Abstract
Objective:
Several studies report that chronic stress results in impaired spatial learning and working memory and enhanced anxiety-like behavior. However, not many studies have looked into the possible ways of reversing stress-induced deficits. Celastrus paniculatus (CP), a traditional ayurvedic herbal medicine, was used to treat cognitive deficits in mentally retarded children. CP oil has been reported to have neuroprotective and antioxidant activities. However, the effects of CP oil on chronic stress-induced cognitive deficits are unclear. In the present study, we intended to analyze the neuroprotective effects of CP oil on stress-associated cognitive dysfunctions.
Materials and Methods:
Chronic stress was induced by subjecting rats to restrainers for 6 h a day for 21 days. CP oil (400, 600 mg/kg) or vehicle was administered intraperitoneally (i.p.) after stress protocol once a day over the next 14 days. Groups used in the present study: normal control, stress, stress + vehicle, stress + CP oil at 2 different doses (400 and 600 mg/kg, i.p.). After the drug treatment, open field and elevated plus maze (EPM) were used to analyze anxiety-like behavior, and partially baited radial arm maze (RAM) and T-maze were used to evaluate spatial learning and memory capabilities. Analysis has been done using two-way ANOVA followed by Bonferroni's post hoc test and one-way ANOVA followed by Tukey's post hoc test.
Results:
Stressed rats showed enhanced anxiety-like behavior in EPM (P < 0.001) and impaired performance in RAM (P < 0.001) and T-maze tasks (P < 0.001) compared to normal animals. In contrast, CP oil treatment to these rats improved their performance in both RAM (P < 0.001) and T-maze (P < 0.001). In addition, CP oil significantly reduced stress-induced anxiety behavior (P < 0.001).
Conclusion:
Chronic treatment with CP oil is to improve cognitive abilities in chronically stressed rats. The current study provides a novel perspective on beneficial effect of herbal therapy on stress-induced cognitive dysfunctions.
Key words: Anxiety, Celastrus paniculatus, chronic stress, cognitive deficits, herbal medicine
The burden of stress-associated disorders, including depression and anxiety, is rapidly rising globally.[1] Chronic stress markedly promotes aging and other neurodegenerative diseases.[2] Repeated stress is also associated with severe deficits in cognitive abilities.[3,4,5,6] Chronic stress induces morphological changes such as dendritic atrophy in the hippocampus.[6] Further, previous studies demonstrated that anxiety is a result of negative emotion caused by repetitive stress-like restraint or immobilization.[7,8] There is a limited ability to treat effectively the associated cognitive decline in chronic stressful conditions. In view of the side effects of synthetic drugs, there is a need for natural remedies which are safe and effective. Celastrus paniculatus (CP), also called as Jyotishmati, a traditional ayurvedic herbal medicine, was used to treat cognitive deficits in mentally retarded children.[9] CP oil has been reported to have neuroprotective and antioxidant activities.[10] However, effect of CP oil treatment on stress-induced cognitive deficits is unclear. Hence, the current study was performed to examine the beneficial effects of CP oil treatment on chronic stress-induced spatial learning and memory impairment and chronic stress-enhanced anxiety-like behavior.
Materials and Methods
Animals
All experiments were designed in strict adherence to the guidelines of the Institutional Animal Ethics Committee (Approval number: AEC/51/318/N.P dated 06/04/2013). Studies were conducted on adult male Wistar rats (180–200 g). The animals were housed in group of 3 in polypropylene cages under standard housing condition with 12-h light and dark cycle and food and water ad libitum.
Chronic-restraint Stress
Restraint stress lasting for 21 days was produced in male Wistar rats using previously established method.[3,4,6,11] Animals were restrained in wire mesh restrainers daily for 6 h/day (9.00 am – 3.00 pm) over a period of 21 days without food and water during the stress period. After the stress protocol, animals were returned to their home cage with food and water ad libitum.
Administration of Celastrus paniculatus
In the present study, we used 400 and 600 mg/kg dose of CP oil and injected intraperitoneally daily for 14 days after stress protocol [Figure 1]. The doses of CP oil were selected based on previous studies.[12,13] After the treatment protocol, all animals were subjected to behavioral evaluation [Figure 1]. CP was solubilized in 1% Tween 20 and 5% dimethyl sulfoxide, and drug solution was prepared freshly.[12,14]
Figure 1.
