ABSTRACT
The delayed flowering phenotype caused by nitrogen (N) fertilizer application has been known for a long time, but we know little about the specific molecular mechanism for this phenomenon before. Our study indicated that low nitrogen increases the NADPH/NADP+ and ATP/AMP ratios which affect adenosine monophosphate-activated protein kinase (AMPK) activity and phosphorylation and abundance of nuclear CRY1 protein. Then CRY1 acts in the N signal input pathway to the circadian clock. Here we further discuss: (1) the role of C/N ratio in flowering, (2) circadian oscillation of plant AMPK transcripts and proteins, (3) conservation of nutrition-mediated CRY1 phosphorylation and degradation, and (4) crosstalks between nitrogen signals and nitric oxide (NO) signals in flowering.
KEYWORDS: Adenosine monophosphate-activated protein kinase, circadian clock, cryptochrome 1, nitrate reductase, nitrogen-regulated flowering
Cryptochrome 1 (CRY1) is a blue light receptor which works upstream of the photoperiod pathway and controls plants flowering through interaction with other light signaling components. CRY1 regulates the expression of flowering-related genes CONSTANS (CO) and FLOWERING LOCUS T (FT).1-2 Here in our study, CRY1 has been identified as the pivotal gene in the nitrogen (N)-regulated flowering pathway.3 N mediates the central oscillator through the CRY1 input pathway.3 Carbohydrates (C) generate another nutrition signal, which also regulates flowering. A recent report found that sugars repress the expression of the morning-expressed gene PSEUDORESPONSE REGULATOR 7 (PRR7) and activate the key component the central oscillator CCA1.4 Similar to the high N condition, nitric oxide (NO) also causes the inhibition of floral transition through repressing circadian-clock output genes CO and GIGANTEA (GI).5 In summary, N, C and NO may influence the circadian clock through the input pathway, the central oscillator pathway and the output pathway, respectively (Fig. 1). Furthermore, circadian-clock output signals in turn give a feedback to the input pathway.
Figure 1.
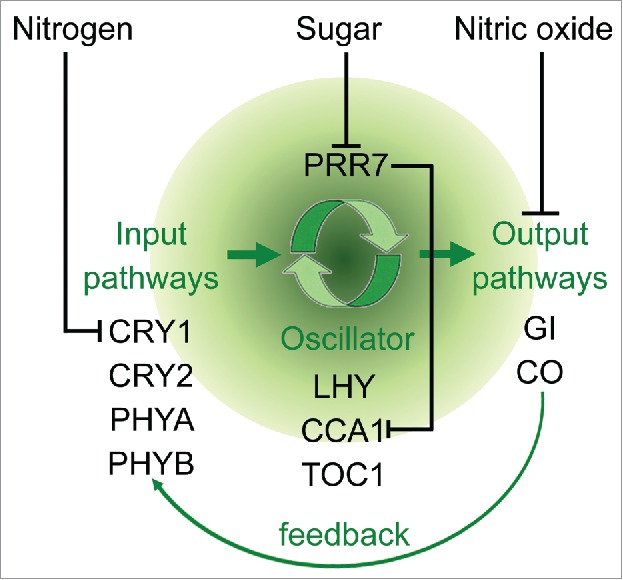
Diagram of the effects of N, sugar, NO on circadian clock. The input pathway may also give a feedback to the input pathway.
C/N ratio may also affect flowering
C and N are indispensable elements among various nutrients and they are affected by many environmental cues such as abiotic and biotic stresses, atmospheric CO2, circadian rhythm and so on.6-10 The change of N must be accompanied by a change of C/N ratio. Therefore, our previous study did not rule out the side-effect of altered C/N ratios. However, both the low C condition (MS media without sucrose) and the high C condition (MS media with 5% sucrose) show late-flowering phenotypes (our unpublished data). Thus, C and N may regulate flowering through different pathways. Given that nitrogen levels regulate ferredoxin-NADP+-oxidoreductase and ATP synthesis rate (energy metabolism flow),3,11 nitrogen metabolism and carbon metabolism may be tightly linked with each other. The crosstalk between C metabolite signals and N metabolite signals needs further investigations.
