Abstract
Introduction and hypothesis
Pelvic organ prolapse is a common condition impacting the quality of life of millions of women worldwide. Although vaginal estrogen is widely used in women with prolapse, little is known regarding estrogen’s benefits on the biomechanical properties of vaginal tissue. Here, we aimed to examine the effect of prolapse on the biomechanical properties of the vagina and determine alterations in vaginal mechanics in the presence and absence of hormone therapy (HT).
Methods
We characterized the viscoelastic properties of vaginal biopsies from age-matched premenopausal women without (n=12) and with prolapse (n=8) and postmenopausal women with prolapse on (n=18) and off HT (n=9). Utilizing a single-lap shear testing protocol, full-thickness anterior vaginal biopsies were subjected to ±10 % shear strain over a range of frequencies (1–90 Hz). This applied energy is either dissipated (viscous) or stored (elastic) as a function of frequency due to compositional or structural differences in the tissue.
Results
Prolapsed tissue was more stiff (higher complex modulus) under shear deformation resulting from increases in both elastic (elastic modulus) and viscous (loss modulus) contributions, with non-prolapsed premenopausal women being the least stiff. Postmenopausal women with prolapse currently on HT were the most stiff of all the groups.
Conclusions
These data suggest that prolapsed tissue has an increased elastic contribution likely resulting from changes in biochemical constituents, and hormones increase the viscous contribution of prolapsed tissue. Overall, this study design characterized the viscoelastic properties of vaginal biopsies and may be utilized to conduct longitudinal studies to better understand the mechanisms of prolapse development and progression.
Keywords: Prolapse, Rheology, Vagina, Viscoelastic
Introduction
A normally supported vagina provides support to the bladder, urethra, uterus, and rectum. Disruption of vaginal support or a decline in the mechanical integrity of the vagina can cause the pelvic organs to descend into the vagina. The descent of the pelvic organs commonly results in a condition known as pelvic organ prolapse (POP). POP is associated with symptoms of pelvic organ dysfunction including urinary and fecal incontinence, pain, psychological and emotional distress, and social isolation [1, 2]. Although age and menopause may increase the risk of POP, epidemiological studies have found that parity is the greatest risk factor for the development of prolapse later in life. This may be related to a maternal birth injury sustained by the levator ani muscles, surrounding connective tissue, or vaginal tissue; however, sound scientific data supporting this presumption are lacking [3, 4]. Interestingly, most parous women never develop symptomatic prolapse during their lifetime and those that do often do not become symptomatic until years, often decades, after vaginal delivery [4].
The reason certain women develop symptomatic prolapse while others maintain pelvic organ support is not clear. Studies from our laboratory have suggested that, while parity sets the stage, hormones play a role by protecting pelvic soft tissue supportive structures from degradation by matrix metalloproteinases (MMPs). This was demonstrated in the rodent model by showing that hormone therapy (HT) or an MMP inhibitor equally obviated the decline in mechanical integrity following surgical menopause (ovariectomy) [5]. Using vaginal fibroblasts subjected to cyclic stretching from human subjects, this mechanism was further elucidated by showing an increase in active MMP in the presence of stretch and suppression of this response in the presence of hormones [6, 7]. These data support other findings, which have demonstrated decreased collagen content, altered collagen subtypes, and an increase in MMP activity in the vaginal wall compared to women without prolapse [8].
These biochemical changes are important to measure, because they impact the mechanical integrity of tissue, which ultimately determines whether a change results in pathology. Thus, functional assessments of the mechanical properties of tissue are critical. Previous studies have demonstrated a change in the mechanical integrity of the vaginal wall after the development of prolapse. A study by Lei et al. that utilized strips of vagina demonstrated a higher elastic modulus in women with prolapse independent of menopausal status [9]. A separate study comparing patients with prolapse relative to non-prolapsed women observed that, in response to uniaxial tension, vaginal strips from patients with prolapse had increased rigidity based on a hyperelastic model [10]. These testing methodologies often require larger tissue samples, which are difficult to obtain from women unless a partial or complete colpectomy is performed. Tissue samples from patients at a single point in time also preclude the ability to delineate the cause and effect of prolapse. Ideally, both the biochemical and biomechanical characteristics of a tissue could be obtained from multiple small (5 mm) biopsies over time. Unfortunately, while biochemical data can be obtained from small tissues, mechanical testing protocols that can analyze the properties of such small samples are limited.
