ABSTRACT
In order to provide more insight into the function of aquaporins during pollination, we characterized NIP4;1 and NIP4;2, 2 pollen-specific aquaporins of Arabidopsis thaliana. NIP4;1 and NIP4;2 displayed high amino acid identity. RT-PCR and GUS promoter analysis showed that they have different expression patterns. NIP4;1 is expressed at low levels in mature pollen, while NIP4;2 is highly expressed only during pollen tube growth. Single T-DNA nip4;1 and nip4;2 mutants and double amiRNA nip4;1 nip4;2 knockdowns showed reduced male fertility due to deficient pollen germination and pollen tube length. Functional assays in oocytes showed that NIP4;1 and NIP4;2 transport water and nonionic solutes. Here, the participation of the different pollen aquaporins in pollen hydration and pollen tube growth is discussed.
KEYWORDS: Aquaporin, pollen, pollen hydration, pollination
A successful fertilization in plants starts when the pollen grain contacts the surface of the stigma and hydrates. After hydration, the internal hydrostatic pressure of the pollen grain increases and a pollen tube is developed. Then, the pollen tube grows through the papilla cell wall, penetrates the transmitting tract of the style, elongates and reaches the embryo sac where double fertilization takes place.
In dry stigmas, pollen hydration is a highly regulated process and takes place only if a compatible communication between the pollen and the stigma cells is established. It is the checkpoint where self-incompatible and incongruous pollen grains are not hydrated and finally rejected due to the lack of a compatible recognition. This specificity is based on controlling the movement of water and solutes from stigma to the pollen grains. Later, during pollen tube growth, water and osmotic solutes have to move very fast into the pollen tube to maintain the required turgor pressure.1 Under these scenarios, aquaporins become good candidates in regulating the flow of water and osmotic solutes across the pollen membranes.2-4
We decide to study how Arabidopsis thaliana pollen specific aquaporins would contribute to transport water and/or solutes during pollination and pollen tube growth. We first characterized 2 Arabidopsis pollen aquaporins, TIP1;3 and TIP5;1, which belong to the tonoplast intrinsic proteins (TIPs) subclass. Both TIPs transported not only water but also urea at high permeability levels.5 Single tip1;3 and tip5;1 mutant pollen showed normal growth rate6 while double mutant tip1;3 tip5;1 showed high numbers of sterile pods, especially under water- or nutrient-deficient conditions.7
We then study the other 2 remaining pollen specific aquaporin genes, NIP4;1 and NIP4;2.8 They both belong to the nodulin26-like intrinsic proteins (NIPs) subclass. Our molecular genetic and functional analysis demonstrated that NIP4;1 and NIP4;2 are important players in pollen development and pollination. NIP4;1 and NIP4;2 are paralog genes found exclusively in Angiosperms. Even though they have high amino acid identity (84%), they showed different expression patterns. RT-PCR and GUS promoter analysis showed that while NIP4;1 is expressed at low levels only in mature pollen, NIP4;2 is highly expressed exclusively during pollen tube growth.
We found that pollen from single T-DNA nip4;1 and nip4;2 mutants and double amiRNA nip4;1 nip4;2 knockdowns had reduced fertility and lower pollen germination and pollen tube length compared to wild type pollen. When we analyzed the kinetics of in vitro pollen germination, we found that wild type pollen germinated faster than T-DNA nip4;1 and nip4;2 mutant pollen grains (Fig. 1). This result is in agreement with the defects found in pollination for the NIP mutants. Coincidently to their different expression pattern, we showed that NIP4;1 functions during pollen development and germination, while NIP4;2 does it exclusively during pollen tube growth.
Figure 1.
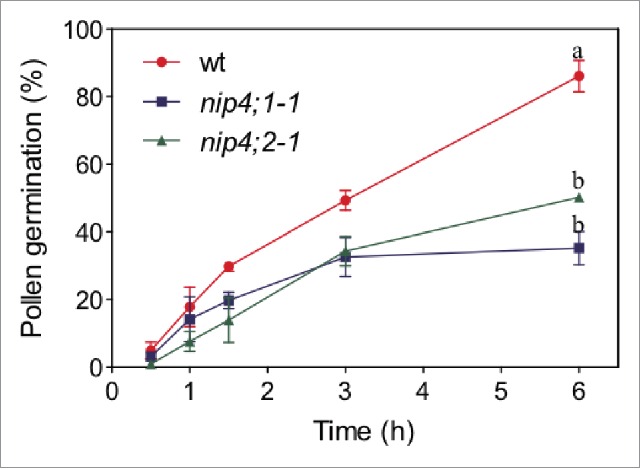
In vitro pollen germination of wild type (wt) and single mutants nip4;1-1 and nip4;1-2 plants. Assays were performed in 3 replicates and 100 pollen grains were measured for each replicate. Different letters indicate statistically significant differences (P < 0.05, 2-way ANOVA, Tukey's test).
