PLOR program (Brünger, 1993). NOE distance and dihedral angle restraints were treated with a square-well potential of 50 kcal mol−1 Å−2. A total of 2448 manually assigned NOE-derived distance restraints were obtained from the 15N- or 13C-edited NOESY data. Included in this figure are 251 intrapeptide and 258 intermolecular distance restraints. Additionally, 255 unambiguous and 52 ambiguous distance restraints were identified from the NOE data by using ARIA (Nilges and O’Donoghue, 1998). The final structure calculations employed a total of 2755 NOE restraints obtained from the manual and the ARIA-assisted assignments, 2703 of which were unambiguously assigned NOE-derived distance restraints that comprise 1072 intraresidue, 466 sequential, 216 medium-range, and 949 long-range NOEs. In addition, 70 hydrogen-bond distance restraints for 35 hydrogen bonds and 19 ϕ-angle restraints were also used in the structure calculations. For the ensemble of the final 20 structures, no distance or torsional angle restraint was violated by more than 0.4 Å or 5°, respectively. The distance-violation, dihedral-violation, and total energies were 74.4 ± 1.7 kcal mol−1, 0.82 ± 0.08 kcal mol−1, and 262.0 ± 6.0 kcal mol−1, respectively. The Lennard-Jones potential, which was not used during any refinement stage, was −659.3 ± 23.1 kcal mol−1 for the final structures. Ramachandran plot analysis by Procheck-NMR (Laskowski et al., 1996) showed that in the final structures of the complex, 98.1% of the backbone geometries of the non-Gly and non-Pro residues in the complex (protein residues 18–116 and peptide residues 412–430) and nearly 100% in the secondary structure (protein residues 19–24, 35–40, 45–49, 52–57, 63–68, 71–76, 85–90, 94–107, and 111–115 and peptide residues 426–430) lie within energetically favorable or allowed regions.
Mutagenesis and Yeast Two-Hybrid Binding Assays
The yeast two-hybrid binding studies of the binding of the SNT-1 PTB domain to hFGFR1 or tyrosine-phosphorylated TRK were performed as described previously (Xu et al., 1998). Briefly, SNT-1 cDNA fragments were cloned into the pACT2 expression vector (Clontech) for expression as GAL4 DNA activation domain (AD) fusion proteins followed by C-terminal AU1-epitope tags. The juxtamembrane region of hFGFR1 was cloned into the pAS2-1 expression vector (Clontech) for expression as a GAL4 DNA binding domain (BD) fusion protein. The QuikChange kit (Stratagene) was used, with this plasmid serving as a template for site-directed mutagenesis of hFGFR1. DNA sequencing confirmed the mutations. AD and BD plasmids were cotransformed into Saccharomyces cerevisiae strain pJ69–4A and plated onto selective media. The synthetic medium lacking the amino acids leucine and tryptophan (Leu−, Trp−) selected for plasmid uptake. Medium lacking histidine, leucine, and tryptophan (His−, Leu−, Trp−) but containing 3 mM 3-aminotriazole was used to select for interaction of the AD and BD fusion proteins. Levels of protein interaction were scored according to relative colony growth on these plates. Expression of the SNT-1 protein was confirmed by immunoprecipitation from yeast lysates with an anti-AU1 monoclonal antibody (BAbCo). Western blotting was performed with anti-AD antibody (Santa Cruz Biotech) and goat anti-mouse IgG conjugated with horseradish-peroxidase and developed by chemiluminescence. Similarly, expression of wild-type and mutant hFGFR1 was detected by immunoprecipitation with a rabbit polyclonal antibody specific for BD (Santa Cruz Biotech) and Western blotting with mouse monoclonal anti-BD antibody (Santa Cruz Biotech).
Supplementary Material
Acknowledgments
We thank I. Wolf for peptide synthesis and A. K. Aggarwal, A. Farooq, D. Logothetis, L. Shapiro, and H. Weinstein for helpful suggestions and critical reading of the manuscript. This work is supported by grants from the National Institutes of Health (M. P. G. and M.-M. Z.). K. Y. and K. W. L. are supported by National Institutes of Health predoctoral training grants. M. K. is a recipient of a National Institutes of Health postdoctoral fellowship.
