Abstract
In children with Sickle Cell Disease, the combination of risk stratification with Transcranial Doppler Ultrasound (TCD) and selective chronic red cell transfusion (CRCT—the STOP Protocol) is one of the most effective stroke prevention strategies in medicine. How fully it is being implemented is unclear.
Nineteen of 26 sites that conducted the two pivotal clinical trials (STOP and STOP II) participated in Post STOP, a comprehensive medical records review assessing protocol implementation in the 10-15 years since the trials ended. Professional abstractors identified medical records in the Post STOP era in 2851 74% of the 3840 children who took part in STOP and/or STOP II, and documented TCD rescreening, maintenance of CRCT in those at risk, and stroke.
Among 1896 children eligible for TCD rescreening (target group), evidence of any rescreening was found in 1090 (57%). There was wide site variation in TCD rescreening ranging from 18% to 91% of eligible children. Both younger age and having a conditional TCD during STOP/II were associated with a higher likelihood of having a TCD in Post STOP. Sixty eight new abnormal, high risk cases were identified.
Despite clear evidence of benefit the STOP protocol is not fully implemented even at experienced sites. Site variation suggests that system improvements might remove barriers to implementation and result in even greater reduction of ischemic stroke in children with SCD.
Keywords: sickle cell anemia, transcranial Doppler, stroke, hydroxyurea
INTRODUCTION
The primary prevention of ischemic stroke in children with Sickle Cell Disease was made possible by stroke risk stratification using transcranial Doppler ultrasound (TCD) (1). When high risk cases identified by TCD received chronic red cell transfusion (CRCT) in a randomized, controlled, multicenter clinical trial (Stroke Prevention in Sickle Cell Anemia—the STOP Study) there was a marked reduction (> 90%) in first stroke compared to standard care(2). A second trial, Optimizing Stroke Prevention in Sickle Cell Disease- STOP II) failed to show that transfusion could be withdrawn safely even after 30 or more months of CRCT (3). Between 1995 and 2005, STOP and STOP II, were performed in the US and Canada and involved 26 centers specializing in the care of children with sickle cell disease (2)(3). The centers identified children with Hemoglobin SS or Sbeta0thal who were then consented to have TCD. The role of TCD, methods of performance, interpretation, and the design of the studies have been described elsewhere (4). TCD stratifies stroke risk on the basis of blood flow velocity in the internal carotid or middle cerebral arteries. Categories derived from a large single center prospective study (1) were used: Time averaged mean of the maximum (TAMM) < 170 cm/sec normal; 170-199 cm/sec conditional and 200 cm/sec or greater for abnormal eligible for randomization in the treatment (CRCT vs standard care) part of the trial. Criteria for STOP II (randomized withdrawal of CRCT) were that the subject had to have had a high risk TCD followed by at least 30 months of CRCT and have a low risk TCD at randomization. Participation in these studies ranged from a single screening TCD to randomization. STOP II also had an observational arm for children on CRCT whose TCD had not reverted to normal.
This protocol, known as the STOP Protocol, became widely recommended in 1998 and since then reduction in stroke in children with Sickle Cell Disease (SCD) has been reported in clinic series (5)(6)(7), and from hospitalization data when time frames after 1998 are compared to years prior to 1998 (8)(9)(10)(11). In addition, a marked reduction in the black-white childhood disparity in the risk of dying from ischemic stroke (from 1.74 to 1.27 Relative Risk blacks more likely than whites) has been attributed to the widespread implementation of the STOP Protocol (12). These data suggest that substantial implementation of the stroke prevention protocol has taken place and stroke reduction has been realized, but it is not clear to what extent the benefits of this research have extended to all children at risk.