Related to their water and solutes transport activity, while both NIP4;1 and NIP4;2 have moderate water transport, they both showed high osmotic solute (glycerol) permeability when expressed in Xenopus oocytes. Survival analysis in yeast confirmed water transport Xenopus results and also showed that NIP4;1 has the capacity of transporting ammonia, urea, boric acid and H2O2. Using mass spectrometry, we also found that a specific serine (Ser267) of the C-termini of NIP4;1 and NIP4;2 was phosphorylated in vitro by CPK34, a pollen-specific calcium-dependent protein kinase essential for pollen tube growth.9 Mutants where Ser267 was replaced by an alanine showed lower levels of water permeability when compared to their wild type versions, suggesting a potential role for S267-phosphorylation in the regulation of the water transport of NIP4;1 and NIP4;2. This result agrees with previous studies in which phosphorylation of soybean NOD26 at Ser-262 enhanced water transport and appeared with the establishment of the symbiosomes involved in nitrogen fixation.10
The main question is why Arabidopsis expresses 4 specific aquaporin genes during pollen development and pollen tube growth, but none of them seems to be totally essential for pollen life. Both combination of double mutants, tip1;3 tip5;1 and nip4;1 nip4;2, did not show a fully sterile male phenotype. Gene redundancy is always a good explanation for that, but in this case it seems to be unlikely because TIP1;3, TIP5;1, NIP4;1 and NIP4;2 belong to 2 different subclasses (2 TIPs and 2 NIPs) that are also differentially expressed: while TIP1;3, TIP5;1 and NIP4;1 are expressed in mature pollen, NIP4;2 is exclusively expressed after pollen germination. Lastly, according to our Xenopus results, they transport different solutes.
Despite the fact that the double amiRNA nip4;1 nip4;2 knockdown showed affected pollen fitness, the lack of a completely unfertile phenotype could be explained by the presence of other pollen -not specific- aquaporins that indeed would control the flux of water. SIP1;1 and SIP2;1 are good candidates because they are expressed in pollen although also in most of the vegetative tissues.11 SIP1;1, but not SIP2;1, showed water transport activity in yeast membrane vesicles by stopped-flow light scattering assays.12 However, the fact that SIP1;1 and SIP2;1 are both expressed in the ER of stems of Arabidopsis plants, makes it difficult to conceive how they would transport water from the female tissue into the pollen.13
Perhaps the simplest hypothesis is that these 4 pollen specific aquaporins transport different solutes propelling the flux of water from female tissues thus increasing the water potential of pollen grains and pollen tubes turgor pressure, necessary for pollen hydration and pollen tube tip growth, respectively.1,14 In this direction, it has been suggested that plasma membrane aquaporins could act as osmosensors controlling turgor pressure of pollen tubes while they grow toward the ovary.14
Disclosure of potential conflicts of interest
No potential conflicts of interest were disclosed.