Footnotes
BioMagResBank Accession Number
Chemical shift assignments of the SNT PTB domain and the hFGFR1 peptide have been deposited in the BioMagResBank (BMRB) under accession number 4790.
References
- Birren SJ, Anderson DJ. A v-myc immortalized sympathoadrenal progenitor cell line in which neuronal differentiation is initiated by FGF but not NGF. Neuron. 1990;4:189–201. doi: 10.1016/0896-6273(90)90094-v. [DOI] [PubMed] [Google Scholar]
- Blaikie P, Immanuel D, Wu J, Li N, Yajnik V, Margolis B. A region in Shc distinct from the SH2 domain can bind a tyrosine phosphorylated growth factor receptor. J Biol Chem. 1994;269:32031–32034. [PubMed] [Google Scholar]
- Borg JP, Ooi J, Levy E, Margolis B. The phosphotyrosine interaction domains of X11 and Fe65 bind to distinct sites on the YENPTY motif of amyloid precursor protein. Mol Cell Biol. 1996;16:6229–6241. doi: 10.1128/mcb.16.11.6229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brünger AT. X-PLOR Version 3.1:ASystem for X-Ray Crystallography and NMR, Version 3.1 Edition. New Haven, CT: Yale University Press; 1993. [Google Scholar]
- Carson M. Ribbons 2.0. J Appl Crystallogr. 1991;24:958–961. [Google Scholar]
- Chien CT, Wang S, Rothenberg M, Jan LY, Jan YN. Numb-associated kinase interacts with the phosphotyrosine-binding domain of Numb and antagonizes the function of Numb in vivo. Mol Cell Biol. 1998;18:598–607. doi: 10.1128/mcb.18.1.598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clore GM, Gronenborn AM. Multidimensional heteronuclear nuclear magnetic resonance of proteins. Methods Enzymol. 1994;239:249–363. doi: 10.1016/s0076-6879(94)39013-4. [DOI] [PubMed] [Google Scholar]
- Delaglio F, Grzesiek S, Vuister GW, Zhu G, Pfeifer J, Bax A. NMRPipe: a multidimensional spectral processing system based on UNIX pipes. J Biomol NMR. 1995;6:277–293. doi: 10.1007/BF00197809. [DOI] [PubMed] [Google Scholar]
- Eck MJ, Dhe-Paganon S, Trüb T, Nolte R, Shoelson SE. Structure of the IRS-1 PTB domain bound to the juxtamembrane region of the insulin receptor. Cell. 1996;85:695–705. doi: 10.1016/s0092-8674(00)81236-2. [DOI] [PubMed] [Google Scholar]
- Forman-Kay JD, Pawson T. Diversity in protein recognition by PTB domains. Curr Opin Struct Biol. 1999;9:690–695. doi: 10.1016/s0959-440x(99)00031-7. [DOI] [PubMed] [Google Scholar]
- Gustafson TA, He W, Craparo A, Schaub CD, O’Meill TJ. Phosphotyrosine-dependent interaction of Shc and insulin receptor substrate 1 with the NPEY motif of the insulin receptor via a novel non-SH2 domain. Mol Cell Biol. 1995;15:2500–2508. doi: 10.1128/mcb.15.5.2500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hadari YR, Kouhara H, Lax I, Schlessinger J. Binding of Shp2 tyrosine phosphatase to FRS2 is essential for fibroblast growth factor-induced PC12 cell differentiation. Mol Cell Biol. 1998;18:3966–3973. doi: 10.1128/mcb.18.7.3966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Howell BW, Kanier LM, Frank R, Gertler FB, Cooper JA. The disabled 1 phosphotyrosine-binding domain binds to the internalization signals of transmembrane glycoproteins and to phospholipids. Mol Cell Biol. 1999;19:5179–5188. doi: 10.1128/mcb.19.7.5179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ip NY, Boulton TG, Li Y, Verdi JM, Birren SJ, Anderson DJ, Yancopoulos GD. CNTF, FGF, and NGF collaborate to drive the terminal differentiation of MAH cells into postmitotic neurons. Neuron. 1994;13:443–455. doi: 10.1016/0896-6273(94)90359-x. [DOI] [PubMed] [Google Scholar]
