Abstract
Objectives:
Inflammation plays a critical role in the progression of diabetic complications such as neurological disorders. Previous reports have indicated the memory-improving effect of troxerutin, in rat hippocampus, but the underlying mechanisms are unclear. Hence, we have investigated the effect of troxerutin pretreatment on gene expressions of inflammation-related microRNAs (miRs), miR-146a and miR-155, and nuclear factor-kappa B (NF-κB) signaling pathway in the hippocampus of healthy and diabetic rats.
Materials and Methods:
Wistar rats were randomly divided into four groups (control, control + troxerutin, diabetic, and diabetic + troxerutin). Diabetes was induced by a single i.p. injection of streptozotocin (50 mg/kg). Troxerutin (150 mg/kg) was orally administered in animals for 1 month. After 10 weeks of diabetes, animals were anesthetized and decapitated for the isolation of hippocampus. The expression of miR-146a and miR-155 and the messenger RNA (mRNA) expressions of NF-κB, interleukin-1 receptor-associated kinase-1 (IRAK-1), and tumor necrosis factor receptor-associated factor-6 (TRAF-6) were analyzed by real-time polymerase chain reaction.
Results:
Diabetes significantly increased hippocampal mRNA levels of NF-κB, IRAK-1, and TRAF-6 compared with nondiabetic rats (P < 0.05); however, pretreatment with troxerutin decreased them in both diabetic and nondiabetic animals, independent of its glycemic effect (P < 0.05). The expression levels of miR-146a and miR-155 were decreased in diabetic group as compared to the control (P < 0.01).
Conclusion:
These findings showed that troxerutin could inhibit the inflammatory NF-κB pathway in the hippocampus of diabetic rats, which may be due to the negative feedback loop regulated by miR-146a.
Key words: Diabetes, inflammation, microRNA-146a, nuclear factor-kappa B, troxerutin
Diabetes mellitus is a growing health problem worldwide and its incidence has been increased by 50% during the last 10 years.[1] Diabetes is a multifactorial disorder, which can be clinically diagnosed by an increase in the blood glucose level from normal values.[2] Prolonged hyperglycemia can lead to micro and macrovascular complications. Macrovascular complications include atherosclerosis, hypertension, cardiovascular disease, coronary artery disorders, and ischemic brain disease. Microvascular complications of diabetes include retinopathy, nephropathy, and neuropathy.[3] The structural complexity of the hippocampus has made it more vulnerable to pathologic alterations of diabetes mellitus.[4] Hippocampus, the crucial part of the limbic system with essential roles in memory and cognition, is one of the sensitive regions of the brain to inflammation and oxidative stresses.[5] Furthermore, recent studies have highlighted the role of inflammation and pro-inflammatory cytokines such as nuclear factor-kappa B (NF-κB) and related adapter molecules in the progress of diabetic complications.[6] In addition, inflammation is a main player in cognitive impairment and brain dementia.[7]
MicroRNAs (miRs) are a novel group of ≈22 nucleotides, single-strand noncoding RNAs regulating gene expression in the posttranscriptional level by dissociation or suppression of transcription of target messenger RNAs (mRNAs).[8] Recent studies have shown a clear relation between diabetic complications and altered miR expression.[9] MiR-155 and miR-146a are involved in the inflammatory processes. In addition, these miRs show different patterns of expression during diabetes and its complications. For example, the miR-155 expression level was increased in diabetic nephropathy[10] and retinopathy.[11] It is also reported that both miRs are decreased in peripheral mononuclear cells of patients with diabetes.[12] The exact roles of these miRs on the expression and activity of NF-κB pathway molecules are not known completely yet.
Troxerutin, a naturally occurring flavonoid, is known mainly because of its anti-inflammatory, antioxidative, antithrombotic, and antineoplastic activities.[13,14] The tissue protective effects of troxerutin in kidney,[15] liver,[16] and brain[17] injuries have been reported in previous studies. We previously reported that it prevents diabetic vascular abnormalities through the reduction of lipid peroxidation and improvement of endogenous antioxidative activity.[18] We have also shown that this agent has antiapoptotic potential in myocardial reperfusion injury of diabetic rats.[14] In two recent works, the neuroprotective and memory-enhancing effects of troxerutin have been shown in the hippocampus of mice fed with a high cholesterol diet[19] and rats with β-amyloid-induced spatial learning impairment.[20] However, the corresponding mechanisms, especially in cognitive deficit in the context of diabetes, have not been studied yet. Considering the role of inflammation in memory impairment induced by diabetes and involvement of miRs in the regulation of signaling pathways elements, in the present study, we examined the effect of troxerutin pretreatment on gene expression levels of inflammatory NF-κB and its adapter molecules, interleukin 1 receptor-associated kinase-1 (IRAK-1) and tumor necrosis factor receptor-associated factor-6 (TRAF-6), as well as miR-146a and miR-155 levels in the hippocampus of healthy and diabetic rats.