Rheology, defined as the study of the flow of materials, is a methodology that has the potential to make mechanical measurements on small sized tissues (5×5 mm). The properties or flow of a material are dependent on the type of molecules and their distribution within it. Biological tissues are viscoelastic, which means they are composed of both a solid (elastic) and a fluid (viscous) phase. By utilizing a rheological approach and subjecting specimens to oscillating strains (cyclical deformation) at varying frequencies, we can characterize these viscoelastic properties as a function of frequency. Further, rheological properties are directly related to the molecules and their distribution within a tissue; therefore, this methodology has the potential to provide us with some insight as to whether specific risk factors or biochemical changes have altered the tissue’s mechanical behavior [11, 12].
Thus, the objective of this study was to utilize a rheological testing protocol to characterize the viscoelastic properties of small vaginal biopsies from age-matched premenopausal women with and without prolapse and age-matched postmenopausal women with prolapse who are on and off HT. To accomplish this, we utilized a single-lap shear test in which the rheological properties of full-thickness vaginal biopsies were defined. We hypothesized that the remodeled vaginal tissue from women with prolapse would be more resistant to shear deformations relative to control tissue based on the findings of the aforementioned studies [9, 10, 13]. Further we hypothesize that, after menopause, women with prolapse receiving HT would have rheological properties similar to those of premenopausal women with prolapse. Although in this study we examined tissues from women obtained at a single point in time, we hoped to establish the usefulness of the methodology described here as a means to follow women through time to track the development and progression of prolapse.
Materials and methods
Full-thickness biopsy samples obtained from the proximal one fourth of the vagina (5–7 mm2) were collected at the time of prolapse or an unrelated gynecological surgery with approval from the Institutional Review Board at the University of Pittsburgh and following patient consent. Demographic data including age, body mass index (BMI), gravidity, parity, menopausal status (pre- and postmenopausal), and stage of prolapse were collected according to previously established criteria [6, 7] at the time biopsies were retrieved (Table 1). Prolapse was assessed by an independent physician using the Pelvic Organ Prolapse Quantification (POP-Q) scale. Patients with stage 0 or I were considered controls, or non-prolapsed women, while women who presented with prolapse to the hymen or greater were considered to have prolapse. Women were divided into four groups for this study: non-prolapsed, premenopausal women (n=12), age-matched premenopausal women with prolapse (n=8), postmenopausal women with prolapse who are not on HT (n=18), and age-matched postmenopausal women with prolapse who are on HT (n=9). Women were considered postmenopausal if they were over 50 years of age and had not had a menses over the previous 12 months. After the biopsies were excised, the longitudinal and circumferential directions of these samples were marked for orientation. The tissue was immediately transported and prepared for histological and biomechanical analysis.
Table 1.
Demographic data of each patient group examined
| Group | Age (years) | BMI | Gravidity | Parity | Stage of prolapse |
|---|---|---|---|---|---|
| Premenopausal, no prolapse (n=12) | 43±5.0 | 28±4.9 | 2 (1–3.75) | 1.5 (1–2) | 0 (0–0) |
| Premenopausal, prolapsed (n=8) | 47±3.9 | 28±6.3 | 3 (2–4) | 2 (2–3) | 3 (2.3–3) |
| Postmenopausal, prolapsed, no HT (n=18) | 72±8.7 | 28±5.1 | 3 (3–4) | 2 (1–3) | 3 (3–3) |
| Postmenopausal, prolapsed, HT (n=9) | 69±9.5 | 29±3.8 | 3 (2–5) | 3 (1.5–4) | 3 (3–4) |
| p value | <0.001 | 0.96 | 0.31 | 0.22 | <0.001 |
Age, BMI, gravidity, parity, and stage of prolapse are represented as median (interquartile range)
Trichrome staining
Histomorphological imaging was utilized to ensure full-thickness (epithelium, subepithelium, muscularis, and adventitia) and orientation of each vaginal tissue prior to mechanical testing. Briefly, tissue was embedded and frozen in optimal cutting temperature compound (OCT, Sakura, Tokyo, Japan). Sections of the vaginal cross section were cut onto slides roughly 5–8 μm thick. In short, slides were fixed using Bouin’s solution and then placed in hematoxylin followed by trichrome stain. Slides were then submerged twice into 100 % EtOH and three steps of xylene. Slides were rinsed in water after each step of the protocol. Orientation was determined by examining the direction of the outer most muscle fibers. Samples were then aligned to test along the longitudinal axis of each specimen. Only those samples that were confirmed to be full-thickness and with a validated orientation were utilized.
