ABSTRACT
In plants, cell wall components including cellulose, hemicelluloses, and pectins interact with each other to form complex extracellular network structures that control cell growth and maintain cell shape. However, it is still not clear exactly how different wall polymers interact, how the conformations and interactions of cell wall polymers relate to wall mechanics, and how these factors impinge on intracellular structures such as the cortical microtubule cytoskeleton. Here, based on studies of Arabidopsis thaliana xxt1 xxt2 mutants, which lack detectable xyloglucan in their walls and display aberrant wall mechanics, altered cellulose patterning and biosynthesis, and reduced cortical microtubule stability, we discuss the potential relationships between cell wall biosynthesis, wall mechanics, and cytoskeletal dynamics in an effort to better understand their roles in controlling plant growth and morphogenesis.
KEYWORDS: Arabidopsis thaliana, cellulose, microtubules, plant cell walls, wall mechanics, xyloglucan
Abbreviations
- AFM
atomic force microscopy
- CESA
cellulose synthase
- CMF
cellulose microfibril
- CSCs
cellulose synthase complexes
- FESEM
field emission scanning electron microscopy
- HERK1
HERCULES RECEPTOR KINASE1
- MAP
microtubule-associated protein
- MT
microtubule
- PRC
PROCUSTE
- TEM
transmission electron microscopy
- WAK1
WALL ASSOCIATED KINASE1
- WIS
wall integrity signaling
- XXT
XYLOGLUCAN XYLOSYLTRANSFERASE
- XyG
Xyloglucan
The primary cell walls of plants are mainly composed of cellulose, hemicelluloses and pectins.1 Xyloglucan (XyG) is the predominant hemicellulose, and is composed of a β-1,4-glucan backbone that is substituted with α-1,6-xylosyl residues, which may be further decorated with galactose and fucose.2,3 XyG is thought to interact with cellulose to form load-bearing networks, but the detailed interacting modes of these and other wall components are not entirely clear.1,4 Several Arabidopsis thaliana (Arabidopsis) XYLOGLUCAN XYLOSYLTRANSFERASEs (XXTs) have been identified and display enzymatic activity in vitro5,6 and in vivo.7 Arabidopsis double mutants lacking two of these, XXT1 and XXT2, lack detectable XyG in their walls, and display altered growth morphology including root hair defects, small leaves, short hypocotyls, and bent stems,7-9 indicating that XXT1 and XXT2 are required for XyG biosynthesis and normal plant growth.
Our recent study9 analyzed the effects of XyG deficiency on cellulose microfibril (CMF) patterning, cellulose biosynthesis by cellulose synthesis complexes (CSCs), and cortical microtubule (MT) dynamics, to investigate the links between wall synthesis, wall mechanical integrity, and cytoskeleton function. Several microscopic techniques were used to observe CMF patterns: atomic force microscopy (AFM), field emission scanning electron microscopy (FESEM), transmission electron microscopy (TEM), and confocal microscopy with Pontamine Fast Scarlet 4B (S4B) staining10-13 revealed that CMFs in xxt1 xxt2 walls are more highly aligned and bundled than in wild type controls. We also found that S4B-stained fibers in xxt1 xxt2 hypocotyl cells, which likely represent larger bundles of cellulose, have wider spacing than wild type, consistent with previous results in roots,10 supporting the hypothesis that XyG regulates cellulose spacing. Also, the loss of XyG resulted in reduced CESA particle density, CESA particle motility, and cellulose content in xxt1 xxt2 plants.9 Because MTs direct CMF deposition by guiding CSCs in the plasma membrane14 and can respond to mechanical force,15 the growth and mechanical defects observed in xxt1 xxt2 mutants7-9 motivated us to observe MT dynamics in this mutant. We labeled MTs by introducing the GFP-MAP4 MT marker16 into xxt1 xxt2 and wild type plants. MT patterning in xxt1 xxt2 cells differed from that in wild type cells, and MTs were more dynamic, with slower growth and faster shrinkage rates, and more sensitive to the MT-depolymerizing drug oryzalin and mechanical stress.9 Together, these data suggest that mechanical and/or signaling links between the cell wall and the MT cytoskeleton are active in xxt1 xxt2 plants.