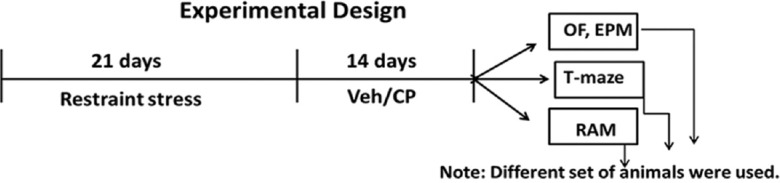
Illustration of experimental design. Veh = Vehicle, CP = Celastrus paniculatus, OF = Open-field test, EPM = Elevated plus maze test, RAM = Radial arm maze task
Evaluation of Exploratory Behavior in Open-field Test
The open field was a square arena (100 cm × 100 cm × 40 cm) whose floor and inner walls are painted black. The floor was divided into 25 squares (20 cm × 20 cm) using thin white lines. The arena was illuminated using overhead tube lights. Experiments were recorded using a video camera from a suitable vantage point so as to cover the entire open field. The rat was placed in the center of the open-field arena. Each rat was allowed to explore the open field for 5 min. The behavior was recorded using a video camera. At the end of 5 min, the rats were returned to their home cage. The open field was cleaned with 70% alcohol between trials to minimize olfactory odors. The recorded video was coded and then subjected to analysis by experimenter. The total number squares crossed were counted as a measure of locomotor behavior.[15] A zone entry was scored when all four paws were in the specified zone.
Evaluation of Anxiety-like Behavioral in Elevated Plus Maze
The test was performed 1 day after the open-field test. Behavioral testing was conducted in a quiet and dimmed room that provided constant illumination along the two open arms of the maze. Behavior during the 5 min test period was monitored and automatically recorded by video camera. Anxiety behavior was studied in the elevated plus maze (EPM). The EPM was a plus-shaped platform elevated 60 cm above the floor.[16] The maze has four arms. Two arms were enclosed by 40 cm high walls, and the other two were open arms without any enclosure. The dimensions of the closed arms are 50 cm × 10 cm × 40 cm with a central platform of 10 cm × 10 cm area. The EPM was illuminated by overhead light source.
The experimental procedure was as follows – rodents were placed in the center of the maze facing the closed arm and were allowed to explore the maze freely for 5 min. The behavior of the animals was video recorded and videos were analyzed offline for a number of parameters such as time spent and number of entries in the open and closed arms. The EPM was cleaned with 70% alcohol after every trial to minimize olfactory cues. The time spent and number of entries in the open arm are the measures of anxiety-like behavior. It reflects a conflict between the animal's fear of open high areas and its natural tendency to explore novel environments. Data were expressed as the percentage of time spent in the open arms (100 × [time in the open arms/total time spent in both arms]) as an index of anxiety-like behavior and the total number of closed-arm entries as an index of overall locomotor activity.
Spatial Learning and Memory Evaluation in Partially Baited Radial Arm Maze Task
The partially baited radial arm maze (RAM) task for spatial learning and memory was evaluated as described previously.[4,11,12,15,17] Rats were placed on the central platform and allowed to obtain reward within 5 min duration, and trail was terminated immediately after all four baits had been consumed or when 5 min had passed. Each rat was given two trails per day. Data from four trials were averaged and expressed as blocks. The data were analyzed for percentage correct choice, reference memory errors (RMEs), and working memory errors (WMEs). An entry into an unbaited arm was considered an RME, and any re-entry was considered as a WME. A re-entry into a baited arm or an unbaited arm was considered as WME correct (WMEC) or WME incorrect (WMEI), respectively.
Spatial Working Memory Evaluation in T-maze Rewarded Alternation Task
Spatial working memory was assessed by T-maze rewarded alternation task. The test was performed as previously described.[6] The T-maze consists of a start box (16.5 cm × 16.5 cm), stem (arm 1) length was 71.1 cm, goal areas (arms 2 and 3) lengths were 45.7 cm, arm width was 10.2 cm, and arm height was 10.2 cm (Columbus Instruments, USA). The stem and start box were separated by a sliding door, and T-maze is kept in a dimly lit, sound-attenuated room.
Procedure
Behavioral evaluation was performed in three phases:
Acclimatization: This session was to familiarize the rats with the T-maze. Rats were partially starved for 24 h and allowed to explore the T-maze for 15 min. Rats were placed in the start box for 30 s. Sliding door was opened to allow rats to explore the T-maze for 15 min and to eat food pellets in each goal area. Rats were then returned to the start box
Acquisition: After the 1st day of acclimatization, the rats were trained for the rewarded alternation task. The same procedure was followed during acquisition as the acclimatization session, except that one arm was baited per trial. The rat has to alternate the arms for the reward. The placement of the reward in the subsequent trial was opposite to the rat's previous arm entry. Each rat received ten trials per day. The inter-trial interval was 30 s during which the maze was cleaned with 70% alcohol to remove odor cues. The number of days each rat took for 80% correct choice (Eight correct entries to the baited arm out of ten trials) was noted
Retention test: 2 days after the last training session, memory retention test was carried out. Rats were given ten trials continuously, with an inter-trial interval of 30 s. The numbers of errors, i.e., entry into the non-rewarded arm in ten trials, were noted.