Materials and Methods
Animals and Materials
In this study, 24 male Wistar rats weighing 200–250 g were housed in a room temperature (22°C–25°C), free access to water and food, and 12 h light/12 h dark cycle. The procedures were performed in accordance with the Guide for the Care and Use of Laboratory Animals published by the US National Institutes of Health (NIH publication. No 85-23, revised 1996) and approved by the Local Animal Care Committee. Streptozotocin (STZ) and troxerutin (EC number 230-389-4) were purchased from Sigma (St. Louis, MO, USA) and all other chemicals and reagents were obtained from commercial sources in the highest quality available.
Induction of Diabetes
Diabetes was induced by a single i.p. injection of STZ (50 mg/kg) dissolved in 0.1 M of citrate buffer. Control animals received the same volume of citrate buffer. Seventy-two hours after diabetes induction, a blood drop was obtained from the tail vein of the rats through a small lancet scratch and then used for measuring of blood glucose level with a glucometer (Boehringer Mannheim, Indianapolis, USA), and rats with blood glucose levels higher than 300 mg/dl were considered as Type 1 diabetes.[21] The period of diabetes was 10 weeks to mimic the chronic nature of the disease. The body weights and blood glucose levels were assayed at the beginning and the end of the experiment in all diabetic and nondiabetic rats.
Experimental Design
Rats were randomly divided into four groups (six in each) including: control (Cont); diabetic (Diab); control + troxerutin (Cont + TXR); and diabetic + troxerutin (Diab + TXR). Rats in Cont + TXR and Diab + TXR groups received oral administration of troxerutin with the dose of 150 mg/kg daily for 1 month, from 7th to 10th weeks.[15,16] Ten weeks after diabetes induction, diabetic and nondiabetic animals were anesthetized with a mixture of ketamine (60 mg/kg) and xylazine (10 mg/kg), and after decapitation, their hippocampus was carefully isolated, weighed, and frozen for further assays.
Preparation of Tissue Homogenates
Certain weights of tissue sample were homogenized on ice in 1 ml of lysis buffer at pH 7.4 in the presence of protease inhibitor cocktail (Sigma-Aldrich, St. Louis, MO, USA), with a Polytron PT-10/ST homogenizer. Then, the homogenate was centrifuged (10,000 g for 10 min at 4°C) and the supernatants were removed and quickly frozen at −80°C until use.
RNA Extraction and Real-time Polymerase Chain Reaction
Total RNA was extracted from the samples using miRCURY™ RNA isolation kit (Exiqon, Vedbaek, Denmark) according to the manufacturer's protocol.[22] The RNA content and purity were measured using the NanoDrop 1000 spectrophotometer (Thermo Scientific, Wilmington, DE 19810, USA). The expression profiles of miR-146a and miR-155 were performed on total RNA extracted using the Universal cDNA Synthesis Kit. Briefly, total RNA containing miRNA was polyadenylated and cDNA was synthesized using a poly (T) primer with a 30 degenerate anchor and a 50 universal tag (Exiqon, Vedbaek, Denmark). RevertAid First Strand cDNA Synthesis Kit (Fermentas GmBH, Leon-Rot, Germany) with the aid of random hexamer primers and MMLV reverse transcriptase (as a complete system for efficient synthesis of first-strand cDNA from mRNA or total RNA templates) were used for determination of IRAK-1, TRAF-6, and NF-ΚB mRNA expression levels. Real-time polymerase chain reaction reactions were performed on a Bio-Rad iQ5 Detection System (Bio-Rad, Richmond, CA, USA). The 2−(ΔΔCt) method was used to determine the relative-quantitative levels of individual mRNAs and miRs. The results were expressed as the fold-difference to the relevant controls.
Statistical Analysis
All values were expressed as mean ± standard error of the mean. The data of parametric variables between different groups were analyzed using one-way ANOVA, followed by Tukey post hoc test. Differences were considered statistically significant when P < 0.05.