References
- 1.Firon N, Nepi M, Pacini E. Water status and associated processes mark critical stages in pollen development and functioning. Ann Bot 2012; 109:1201-14; PMID:22523424; http://dx.doi.org/ 10.1093/aob/mcs070 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Abascal F, Irisarri I, Zardoya R. Diversity and evolution of membrane intrinsic proteins. Biochim Biophys Acta 2014; 1840:1468-81; PMID:24355433; http://dx.doi.org/ 10.1016/j.bbagen.2013.12.001 [DOI] [PubMed] [Google Scholar]
- 3.Pérez Di Giorgio J, Soto G, Alleva K, Jozefkowicz C, Amodeo G, Muschietti JP, Ayub ND. Prediction of aquaporin function by integrating evolutionary and functional analyses. J Membr Biol 2014; 247:107-25; PMID:24292667; http://dx.doi.org/ 10.1007/s00232-013-9618-8 [DOI] [PubMed] [Google Scholar]
- 4.Soto G, Alleva K, Amodeo G, Muschietti J, Ayub ND. New insight into the evolution of aquaporins from flowering plants and vertebrates: orthologous identification and functional transfer is possible. Gene 2012; 503:165-76; PMID:22561693; http://dx.doi.org/ 10.1016/j.gene.2012.04.021 [DOI] [PubMed] [Google Scholar]
- 5.Soto G, Alleva K, Mazzella MA, Amodeo G, Muschietti JP. AtTIP1;3 and AtTIP5;1, the only highly expressed Arabidopsis pollen-specific aquaporins, transport water and urea. FEBS Lett 2008; 582:4077-82; PMID:19022253; http://dx.doi.org/ 10.1016/j.febslet.2008.11.002 [DOI] [PubMed] [Google Scholar]
- 6.Soto G, Fox R, Ayub N, Alleva K, Guaimas F, Erijman EJ, Mazzella A, Amodeo G, Muschietti J. TIP5;1 is an aquaporin specifically targeted to pollen mitochondria and is probably involved in nitrogen remobilization in Arabidopsis thaliana. Plant J 2010; 64:1038-47; PMID:21143683; http://dx.doi.org/ 10.1111/j.1365-313X.2010.04395.x [DOI] [PubMed] [Google Scholar]
- 7.Wudick MM, Luu DT, Tournaire-Roux C, Sakamoto W, Maurel C. Vegetative and sperm cell-specific aquaporins of Arabidopsis highlight the vacuolar equipment of pollen and contribute to plant reproduction. Plant Physiol 2014; 164:1697-706; PMID:24492334; http://dx.doi.org/ 10.1104/pp.113.228700 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Pérez Di Giorgio J, Bienert GP, Ayub N, Yaneff A, Barberini ML, Mecchia MA, Amodeo G, Soto GC, Muschietti JP. Pollen-specific aquaporins NIP4;1 and NIP4;2 are required for pollen development and pollination in Arabidopsis thaliana. Plant Cell 2016; 28:1053-77; PMID:27095837; http://dx.doi.org/ 10.1105/tpc.15.00776 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Myers C, Romanowsky SM, Barron YD, Garg S, Azuse CL, Curran A, Davis RM, Hatton J, Harmon AC, Harper JF. Calcium-dependent protein kinases regulate polarized tip growth in pollen tubes. Plant J 2009; August; 59(4):528-39; PMID:19392698; http://dx.doi.org/ 10.1111/j.1365-313X.2009.03894.x [DOI] [PubMed] [Google Scholar]
- 10.Guenther JF, Chanmanivone N, Galetovic MP, Wallace IS, Cobb JA, Roberts DM. Phosphorylation of soybean nodulin 26 on serine 262 enhances water permeability and is regulated developmentally and by osmotic signals. Plant Cell 2003; April; 15(4):981-91; PMID:12671092; http://dx.doi.org/ 10.1105/tpc.009787 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Honys D, Twell D. Transcriptome analysis of haploid male gametophyte development in Arabidopsis. Genome Biol 2004; 5:R85; PMID:15535861; http://dx.doi.org/ 10.1186/gb-2004-5-11-r85 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Ishikawa F, Suga S, Uemura T, Sato MH, Maeshima M. Novel type aquaporin SIPs are mainly localized to the ER membrane and show cell-specific expression in Arabidopsis thaliana. FEBS Lett 2005; 579:5814-20; PMID:16223486; http://dx.doi.org/ 10.1016/j.febslet.2005.09.076 [DOI] [PubMed] [Google Scholar]
- 13.Maeshima M, Ishikawa F. ER membrane aquaporins in plants. Pflugers Arch 2008; 456:709-16; PMID:17924135; http://dx.doi.org/ 10.1007/s00424-007-0363-7 [DOI] [PubMed] [Google Scholar]
- 14.Hill AE, Shachar-Hill B, Skepper JN, Powell J, Shachar-Hill Y. An osmotic model of the growing pollen tube. PLoS One 2012; 7:e36585; PMID:22615784; http://dx.doi.org/ 10.1371/journal.pone.0036585 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Shachar-Hill B, Hill AE, Powell J, Skepper JN, Shachar-Hill Y. Mercury-sensitive water channels as possible sensors of water potentials in pollen. Journal of Exp Botany 2013; 64:5195-205; PMID:24098048; http://dx.doi.org/ 10.1093/jxb/ert311 [DOI] [PMC free article] [PubMed] [Google Scholar]