Statistical Analysis of the Data
Data were analyzed using GraphPad PRISM 6.0 statistical software (GraphPad Software, Inc. CA, USA). Data sheets and videos were coded before analysis, and the experimenter was blind to the treatment conditions. Data from the EPM, open field, T-maze were analyzed using one-way ANOVA followed by Tukey's post hoc test. Percentage correct choice and RME in the RAM was analyzed using one-way ANOVA followed by Tukey's post hoc test and two-way ANOVA followed by Bonferroni's post hoc test. The data were expressed as mean ± standard error of the mean, and P < 0.05 was considered statistically significant.
Results
Anxiolytic Effect of Celastrus paniculatus Oil in Stressed Animals
In the EPM, percentage time spent in the open arms declined significantly [F4, 55 = 279.1, P < 0.001, Figure 2a], whereas percentage time spent in closed arms drastically increased in stressed animals compared to control animals [F4, 55 = 240.3, P < 0.001, Figure 2c]. CP oil treatment resulted in anxiolytic activity in stressed animals by increasing time spent in open arms [F4, 55 = 279.1, P < 0.001, Figure 2a] and decreasing time spent in closed arms [F4, 55 = 240.3, P < 0.001, Figure 2c].
Figure 2.
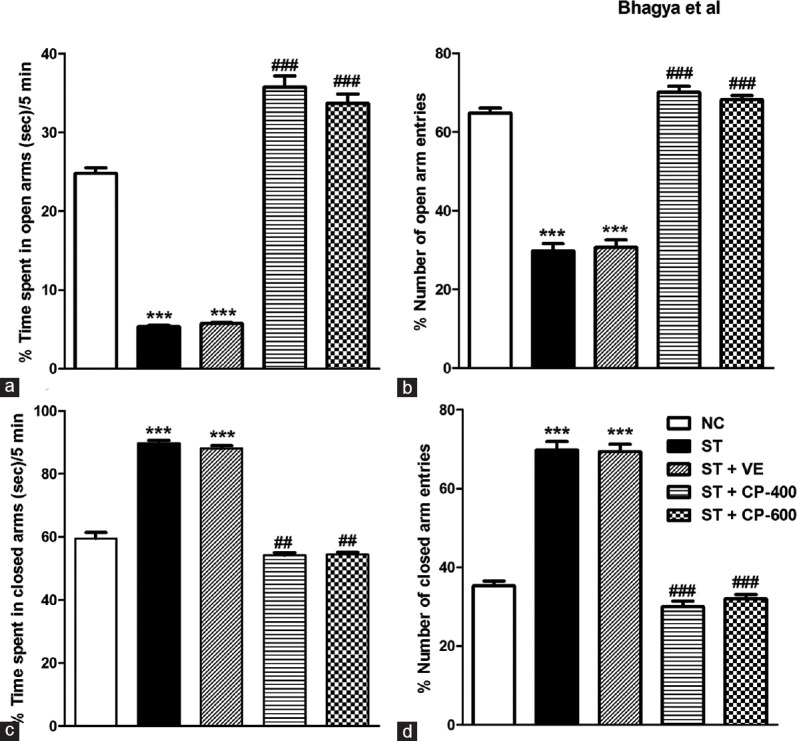
Chronic stress increased anxiety-like behavior in elevated plus maze and chronic Celastrus paniculatus treatment to stressed rats decreased anxiety behavior in these animals. (a) Percentage time spent in open arms; (b) percentage number of open arms entries; (c) percentage time spent in closed arms; (d) percentage number of closed arms entries. Data are represented as mean ± standard error of the mean. NC: Normal control (n = 12). ST: Rats subjected to restraint stress for 21 days (6 h/day) (n = 12). ST + VE (n = 12), ST + CP-400 (n = 12), and ST + CP-600 (n = 12): Stressed rats subjected to 14 days of treatment with vehicle, Celastrus paniculatus 400 or 600 mg/kg, intraperitoneally, respectively. One-way ANOVA followed by Tukey's post hoc test. ***P < 0.001 normal versus stress. ##P < 0.01, ###P < 0.001 stress versus stress + drug treatment
The number of open arm entries reduced significantly [F4, 55 = 168.9, P < 0.001, Figure 2b] and number of closed arm entries increased in stressed condition [F4, 55 = 163.8, P < 0.001, Figure 2d], whereas the total number of arm entries did not alter significantly, indicating that overall locomotor activity was unaltered across different groups of rats (F4, 55 = 1.99, P > 0.05). CP restored open [F4, 55 = 168.9, Figure 2b] and closed arm entries [F4, 55 = 163.8, P < 0.001, Figure 2d] in ST group without affecting total arm entries (F4, 55 = 1.99, P > 0.05). As the total number of entries did not change among groups, it indicates that motor activity is spared by chronic stress and drug treatment.
Effect of Chronic Stress and Celastrus paniculatus Treatment on Locomotor and Exploratory Behavior in Open-field Test
Locomotor and exploratory behavior was evaluated in open field. Both chronic stress and CP oil treatment did not affect locomotor and exploratory behavior. Total time spent by rats in the periphery [F4, 55 = 0.78, P > 0.05, Figure 3a] and the number of crossings in the open-field test did not change in all groups studied [F4, 55 = 3.43, P > 0.05, Figure 3b].