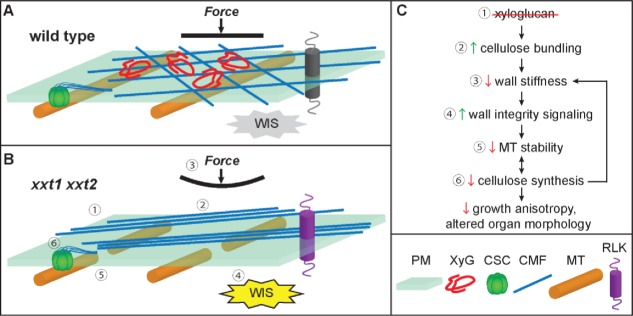
However, the precise causal relationships between XyG, cellulose, wall stiffness, MT stability, and the regulation of morphology remain unclear. Based on our work and previous studies of xxt1 xxt2 mutants, we propose a model to explain these relationships (Fig. 1). Compared with wild type, in xxt1 xxt2 mutant plants, the loss of XyG makes CMFs more prone to aggregate and form parallel bundles, and it is this CMF aggregation that results in mechanically weaker walls.8 Increased bundling could have this effect due to either reduction of recently-hypothesized biomechanical hotspots,4 or decreased exposure of cellulose surface area, which would cause weaker interactions between tethering matrix polysaccharides and CMFs.1,4 In addition, the unitary orientation of wall patterning in xxt1 xxt2 mutants might make their walls weaker. Distinct alterations in wall composition and ultrastructure have been implicated in determining different mechanical properties. As observed in xxt1 xxt2 mutants, mur3 mutants lacking a XyG galactosyltransferase have reduced wall tensile strength,17 implying that XyG galactosylation is required for wall integrity. An Arabidopsis cellulose-deficient mutant, cesa6procuste1-1 (prc1-1), displays decreased tensile stiffness.18 Additionally, the stems of mutants lacking PECTIN METHYLESTERASE35 show less mechanical strength and pendant phenotypes.19 Decreased mechanical strength might trigger wall integrity signaling (WIS) and changes in MT stability. In xxt1 xxt2 mutants, we found that the expression levels of receptors implicated in wall integrity signaling, such as FEI1, FEI2, FERONIA, HERCULES RECEPTOR KINASE1 (HERK1) and WALL ASSOCIATED KINASE1 (WAK1),20 are altered.9 Additionally, the expression of several microtubule-associated proteins (MAPs), including MAP20,21 MAP70-5,22 and CLASP23 have decreased gene expression in xxt1 xxt2 mutants,9 possibly contributing to the observed decrease in MT stability. However, we cannot exclude the effects of other MAPs on MT stability in xxt1 xxt2, since no stem bending phenotypes are observed in MAP70-5 RNAi plants or clasp-1 mutants.22,23 In several cases in plants, MT organization and stability have been linked to organ morphogenesis. Arabidopsis MAP65-1 and MAP65-2 regulate MT bundling, growth, and shrinkage and modulate cell growth and hypocotyl length.24 Arabidopsis spiral1 (spr1) mutants show right-handed helical growth and altered MT organization,25 and overexpression of MAP70-5 induces right-handed organ twisting.22 SPIRAL2 determines MT organization in leaf pavement cells and petiole cells by modulating MT severing.26
Figure 1.
A proposed model of how xyloglucan affects cellulose and cortical MTs to control plant cell expansion and organ morphology. Compared with wild type (A), in xxt1 xxt2 mutants (B and C), the loss of XyG (1) promotes CMF aggregation and bundling (2), reducing wall stiffness (3). Decreased wall stiffness triggers wall integrity signaling (WIS) (4), possibly triggering changes in the expression of MT-associated proteins, resulting in unstable MTs (5). Importantly, cortical MTs can respond changes in wall mechanics directly or through modulations in MT-associated protein abundance or activity. MTs guide CSCs biosynthesis at the plasma membrane, and reductions in MT stability result in reduced cellulose synthesis (6). Together, these changes reduce growth anisotropy and alter plant organ morphology. PM, plasma membrane; XyG, xyloglucan; CSC, Cellulose Synthesis Complex; CMF, cellulose microfibril; MT, microtubule; RLK, receptor-like kinase.
