Abstract
Objectives:
The aim of this study is to evaluate the effect of four triterpenoids such as oleanolic acid, ursolic acid, cycloastragenol, and beta-boswellic acid alone and in combination with antibiotics against Staphylococcus aureus strains.
Materials and Methods:
Sixteen clinical strains of S. aureus from infected wounds were isolated. Eight were methicillin-sensitive S. aureus (MSSA), and the other eight were methicillin-resistant S. aureus (MRSA). The activity was also seen in reference S. aureus American Type Culture Collection™ strains. The activity of all the triterpenoids and antibiotics against S. aureus was evaluated by broth microdilution method. The effectiveness was judged by comparing the minimum inhibitory concentrations (MICs) of the compounds with antibiotics. The combination of antibiotics with compounds was evaluated by their fractional inhibitory concentrations (FIC).
Results:
Against both clinical and reference MSSA strains, none of the compounds exhibited comparable activity to antibiotics vancomycin or cefradine except for ursolic acid (MIC 7.8 μg/ml). Against MRSA, all compounds (MIC 16–128 μg/ml) showed lesser activity than vancomycin (MIC 5.8 μg/ml). Among triterpenoid-antibiotic combinations, the most effective were ursolic acid and vancomycin against clinical strain MSSA (FICS 0.17). However, overall, different combinations between triterpenoids and antibiotics showed 95%–46% (P < 0.05) reduction in MICs of antibiotics compared to when antibiotics were used alone. Cefradine, a drug not suitable for treating MRSA (MIC = 45 μg/ml), showed a remarkable decrease in its MIC (87% P< 0.01) when it was used in combination with oleanolic acid or ursolic acid in both clinical and reference strains.
Conclusion:
The tested triterpenoids are relatively weaker than antibiotics. However, when used in combination with antibiotics, they showed remarkable synergistic effect and thus can help in prolonging the viability of these antibiotics against S. aureus infections. Furthermore, reduction in MIC of cefradine with oleanolic acid indicates their potential use against MRSA.
Key words: Antibiotics, antimicrobial activity, Staphylococcus aureus, synergism, triterpenoids
Staphylococcus aureus is an important pathogen, causing nosocomial and postsurgical infections. Unfortunately, S. aureus has become resistant to most of the antibiotics because of their extensive use. The high degree of resistance in S. aureus is a worldwide problem that demands new drugs to combat it.
Triterpenoids are a class of naturally occurring organic chemicals derived from six isoprenoid units (30 carbons) that are produced by plants and are reported to possess antimicrobial activity among other attributes.[1] The four triterpenoids in this study are oleanolic acid, ursolic acid, beta-boswellic acid, and cycloastragenol. Research has been done on the antistaphylococcal properties of oleanolic acid, ursolic acid, and beta-boswellic acid,[2,3] but only against reference strains of S. aureus and not against clinical isolates. No source could be found in literature regarding the antibacterial properties of cycloastragenol.
Synergistic combinations of different antimicrobials can help counter the resistance in bacteria and reduce risks of toxicity.[4] Synergism is defined as when the effect of the drugs combines together for a greater effect while antagonism occurs when a combination of drugs worsens activity.[5] Research has also been done to evaluate interactions between plant products and antibiotics,[6] but the four compounds in this study have not been tested for interaction with antibiotics against any bacteria.
This study aims to judge the antistaphylococcal activity of all these four compounds in clinical isolates of S. aureus and to evaluate their interaction with antibiotics in combination studies.
Materials and Methods
Compounds and Antibiotics
The four triterpenoids were all acquired from Sigma-Aldrich (USA) while the analytical grade antibiotic cefradine and vancomycin was used. The potency of every compound and antibiotic was measured as per the guidelines of Clinical and Laboratory Standards Institute (CLSI),[7] using the formula:
Potency = (Assay purity) × (Active fraction) × (1 − Water Content)
The information of assay purity and water content was provided by the manufacturer for each of the compound and antibiotic. The potency of four compounds was 919.125 μg/mg for beta-boswellic acid, 942.7 μg/mg for ursolic acid, 945.7 μg/mg for cycloastragenol, and 960.4 μg/mg for oleanolic acid. Cefradine and vancomycin had a potency of 902 μg/mg and 900 μg/mg, respectively.
Bacterial Strains
Sixteen bacterial strains were obtained from microbiology laboratory and were isolated from infected wounds, abscesses, rectal specimens, and blood. Eight of the bacterial strains tested were methicillin sensitive while the other eight strains tested were methicillin resistant. The criteria used for judging resistance were as per the guidelines of CLSI.[7] Methicillin-resistant S. aureus/methicillin-sensitive S. aureus (MRSA/MSSA) typing was assessed using cefoxitin whose minimum inhibitory concentration (MIC) came out to be 8 μg/ml against MRSA strain and 2 μg/ml against MSSA strain using broth microdilution method (MIC >4 μg/ml indicates mec-A-mediated resistance and hence MRSA). Two reference strains from American Type Culture Collection (ATCC™) were used. Reference MSSA strain used was S. aureus subsp. aureus (ATCC® 29213™). Reference MRSA strain used was S. aureus subsp. aureus (ATCC® 43300™). Hence, a total of eighteen strains were used.