Problems with access to, or compliance with, TCD screening remain important barriers to full dissemination and implementation of the STOP protocol (13)(14). Guidelines called for yearly TCD from age 2-16 years if the results remained in the normal range. More frequent TCD's should be performed if conditional results were observed, especially in younger children with velocities closer to 200 cm/sec (15)(16). Adherence to the guidelines varies widely, from 45% (2004-2006) to 68% (2008) (17) but substantial numbers of children (31%) received few if any TCD examinations over 10 years after publication of the STOP study (18). While Medicaid claims data analysis showed an increase from 22% to 44% from 2005-2010 there was substantial variation across states and overall screening rates remain low (19).
The participation of almost 4000 children with SCD in either or both the STOP or STOP II studies represents a unique opportunity to examine real world stroke prevention practices after the clinical trials ended in centers that conducted the studies. This is the initial report from the Post STOP Study, an NIH funded project which sought to re-identify and locate (in the medical record) as many of the participants of STOP and/or STOP II as possible to compare: 1) actual TCD screening patterns/practices after the trials ended and 2) initiation and maintenance of CRCT when high risk was discovered, to the STOP protocol guidelines (idealized implementation). A third aim was to categorize the Post STOP ischemic strokes as either presumed failures of risk detection (with TCD) or failures of risk mitigation (with CRCT). This paper reports TCD rescreening in those younger subjects eligible for TCD by protocol.
These data will inform efforts to further reduce stroke and advance the NHLBI goal of a “stroke free generation” in Sickle Cell Disease (20) by identifying gaps in implementation or protocol efficacy leading to targeted intervention strategies.
METHODS
For all participants of STOP/II a date was identified at which they had their last encounter/data entry in these studies. The period of study for Post STOP was individually determined and extended from this date to the date when records of care were abstracted at the study sites. No effort was made to locate records of care from outside centers.
Definition of Idealized STOP TCD Screening Implementation:
-
1)Children age 2-16 years get yearly TCD unless:
- Conditional TCD is detected, which should prompt more frequent TCD (not otherwise specified except that frequency should be based on age—younger children and those with TCD velocities closer to 200 cm/sec getting more TCD).
- If abnormal TCD is detected, either CRCT should be initiated or early repeat (within 4 weeks) TCD should be performed and if abnormal CRCT initiated or if conditional TCD should be repeated. Initially the STOP Protocol called for repeating an Abnormal TCD within 4 weeks to confirm high risk prior to starting transfusion. However, in both STOP and STOP II strokes were documented in the period between tests leading to recommendations for transfusion after a single abnormal. Because of this variance either transfusion or early repeat of TCD are considered consistent with idealized implementation.
-
2)
Inadequate TCD, caused by either technical problems or severe arterial disease with occlusion of the arteries of interest, provides no clear indication of risk. While repeat TCD or alternate methods of evaluation such as magnetic resonance angiography are often performed guidelines in the Post STOP era do not provide specific recommendations.
Chart Abstraction
Institutional Review Board approval was obtained from participating sites. Subjects who participated in STOP or STOP II were identified from their study acrostic. A data abstraction team visited each site and examined all available inpatient and outpatient records for that site's subjects from the Post STOP date up to the visit date or far as possible for those no longer being cared for at that site. Abstraction visits began in January 2012 and ended in May 2014. All TCD data, any brain neuroimaging test results, and all available written materials pertaining to any neurological events were de-identified and retrieved for later analysis.
Post STOP TCD results were classified from written reports into STOP Protocol categories: Normal (< 170 cm/sec Time averaged Mean of the Maximum); Conditional (170-199 cm/sec); Abnormal (≥ 200 cm/sec) or Inadequate. Cases where the report included velocities were interpreted and classified by the investigative team according to STOP criteria regardless of the local reading.
Statistical Methods
Descriptive statistics were performed using chi-square and t-tests for categorical and continuous data, respectively. Last STOP/ II visit was considered to be the start of Post STOP study. As such, age at start of Post STOP was calculated as age at last STOP/II visit. Follow-up time subsequently calculated as the time from last STOP/II visit to last encounter in medical charts during Post STOP era.