On a subgroup of samples, blinded technicians examined additional sections of non-prolapsed, premenopausal control (n=5), premenopausal prolapse (n=7), postmenopausal without HT (n=16), and postmenopausal with HT (n=9) vagina for any relative changes in the thickness. The entire cross section was imaged and post-processed. The total thickness was determined by defining a relationship between each pixel of the image and a distance. Briefly, the total length and width of each image was calculated and then utilized to determine the length to pixel (length/pixels) ratio and utilized to determine the distance in μm of each pixel. For each specimen, a blinded researcher examined the number of pixels accounting for the distance of the total cross section. This process was performed in duplicate on each sample. These measurements were then averaged to create a single pixel value, which could be converted to a distance dimension (μm) representing the total thickness of each sample.
Biomechanics
Rheological evaluation was performed using a single-lap, sinusoidal, oscillatory shear test that has been modified from the design of Xu et al. [14]. The luminal surface of the vaginal biopsy was secured to a stationary plate with a very small amount of cyanoacrylate. Tissue length and width measurements were made using digital calipers (Mitutoyo Corp., Aurora, IL, USA). The basic experimental setup can be seen in Fig. 1. In short, the vagina was placed on the bottom plate and aligned below the upper plate. Then the lower plate was raised until the tissue contacted the top plate, and the compressed tissue thickness (roughly 10 % compressive strain) was measured using digital calipers. The upper plate, which was attached to an EnduraTEC ElectroForce Mechanical Testing System (Model ELF 3200, Bose, Minnetonka, MN, USA), was used to apply the oscillating strain to the tissue. The load response of the tissue was measured using a 10 N Sensotec Miniature Load Cell (Model 31, Honeywell, Columbus, OH, USA) with a resolution of 0.01 N.
Fig. 1.
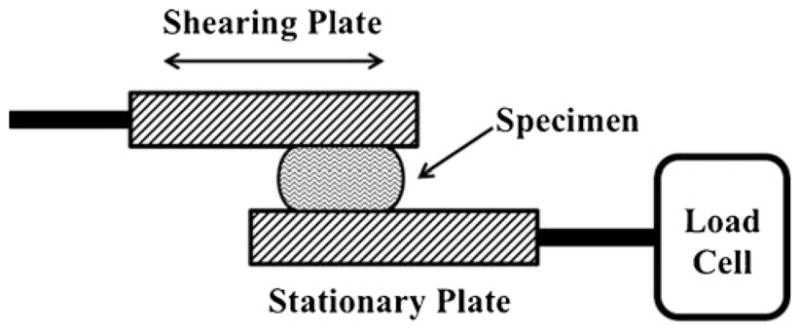
Linear lap shear test schematic. The bottom plate remains stationary and is attached in series with a load cell. The tissue sample is placed onto the bottom plate and is raised until it contacts the top plate. The top plate is connected to the actuator and applies the shear deformation to the specimen
The tissue was kept at room temperature and was surrounded by 0.9 % saline, which sat on the surface of the bottom plate, throughout the testing protocol [15]. All specimens were sheared along the longitudinal axis of the vagina. Vaginal samples were subjected to 10 % shear strain. To calculate the required displacements, the desired shear strain (10 %) was multiplied by the compressed tissue thickness. The tissue was subjected to oscillations of this strain level at a range of frequencies (1.0–90 Hz). Inertial effects were assumed to be negligible at this strain level based on the elastic modulus and sample thickness [14]. For validation of the testing protocol and testing setup, the rheological properties of a known material (Radiesse™) were measured and found to match what has been reported in the literature (data not shown).