Figure 3.
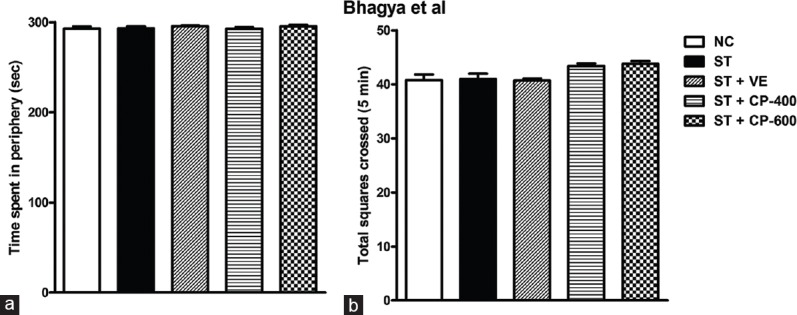
Both stress and Celastrus paniculatus treatment did not affect locomotor and exploratory behavior in open-field test. (a) Time spent in periphery (sec); (b) total squares crossed (5 min) in open filed. Data are represented as mean ± standard error of the mean. NC: normal control (n = 12). ST: rats subjected to restraint stress for 21 days (6 h/day) (n = 12). ST + VE (n = 12), ST + CP-400 (n = 12), and ST + CP-600 (n = 12): stressed rats subjected to 14 days of treatment with vehicle, Celastrus paniculatus 400 or 600 mg/kg, intraperitoneally, respectively
Celastrus paniculatus Ameliorated Spatial Learning and Memory Deficits in Stressed Rats
Two-way ANOVA with repeated measures revealed significant main effect of groups [F4, 327 = 61.23, P < 0.001, Figure 4a] in the RAM. Chronically restrained rats exhibited impaired spatial learning and memory. They showed significantly lower percentage correct choices in comparison with normal control animals. Stressed rats showed only 57.5% ±1.1% correct choices even after 16 days of training (normal control: 88.33% ±1.8% correct choices). Vehicle treatment did not improve the spatial learning in ST rats. On the other hand, chronic daily treatment with two doses of CP oil (400 mg/kg and 600 mg/kg) for 14 days produced a significant increase in percentage correct choices [F4, 327 = 61.23, P < 0.001, Figure 4a]. Post hoc analysis indicated that significant difference was observed between stressed and treated groups from the 5th block of RAM. CP-treated animals performed better when compared to stressed animals in learning partially baited RAM task. At the end of training, the percentage correct choices of ST + CP-400 and ST + CP-600 groups of animals were significantly higher than ST and ST + VE groups in block 7 [F4, 43 = 27.69, P < 0.001, Figure 4b] and block 8 [F4, 43 = 36.66, P < 0.001, Figure 4b]. However, there was no difference between 400 and 600 mg doses of CP oil [P > 0.05; Figure 4b].
Figure 4.
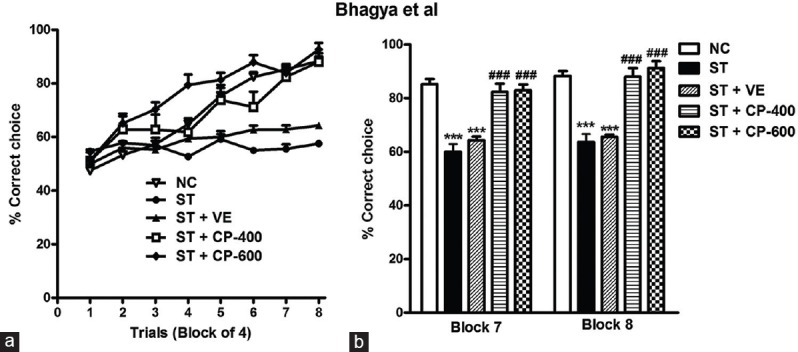
The effect of chronic Celastrus paniculatus oil treatment on performance of a partially baited task in 8-arm radial maze in different groups. (a) The acquisition (percentage correct choice) of the radial arm maze task across trials; (b) performance in block 7 and 8 (percentage correct choice). Data are represented as mean ± standard error of the mean. NC: Normal control (n = 14). ST: Rats subjected to restraint stress for 21 days (6 h/day) (n = 8). ST + VE (n = 8), ST + CP-400 (n = 8), and ST + CP-600 (n = 8): Stressed rats subjected to 14 days of treatment with vehicle, Celastrus paniculatus 400 or 600 mg/kg, intraperitoneally, respectively. One-way ANOVA followed by Tukey's post hoc test. ***P < 0.001 normal versus stress. ###P < 0.001 stress versus stress + drug treatment
Reference Memory Impairment in Stressed Rats was Rescued by Celastrus paniculatus Oil Treatment
Entries into unbaited arms were measured as RMEs. A post hoc analysis showed that the number of RMEs in the ST and ST + VE groups was significantly higher than NC group [F4, 327 = 45.21, P < 0.001, Figure 5a] and chronic CP treatment considerably counteracted stress-induced effect on reference memory [F4, 327 = 45.21, P < 0.001, Figure 5]. Both treated groups made fewer RMEs compared to ST group. One-way ANOVA reveals that the number of RMEs in block 7 [F4, 43 = 44.90, P < 0.001, Figure 5b] and block 8 [F4, 43 = 103.3, P < 0.001, Figure 5b] was significantly lower in ST + CP-400 and ST + CP-600 groups compared to ST group. Both doses of CP appeared to be effective in ameliorating stress-induced learning and memory deficits, and as such, there was no obvious dose–response relationship.
