ABSTRACT
Land plants face the perpetual dilemma of using atmospheric carbon dioxide for photosynthesis and losing water vapors, or saving water and reducing photosynthesis and thus growth. The reason behind this dilemma is that this simultaneous exchange of gases is accomplished through the same minute pores on leaf surfaces, called stomata. In a recent study we provided evidence that pigweed, an aggressive weed, attenuates this problem exploiting large crystals of calcium oxalate as dynamic carbon pools. This plant is able to photosynthesize even under drought conditions, when stomata are closed and water losses are limited, using carbon dioxide from crystal decomposition instead from the atmosphere. Abscisic acid, an alarm signal that causes stomatal closure seems to be implicated in this function and for this reason we named this path “alarm photosynthesis.” The so-far “enigmatic,” but highly conserved and widespread among plant species calcium oxalate crystals seem to play a crucial role in the survival of plants.
KEYWORDS: Abscisic acid, alarm photosynthesis, calcium oxalate, drought, stomata
The role of calcium oxalate crystals (Fig. 1) in plants puzzled plant scientists for ages. Even though crystals are widespread among plant species, often reaching up to 80% of dry biomass, our knowledge on the formation and the possible roles of these crystals in plant function remained limited. It was hypothesized that crystals are formed for (a) the regulation or sequestration of calcium ions, (b) ion balance, (c) the detoxification from oxalate and/or heavy metals, (d) light reflection and (e) plant protection against herbivores.1,2,3,4 Most of these hypotheses focused on the inorganic part of the crystals (calcium), whereas the organic part (oxalate) was mostly considered as a counter ion required for calcium binding.
Figure 1.
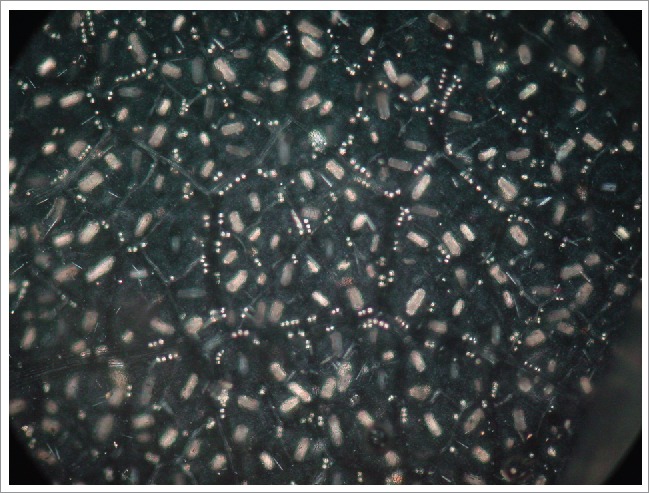
A chlorine-bleached mature grapevine leaf under polarized light field. Abundant calcium oxalate crystals in the form of raphides in the mesophyll and drusses in the bundle sheaths are visible as bright spots.
A different approach focusing on the possible exploitation of the organic part of the crystals, led us to the formulation of a novel hypothesis concerning their functional role. Our working hypothesis was developed on the basis that oxalate represents a rich source of carboxy groups that could be converted to CO2 by the enzyme oxalate oxidase when the entry of atmospheric CO2 is limited, e.g. under drought conditions. Indeed, according to the results of our recent study, we provided evidence that calcium oxalate crystals represent dynamic carbon pools, supplying CO2 to photosynthetic cells when stomata are partially or totally closed. This biochemical appendance of the photosynthetic machinery is a means to alleviate the perpetual plant dilemma of using atmospheric carbon dioxide for photosynthesis and losing water vapors, or saving water and reducing photosynthesis. Given that under drought conditions carbon acquisition from the atmosphere can become very expensive as well as hazardous to survival in terms of water loss, the ability to utilize an alternative internal carbon pool that allows photosynthetic function even when stomatal conductance is diminished i.e. saving water, can become crucial for survival under adverse conditions.
Our first goal was to examine calcium oxalate crystals as a dynamic system. Do the dynamics of this system change and how? What is the origin of the organic part of the crystals? Our study showed that partial stomatal closure during the day is accompanied by a gradual decrease in crystal volume, which is recovered during the night, confirming that calcium oxalate crystals are indeed a dynamic system. In addition, the stable carbon isotope composition of isolated crystals revealed that they are not of atmospheric origin and the metabolic profiling showed the presence of oxalate in the xylem sap. The above results indicated that the organic part of the crystals (oxalate) may derive from leaf dark respiration or from the roots.