How do changes in MT organization and/or stability translate to changes in wall synthesis and organization? Cortical MTs guide CSCs in the plasma membrane to direct CMF deposition.27 However, drug treatments with the cellulose synthesis inhibitors isoxaben and 2,6-dichlorobenzonitrile alter MT organization,28,29 and cortical MT orientation is also altered in two cellulose synthesis-deficient mutants, CESA652-isx and kor1-3.14 Thus, cellulose synthesis can feedback on MT organization, and a bidirectional relationship may exist between MT dynamics and cellulose synthesis. We hypothesize that in xxt1 xxt2 mutants with less-stable MTs, CSC insertion in the plasma membrane and guidance by MTs is reduced, affecting CSC biosynthetic activity and ultimately resulting in less cellulose production, further reducing wall mechanical integrity. This feedback loop might ultimately inhibit cellular growth anisotropy.30,31
Several key unanswered questions remain: 1) How does the absence of XyG result in altered stiffness? Cellulose, XyG, and pectins can all affect tissue mechanical properties, based on mutant analyses.8,18,19 However, it is difficult to determine whether a single wall component or the combined action of multiple components controls stiffness. We found that in xxt1 xxt2 mutants, both XyG and cellulose are altered,9 suggesting that changes in multiple cell wall components contribute to changes in wall stiffness in this mutant. 2) How does reduced wall stiffness trigger wall integrity signaling and changes in MT behavior, and to what extent do cortical MTs respond directly to mechanical stress?15,32 3) What are the detailed functional mechanisms by which MTs guide CSCs? Multiple proteins, including CESA INTERACTIVE (CSI)33 and COMPANION OF CELLULOSE SYNTHASE (CC)34 proteins, are hypothesized to connect MTs and CSCs. The complexity of wall matrix polymers in composition and structure implies complex cross-linking between different cell wall components or polymer chains. Our model proposes links between cell wall biosynthesis, wall mechanical integrity, and the cytoskeleton in regulating organ morphogenesis and can serve as a basis for further investigation of these links.
Disclosure of potential conflicts of interest
No potential conflicts of interest were disclosed.
Acknowledgment
Thanks to Daniel McClosky for helpful comments.
Funding
This work was supported as part of The Center for Lignocellulose Structure and Formation, an Energy Frontier Research Center funded by the US. Department of Energy, Office of Science, Basic Energy Sciences (award no. DE– SC0001090).
References
- 1.Carpita NC, Gibeaut DM. Structural models of primary cell walls in flowering plants - consistency of molecular structure with the physical properties of the walls during growth. Plant J 1993; 3:1-30; PMID:8401598; http://dx.doi.org/ 10.1111/j.1365-313X.1993.tb00007.x [DOI] [PubMed] [Google Scholar]
- 2.Hoffman M, Jia Z, Pena MJ, Cash M, Harper A, Blackburn AR 2nd, Darvill A, York WS. Structural analysis of xyloglucans in the primary cell walls of plants in the subclass Asteridae. Carbohydr Rese 2005; 340:1826-40; PMID:15975566; http://dx.doi.org/ 10.1016/j.carres.2005.04.016 [DOI] [PubMed] [Google Scholar]
- 3.Pauly M, Gille S, Liu L, Mansoori N, de Souza A, Schultink A, Xiong G. Hemicellulose biosynthesis. Planta 2013; 238:627-42; PMID:23801299; http://dx.doi.org/ 10.1007/s00425-013-1921-1 [DOI] [PubMed] [Google Scholar]
- 4.Park YB, Cosgrove DJ. A revised architecture of primary cell walls based on biomechanical changes induced by substrate-specific endoglucanases. Plant Physiol 2012; 158:1933-43; PMID:22362871; http://dx.doi.org/ 10.1104/pp.111.192880 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Cavalier DM, Keegstra K. Two xyloglucan xylosyltransferases catalyze the addition of multiple xylosyl residues to cellohexaose. J Biol Chem 2006; 281:34197-207; PMID:16982611; http://dx.doi.org/ 10.1074/jbc.M606379200 [DOI] [PubMed] [Google Scholar]