Figure 5.
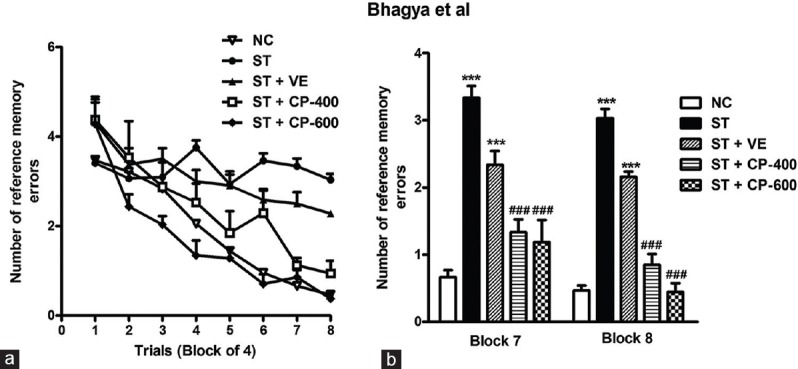
The effect of chronic Celastrus paniculatus oil treatment on reference memory in radial arm maze task. (a) The number of reference memory errors during acquisition of the radial arm maze task across trials; (b) reference memory errors in block 7 and 8 (reference memory errors). Data are represented as mean ± standard error of the mean. NC: Normal control (n = 14). ST: Rats subjected to restraint stress for 21 days (6 h/day) (n = 8). ST + VE (n = 8), ST + CP-400 (n = 8), and ST + CP-600 (n = 8): Stressed rats subjected to 14 days of treatment with vehicle, Celastrus paniculatus 400 or 600 mg/kg, intraperitoneally, respectively. One-way ANOVA followed by Tukey's post hoc test. ***P < 0.001 normal versus stress. ###P < 0.001 stress versus stress + drug treatment
There was no significant difference observed between different groups in working memory component of the RAM task. Both WMEC and WMEI were same in all groups (Data not shown).
Effect of Stress and Celastrus paniculatus Oil Treatment on Retention of the Radial Arm Maze Task
We did retention test 10 days after the acquisition of the task to assess whether the effect of CP oil was restricted to learning or it had effect on memory as well. In the retention test, both ST and ST + VE rats continued to show the impairment seen during the acquisition of the RAM task [F4, 43 = 36.95; P < 0.001, Figure 6a]. CP-treated groups of rats showed the same level of performance in the retention test as that of acquisition. The percentage correct choice was comparable to normal control [P > 0.05; Figure 6a]. Similarly, an increase in the number of RMEs seen in the acquisition in the ST and ST + VE groups was persistent in the retention test [F4, 43 = 30.48; P < 0.001, Figure 6b]. Interestingly, treated groups made less RMEs indicating preservation of the learned information. As seen in the acquisition, there was no difference with regard to WMEs for all the groups in the retention test.
Figure 6.
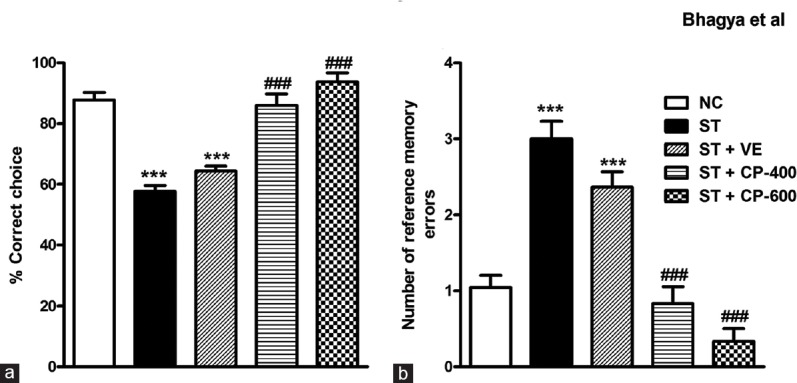
The effect of chronic Celastrus paniculatus oil treatment on retention (memory) in radial arm maze task. (a) The percentage correct choices; (b) number of reference memory errors in the retention test. Data are represented as mean ± standard error of the mean. NC: Normal control (n = 14). ST: Rats subjected to restraint stress for 21 days (6 h/day) (n = 8). ST + VE (n = 8), ST + CP-400 (n = 8), and ST + CP-600 (n = 8): Stressed rats subjected to 14 days of treatment with vehicle, Celastrus paniculatus 400 or 600 mg/kg, intraperitoneally, respectively. One-way ANOVA followed by Tukey's post hoc test. ***P < 0.001 normal versus stress. ###P < 0.001 stress versus stress + drug treatment
Spatial Working Memory Deficits in Stressed Rats was Rescued by Celastrus paniculatus Oil Treatment
In the learning phase of the T-maze rewarded alteration task, stressed animals showed poor performance compared to normal control animals. ST and ST + VE groups took more days to reach 80% criterion in comparison with NC [F4, 45 = 18.91; P < 0.001, Figure 7a]. Strikingly, the CP-treated animals took fewer days to reach criterion compared to ST group. The improved performance of CP-treated groups in learning T-maze rewarded alternation task was statistically significant compared to ST group [F4, 45 = 18.91; P < 0.001, Figure 7a].