Our next step was to examine the relationship between stomatal closure and the observed decrease in crystal volume. Therefore, we exogenously applied abscisic acid (ABA) on cut leaves. Interestingly, we saw that in ABA-treated leaves, opposite to the control ones, stomata remained closed throughout a 6 h period and a gradual reduction of the crystal volume was recorded. The degradation of crystals was not accompanied by the release of considerable amounts of oxalate in ABA-treated leaves, suggesting its rapid breakdown into CO2 and H2O2 by the enzyme oxalate oxidase.5 Indeed, through a histochemical detection of this enzyme we observed that it was mainly localized in the spongy mesophyll cells and its activity was increased due to the ABA treatment. In addition, the histochemical detection of catalase, the enzyme that cleaves H2O2 to H2O and O2, showed strong staining in both the control and the ABA-treated leaves. These observations further supported our hypothesis that crystal degradation is correlated with oxalate oxidase and catalase activities suggesting that the produced CO2 could be assimilated in Calvin cycle reactions while the reactive H2O2 could be scavenged by catalase. In such case, the photosynthetic metabolism in the ABA-treated leaves would remain active, in spite of their closed stomata and the accompanied CO2 starvation. The metabolomic analysis showed that despite stomatal closure, the ABA-treated leaves did not exhibit any substantial metabolic decline compared to the control leaves. Additionally, chlorophyll fluorescence measurements showed that the operational efficiency of the photosystem II photochemistry (ΔF/Fm´) in the ABA-treated leaves remained substantial, despite the CO2 starvation conditions.
In order to consolidate this relationship between stomatal closure and the calcium oxalate crystal degradation, we conducted a drought stress experiment. We selected 4 plant species possessing calcium oxalate crystals and representing different functional groups (Dianthus chinensis and Pelargonium peltatum, C3 plants, Amaranthus hybridus, C4 plant and Portulacaria afra, succulent, probably CAM) and exposed them to drought under controlled conditions. As expected, water deficit caused a reduction in leaf water potential and a decrease in stomatal conductance. This decrease was accompanied by a significant reduction in the crystal volume and an increase of oxalate oxidase activity in all 4 plant species (in D. chinensis the activity of the enzyme was very high irrespectively of the treatment), confirming our previous observations.
Based on the above observations, we propose that alarm photosynthesis acts as a diurnally 2-phase biochemical appendance by: (a) collecting and saving carbon in the form of calcium oxalate crystals during the night and (b) providing subsidiary CO2 for photosynthetic assimilation during the day through the degradation of crystals and the subsequent breakdown of oxalate by oxalate oxidase, especially under water deficit conditions (Fig. 2). The function of alarm photosynthesis seems to be rather ancillary to the overall photosynthetic performance by supporting a low photosynthetic rate aiming to the maintenance and survival and not to growth. According to our estimations in pigweed, the amount of CO2 that can be released from the complete crystal decomposition could support a low photosynthetic rate of 3 μmol CO2 m−2 s−1 for 4–5 hrs. This biochemical appendance seems to provide a number of adaptive advantages that can justify its vast presence in the plant kingdom:
Figure 2.
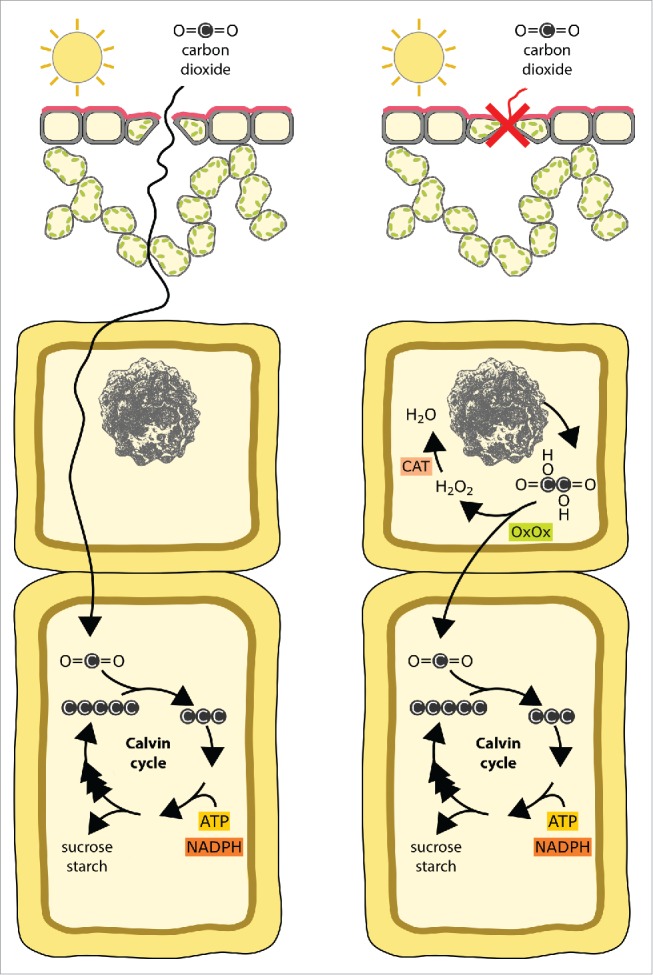
A simplified scheme of the biochemical reactions involved in alarm photosynthesis. Under water adequacy conditions (left), atmospheric CO2 entering from stomatal pores is fixed by RubiSCO or PEPcase (in C4 and CAM plants, not shown for simplicity) and converted to photosynthetic products through the Calvin cycle. Water deficit (right) causes stomatal closure, creating carbon starvation within mesophyll. Under these conditions oxalate released from calcium oxalate crystals is converted to CO2 by oxalate oxidase and the produced CO2 can support the Calvin cycle reactions. The reactive H2O2, the byproduct of oxalate oxidase reaction, can be neutralized by catalase. CAT: Catalase; OxOx: Oxalate oxidase.