Assuming linear viscoelasticity, the resulting shear strain and stress data were utilized to calculate parameters describing the viscoelastic behavior of vaginal tissue [16–18]. The complex shear modulus is a measure of resistance to shear deformation and would be the stiffness felt if a sample were rubbed between one’s fingers and is the result of the combined elastic and viscous components of the tissue [17, 18]. Based on theory, the complex modulus can be decomposed into its elastic and viscous contributions according to:
where E* is the complex modulus, E′ is the storage modulus (aka elastic modulus) providing a metric of how well the sample is able to store energy as if it were an elastic solid, and E″ is the loss modulus providing a metric of how well the sample can dissipate energy as if it were a viscous fluid.
To quantify how each of these parameters were changing with frequency, each was fit to a power-law function of the form:
where y is our parameter, A is an arbitrary coefficient representing the value of the parameter at low frequencies, and γ represents the rate at which the parameter changes with increasing frequency.
From this analysis, it was observed that the tissue samples displayed shear-thinning behavior, which was linear on a log-log scale with a flow index between −0.78 and −0.8. This was consistent with what has been previously found on other biological soft tissues confirming our testing methods [16–20]. While E*, E′, and E″ all increased with frequency, the rates of these respective increases were consistent across all groups, so only the coefficients are reported below to represent E*, E′, and E″, respectively.
Statistics
Age and BMI were found to be normally distributed and are reported as mean ± standard deviation. The overall cross-sectional thickness was also normally distributed. For these parameters, a one-way analysis of variance (ANOVA) was performed. A Dunnett’s T3 or Bonferroni post hoc was performed based on a Levene test for the homogeneity of variances. The remaining demographic data (gravidity, parity, and stage of prolapse) and the rheological parameters represented as the power-law coefficients (complex modulus, storage modulus, and loss modulus) were found to be nonparametric and are reported as median and interquartile ranges (75th–25th). Statistical analysis was performed using the nonparametric Kruskal-Wallis with a Mann-Whitney post hoc test. Due to the larger variances associated with human shear properties all significance levels were set a priori to p=0.1 [21, 22].
Results
No significant difference in patient age was observed between premenopausal women with or without prolapse (p= 1.0). Although there was no significant difference in age between the two postmenopausal groups (p=0.98), there was a difference between both premenopausal groups compared to postmenopausal groups, this was expected due to the onset of menopause occurring at an older age (p<0.001, Table 1). There were no significant differences among any of the groups in gravidity (p=0.31), parity (p=0.22), and BMI (p=0.96). Among women with prolapse, the median stage of prolapse was III with no significant differences between groups for stage of prolapse (p=1.0). The total thickness of the samples was not found to be significantly different between any of the groups (Table 2, p=0.12). All viscoelastic parameters are displayed in Table 2 and statistical p values in Table 3. The complex modulus (a measure of the stiffness of a material as a result of the elastic and viscous contributions) was different across the groups (p= 0.008). The complex modulus was 52 % lower in premenopausal women without prolapse than premenopausal women with prolapse (p=0.016). Therefore, the premenopausal women with prolapse were more resistant to shear deformation compared to non-prolapsed women. In addition, the complex modulus in premenopausal women without prolapse was significantly lower by 38 and 64 % compared to postmenopausal women off (p=0.044) and on HT (p= 0.004). No difference was observed between premenopausal women and postmenopausal women on and off HT (p=0.28 and p=0.28, respectively). Lastly, postmenopausal women without HT had a 69 % lower complex modulus than women on HT (p=0.044).
Table 2.
Viscoelastic parameters calculated from the linear lap shear test including the complex modulus, storage modulus, and loss modulus
| Group | Complex modulus (E*) | Storage modulus (E′) | Loss modulus (E″) | Total thickness (μm) |
|---|---|---|---|---|
| Premenopausal, no prolapse (n=12) | 2,270 (1,520–4,131) | 2,029 (1,319–3,502) | 1,186 (536–1,739) | 2,325±1,208 |
| Premenopausal, prolapsed (n=8) | 4,780 (3,498–8,338) | 3,655 (3,039–5,059) | 2,663 (830–3,316) | 3,246±326 |
| Postmenopausal, prolapsed, no HT (n=18) | 3,878 (2,587–7,154) | 3,362 (2,721–6,470) | 1,620 (1,064–2,134) | 2,139±87 |
| Postmenopausal, prolapsed, HT (n=9) | 6,737 (4,955–11,580) | 6,414 (5,196–11,080) | 2,305 (1,627–3,202) | 2,584±1,007 |
| p value | 0.008 | 0.011 | 0.034 | 0.12 |
All data are presented as median (interquartile range). The total thickness of specimen represented as mean ± standard deviation; p values for subsequent post hoc analyses are provided in Table 3
Table 3.