Figure 7.
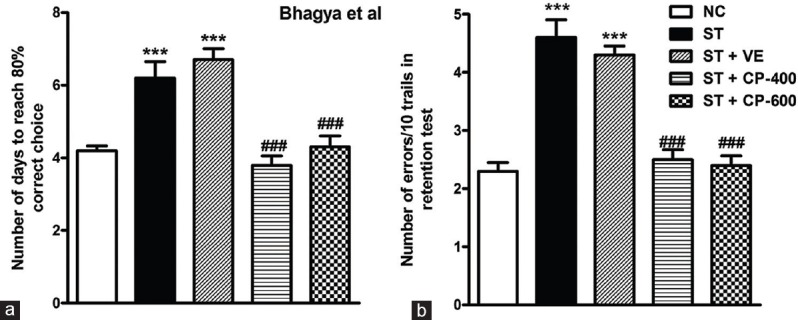
Chronic stress resulted in impaired spatial working memory in T-maze task and chronic Celastrus paniculatus treatment to stressed rats ameliorated working memory deficits in these animals. (a) Number of days to reach 80% correct choice; (b) number of errors per 10 trials in the retention test of T-maze rewarded alteration task. Data are represented as mean ± standard error of the mean. NC: Normal control (n = 10). ST: Rats subjected to restraint stress for 21 days (6 h/day) (n = 10). ST + VE (n = 10), ST + CP-400 (n = 10), and ST + CP-600 (n = 10): Stressed rats subjected to 14 days of treatment with vehicle, Celastrus paniculatus 400 or 600 mg/kg, intraperitoneally, respectively. One-way ANOVA followed by Tukey's post hoc test. ***P = 0.01 normal versus stress, ###P < 0.001 stress versus stress + drug treatment
In addition, stressed animals made more errors (number of errors/10 trails in retention test) compared to NC. Both ST + CP-400 and ST + CP-600 groups made less errors compared to ST group [F4, 45 = 32.84; P < 0.001, Figure 7b]. This indicates that the CP-treated animals have better spatial working memory.
Discussion
The current study intended to assess the beneficial effect of CPoil treatment on spatial learning and memory and anxiety in chronically stressed rats. We showed that chronic restraint stress for 21 days resulted in impaired spatial learning and memory in RAM task. Furthermore, stressed animals exhibited working memory impairment in T-maze task. Chronic stress resulted in enhanced anxiety-like behavior in the EPM. The data are in consistent with the previous studies suggesting compromised memory functions and enhanced anxiety in chronic stress models.[3,4,5,6]
Interestingly, chronic treatment with CP oil for 14 days reversed stress-induced spatial learning and memory impairment in RAM task as shown by enhanced percentage correct choices and reduced RMEs. Working memory is also restored back to normal after CP oil treatment. CP oil showed anxiolytic action in this chronic stress model by increasing number of entries and time spent in the open arms of EPM. Interestingly, vehicle treatment to stressed rats did not show any improvement in spatial learning, working memory, and anxiety behavior. This confirms the beneficial effect of CP on stress-induced cognitive deficits.
The precise mechanism responsible for beneficial effects of CP oil against chronic stress-induced cognitive dysfunctions is not known, but it may be due to its antioxidant and neuroprotective effects.[10,18,19] Previous studies examined the effects of CP on learning and memory in different behavioral tasks.[12,20,21] When tested in a raised platform shock-avoidance task, CP-treated group (400 mg/kg once daily for 3 days) displayed a significantly improved learning compared to vehicle control group.[20] Another study by Nalini et al. reported that CP oil treatment for 15 days (850 mg/kg once daily) enhanced retention in passive avoidance task.[21] In addition, previous study from our laboratory demonstrated cognitive-enhancing effect of CP in naive animals.[12] Hence, the current study is consistent with previous studies with regard to the ability of CP in augmenting cognitive performance in different memory tasks.