Results
The Effect of Troxerutin on Body Weight and Blood Glucose Levels
The values of body weights at the beginning and end of the experiment, first and last days, respectively, and blood glucose levels are shown in Table 1. Induction of diabetes significantly decreased the body weight of rats in comparison with those of control group after 10 weeks (P < 0.05). Treatment with troxerutin comparatively recovered the diabetes-induced decline in body weight at the end of experiment. On the other hand, 10-week diabetes significantly increased the blood glucose levels as compared with controls, but troxerutin pretreatment could not significantly reduce the diabetes-induced elevation of glucose levels [Table 1].
Table 1.
The effect of troxerutin on body weight and blood glucose levels in rats
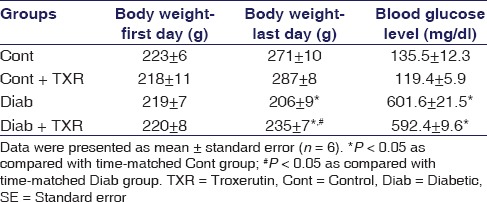
The Effect of Troxerutin on Nuclear Factor-kappa B Messenger RNA Expression Level
Diabetes significantly increased the NF-κB mRNA expression level as compared with the control group (P < 0.05). Administration of troxerutin decreased NF-κB mRNA level in both diabetic and nondiabetic groups in comparison with corresponding controls [Figure 1].
Figure 1.
The messenger RNA expression levels of nuclear factor-kappa B in the rat hippocampus. Data were presented as mean ± standard error (n = 6). *P < 0.05 versus Cont group and #P < 0.01 versus Diab group. TXR = troxerutin, Cont = Control, Diab = Diabetic
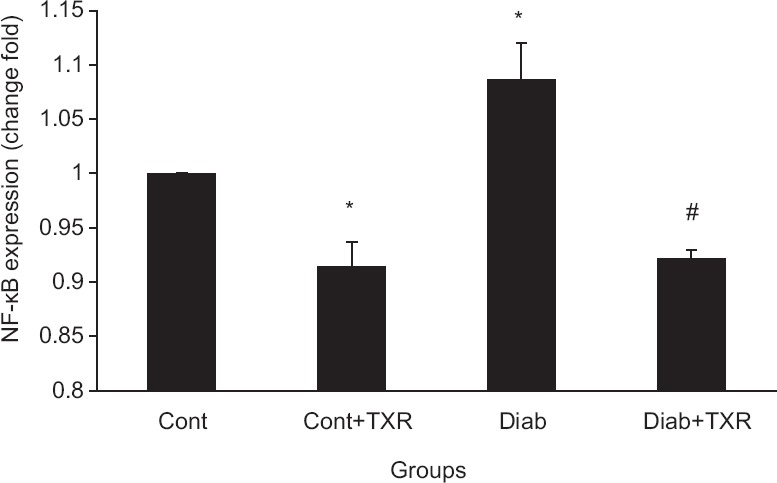
The Effect of Troxerutin on Interleukin 1 Receptor-associated Kinase-1 Messenger RNA Expression Level
The mRNA expression level of IRAK-1 in the hippocampus of diabetic rats was greater than those of the control group [Figure 2]. Pretreatment with troxerutin decreased IRAK-1 mRNA level in the diabetic group but had no significant effect in the control group.
Figure 2.
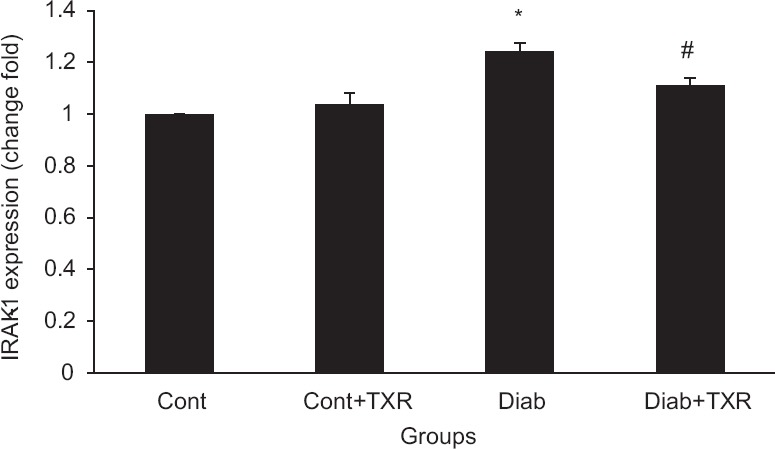
The messenger RNA expression levels of interleukin 1 receptor-associated kinase-1 in the rat hippocampus. Data were presented as mean ± standard error (n = 6). *P < 0.05 versus Cont group and #P < 0.01 versus Diab group. TXR: troxerutin, Cont = Control, Diab = Diabetic
The Effect of Troxerutin on Tumor Necrosis Factor Receptor-associated Factor-6 Messenger RNA Expression Level
Diabetes significantly increased the mRNA expression level of TRAF-6 as compared with the control group [Figure 3]. Administration of troxerutin significantly decreased TRAF-6 mRNA level in both diabetic and control groups (P < 0.05).