- 6.Culbertson A, Chou YH, Smith AL, Young ZT, Tietze AA, Cottaz S, Faure R, Zabotina OA. Enzymatic activity of Arabidopsis xyloglucan xylosyltransferase 5. Plant Physiol 2016; 171:1893-904; PMID:27208276; In press [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Cavalier DM, Lerouxel O, Neumetzler L, Yamauchi K, Reinecke A, Freshour G, Zabotina OA, Hahn MG, Burgert I, Pauly M, et al.. Disrupting two Arabidopsis thaliana xylosyltransferase genes results in plants deficient in xyloglucan, a major primary cell wall component. Plant Cell 2008; 20:1519-37; PMID:18544630; http://dx.doi.org/ 10.1105/tpc.108.059873 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Park YB, Cosgrove DJ. Changes in cell wall biomechanical properties in the xyloglucan-deficient xxt1/xxt2 mutant of Arabidopsis. Plant Physiol 2012; 158:465-75; PMID:22108526; http://dx.doi.org/ 10.1104/pp.111.189779 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Xiao C, Zhang T, Zheng Y, Cosgrove DJ, Anderson CT. Xyloglucan deficiency disrupts microtubule stability and cellulose biosynthesis in Arabidopsis, altering cell growth and morphogenesis. Plant Physiol 2016; 170:234-49; PMID:26527657; http://dx.doi.org/ 10.1104/pp.15.01395 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Anderson CT, Carroll A, Akhmetova L, Somerville C. Real-time imaging of cellulose reorientation during cell wall expansion in Arabidopsis roots. Plant Physiol 2010; 152:787-96; PMID:19965966; http://dx.doi.org/ 10.1104/pp.109.150128 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Derbyshire P, McCann MC, Roberts K. Restricted cell elongation in Arabidopsis hypocotyls is associated with a reduced average pectin esterification level. BMC Plant Biol 2007; 7:1-12; PMID:17572910; http://dx.doi.org/18583534 10.1186/1471-2229-7-31 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Hodick D, Kutschera U. Light-induced inhibition of elongation growth in sunflower hypocotyls - biophysical and ultrastructural investigations. Protoplasma 1992; 168:7-13; http://dx.doi.org/ 10.1007/BF01332645 [DOI] [Google Scholar]
- 13.Zhang T, Mahgsoudy-Louyeh S, Tittmann B, Cosgrove DJ. Visualization of the nanoscale pattern of recently-deposited cellulose microfibrils and matrix materials in never-dried primary walls of the onion epidermis. Cellulose 2014; 21:853-62; http://dx.doi.org/ 10.1007/s10570-013-9996-1 [DOI] [Google Scholar]
- 14.Paredez AR, Persson S, Ehrhardt DW, Somerville CR. Genetic evidence that cellulose synthase activity influences microtubule cortical array organization. Plant Physiol 2008; 147:1723-34; PMID:18583534; http://dx.doi.org/ 10.1104/pp.108.120196 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Hamant O, Heisler MG, Jonsson H, Krupinski P, Uyttewaal M, Bokov P, Corson F, Sahlin P, Boudaoud A, Meyerowitz EM, et al.. Developmental patterning by mechanical signals in Arabidopsis. Science 2008; 322:1650-5; PMID:19074340; http://dx.doi.org/ 10.1126/science.1165594 [DOI] [PubMed] [Google Scholar]
- 16.Marc J, Granger CL, Brincat J, Fisher DD, Kao TH, McCubbin AG, Cyr RJ. A GFP-MAP4 reporter gene for visualizing cortical microtubule rearrangements in living epidermal cells. Plant Cell 1998; 10:1927-39; PMID:9811799; http://dx.doi.org/14730072 10.1105/tpc.10.11.1927 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Pena MJ, Ryden P, Madson M, Smith AC, Carpita NC. The galactose residues of xyloglucan are essential to maintain mechanical strength of the primary cell walls in Arabidopsis during growth. Plant Physiol 2004; 134:443-51; PMID:14730072; http://dx.doi.org/ 10.1104/pp.103.027508 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Saxe F. Significance of cell-wall ultrastructure for growth and mechanical properties of Arabidopsis thaliana. Max Planck Institute 2012 [Google Scholar]
- 19.Hongo S, Sato K, Yokoyama R, Nishitani K. Demethylesterification of the primary wall by PECTIN METHYLESTERASE35 provides mechanical support to the Arabidopsis stem. Plant Cell 2012; 24:2624-34; PMID:22693281; http://dx.doi.org/ 10.1105/tpc.112.099325 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Wolf S, Hematy K, Hofte H. Growth Control and Cell Wall Signaling in Plants. Annual Review of Plant Biology 2012; 63(63):381-407; PMID:22224451; http://dx.doi.org/ 10.1146/annurev-arplant-042811-105449 [DOI] [PubMed] [Google Scholar]