- Johnson BA, Blevins RA. NMRView: acomputer program for the visualization and analysis of NMR data. J Biomol NMR. 1994;4:603–614. doi: 10.1007/BF00404272. [DOI] [PubMed] [Google Scholar]
- Kavanaugh WM, Williams LT. An alternative to SH2 domains for binding tyrosine-phosphorylated proteins. Science. 1994;266:1862–1865. doi: 10.1126/science.7527937. [DOI] [PubMed] [Google Scholar]
- Kavanaugh WM, Turck CW, Williams LT. PTB domain binding to signaling proteins through a sequence motif containing phosphotyrosine. Science. 1995;268:1177–1179. doi: 10.1126/science.7539155. [DOI] [PubMed] [Google Scholar]
- Kouhara H, Hadari YR, Spivak-Kroizman T, Schilling J, Bar-Sagi D, Lax I, Schlessinger J. A lipid-anchored Grb2- binding protein that links FGF-receptor activation to the Ras/MAPK signaling pathway. Cell. 1997;89:693–702. doi: 10.1016/s0092-8674(00)80252-4. [DOI] [PubMed] [Google Scholar]
- Laskowski RA, Rullmannn JA, MacArthur MW, Kaptein R, Thornton JM. AQUA and PROCHECK-NMR: programs for checking the quality of protein structures solved by NMR. J Biomol NMR. 1996;8:477–486. doi: 10.1007/BF00228148. [DOI] [PubMed] [Google Scholar]
- Lemmon MA, Ferguson KM, Schlessinger J. PH Domains: diverse sequences with a common fold recruit signaling molecules to the cell surface. Cell. 1996;85:621–624. doi: 10.1016/s0092-8674(00)81022-3. [DOI] [PubMed] [Google Scholar]
- Li SC, Songyang Z, Vincent SJF, Zwahlen C, Wiley S, Cantley L, Kay LE, Forman-Kay J, Pawson T. High-affinity binding of the Drosophila Numb phosphotyrosine-binding domain to peptides containing a Gly-Pro-(p)Tyr motif. Proc Natl Acad Sci USA. 1997;94:7204–7209. doi: 10.1073/pnas.94.14.7204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li SC, Zwahlen C, Vincent SJ, McGlade CJ, Kay LE, Pawson T, Forman-Kay JD. Structure of a Numb PTB domain-peptide complex suggests a basis for diverse binding specificity. Nat Struct Biol. 1998;5:1075–1083. doi: 10.1038/4185. [DOI] [PubMed] [Google Scholar]
- Meakin SO, MacDonald JIS, Gryz EA, Kubu CJ, Verdi JM. The signaling adapter FRS-2 competes with Shc for binding to the nerve growth factor receptor TrkA. J Biol Chem. 1999;274:9861–9870. doi: 10.1074/jbc.274.14.9861. [DOI] [PubMed] [Google Scholar]
- Nicholls A, Bharadwj R, Honig B. GRASP: graphical representation and analysis of surface properties. Biophys J. 1993;64:166–170. [Google Scholar]
- Nilges M, O’Donoghue S. Ambiguous NOEs and automated NOE assignment. Prog NMR Spectroscopy. 1998;32:107–139. [Google Scholar]
- Ong SH, Guy GR, Hadari YR, Laks S, Gotoh N, Schlessinger J, Lax I. FRS2 proteins recruit intercellular signaling pathways by binding to diverse targets on fibroblast growth factor and nerve growth factor receptors. Mol Cell Biol. 2000;20:979–989. doi: 10.1128/mcb.20.3.979-989.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pawson T, Scott JD. Signaling through scaffold, anchoring, and adaptor proteins. Nature. 1997;278:2075–2080. doi: 10.1126/science.278.5346.2075. [DOI] [PubMed] [Google Scholar]