Figure 3.

The messenger RNA expression levels of tumor necrosis factor receptor-associated factor-6 in the rat hippocampus. Data were presented as mean ± standard error (n = 6). *P < 0.05 versus Cont group and #P < 0.01 versus Diab group. TXR: Troxerutin, Cont = Control, Diab = Diabetic
The Effect of Troxerutin on MicroRNA-146a Expression Level
Ten-week diabetes decreased the miR-146a expression level significantly as compared with those of control group (P < 0.01) [Figure 4]. Pretreatment of control nondiabetic rats with troxerutin had no significant effect on the miR-146a expression level, whereas it increased its level significantly in diabetic rats [Figure 4].
Figure 4.
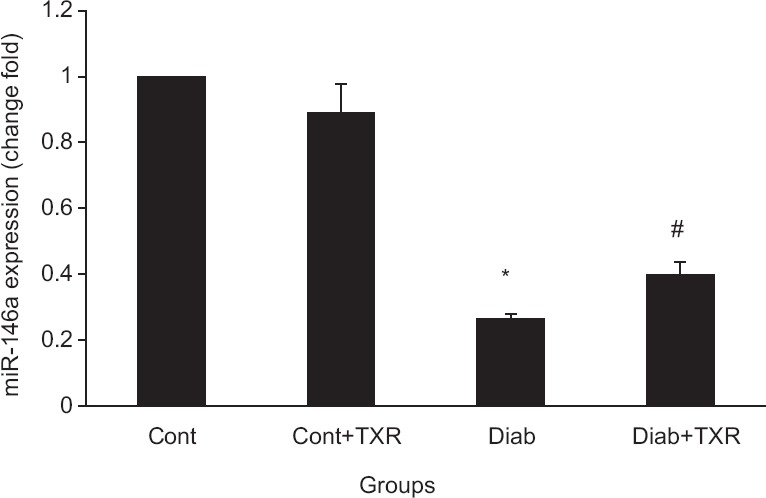
Real-time quantitative polymerase chain reaction analysis of microRNA-146a expression levels in the rat hippocampus. Data were presented as mean ± standard error (n = 6). *P < 0.05 versus Cont group and #P < 0.01 versus Diab group. TXR: Troxerutin, Cont = Control, Diab = Diabetic
The Effect of Troxerutin on MicroRNA-155 Expression Level
The hippocampal expression level of miR-155 was decreased in diabetic rats compared with control group (P < 0.01). After administration of troxerutin, miR-155 expression levels remained unchanged in both nondiabetic control and diabetic rats, the alterations were not statistically significant [Figure 5].
Figure 5.

Real-time quantitative polymerase chain reaction analysis of microRNA-155 expression levels in the rat hippocampus. Data were presented as mean ± standard error (n = 6). *P < 0.05 versus Cont group. TXR: Troxerutin, Cont = Control
Discussion
In the present study, we found that troxerutin affected differently on miR-146a and miR-155 expression levels in the hippocampus of diabetic and nondiabetic rats. In addition, troxerutin pretreatment decreased the mRNA levels of NF-κB, IRAK-1, and TRAF-6 in both conditions, indicating the anti-inflammatory properties of troxerutin. Only in diabetic rats, this action of troxerutin was associated with increased level of miR-146a that had been reduced by chronic diabetes.