Water economy and drought resistance: Alarm photosynthesis allows the utilization of large calcium oxalate crystals as dynamic internal carbon pools, irrespectively of the availability of the atmospheric CO2 and thus preventing water losses. Perhaps this could explain why large quantities of calcium oxalate crystals are present in xerophytes such as cacti,6 as well as why at the intraspecific level the number of crystals in the leaves increases with aridity.7
A lower risk of photoinhibition: Alarm photosynthesis could act as a quenching valve for the energy excess accumulated in the electron transport chain, when the light reactions are not in pace with the photosynthetic CO2 assimilation from the atmosphere under drought conditions.
Metabolic advantages: CaOx crystals represent carbon deposits which are metabolically and osmotically inactive. Only if needed, CO2 and calcium ions can be released.
Alarm photosynthesis represents an unknown photosynthetic variation to be added to the already known C4 and CAM pathways. However, alarm photosynthesis, in contrast to the above pathways, operates as a biochemical pump that collects carbon from the organ interior (or from the soil) and not from the atmosphere.
Apart from the importance of the discovery of alarm photosynthesis as a novel possible explanation justifying the extensive occurrence of CaOx crystals in the Plant Kingdom, the potential adaptive advantages of alarm photosynthesis may also be of great signigicance if we are to understand more about drought tolerance. As climate change scenarios predict intensified drought conditions in many parts of the world and concomitant yield reduction,8 the revelation of such a function could have a great contribution to the comprehension of drought resistance mechanisms of wild and cultivated plants, including crops9 (spinach, grapevine, etc, see Fig. 1) and weeds (pigweed, Chenopodium, Conyza, Oxalis, Portulaca etc). The calcium oxalate carbon cycling mechanism could also be further explored in efforts aiming to the development of drought-resistant plant cultivars and/or weed control.
Disclosure of potential conflicts of interest.
No potential conflicts of interest were disclosed.
References
- 1.Nakata PA. Plant calcium oxalate crystal formation, function, and its impact on human health. Front Biol 2012; 7:254-66; http://dx.doi.org/ 10.1007/s11515-012-1224-0 [DOI] [Google Scholar]
- 2.Webb MA. Cell-mediated crystallization of calcium oxalate in plants. Plant Cell 1999; 11:751-61; PMID:10213791; http://dx.doi.org/ 10.1105/tpc.11.4.751 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Franceschi VR, Loewus F. Oxalate biosynthesis and function in plants and fungi In Oxalate in Biological Systems, Boca Raton: CRC Press; Khan SR. (ed), 1995. [Google Scholar]
- 4.He H, Veneklaas EJ, Kuo J, Lambers H. Physiological and ecological significance of biomineralization in plants. Trends Plant Sci 2013; 19:166-74; PMID:24291440; http://dx.doi.org/ 10.1016/j.tplants.2013.11.002 [DOI] [PubMed] [Google Scholar]
- 5.Lane BG. Oxalate, germin and the extracellular matrix of higher plants. FASEB J 1994; 8:294-301; PMID: 143935 [DOI] [PubMed] [Google Scholar]
- 6.Garvie LAJ. Decay of cacti and carbon cycling. Naturwissenschaften 2006; 93:114-18; PMID:16453105; http://dx.doi.org/ 10.1007/s00114-005-0069-7 [DOI] [PubMed] [Google Scholar]
- 7.Brown SL, Warwick NWM, Prychid CJ. Does aridity influence the morphology, distribution and accumulation of calcium oxalate crystals in Acacia (Leguminosae: Mimosoideae)? Plant Physiol Biochem 2013; 73:219-28; PMID:24157700; http://dx.doi.org/ 10.1016/j.plaphy.2013.10.006 [DOI] [PubMed] [Google Scholar]
- 8.Long SP, Ort DR. More than taking the heat: crops and global change. Curr Opin Plant Biol 2010; 13:241-48; PMID:20494611; http://dx.doi.org/ 10.1016/j.pbi.2010.04.008 [DOI] [PubMed] [Google Scholar]
- 9.Libert B, Franceschi VR. Oxalate in crop plants. J Agr Food Chem 1987; 35:926-38; http://dx.doi.org/ 10.1021/jf00078a019 [DOI] [Google Scholar]