The significance level (p value) obtained from post hoc analyses of the viscoelastic parameters (Table 2) between each experimental group
| Group | Complex modulus (E*) | Storage modulus (E′) | Loss modulus (E″) |
|---|---|---|---|
| Premenopausal, no prolapse vs premenopausal prolapsed | 0.016 | 0.098 | 0.056 |
| Premenopausal, no prolapse vs postmenopausal prolapsed, no HT | 0.044 | 0.048 | 0.087 |
| Premenopausal, no prolapse vs postmenopausal prolapsed, HT | 0.004 | 0.003 | 0.015 |
| Premenopausal prolapse vs postmenopausal prolapsed, no HT | 0.28 | 0.61 | 0.3 |
| Premenopausal prolapse vs postmenopausal prolapsed, HT | 0.28 | 0.093 | 1 |
| Postmenopausal prolapse, no HT vs postmenopausal prolapsed, HT | 0.044 | 0.041 | 0.085 |
Next, recalling that many biological tissues have an elastic, or solid phase, and viscous, or fluid phase, we examined the storage modulus of the tissue, which describes the elastic portion of the viscoelastic response. There was a significant difference in the elastic response between each of the groups (p=0.011, Table 2). Non-prolapsed, premenopausal women had the lowest storage modulus (E′), which increased 1.8-fold in premenopausal women with prolapse (p=0.098) and 1.6- and 2.9-fold in postmenopausal with prolapse on (p=0.003) and off HT (p=0.048). This indicates that the premenopausal women with prolapse and both groups of postmenopausal women had a larger elastic response compared to premenopausal women without prolapse. Interestingly, postmenopausal women on HT were found to have a 1.6- and 1.8-fold increase in the storage modulus compared to premenopausal women with prolapse (p=0.093) and postmenopausal women not on HT (p=0.041). This distinct increase from the samples from postmenopausal women without HT illustrates similar changes in the elastic contribution that reflect those found for the complex modulus.
Lastly, we examined the loss modulus (E″), where we found significant differences (p=0.034) between each group with non-prolapsed women having the smallest loss modulus (Table 2). Premenopausal women without prolapse were found to have 49 % lower loss modulus compared to premenopausal women with prolapse (p=0.056). Interestingly, premenopausal women without prolapse were also found to have a 28 % lower loss modulus than postmenopausal women not on HT (p=0.087). Premenopausal women without prolapse also had a 52 % lower loss modulus compared to postmenopausal women on HT (p=0.015). Interestingly, no significant differences in the viscous response were found between the premenopausal women with prolapse and the postmenopausal women with prolapse on (p=1.0) and off HT (p=0.3). However, the loss modulus was 32 % decreased in postmenopausal women with prolapse not on HT compared to their counterparts on HT (p=0.085).
Discussion
In this study, we examined the viscoelastic properties of full-thickness vaginal biopsies in premenopausal women with and without prolapse and postmenopausal women with prolapse on and off HT. Our major finding was that biopsies from women with stage II prolapse or greater, regardless of age or hormonal status, had a significantly higher complex modulus resulting from increases in both the storage and loss moduli, relative to women without prolapse. However, increases in the loss modulus seem to be more pronounced in patients with prolapse that were either premenopausal or postmenopausal on HT. These data suggest that the vagina is rapidly remodeling in the presence of prolapse and that hormones do not appear to result in any degree of restoration in tissue quality for patients with prolapse, thereby refuting our hypothesis that HT would have a restorative or protective effect in patients with prolapse. Most importantly, this study demonstrated that meaningful mechanical data can be obtained from small, full-thickness human vaginal biopsies, perhaps allowing for future studies to address questions related to prolapse development and the impact of hormones to be probed with a more rigorous experimental design.