Diabetes is associated with the impairment of cognition and loss of memory in human and experimental animals.[23] Administration of troxerutin has reversed the excitotoxic brain damage and improved memory and cognitive impairment in the hippocampus of nondiabetic mice[13] and enhanced the spatial learning in rats.[20] However, the underlying mechanisms of these protective actions of troxerutin have not been yet fully understood. In this study, we suggested an NF-κB-dependent anti-inflammatory mechanism for the protective effects of troxerutin in rat hippocampus. In this line, the antioxidant effect of troxerutin on the protection of hepatic injury induced by D-galactose[16] has been reported before. Similarly, another investigation has shown its positive influence on mouse kidney injury through anti-inflammatory and antioxidative mechanisms and suppressing the expression of inducible nitric oxide synthase and cyclooxygenase-2.[17] In addition, we have previously showed the protective effects of troxerutin on reduction of diabetes-induced vascular[18] and myocardial[14,24] injuries through antioxidant and antiapoptotic mechanisms. These results support our hypothesis and findings about the neuroprotective and inhibitory effects of troxerutin on NF-κB-mediated inflammatory response.
We have also shown that troxerutin could mediate its anti-inflammatory role somewhat by altering miRs expression levels. MiR-146a and miR-155 have been known as the inflammation-related miRs in the previous studies.[10] In our study, diabetes decreased the levels of miR-155 and miR-146a expressions in the hippocampus of diabetic rats. Downregulation of miR-146a in diabetic condition was responsible for the abnormal inflammatory response and production of extracellular matrix proteins such as fibronectin,[25] developing the diabetes-induced vascular complications in Type 1 and Type 2 diabetic animals.[26] In addition, the basal expression of miR-155 was downregulated in peripheral blood mononuclear cells of patients with Type 2 diabetes.[12] In contrast, miR-155 has been upregulated in retinal cells of diabetic rats.[11] This expression discrepancy implies that the expression levels of miR-155 and miR-146a likely be dependent on the tissue type, duration of diabetes, grade of inflammation, and ligands that induce the inflammatory response.
Our study revealed that the anti-inflammatory action of troxerutin on NF-κB-mediated pathway was partially associated with its effect on the miR-146a restoration in diabetic rats. Thus, troxerutin may inhibit the expression levels of NF-κB and its adaptor proteins TRAF-6 and IRAK-1 by targeting their regulatory miR-146a in diabetic hippocampus. A regulatory role has been suggested for miR-146a and miR-155 in modulating of cytokines and toll-like receptor signaling through a negative feedback loop, in which downregulation of IRAK-1 and TRAF-6 induces miRs expression.[25] In our study, we observed downregulation of these miRs in the hippocampus of diabetic rats and, at the same time, up-regulation of NF-κB-mediated pathway expression; administration of troxerutin increased miR-146a level and reduced NF-κB, TRAF-6, and IRAK-1 mRNA levels, thus we can attribute these effects to relative amplification of the negative feedback loop between miR-146a and NF-κB. However, the hippocampal expression level of miR-155 was not changed in this scenario, indicating that troxerutin does not affect this miR.
On the other hand, the observed positive effects of troxerutin in diabetic rats were not associated with its effect on hyperglycemia in our model of STZ-induced diabetes; therefore, these are likely due to the effects other than a hypoglycemic mechanism. To support this hypothesis, restoration of normoglycemia could not reduce upregulation of free radical generation and vascular dysfunction in diabetic mice and human endothelial cells.[27] Hyperglycemia-induced damages are mediated through several mechanisms, including increased formation of advanced glycation end products, activation of protein kinase C isoforms, endoplasmic reticulum stress, mitochondrial dysfunction, apoptosis, as well as free radicals formation, that all can be the cellular targets of troxerutin.[18]
As some limitations of this study, we did not investigate the role of troxerutin on glucose metabolism for clear understanding of the correlation between troxerutin anti-inflammatory effects and blood glucose levels. However, we could not show any correlation (glucose levels remained unchanged); thus, it can be assumed that troxerutin may influence signaling mediators through its multimechanistic actions independent of its glycemic effect. Even though the memory-enhancing effect of troxerutin with our tested dosage (150 mg/dl) was reported previously, the cognitive improvement by troxerutin was not assessed in the present study. In addition, comparing the effect of troxerutin with a standard anti-inflammatory drug or with insulin as a main therapy for Type 1 diabetes would improve the reliability of the study.
Conclusion
STZ-induced diabetes increased the mRNA levels of NF-κB and its two adapter molecules IRAK-1 and TRAF-6, while reduced the expression levels of miRs in the hippocampus of diabetic rats. Pretreatment with troxerutin inhibited the inflammatory NF-κB-mediated pathway targets by partial restoration of miR-146a expression level, presumably through a regulatory feedback in diabetic rats. However, further study is required to understand the exact roles of miRs in anti-inflammatory responses.