- 21.Rajangam AS, Kumar M, Aspeborg H, Guerriero G, Arvestad L, Pansri P, Brown CJ, Hober S, Blomqvist K, Divne C, et al.. MAP20, a microtubule-associated protein in the secondary cell walls of hybrid aspen, is a target of the cellulose synthesis inhibitor 2,6-dichlorobenzonitrile. Plant Physiol 2008; 148:1283-94; PMID:18805954; http://dx.doi.org/ 10.1104/pp.108.121913 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Korolev AV, Buschmann H, Doonan JH, Lloyd CW. AtMAP70-5, a divergent member of the MAP70 family of microtubule-associated proteins, is required for anisotropic cell growth in Arabidopsis. J Cell Sci 2007; 120:2241-7; PMID:17567681; http://dx.doi.org/ 10.1242/jcs.007393 [DOI] [PubMed] [Google Scholar]
- 23.Ambrose JC, Shoji T, Kotzer AM, Pighin JA, Wasteneys GO. The Arabidopsis CLASP gene encodes a microtubule-associated protein involved in cell expansion and division. Plant Cell 2007; 19:2763-75; PMID:17873093; http://dx.doi.org/ 10.1105/tpc.107.053777 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Lucas JR, Courtney S, Hassfurder M, Dhingra S, Bryant A, Shaw SL. Microtubule-associated proteins MAP65-1 and MAP65-2 positively regulate axial cell growth in etiolated Arabidopsis hypocotyls. Plant Cell 2011; 23:1889-903; PMID:21551389; http://dx.doi.org/ 10.1105/tpc.111.084970 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Nakajima K, Kawamura T, Hashimoto T. Role of the SPIRAL1 gene family in anisotropic growth of Arabidopsis thaliana. Plant Cell Physiol 2006; 47:513-22; PMID:16478750; http://dx.doi.org/ 10.1093/pcp/pcj020 [DOI] [PubMed] [Google Scholar]
- 26.Wightman R, Chomicki G, Kumar M, Carr P, Turner SR. SPIRAL2 Determines plant microtubule organization by modulating microtubule severing. Curr Biol 2013; 23:1902-7; PMID:24055158; http://dx.doi.org/ 10.1016/j.cub.2013.07.061 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Paredez AR, Somerville CR, Ehrhardt DW. Visualization of cellulose synthase demonstrates functional association with microtubules. Science 2006; 312:1491-5; PMID:16627697; http://dx.doi.org/ 10.1126/science.1126551 [DOI] [PubMed] [Google Scholar]
- 28.Fisher DD, Cyr RJ. Extending the microtubule/microfibril paradigm. Cellulose synthesis is required for normal cortical microtubule alignment in elongating cells. Plant Physiol 1998; 116:1043-51; PMID:9501137; http://dx.doi.org/ 10.1104/pp.116.3.1043 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Himmelspach R, Williamson RE, Wasteneys GO. Cellulose microfibril alignment recovers from DCB-induced disruption despite microtubule disorganization. Plant J 2003; 36:565-75; PMID:14617086; http://dx.doi.org/ 10.1046/j.1365-313X.2003.01906.x [DOI] [PubMed] [Google Scholar]
- 30.Peaucelle A, Wightman R, Hofte H. The control of growth symmetry breaking in the Arabidopsis hypocotyl. Curr Biol 2015; 25:1746-52; PMID:26073136; http://dx.doi.org/ 10.1016/j.cub.2015.05.022 [DOI] [PubMed] [Google Scholar]
- 31.Slabaugh E, Scavuzzo-Duggan T, Chaves A, Wilson L, Wilson C, Davis JK, Cosgrove DJ, Anderson CT, Roberts AW, Haigler CH. The valine and lysine residues in the conserved FxVTxK motif are important for the function of phylogenetically distant plant cellulose synthases. Glycobiology 2016; 26:509-19; PMID:26646446; http://dx.doi.org/ 10.1093/glycob/cwv118 [DOI] [PubMed] [Google Scholar]
- 32.Sampathkumar A, Krupinski P, Wightman R, Milani P, Berquand A, Boudaoud A, Hamant O, Jonsson H, Meyerowitz EM. Subcellular and supracellular mechanical stress prescribes cytoskeleton behavior in Arabidopsis cotyledon pavement cells. eLife 2014; 3:e01967; PMID:24740969; http://dx.doi.org/ 10.7554/eLife.01967 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Li SD, Lei L, Somerville CR, Gu Y. Cellulose synthase interactive protein 1 (CSI1) links microtubules and cellulose synthase complexes. Proc Natl Acad Sci USA 2012; 109:185-90; PMID:22190487; http://dx.doi.org/ 10.1073/pnas.1118560109 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Endler A, Kesten C, Schneider R, Zhang Y, Ivakov A, Froehlich A, Funke N, Persson S. A mechanism for sustained cellulose synthesis during salt stress. Cell 2015; 162:1353-64; PMID:26343580; http://dx.doi.org/ 10.1016/j.cell.2015.08.028 [DOI] [PubMed] [Google Scholar]