The role of AMPK in plant cells
AMPK is the key molecular in biological energy metabolism. AMPK is a heterotrimeric protein kinase consisting of a catalytic (α) subunit and 2 regulatory (β, γ) subunits.12 Under conditions of hypoxia, exercise, ischemia, heat shock, and low glucose, AMPK is activated allosterically by rising cellular AMP and by phosphorylation of the catalytic α subunit.12 In mammalian cells, AMPK plays a broader role in regulating whole-body energy metabolism and glucose homeostasis through the regulation of processes like muscle glucose uptake, insulin production and secretion, management of body lipids, and appetite.13 But its functions in plants were rarely reported. The SnRK family in Arabidopsis is the homologous to mammalian AMPK, which comprised of 3 distinct subfamilies.14 Arabidopsis AMPKα1 shows inhibitory responses to sugar metabolites, especially the trehalose-6-phosphate (T6P).15 But no clear correction between plant SnRK family proteins and sugar signaling or other nutritional signaling in plant cells has been identified. Here we find that the high level of AMP under the high N condition activates Arabidopsis AMPKα1.3 Similar to mammal AMPKs, the AMPKα1 protein in plant cell nuclei also shows a robust rhythm.3 Furthermore, nuclear activity of AMPKα1 was also higher in the day than at night. In mammal cells, circadian expression of AMPKβ2 transcript has been observed.16 Because the subunit composition of AMPK complexes regulates its localization, oscillating AMPKβ2 could diurnally regulate the nuclear import of AMPK.16 Circadian expression of AMPKβ2 mRNA and the diurnal AMPK import should be further verified in plant cells.
Conservation of AMPK-CRY1 pathway in nutritional signaling
Previous studies in mammalian cells have demonstrated that AMPK regulates CRY1 stability. Active nuclear AMPK phosphorylates cryptochromes, thus increasing their interaction with F-box and leucine-rich repeat protein 3 (FBXL3) and leading to ubiquitin-dependent proteasomal degradation.16,17 However in Arabidopsis cells, blue-light-induced CRY1 phosphorylation is not accompanied by a decrease in its protein steady-state-level.18 Indeed, Arabidopsis CRY1 is a light-stable protein; while CRY2 is a light-labile protein.18,19 Experiments in Arabidopsis demonstrated that the blue-light-dependent phosphorylation of CRY2 (but not CRY1) triggers its degradation.19 Different subcellular localization of Arabidopsis CRY1 between light conditions (mainly in the cytosol) and dark conditions (mainly in the nucleus) might be the reason.20 In plant cells, nuclear AMPK induce nuclear CRY1 phosphorylation.3 While phosphorylated nuclear CRY1 promotes its ubiquitin-dependent degradation (the localization of ubiquitin-dependent proteasomes exist both in cytosol and the nuclear).3,16 Thus, the nutritional status – AMPK – CRY1 – circadian clock pathway may represent a conserved mechanism in higher eukaryotes.3,16
NO may participate in N-regulated flowering
Nitrate reductase (NR) is the key enzyme in N assimilation that catalytic reduction from nitrate to nitrite. NR produces NO from nitrite by NAD(P)H-dependent manner.21-23 Therefore, our previous study cannot rule out the side-effect of NO generated by the N assimilation. NO is involved in photoperiod and autonomous flowering pathway (NO represses the amplitudes of output pathway components CO and GI).5 These correlations imply that N-regulated flowering pathways may be very complex. We should consider more about carbon metabolite signals and NO signals in the future studies.
Disclosure of potential conflicts of interest
No potential conflicts of interest were disclosed.
Funding
This work was supported by the Preeminent Youth Fund of Sichuan Province (grant number 2015JQO045) to S.Y.