Financial Support and Sponsorship
Nil.
Conflicts of Interest
There are no conflicts of interest.
Acknowledgment
This study was derived from the Msc. Thesis of Raana Yavari (No: 92.12-3) and supported by a grant from Neurosciences Research Center, Tabriz University of Medical Sciences.
References
- 1.Shaw JE, Sicree RA, Zimmet PZ. Global estimates of the prevalence of diabetes for 2010 and 2030. Diabetes Res Clin Pract. 2010;87:4–14. doi: 10.1016/j.diabres.2009.10.007. [DOI] [PubMed] [Google Scholar]
- 2.Mathis D, Vence L, Benoist C. Beta-cell death during progression to diabetes. Nature. 2001;414:792–8. doi: 10.1038/414792a. [DOI] [PubMed] [Google Scholar]
- 3.Kato M, Castro NE, Natarajan R. MicroRNAs: Potential mediators and biomarkers of diabetic complications. Free Radic Biol Med. 2013;64:85–94. doi: 10.1016/j.freeradbiomed.2013.06.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Lobnig BM, Krömeke O, Optenhostert-Porst C, Wolf OT. Hippocampal volume and cognitive performance in long-standing Type 1 diabetic patients without macrovascular complications. Diabet Med. 2006;23:32–9. doi: 10.1111/j.1464-5491.2005.01716.x. [DOI] [PubMed] [Google Scholar]
- 5.Aragno M, Mastrocola R, Brignardello E, Catalano M, Robino G, Manti R. Dehydroepiandrosterone modulates nuclear factor-kB activation in hippocampus of diabetic rats. Endocrinology. 2002;143:3250–8. doi: 10.1210/en.2002-220182. [DOI] [PubMed] [Google Scholar]
- 6.Kaul K, Hodgkinson A, Tarr JM, Kohner EM, Chibber R. Is inflammation a common retinal-renal-nerve pathogenic link in diabetes? Curr Diabetes Rev. 2010;6:294–303. doi: 10.2174/157339910793360851. [DOI] [PubMed] [Google Scholar]
- 7.Campbell IL, Stalder AK, Chiang CS, Bellinger R, Heyser CJ, Steffensen S, et al. Transgenic models to assess the pathogenic actions of cytokines in the central nervous system. Mol Psychiatry. 1997;2:125–9. doi: 10.1038/sj.mp.4000225. [DOI] [PubMed] [Google Scholar]
- 8.Zhang B, Farwell MA. MicroRNAs: A new emerging class of players for disease diagnostics and gene therapy. J Cell Mol Med. 2008;12:3–21. doi: 10.1111/j.1582-4934.2007.00196.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Kantharidis P, Wang B, Carew RM, Lan HY. Diabetes complications: The microRNA perspective. Diabetes. 2011;60:1832–7. doi: 10.2337/db11-0082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Liu F, Huang Y, Fu P. Involvement of inflammation-related microRNAs: miR-155 and miR-146a in diabetic nephropathy: Implication in inflammation mediated endothelial injury. Nephrol Dial Transplant. 2012;27:34–5. [Google Scholar]
- 11.Kovacs B, Lumayag S, Cowan C, Xu S. MicroRNAs in early diabetic retinopathy in streptozotocin-induced diabetic rats. Invest Ophthalmol Vis Sci. 2011;52:4402–9. doi: 10.1167/iovs.10-6879. [DOI] [PubMed] [Google Scholar]
- 12.Corral-Fernández NE, Salgado-Bustamante M, Martínez-Leija ME, Cortez-Espinosa N, García-Hernández MH, Reynaga-Hernández E, et al. Deregulated miR-155 expression in peripheral blood mononuclear cells from patients with type 2 diabetes. Exp Clin Endocrinol Diabetes. 2013;121:347–53. doi: 10.1055/s-0033-1341516. [DOI] [PubMed] [Google Scholar]
- 13.Liu F, Huang Y, Fu P. Troxerutin Counteracts domoic acid–induced memory deficits in mice by inhibiting CCAAT/Enhancer binding protein β-mediated inflammatory response and oxidative stress. J Immunol. 2013;190:3466–79. doi: 10.4049/jimmunol.1202862. [DOI] [PubMed] [Google Scholar]