We initially hypothesized that HT in postmenopausal women would help restore the rheological properties of the vagina to similar values as premenopausal prolapsed women; however, we found that postmenopausal women on HT had distinct rheological properties. Interestingly, it appears that the presence of hormones in patients with prolapse seems to also contribute to a higher instantaneous stiffness of the vagina as shown from the increases in the loss modulus. However, unlike the storage or elastic contribution, the tissue can dissipate this energy over time. In other words, this tissue is better able to “stretch out” or flow over time when a force is applied to it. This is called “creep behavior” in the field of viscoelasticity and is a characteristic of viscous fluids. This may be a reflection of why the administration of HT is recommended for vaginal atrophy. The therapy results in improved tissue hydration along with a thicker and more vascularized epithelium. This effect may be the explanation for the increased loss moduli observed in women on HT. However, whether this is beneficial in terms of slowing the progression of prolapse in unclear based on this study.
The increased storage moduli in patients with prolapse is likely related to changes in collagen and elastin, which could either be causal or secondary to the development of prolapse. These results were consistent with a study performed by Lei et al. who examined vaginal strips and found an increase in storage modulus in pre- or postmenopausal women with prolapse relative to their respective controls (non-prolapsed women), and uniaxial testing showing the vaginal wall is stiffer in prolapsed women compared to non-prolapsed women [9]. A previous study performed in our laboratory examined the biochemical properties of the vagina in the same defined groups of women. This study reported evidence of accelerated remodeling with increased collagen III, increased tropoelastin and desmosine, and increases in the active form of MMPs-1, -2 and -9 in women with prolapse relative to controls independent of age or menopausal status [23]. Thus, the combined data suggest that there is a significant degree of vaginal remodeling, indicated by the biochemical alterations, which then manifest in a stiffer, less compliant vagina after the development of prolapse that is less than ideal in terms of function of the vagina.
It is important to understand that there are many other factors that could have influenced the viscoelastic properties of this rheological protocol. It is well known that muscle is significantly more compliant than collagen under passive conditions and a decrease in vaginal muscle composition could result in some of the observed changes in the viscoelastic properties observed in this study. Previous studies have shown that the vaginal muscularis of prolapsed women was significantly thinner compared to non-prolapsed patients [11, 19, 20], which may account for our observed changes in viscoelastic parameters. Future studies that better quantify the percentage of smooth muscle relative to the other sublayers of the vagina may improve our understanding of how each relates to the biomechanical properties of the tissue.
The current study examined women that did not have significant differences in BMI, gravidity, parity, and the degree of prolapse. This is an advantage of the current study design because it reduces the effects of possible confounding risk factors for the development of prolapse. However, the differences in age between pre- and postmenopausal patients, which is another risk factor associated with the development of prolapse, do not allow for a concrete clinical interpretation of these data. Moreover, for those on HT, it is unclear when the therapy was administered, i.e., at the time of menopause or later, as well as pre- or post-prolapse development. Finally, some of the women in the premenopausal group may actually be perimenopausal. Nevertheless, recognizing these limitations, the mechanical data do appear to be consistent with previous literature as well as consistent with the expected impact of hormones on the quality of tissue in the vaginal wall as described above.
Other major limitations of this study are that we are assuming a small biopsy sample is representative of changes occurring throughout the entire vagina. To reduce variability vaginal biopsies were taken from a consistent location, but additional studies on the variability of these properties throughout the vagina are needed to better put these data into context. Lastly, our analysis was done with a significance level of p=0.1 determined during the initial design of this study as previous research has shown large variances in rheological properties of human tissue [17, 21, 22]. Upon post hoc analysis many viscoelastic parameters did reach the traditional p<0.05 level (Table 3); however, a post hoc power analysis determined that a sample size of between 28 and 37 specimens would be required for this study to be appropriately powered for those parameters that did not reach the p<0.05 significance level.