References
- 1.Yu JW, Rubio V, Lee NY, Bai SL, Lee SY, Kim SS, Liu LJ, Zhang YY, Irigoyen ML, Sullivan JA, et al.. COP1 and ELF3 control circadian function and photoperiodic flowering by regulating GI stability. Mol Cell 2008; 32:617-30; PMID:19061637; http://dx.doi.org/ 10.1016/j.molcel.2008.09.026 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Liu H, Wang Q, Liu Y, Zhao XY, Imaizumi T, Somers D, Tobin E, Lin CT. Arabidopsis CRY2 and ZTL mediate blue-light regulation of the transcription factor CIB1 by distinct mechanisms. Proc Natl Acad Sci USA 2013; 110:17582-7; PMID:24101505; http://dx.doi.org/ 10.1073/pnas.1308987110 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Yuan S, Zhang ZW, Zheng C, Zhao ZY, Wang Y, Feng LY, Niu G, Wang CQ, Wang JH, Feng H, et al.. Arabidopsis cryptochrome 1 functions in nitrogen regulation of flowering. Proc Natl Acad Sci USA 2016; 113:7661-6; PMID:27325772; http://dx.doi.org/ 10.1073/pnas.1602004113 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Haydon MJ, Mielczarek O, Robertson FC, Hubbard KE, Webb AA. Photosynthetic entrainment of the Arabidopsis thaliana circadian clock. Nature 2013; 502:689-92; PMID:24153186; http://dx.doi.org/ 10.1038/nature12603 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.He Y, Tang RH, Hao Y, Stevens RD, Cook CW, Ahn SM, Jing L, Yang Z, Chen L, Guo F, et al.. Nitric oxide represses the Arabidopsis floral transition. Science 2004; 305:1968-1971; PMID:15448272; http://dx.doi.org/ 10.1126/science.1098837 [DOI] [PubMed] [Google Scholar]
- 6.Gibon Y, Bläsing OE, Palacios-Rojas N, Pankovic D, Hendriks JHM, Fisahn J, Hohne M, Günther M, Stitt M. Adjustment of diurnal starch turnover to short days: depletion of sugar during the night leads to a temporary inhibition of carbohydrate utilization, accumulation of sugars and post-translational activation of ADP-glucose pyrophosphorylase in the following. Plant J 2004; 39:847-62; PMID:15341628; http://dx.doi.org/ 10.1111/j.1365-313X.2004.02173.x [DOI] [PubMed] [Google Scholar]
- 7.Klotke J, Kopka J, Gatzke N, Heyer AG. Impact of soluble sugar concentrations on the acquisition of freezing tolerance in accessions of Arabidopsis thaliana with contrasting cold adaptation - evidence for a role of raffinose in cold acclimation. Plant Cell Environ 2004; 27:1395-404; http://dx.doi.org/ 10.1111/j.1365-3040.2004.01242.x [DOI] [Google Scholar]
- 8.Roitsch T, Gonz alez MC. Function and regulation of plant invertases: sweet sensations. Trends Plant Sci 2003; 9:606-13; PMID:15564128; http://dx.doi.org/ 10.1016/j.tplants.2004.10.009 [DOI] [PubMed] [Google Scholar]
- 9.Miller AJ, Fan X, Orsel M, Smith SJ, Wells DM. Nitrate transport and signalling. J Exp Bot 2007; 58:2297-306; PMID:17519352; http://dx.doi.org/ 10.1093/jxb/erm066 [DOI] [PubMed] [Google Scholar]
- 10.Smith AM, Stitt M. Coordination of carbon supply and plant growth. Plant Cell Environ 2007; 30:1126-49; PMID:17661751; http://dx.doi.org/ 10.1111/j.1365-3040.2007.01708.x [DOI] [PubMed] [Google Scholar]
- 11.Lintala M, Allahverdiyeva Y, Kidron H, Piippo M, Battchikova N, Suorsa M, Rintamäki E, Salminen TA, Aro EM, Mulo P. Structural and functional characterization of ferredoxin-NADP+-oxidoreductase using knock-out mutants of Arabidopsis. Plant J 2007; 49:1041-52; PMID:17335513; http://dx.doi.org/ 10.1111/j.1365-313X.2006.03014.x [DOI] [PubMed] [Google Scholar]