- 14.Mokhtari B, Badalzadeh R, Alihemmati A, Mohammadi M. Phosphorylation of GSK-3ß and reduction of apoptosis as targets of troxerutin effect on reperfusion injury of diabetic myocardium. Eur J Pharmacol. 2015;765:316–21. doi: 10.1016/j.ejphar.2015.08.056. [DOI] [PubMed] [Google Scholar]
- 15.Fan SH, Zhang ZF, Zheng YL, Lu J, Wu DM, Shan Q, et al. Troxerutin protects the mouse kidney from d-galactose-caused injury through anti-inflammation and anti-oxidation. Int Immunopharmacol. 2009;9:91–6. doi: 10.1016/j.intimp.2008.10.008. [DOI] [PubMed] [Google Scholar]
- 16.Zhang ZF, Fan SH, Zheng YL, Lu J, Wu DM, Shan Q, et al. Troxerutin protects the mouse liver against oxidative stress-mediated injury induced by D-galactose. J Agric Food Chem. 2009;57:7731–6. doi: 10.1021/jf9012357. [DOI] [PubMed] [Google Scholar]
- 17.Lu J, Wu DM, Hu B, Cheng W, Zheng YL, Zhang ZF, et al. Chronic administration of troxerutin protects mouse brain against D-galactose-induced impairment of cholinergic system. Neurobiol Learn Mem. 2010;93:157–64. doi: 10.1016/j.nlm.2009.09.006. [DOI] [PubMed] [Google Scholar]
- 18.Badalzadeh R, Layeghzadeh N, Alihemmati A, Mohammadi M. Beneficial effect of troxerutin on diabetes-induced vascular damages in rat aorta: Histopathological alterations and antioxidation mechanism. Int J Endocrinol Metab. 2015;13:e25969. doi: 10.5812/ijem.25969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Lu J, Wu DM, Zheng ZH, Zheng YL, Hu B, Zhang ZF. Troxerutin protects against high cholesterol-induced cognitive deficits in mice. Brain. 2011;134(Pt 3):783–97. doi: 10.1093/brain/awq376. [DOI] [PubMed] [Google Scholar]
- 20.Babri S, Amani M, Mohaddes G, Alihemmati A, Ebrahimi H. Protective effects of troxerutin on β-amyloid (1-42)-induced impairments of spatial learning and memory in rats. Neurophysiology. 2012;44:387–93. [Google Scholar]
- 21.Badalzadeh R, Mohammadi M, Najafi M, Ahmadiasl N, Farajnia S, Ebrahimi H. The additive effects of ischemic postconditioning and cyclosporine-A on nitric oxide activity and functions of diabetic myocardium injured by ischemia/reperfusion. J Cardiovasc Pharmacol Ther. 2012;17:181–9. doi: 10.1177/1074248411416118. [DOI] [PubMed] [Google Scholar]
- 22.Lässer C, Eldh M, Lötvall J. Isolation and characterization of RNA-containing exosomes. J Vis Exp. 2012;59:e3037. doi: 10.3791/3037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Badalzadeh R, Mokhtari B, Yavari R. Contribution of apoptosis in myocardial reperfusion injury and loss of cardioprotection in diabetes mellitus. J Physiol Sci. 2015;65:201–15. doi: 10.1007/s12576-015-0365-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Heidarzadeh F, Badalzadeh R, Hatami H. The effect of troxerutin on lipid peroxidation and tissue injury induced by myocardial ischemia reperfusion injury in diabetic rat. Razi J Med Sci. 2014;21:37–45. [Google Scholar]
- 25.Xu J, Wu W, Zhang L, Dorset-Martin W, Morris MW, Mitchell ME, et al. The role of microRNA-146a in the pathogenesis of the diabetic wound-healing impairment: Correction with mesenchymal stem cell treatment. Diabetes. 2012;61:2906–12. doi: 10.2337/db12-0145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Feng B, Chen S, McArthur K, Wu Y, Sen S, Ding Q, et al. miR-146a-Mediated extracellular matrix protein production in chronic diabetes complications. Diabetes. 2011;60:2975–84. doi: 10.2337/db11-0478. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Paneni F, Mocharla P, Akhmedov A, Costantino S, Osto E, Volpe M, et al. Gene silencing of the mitochondrial adaptor p66(Shc) suppresses vascular hyperglycemic memory in diabetes. Circ Res. 2012;111:278–89. doi: 10.1161/CIRCRESAHA.112.266593. [DOI] [PubMed] [Google Scholar]