Overall, this study has demonstrated the feasibility of obtaining potentially meaningful mechanical data utilizing only a small tissue biopsy sample. From this rheological protocol, we have illustrated that the vagina is undergoing a significant degree of remodeling that is associated with prolapse and that HT appears to impact the viscous behavior of vaginal tissue. Overall, this provides a step toward better characterizing the biomechanical properties of small tissue biopsies from the vaginal wall, which may be incorporated into a longitudinally designed study aimed at fully understanding the mechanisms of prolapse development and progression as well as the effect of known risk factors.
Acknowledgments
National Institutes of Health (NIH) Support R01HD-045590 and K12HD-043441. In addition, we would like to like Stacy Palcsey for her assistance on several tests within this work.
Footnotes
Conflicts of interest None.
Contributor Information
Andrew Feola, Musculoskeletal Research Center, Department of Bioengineering, University of Pittsburgh, Pittsburgh, PA, USA.
Robert Duerr, Magee-Womens Research Institute, University of Pittsburgh, Pittsburgh, PA, USA.
Pamela Moalli, Division of Urogynecology and Reconstructive Pelvic Surgery, Department of Obstetrics, Gynecology, and Reproductive Sciences, Magee-Womens Hospital, Magee-Womens Research Institute, University of Pittsburgh, Pittsburgh, PA, USA.
Steven Abramowitch, Email: sdast9@pitt.edu, Musculoskeletal Research Center, Department of Bioengineering, University of Pittsburgh, Pittsburgh, PA, USA. Magee-Womens Research Institute, University of Pittsburgh, Pittsburgh, PA, USA. Department of Bioengineering, University of Pittsburgh, 405 Center for Bioengineering, 300 Technology Drive, Pittsburgh, PA 15219, USA.
References
- 1.Barber MD, Visco AG, Wyman JF, et al. Sexual function in women with urinary incontinence and pelvic organ prolapse. Obstet Gynecol. 2002;99(2):281–289. doi: 10.1016/s0029-7844(01)01727-6. [DOI] [PubMed] [Google Scholar]
- 2.Heit M, Rosenquist C, Culligan P, et al. Predicting treatment choice for patients with pelvic organ prolapse. Obstet Gynecol. 2003;101(6):1279–1284. doi: 10.1016/s0029-7844(03)00359-4. [DOI] [PubMed] [Google Scholar]
- 3.DeLancey JO, Kearney R, Chou Q, et al. The appearance of levator ani muscle abnormalities in magnetic resonance images after vaginal delivery. Obstet Gynecol. 2003;101(1):46–53. doi: 10.1016/s0029-7844(02)02465-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Moalli PA, Jones Ivy S, Meyn LA, et al. Risk factors associated with pelvic floor disorders in women undergoing surgical repair. Obstet Gynecol. 2003;101(5 Pt 1):869–874. doi: 10.1016/s0029-7844(03)00078-4. [DOI] [PubMed] [Google Scholar]
- 5.Moalli PA, Debes KM, Meyn LA, et al. Hormones restore biomechanical properties of the vagina and supportive tissues after surgical menopause in young rats. Am J Obstet Gynecol. 2008;199(2):161e1–161.e8. doi: 10.1016/j.ajog.2008.01.042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Zong W, Abramowitch SD, Moalli PA. Hormone therapy obviates the increased expression of a key collagen degrading enzyme with repetitive mechanical stress. J Pelvic Med Surg. 2007;13(5):231. [Google Scholar]
- 7.Zong W, Meyn LA, Moalli PA. The amount and activity of active matrix metalloproteinase 13 is suppressed by estradiol and progesterone in human pelvic floor fibroblasts. Biol Reprod. 2009;80(2):367–374. doi: 10.1095/biolreprod.108.072462. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Nicolle S, Lounis M, Willinger R, et al. Shear linear behavior of brain tissue over a large frequency range. Biorheology. 2005;42(3):209–223. [PubMed] [Google Scholar]