RESULTS
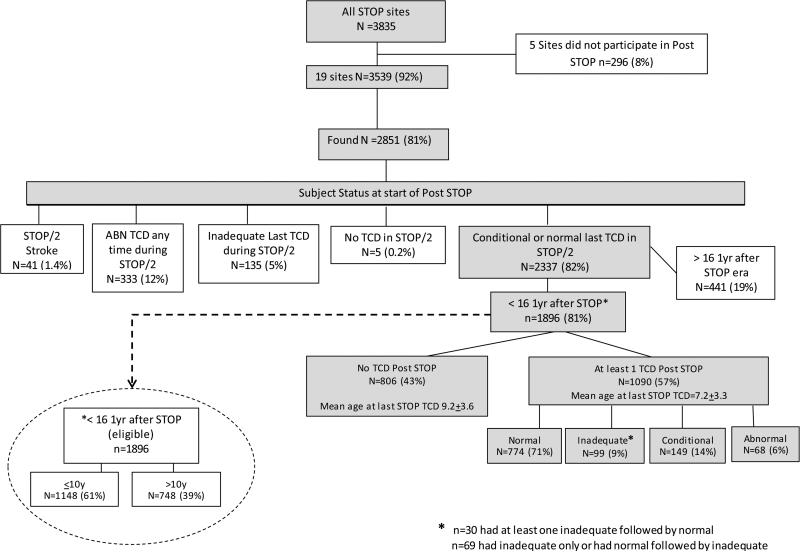
The two study data (STOP and STOP II) bases contained unique records on 3854 children at 26 sites. Nineteen of these sites participated in Post STOP but these sites accounted for 3539 subjects (92%). Of these 3539, records of care at the enrolling sites in the Post STOP era were located 2851 (81% of possible at participating sites) (Figure 1). The mean age of subjects at the start of their individual Post STOP period was 10.5+4.6 (median 10.4 (range 2.0-23.2)). The mean follow-up time from last STOP/II visit was 9.1+3.4 years (median 10.3 (range <1 year-15.4 years)).
Figure 1.
Subject status in Post STOP
TCD Rescreening
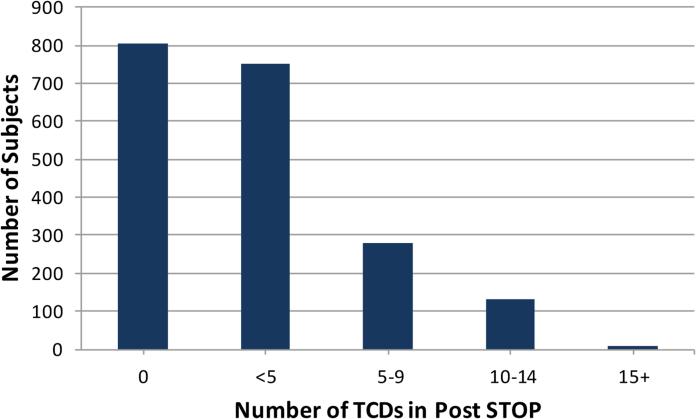
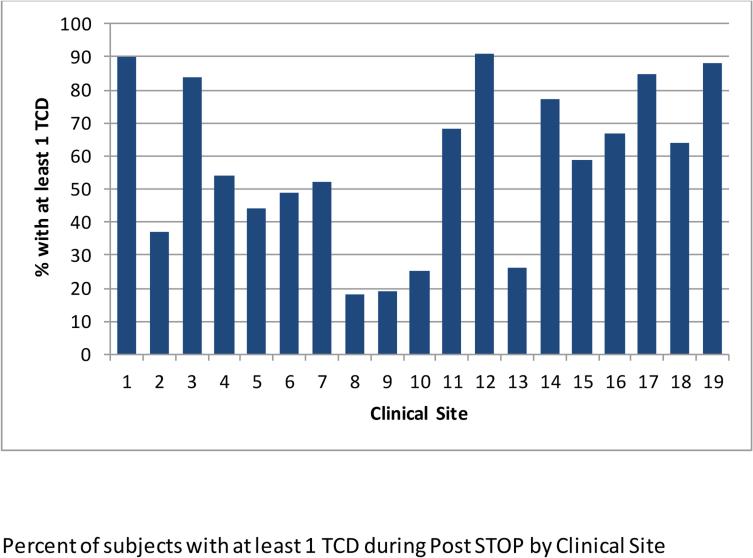
Subjects with conditions or age that precluded TCD rescreening by the protocol were excluded from the following analysis. This included any subject with abnormal TCD or stroke during the STOP/II era and those falling outside age guidelines. Removing subjects entering Post STOP with abnormal TCD (n=333), those whose last TCD was inadequate (n=135), those with stroke (n=41) and subjects within 12 months of their 17th birthday (n=441) reduced the rescreening candidates from 2851 to 1896 (Figure 1). Among these 1896, 49% were female and the mean age was 8.7+3.5 at the start of Post STOP. The last TCD in STOP/II was conditional for 208 (11%) and normal for 1688 (89%). Evidence of at least one Post STOP TCD was found in 1090 (57%). The number of TCD's per subject varied widely (Figure 2). Age (p<0.0001) but not gender (p=0.33) was associated with being rescreened. The percentage of subjects who had TCD rescreening varied by site, ranging from 18% to 91% (Figure 3). To determine if low overall screening rates reflected a bias at some sites to screen only younger children, the rates were determined deleting children who were or would turn 11 years of age or older one year into Post STOP. Rescreening rates ranged from 27% to 98% but the relative performance changed little. The four lowest performing sites and three of the 4 top performing sites were the same in both analyses.
Figure 2.
Number of TCD's in Post STOP
Figure 3.
Percent of subjects with at least 1 TCD during Post STOP by Clinical Site