- Peng X, Greene LA, Kaplan DR, Stephens RM. Deletion of a conserved juxtamembrane sequence in Trk abolishes NGF-promoted neuritogenesis. Neuron. 1995;15:395–406. doi: 10.1016/0896-6273(95)90043-8. [DOI] [PubMed] [Google Scholar]
- Ravichandran KS, Zhou MM, Pratt JC, Harlan JE, Walk S, Fesik SW, Burakoff SJ. Evidence for a requirement for both phospholipid and phosphotyrosine binding via the Shc phosphotyrosine binding domain in vivo. Mol Cell Biol. 1997;17:5540–5549. doi: 10.1128/mcb.17.9.5540. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sattler M, Schleucher J, Griesinger C. Heteronuclear multidimensional NMR experiments for the structure determination of proteins in solution employing pulsed field gradients. Prog NMR Spectroscopy. 1999;34:93–158. [Google Scholar]
- Stemple DL, Mahanthappa NK, Anderson DJ. Basic FGF induces neuronal differentiation, cell division, and NGF dependence in chromaffin cells: a sequence of events in sympathetic development. Neuron. 1988;1:517–525. doi: 10.1016/0896-6273(88)90182-1. [DOI] [PubMed] [Google Scholar]
- Wang JK, Xu H, Li HC, Goldfarb M. Broadly expressed SNT-like proteins link FGF receptor stimulation to activators of Ras. Oncogene. 1996;13:721–729. [PubMed] [Google Scholar]
- Xu H, Lee KW, Goldfarb M. Novel recognition motif on fibroblast growth factor receptor mediates direct association and activation of SNT adaptor proteins. J Biol Chem. 1998;273:17987–17990. doi: 10.1074/jbc.273.29.17987. [DOI] [PubMed] [Google Scholar]
- Yamazaki T, Lee W, Arrowsmith CH, Mahandiram DR, Kay LE. A suite of triple resonance NMR experiments for the backbone assignment of 15N, 13C, 2H labeled proteins with high sensitivity. J Am Chem Soc. 1994;116:11655–11666. [Google Scholar]
- Zambrano N, Buxbaum JD, Minopoli G, Fiore F, Candia PD, Renzis SD, Faraonio R, Sabo S, Cheetham J, Sudol M, Russo T. Interaction of the phosphotyrosine interaction/ phosphotyrosine binding-related domains of FE65 with wild-type and mutant Alzheimer’s β-amyloid precursor protein. J Biol Chem. 1997;272:6399–6409. doi: 10.1074/jbc.272.10.6399. [DOI] [PubMed] [Google Scholar]
- Zhou MM, Ravichandran KS, Olejniczak ET, Petros AP, Meadows RP, Sattler M, Harlan JE, Wade W, Burakoff SJ, Fesik SW. Structure and ligand recognition of the phosphotyrosine binding domain of Shc. Nature. 1995;378:584–592. doi: 10.1038/378584a0. [DOI] [PubMed] [Google Scholar]
- Zhou MM, Huang B, Olejniczak ET, Meadows RP, Shuker SB, Miyazak M, Trüb T, Shoelson SE, Fesik SW. Structural basis of IL-4 receptor phosphopeptide recognition by the IRS-1 PTB domain. Nature Struct Biol. 1996;3:388–393. doi: 10.1038/nsb0496-388. [DOI] [PubMed] [Google Scholar]
- Zwahlen C, Li SC, Kay LE, Pawson T, Forman-Kay JD. Multiple modes of peptide recognition by the PTB domain of the cell fate determinant Numb. EMBO J. 2000;19:1505–1515. doi: 10.1093/emboj/19.7.1505. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