Methanolic extract of CPshowed a dose-dependent free radical scavenging action and a protective effect on H2O2-induced cytotoxicity and DNA damage in human nonimmortalized fibroblasts.[22] Furthermore, CP water extracts protected cultured forebrain neuronal cells by reducing lipid peroxidation and enhancing the antioxidant enzyme catalase activity.[18] CP water extract showed neuroprotective action against glutamate-induced toxicity in neuronal cultures and reversibly inhibited N-methyl-D-aspartate-activated whole currents.[19] Previous study has shown cognitive-enhancing efficacy of CP in normal animals.[12] An improvement in learning and memory in both the shuttle box and step-through paradigm was seen after aqueous seed extract of CP.[23] Chronic oral administration of CP seed oil (50, 200, or 400 mg/kg) for 14 days completely reversed the scopolamine (0.5 mg/kg)-induced learning deficits.[21,13]
Previous studies demonstrated the anti-anxiety activity of CP. The petroleum ether extract of seeds showed anti-anxiety activity by significantly reduced punishment and reward associated inhibition of operant behavior in rats at different doses.[24] CP oil exhibited significant anxiolytic activity in the EPM and thirsty rat conflict paradigm.[24,25] Further, CP did not produce any sedative effect and reversed anxiolytic effect of 5-HT1A partial agonist buspirone in the open-field test, indicating its effect on serotonergic neurotransmission.[25]
Conclusion
CP oil treatment for 14 days showed ameliorating effect on chronic restraint stress-induced cognitive deficits. CP restored spatial learning and memory, showed anti-anxiety activity in stressed condition. In conclusion, this work provides a novel perspective on beneficial effect of herbal therapy on stress-induced cognitive dysfunctions. However, further studies are necessitated to identify cellular and molecular mechanisms of CPon cognitive-enhancing activity in stress and stress-associated disorders.
Financial Support and Sponsorship
Financial support of this work by the Science and Engineering Research Board (SERB: SB/FT/LS-371/2012), Department of Science and Technology, Government of India, to BV; DBT and NIMHANS are gratefully acknowledged.
Conflicts of Interest
There are no conflicts of interest.
References
- 1.Olivier B, Zethof T, Pattij T, van Boogaert M, van Oorschot R, Leahy C, et al. Stress-induced hyperthermia and anxiety: Pharmacological validation. Eur J Pharmacol. 2003;463:117–32. doi: 10.1016/s0014-2999(03)01326-8. [DOI] [PubMed] [Google Scholar]
- 2.Elliott GR, Eisdorfer C. Stress and Human Health. New York: Springer Publishing; 1982. [Google Scholar]
- 3.McLaughlin KJ, Gomez JL, Baran SE, Conrad CD. The effects of chronic stress on hippocampal morphology and function: An evaluation of chronic restraint paradigms. Brain Res. 2007;1161:56–64. doi: 10.1016/j.brainres.2007.05.042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Srikumar BN, Raju TR, Shankaranarayana Rao BS. The involvement of cholinergic and noradrenergic systems in behavioral recovery following oxotremorine treatment to chronically stressed rats. Neuroscience. 2006;143:679–88. doi: 10.1016/j.neuroscience.2006.08.041. [DOI] [PubMed] [Google Scholar]
- 5.Kleen JK, Sitomer MT, Killeen PR, Conrad CD. Chronic stress impairs spatial memory and motivation for reward without disrupting motor ability and motivation to explore. Behav Neurosci. 2006;120:842–51. doi: 10.1037/0735-7044.120.4.842. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Ramkumar K, Srikumar BN, Shankaranarayana Rao BS, Raju TR. Self-stimulation rewarding experience restores stress-induced CA3 dendritic atrophy, spatial memory deficits and alterations in the levels of neurotransmitters in the hippocampus. Neurochem Res. 2008;33:1651–62. doi: 10.1007/s11064-007-9511-x. [DOI] [PubMed] [Google Scholar]
- 7.Hata T, Itoh E, Nishikawa H. Stress-induced anxiety and endogenous anxiogenic substances. Nihon Yakurigaku Zasshi. 2000;115:13–20. doi: 10.1254/fpj.115.13. [DOI] [PubMed] [Google Scholar]
- 8.Gregus A, Wintink AJ, Davis AC, Kalynchuk LE. Effect of repeated corticosterone injections and restraint stress on anxiety and depression-like behavior in male rats. Behav Brain Res. 2005;156:105–14. doi: 10.1016/j.bbr.2004.05.013. [DOI] [PubMed] [Google Scholar]