Having a conditional TCD during STOP/II increased the likelihood of having TCD in Post STOP but only if the last TCD was conditional. For those with any conditional during the two trials 60% had TCD (vs 57% overall, p=.18). However, if the last TCD in STOP/II was conditional 69% had TCD in Post STOP (compare to 56% overall, p=.0003). Among those eligible for rescreening, 68 converted to abnormal TCD (37 from Normal and 31 from Conditional-- last TCD in STOP/II) at a mean age of 9.0+3.3 (range 4.0 to 19.6 years). (Figure 1).
DISCUSSION
Post STOP documented considerable variation in implementation of the STOP TCD Screening Protocol with 5 sites achieving > 80% of eligible children having at least one TCD while 4 sites achieved < 27%. Since these were experienced sites it is likely that results generally may be worse. The results were changed little by looking only at younger children suggesting that some sites have developed very effective ways to carry out TCD rescreening while others have not. No evidence could be found for TCD rescreening in 43% of those eligible. Since these sites all had TCD screening programs, this represents difficulties in implementation rather than dissemination. The advantages of Post STOP are that extended follow up was obtained on a large cohort who had standardized stroke risk assessments as part of a clinical trial that established a baseline for stroke risk. The medical care examined was from centers that participated in the trials and where it is presumed that protocol implementation would be optimal compared to the medical community at large. Trained abstractors visited each site and used consistent survey tools and procedures. Important limitations: 1) chart review can generally only produce positive evidence of, rather than confirm absence of, testing or treatment; 2) Abstractors encountered both paper and electronic records and charts may have been missing important data or abstractors may have missed important information on screening, transfusion decisions and compliance; 3) only about half of the participating centers had integrated pediatric and adult medical records which meant that important information on treatment after transfer to the adult system was missing on subjects at some sites; 4) it is possible but unlikely that substantially different outcomes might have taken place in the 984 children who could not be located.