- 9.Lei L, Song Y, Chen R. Biomechanical properties of prolapsed vaginal tissue in pre- and postmenopausal women. Int Urogynecol J Pelvic Floor Dysfunct. 2007;18(6):603–607. doi: 10.1007/s00192-006-0214-7. [DOI] [PubMed] [Google Scholar]
- 10.Rubod C, Boukerrou M, Brieu M, et al. Biomechanical properties of vaginal tissue: preliminary results. Int Urogynecol J Pelvic Floor Dysfunct. 2008;19(6):811–816. doi: 10.1007/s00192-007-0533-3. [DOI] [PubMed] [Google Scholar]
- 11.Boreham MK, Wai CY, Miller RT, et al. Morphometric analysis of smooth muscle in the anterior vaginal wall of women with pelvic organ prolapse. Am J Obstet Gynecol. 2002;187(1):56–63. doi: 10.1067/mob.2002.124843. [DOI] [PubMed] [Google Scholar]
- 12.Frisén M, Mägi M, Sonnerup I, et al. Rheological analysis of soft collagenous tissue. Part I: theoretical considerations. J Biomech. 1969;2(1):13–20. doi: 10.1016/0021-9290(69)90037-2. [DOI] [PubMed] [Google Scholar]
- 13.Goh JT. Biomechanical properties of prolapsed vaginal tissue in pre- and postmenopausal women. Int Urogynecol J Pelvic Floor Dysfunct. 2002;13(2):76–79. doi: 10.1007/s001920200019. discussion 79. [DOI] [PubMed] [Google Scholar]
- 14.Xu C, Chan R, Tirunagari N. A biodegradable, acellular xenogeneic scaffold for regeneration of the vocal fold lamina propria. Tissue Eng. 2007;13(3):551–566. doi: 10.1089/ten.2006.0169. [DOI] [PubMed] [Google Scholar]
- 15.Rubod C, Boukerrou M, Brieu M, et al. Biomechanical properties of vaginal tissue. Part 1: new experimental protocol. J Urol. 2007;178(1):320–325. doi: 10.1016/j.juro.2007.03.040. discussion 325. [DOI] [PubMed] [Google Scholar]
- 16.Caton T, Thibeault SL, Klemuk S, Smith ME. Viscoelasticity of hyaluronan and nonhyaluronan based vocal fold injectables: implications for mucosal versus muscle use. Laryngoscope. 2007;117(3):516–521. doi: 10.1097/MLG.0b013e31802e9291. [DOI] [PubMed] [Google Scholar]
- 17.Chan RW, Titze IR. Viscoelastic shear properties of human vocal fold mucosa: measurement methodology and empirical results. J Acoust Soc Am. 1999;106(4 Pt 1):2008–2021. doi: 10.1121/1.427947. [DOI] [PubMed] [Google Scholar]
- 18.Chan RW, Titze IR. Viscoelastic shear properties of human vocal fold mucosa: theoretical characterization based on constitutive modeling. J Acoust Soc Am. 2000;107(1):565–580. doi: 10.1121/1.428354. [DOI] [PubMed] [Google Scholar]
- 19.Boreham MK, Miller RT, Schaffer JI, et al. Smooth muscle myosin heavy chain and caldesmon expression in the anterior vaginal wall of women with and without pelvic organ prolapse. Am J Obstet Gynecol. 2001;185(4):944–952. doi: 10.1067/mob.2001.117342. [DOI] [PubMed] [Google Scholar]
- 20.Boreham MK, Wai CY, Miller RT, et al. Morphometric properties of the posterior vaginal wall in women with pelvic organ prolapse. Am J Obstet Gynecol. 2002;187(6):1501–1508. doi: 10.1067/mob.2002.130005. discussion 1508–1509. [DOI] [PubMed] [Google Scholar]
- 21.Goodyer E, Welham NV, Choi SH, et al. The shear modulus of the human vocal fold in a transverse direction. J Voice. 2009;23(2):151–155. doi: 10.1016/j.jvoice.2007.09.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Goodyer E, Hemmerich S, Müller F, et al. The shear modulus of the human vocal fold, preliminary results from 20 larynxes. Eur Arch Otorhinolaryngol. 2007;264(1):45–50. doi: 10.1007/s00405-006-0133-8. [DOI] [PubMed] [Google Scholar]
- 23.Zong W, Stein SE, Starcher B, et al. Alteration of vaginal elastin metabolism in women with pelvic organ prolapse. Obstet Gynecol. 2010;115(5):953–961. doi: 10.1097/AOG.0b013e3181da7946. [DOI] [PMC free article] [PubMed] [Google Scholar]