- 9.Nalini K, Aroor AR, Kumar KB, Rao A. Studies on biogenic amines and their metabolites in mentally retarded children on celastrus oil therapy. Altern Med. 1986;1:355–60. [Google Scholar]
- 10.Godkar PB, Gordon RK, Ravindran A, Doctor BP. Celastrus paniculatus seed oil and organic extracts attenuate hydrogen peroxide- and glutamate-induced injury in embryonic rat forebrain neuronal cells. Phytomedicine. 2006;13:29–36. doi: 10.1016/j.phymed.2003.11.011. [DOI] [PubMed] [Google Scholar]
- 11.Veena J, Srikumar BN, Mahati K, Bhagya V, Raju TR, Shankaranarayana Rao. BS Enriched environment restores hippocampal cell proliferation and ameliorates cognitive deficits in chronically stressed rats. J Neurosci Res. 2009;87:831–43. doi: 10.1002/jnr.21907. [DOI] [PubMed] [Google Scholar]
- 12.Lekha G, Bhagya PK, Rao S, Arockiasamy I, Mohan K. Cognitive enhancement and neuroprotective effect of Celastrus paniculatus Willd. seed oil (Jyothismati oil) on male Wistar rats. J Pharm Sci Technol. 2010;2:130–8. [Google Scholar]
- 13.Gattu M, Boss KL, Terry AV, Jr, Buccafusco JJ. Reversal of scopolamine-induced deficits in navigational memory performance by the seed oil of Celastrus paniculatus. Pharmacol Biochem Behav. 1997;57:793–9. doi: 10.1016/s0091-3057(96)00391-7. [DOI] [PubMed] [Google Scholar]
- 14.Lekha G, Mohan K, Samy IA. Effect of Celastrus paniculatus seed oil (Jyothismati oil) on acute and chronic immobilization stress induced in swiss albino mice. Pharmacognosy Res. 2010;2:169–74. doi: 10.4103/0974-8490.65512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Bhagya V, Srikumar BN, Raju TR, Shankaranarayana Rao BS. The selective noradrenergic reuptake inhibitor reboxetine restores spatial learning deficits, biochemical changes, and hippocampal synaptic plasticity in an animal model of depression. J Neurosci Res. 2015;93:104–20. doi: 10.1002/jnr.23473. [DOI] [PubMed] [Google Scholar]
- 16.Anuradha H, Srikumar BN, Shankaranarayana Rao BS, Lakshmana M. Euphorbia hirta reverses chronic stress-induced anxiety and mediates its action through the GABA (A) receptor benzodiazepine receptor-Cl(-) channel complex. J Neural Transm (Vienna) 2008;115:35–42. doi: 10.1007/s00702-007-0821-6. [DOI] [PubMed] [Google Scholar]
- 17.Bhagya V, Srikumar BN, Raju TR, Rao BS. Chronic escitalopram treatment restores spatial learning, monoamine levels, and hippocampal long-term potentiation in an animal model of depression. Psychopharmacology (Berl) 2011;214:477–94. doi: 10.1007/s00213-010-2054-x. [DOI] [PubMed] [Google Scholar]
- 18.Godkar P, Gordon RK, Ravindran A, Doctor BP. Celastrus paniculatus seed water soluble extracts protect culture rat forebrain neuronal cells from hydrogen peroxide induced oxidative injury. Fitoterapia. 2003;74:658–69. doi: 10.1016/s0367-326x(03)00190-4. [DOI] [PubMed] [Google Scholar]
- 19.Godkar PB, Gordon RK, Ravindran A, Doctor BP. Celastrus paniculatus seed water soluble extracts protect against glutamate toxicity in neuronal cultures from rat forebrain. J Ethnopharmacol. 2004;93:213–9. doi: 10.1016/j.jep.2004.03.051. [DOI] [PubMed] [Google Scholar]
- 20.Karanth KS, Haridas KK, Gunasundari S, Guruswami MN. Effect of Celastrus paniculatus on learning process. Arogya. 1980;6:137–9. [Google Scholar]
- 21.Nalini K, Karanth KS, Rao A, Aroor AR. Effects of Celastrus paniculatus on passive avoidance performance and biogenic amine turnover in albino rats. J Ethnopharmacol. 1995;47:101–8. doi: 10.1016/0378-8741(95)01264-e. [DOI] [PubMed] [Google Scholar]
- 22.Russo A, Izzo AA, Cardile V, Borrelli F, Vanella A. Indian medicinal plants as antiradicals and DNA cleavage protectors. Phytomedicine. 2001;8:125–32. doi: 10.1078/0944-7113-00021. [DOI] [PubMed] [Google Scholar]
- 23.Kumar MH, Gupta YK. Antioxidant property of Celastrus paniculatus willd.: A possible mechanism in enhancing cognition. Phytomedicine. 2002;9:302–11. doi: 10.1078/0944-7113-00136. [DOI] [PubMed] [Google Scholar]
- 24.Jadhav RB, Patwardhan B. Anti-anxiety activity of Celastrus paniculatus seeds. Indian J Nat Prod. 2003;19:16–9. [Google Scholar]
- 25.Rajkumar R, Kumar EP, Sudha S, Suresh B. Evaluation of anxiolytic potential of Celastrus oil in rat models of behaviour. Fitoterapia. 2007;78:120–4. doi: 10.1016/j.fitote.2006.09.028. [DOI] [PubMed] [Google Scholar]