Regarding these limitations
1) it is unlikely that many children underwent TCD screening outside of these centers unless they relocated to other cities with comprehensive SCD centers as access was limited during this period making it unlikely that institutions in the area of participating sites offered competitive services; 2) chart abstraction is imperfect but the team experienced in the exercise for 3 years would be unlikely to miss substantial data when using the same examination template; 3) the lack of visibility into the adult treatment limits conclusions regarding problems during care transition but it does not affect the positive findings of the study because TCD protocol implementation was examined only in children; 4) protocol adherence was probably worse rather than better in those not located unless they transferred care to another SCD Comprehensive Center.
Also during this period use of hydroxyurea increased, but was not likely to have influenced the rescreening rate. Recently the results of the TWiTCH study were published. In this study children with abnormal TCD (but without severe vasculopathy on magnetic resonance angiograpy) were randomized to either continue transfusion after one year or be transitioned to hydroxyurea. No change in TCD was reported after two years on HU and no strokes occurred in the subjects removed from transfusion (21). Their results may spur TCD screening with renewed interest as long-term transfusion may not be needed in all children identified as high risk provided they are identified early before severe vasculopathy becomes established.
While the use of TCD for risk stratification in SCD is not controversial, guidelines acknowledge that the optimal timing and frequency of screening are not evidence based. There is not universal acceptance of the need for yearly TCD in older children and some have advocated not screening older children who have had normal TCD in early childhood. In the French newborn cohort study no first time abnormals were noted over the age of nine years (7). However, both STOP (22) and Post STOP documented a low but non-zero abnormal “find rate” in older children. The French study is not typical in that intensive efforts were made for regular TCD and follow up from an early age, unlike clinical experience outside a research cohort. However, it does suggest that with early and repeat TCD, evidence could indicate that at some point screening beyond a certain age is not necessary. Until such data are available recommendations remain in place for screening from ages 2 -16 years. Nonetheless Post STOP and the Medicaid Claims data (19) both show that older children were less likely to obtain TCD rescreening. However, the marked variation in site performance in Post STOP was not explained by age selectivity at these sites. The care of children with SCD is challenging. These results suggest that institutional programs (such as computerized monitoring of regular TCD screening with outreach to those who miss appointments) may have a substantial impact on STOP TCD Protocol implementation.
Conclusions
Even at experienced sites TCD screening to detect risk for primary stroke is rarely implemented according to protocol. Special programs aimed at improving implementation of TCD screening would be likely to further reduce ischemic stroke in Sickle Cell Disease.
ACKNOWEDGMENTS
The authors thank these individuals for their assistance in locating and abstracting charts: Elliott Vichinsky MD Children's Hospital of Oakland, Oakland CA; Brian Berman MD UH at Case Medical, Rainbow Children's, Cleveland OH; Winfred Wang MD St. Jude Children's Research Hospital, Memphis TN; Ify Osunkwo MD MPH CHOA-Egleston Children's Hospital, Atlanta GA; Beatrice Gee MD CHOA-Hughes Spaulding Children's Hospital, Atlanta GA; Cindy Neunert MD Georgia Regents University, Augusta GA; Beng Fuh MD East Carolina University, Greenville, NC; Ofelia Alvarez MD University of Miami – Miller School of Medicine, Miami FL; Scott Miller MD State University of New York – Downstate Brooklyn NY; Margaret Lee MD Columbia University Medical Center, New York NY; Melanie Kirby-Allen MD The Hospital for Sick Children Toronto ON, Canada; Julie Kanter MD Medical University of South Carolina, Charleston, SC; Emily Meier MD Children's National Medical Center, Washington DC; Karen Kalinyak MD Cincinnati Children's Hospital, Cincinnati OH; Dr. Tathi V Iyer MD University of Mississippi Medical Center, Jackson MS; Lee Hilliard MD University of Alabama at Birmingham, Birmingham AL; R Clark Brown MD PhD CHOA – Children's at Scottish Rite Atlanta GA; Janet Kwiatkowski MD Children's Hospital of Philadelphia, Philadelphia PA. Bea Files MD Atlanta Georgia assisted in study design. Mary Lanier assisted with manuscript preparation. The authors acknowledge the dedication of the patients, families, nurses, TCD examiners, physicians and others who participate in stroke prevention research and clinical practice.