- 12.Tokunaga C, Yoshino K, Yonezawa K. mTOR integrates amino acid- and energy-sensing pathways. Biochem Biophys Res Commun 2004; 313:443-6; PMID:14684182; http://dx.doi.org/ 10.1016/j.bbrc.2003.07.019 [DOI] [PubMed] [Google Scholar]
- 13.Hardie DG, Ross FA, Hawley SA. AMPK: a nutrient and energy sensor that maintains energy homeostasis. Nat Rev Mol Cell Biol 2012; 13:251-62; PMID:22436748; http://dx.doi.org/ 10.1038/nrm3311 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Crozet P, Margalha L, Confraria A, Rodrigues A, Martinhol C, Adamol M, Elias C, Baena-Gonzálezl E. Mechanisms of regulation of SNF1/AMPK/SnRK1 protein kinases. Front Plant Sci 2014; 5:190; PMID:24904600; http://dx.doi.org/ 10.3389/fpls.2014.00190 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Zhang Y, Primavesi LF, Jhurreea D, Andralojc PJ, Mitchell RA, Powers SJ, Schluepmann H, Delatte T, Wingler A, Paul MJ. Inhibition of SNF1-related protein kinase1 activity and regulation of metabolic pathways by trehalose-6-phosphate. Plant Physiol 2009; 149:1860-71; PMID:19193861; http://dx.doi.org/ 10.1104/pp.108.133934 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Lamia KA, Sachdeva UM, DiTacchio L, Williams EC, Alvarez JG, Egan DF, Vasquez DS, Juguilon H, Panda S, Shaw RJ, et al.. AMPK regulates the circadian clock by cryptochrome phosphorylation and degradation. Science 2009; 326:437-40; PMID:19833968; http://dx.doi.org/ 10.1126/science.1172156 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Gatfield D, Schibler U. Proteasomes keep the circadian clock ticking. Science 2007; 316:1135-6; PMID:17495136; http://dx.doi.org/ 10.1126/science.1144165 [DOI] [PubMed] [Google Scholar]
- 18.Shalitin D, Yu X, Maymon M, Mockler T, Lin C. Blue light-dependent in vivo and in vitro phosphorylation of Arabidopsis cryptochrome 1. Plant Cell 2003; 15:2421-9; PMID:14523249; http://dx.doi.org/ 10.1105/tpc.013011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Shalitin D, Yang H, Mockler TC, Maymon M, Guo H, Whitelam GC, Lin C. Regulation of Arabidopsis cryptochrome 2 by blue-light-dependent phosphorylation. Nature 2002; 417:763-7; PMID:12066190; http://dx.doi.org/ 10.1038/nature00815 [DOI] [PubMed] [Google Scholar]
- 20.Yang HQ, Wu YJ, Tang RH, Liu D, Liu Y, Cashmore AR. The C termini of Arabidopsis cryptochromes mediate a constitutive light response. Cell 2000; 103:815-27; PMID:11114337; http://dx.doi.org/ 10.1016/S0092-8674(00)00184-7 [DOI] [PubMed] [Google Scholar]
- 21.Dean JV, Harper JE. The conversion of nitrite to nitrogen oxide(s) by the constitutive NAD(P)H-nitrate reductase enzyme from soybean. Plant Physiol 1988; 88:389-95; PMID:16666314; http://dx.doi.org/ 10.1104/pp.88.2.389 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Yamasaki H, Sakihama Y, Takahashi S. An alternative pathway for nitric oxide production in plants: new features of an old enzyme. Trends Plant Sci 1999; 4:128-9; PMID:10322545; http://dx.doi.org/ 10.1016/S1360-1385(99)01393-X [DOI] [PubMed] [Google Scholar]
- 23.Rockel P, Strube F, Rockel A, Wildt J, Kaiser WM. Regulation of nitric oxide (NO) production by plant nitrate reductase in vivo and in vitro. J Exp Bot 2002; 53:103-10; PMID:11741046; http://dx.doi.org/ 10.1093/jexbot/53.366.103 [DOI] [PubMed] [Google Scholar]