This research was supported by 1R01HL096789-01 from NHLBI.
Contributor Information
Robert J. Adams, Medical University of South Carolina Neurology.
Dan T. Lackland, Medical University of South Carolina Neurology.
Lynette Brown, Tidelands Health Cancer Service.
David Brown, Medical University of South Carolina.
Jenifer Voeks, Medical University of South Carolina Neurology.
Heather J. Fullerton, University of California San Francisco Neurology & Pediatrics.
Julie Kanter, Medical University of South Carolina Pediatrics.
Janet L. Kwiatkowski, Children's Hospital of Philadelphia..
REFERENCES
- 1.Adams R, McKie V, Nichols F, et al. The Use of Transcranial Ultrasonography to Predict Stroke in Sickle Cell Disease. New England Journal of Medicine. 1992;326(9):605–610. doi: 10.1056/NEJM199202273260905. [DOI] [PubMed] [Google Scholar]
- 2.Adams R, McKie V, Hsu L, et al. Prevention of a First Stroke by Transfusions in Children with Sickle Cell Anemia and Abnormal Results on Transcranial Doppler Ultrasonography. New England Journal of Medicine. 1998;339(1):5–11. doi: 10.1056/NEJM199807023390102. [DOI] [PubMed] [Google Scholar]
- 3.Adams R, Brambilla D, for the STOP II Consortium Discontinuing Prophylactic Transfusions Used to Prevent Stroke in Sickle Cell Disease. New England Journal of Medicine. 2005;353(26):2769–2778. doi: 10.1056/NEJMoa050460. [DOI] [PubMed] [Google Scholar]
- 4.Adams RJ, McKie VC, Brambilla DJ, et al. Stroke Prevention Trial in Sickle Cell Anemia (“STOP”): Study Design. Controlled Clinical Trials. 1997;19:110–129. doi: 10.1016/s0197-2456(97)00099-8. [DOI] [PubMed] [Google Scholar]
- 5.McCarville M, Goodin G, Fortner G, et al. Evaluation of a comprehensive transcranial doppler screening program for children with sickle cell anemia. Pediatric Blood Cancer. 2008;50(4):818–821. doi: 10.1002/pbc.21430. [DOI] [PubMed] [Google Scholar]
- 6.Enninful-Eghan H, Moore R, Ichord R, Smith-Whitley K, Kwiatkowski J. Transcranial Doppler Ultrasonography and Prophylactic Transfusion Program Is Effective in Preventing Overt Stroke in Children with Sickle Cell Disease. The Journal of Pediatrics. 2010;157(3):479–484. doi: 10.1016/j.jpeds.2010.03.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Bernaudin F, Verlhac S, Arnaud C, et al. Impact of early transcranial Doppler screening and intensive therapy on cerebral vasculopathy outcome in a newborn sickle cell anemia cohort. Blood. 2010;117(4):1130–1140. doi: 10.1182/blood-2010-06-293514. [DOI] [PubMed] [Google Scholar]
- 8.Fullerton H. Declining stroke rates in Californian children with sickle cell disease. Blood. 2004;104(2):336–339. doi: 10.1182/blood-2004-02-0636. [DOI] [PubMed] [Google Scholar]
- 9.George MG, Tong X, Kulina EV, Labarthe DR. Trends in stroke hospitalizations and risk factors among children and young adults, 1995-2008. Annals of Neurology. 2011;70(5):713–721. doi: 10.1002/ana.22539. [DOI] [PubMed] [Google Scholar]
- 10.Ovbiagele B, Adams R. Trends in comorbid sickle cell disease among stroke patients. Journal of the Neurological Sciences. 2012;313(1-2):86–91. doi: 10.1016/j.jns.2011.09.023. [DOI] [PubMed] [Google Scholar]
- 11.McCavit T, Xuan L, Zhang S, Flores G, Quinn C. National trends in incidence rates of hospitalization for stroke in children with sickle cell disease. Pediatr Blood Cancer. 2012;60(5):823–827. doi: 10.1002/pbc.24392. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Lehman L, Fullerton H. Changing Ethnic Disparity in Ischemic Stroke Mortality in US Children After the STOP Trial. JAMA Pediatrics. 2013;167(8):754. doi: 10.1001/jamapediatrics.2013.89. [DOI] [PubMed] [Google Scholar]
- 13.Fullerton H, Gardner M, Adams R, Lo L, Johnston S. Obstacles to primary stroke prevention in children with sickle cell disease. Neurology. 2006;67(6):1098–1099. doi: 10.1212/01.wnl.0000237398.13545.86. [DOI] [PubMed] [Google Scholar]
- 14.Bollinger L, Nire K, Rhodes M, Chisolm D, O'Brien S. Caregivers’ perspectives on barriers to transcranial doppler screening in children with sickle-cell disease. Pediatr Blood Cancer. 2010;56(1):99–102. doi: 10.1002/pbc.22780. [DOI] [PubMed] [Google Scholar]
- 15.Goldstein L, Adams R, Alberts M, et al. American Heart Association; American Stroke Association Stroke Council. Primary prevention of ischemic stroke: a guideline from the American Heart Association/American Stroke Association Stroke Council: co-sponsored by the Atherosclerotic Peripheral Vascular Disease Interdisciplinary Working Group; Cardiovascular Nursing Council; Clinical Cardiology Council; Nutrition, Physical Activity, and Metabolism Council; and the Quality of Care and Outcomes Research Interdisciplinary Working Group. Stroke. 2011;42:517–584. [Google Scholar]
- 16.The Management of Sickle Cell Disease. National Institute Of Health Web site; www.nhlbi.nih.gov/health-pro/guidelines/current/management-sickle-cell-disease Published 1984. Revised 1989, 1995, and June 2002. [Google Scholar]
- 17.Raphael J, Shetty P, Liu H, Mahoney D, Mueller B. A critical assessment of transcranial doppler screening rates in a large pediatric sickle cell center: Opportunities to improve healthcare quality. Pediatr Blood Cancer. 2008;51(5):647–651. doi: 10.1002/pbc.21677. [DOI] [PubMed] [Google Scholar]
- 18.Eckrich M, Wang W, Yang E, et al. Adherence to transcranial Doppler screening guidelines among children with sickle cell disease. Pediatr Blood Cancer. 2013;60(2):270–274. doi: 10.1002/pbc.24240. [DOI] [PubMed] [Google Scholar]
- 19.Reeves SL, Madden B, Freed GL, Dombkowski KJ. Transcranial Doppler Screening among children and adolescents with Sickle Cell Anemia. JAMA Pediatr. 2016;170(6):550–556. doi: 10.1001/jamapediatrics.2015.4859. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.National Library of Medicine. Department of Health and Human Services [Internet] [2015 Sep 5];2015 Available from: http://WWW.nlm.nih.gov/od/bor/913BORMinutes.pdf.
- 21.Ware RE, Davis BR, Schultz WH, et al. Hydroxycarbamide versus chronic transfusion for maintenance of transcranial doppler flow velocities in children with sickle cell anaemia-TCD With Transfusions Changing to Hydroxyurea (TWiTCH): a multicentre, open-label, phase 3, non-inferiority trial. Lancet. 2016;387(10019):661–70. doi: 10.1016/S0140-6736(15)01041-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Adams R, Brambilla DJ, Granger S, et al. for the STOP Study Investigative Team Stroke and Conversion to High Risk in Children Screened with Transcranial Doppler Ultrasound during the STOP Study. Blood. 2004;103:3689–3694. doi: 10.1182/blood-2003-08-2733. [DOI] [PubMed] [Google Scholar]