Minimum Inhibitory Concentration Determination
All the four triterpenoids and the two antibiotics were tested against the 18 strains of S. aureus by the microdilution broth method. The two antibiotics were dissolved in water to give a stock solution of 8192 μg/ml. None of the four triterpenoids were soluble in water and had to be dissolved in dimethyl sulfoxide (DMSO) to give a stock solution of 16,384 μg/ml for each of the four compounds. Final concentration of DMSO in each microtiter well was <1% which is inactive against bacteria.[8] Serial two-fold dilutions of antibiotics and triterpenoids from their stock solutions resulted in concentrations of 256, 128, 64, 32, 16, 8, 4, 2, and 1 μg/ml. Fifty microliters of antibiotic or triterpenoid from these solutions was then dispensed into the microtiter wells.
The strains of S. aureus were cultured in Mueller Hinton Broth at 37°C ± 1°C and at a pH of 7.4 ± 0.1 for 24 h. The calcium added in the broth was 2.45 mg/100 ml, and 1.22 mg/100 ml of magnesium was also added. After 24 h incubation, 1 ml of cultured broth was suspended in 9 ml of fresh saline and was incubated with the same conditions for 6 h. After second incubation, the turbidity of the broth was compared to 0.5 McFarland standard, and adjustments were made. This gave an approximate bacterial concentration of 1.5 × 108 CFU/ml in the broth. This was further diluted by adding more broth to give an approximate bacterial concentration of 1 × 106 CFU/ml.
Fifty microliters from this adjusted broth was dispensed into microtiter wells containing 50 μl of antibiotic or triterpenoid in particular concentrations. The control wells had 50 μl of bacterial suspension and 50 μl of fresh broth containing 1% DMSO. The concentration of the triterpenoids and antibiotics increased progressively from right to left in the microtiter plates. The final bacterial count in each well was 5 × 105 CFU/ml. The microtiter plates were then incubated for 24 h and MIC was measured as the lowest concentration of antibiotic or triterpenoid at which no turbidity had appeared and at concentrations lower than that turbidity increased consequently. Experiments were performed a single time on each of the eight clinical strains of MSSA and MRSA, respectively. Experiments on the two reference strains were done three times. The values thus reported for clinical isolates are the mean of eight values for MSSA and MRSA, respectively, while for the two reference strains, mean is calculated using three values.
Determination of Interaction between Triterpenoids and Antibiotics
To determine interaction between triterpenoids and antibiotics, checkerboard assay was used.[5] Twenty-five microliters of triterpenoid was added into 25 μl of antibiotic in a microtiter well to form 50 μl. Then, 50 μl of bacterial suspension having a bacterial count of 1 × 106 CFU/ml was added in the well to make the solution 100 μl. The final bacterial count in each well was about 5 × 105 CFU/ml. The control wells had 50 μl of bacterial suspension and 50 μl of fresh broth containing 1% DMSO. In this way, each of the four triterpenoids was used with the two antibiotics against 18 strains of S. aureus. Different concentrations of triterpenoids were used with different concentrations of antibiotics to evaluate the full extent of interaction. The microtiter plates were then incubated for 24 h, and MIC was measured as the lowest concentration of antibiotic or triterpenoid both at which no turbidity had appeared and at concentrations lower than that turbidity increased consequently. Experiments were performed a single time for each of the eight strains of MSSA and MRSA, respectively. The values thus reported for clinical isolates are the mean of eight values of MSSA and MRSA, respectively, while for reference strains, mean is calculated using just the three values.
Fractional inhibitory concentrations (FIC) for the triterpenoids and antibiotics were obtained by the following formula:
FIC of triterpenoid (FICT) = MIC of triterpenoid in combination/MIC of triterpenoid alone
FIC of antibiotic (FICA) = MIC of antibiotic in combination/MIC of antibiotic alone
The two fractional concentrations were then added for each antibiotic-triterpenoid combination and is called the sum of FICS.
FICS = FICT + FICA
Following criteria were then used to classify the interaction:
Synergistic when FICS was 0.5 or <0.5
Additive when FICS was between 0.5 and 4
Antagonistic when FICS was above 4.[5]
Statistical Analysis
The experiments to measure MIC without combination were conducted a single time for each of the eight clinical strains of MSSA and MRSA, respectively, and three times for the two reference strains. The results from clinical isolates are reported as mean of eight values for MSSA and MRSA, respectively, along with standard error of mean. For the two reference strains, mean is calculated from three values. In combination, a single experiment was performed for each of the eight strains of MSSA and MRSA, respectively, and three times for the two reference strains. The mean for clinical isolates is thus calculated out of eight values for MSSA and MRSA, respectively. For reference strains, mean is calculated from three values. Paired Student's t-test was performed by IBM Statistical Package for the social sciences version 19. (IBM, Armonk, New York, United States of America) to evaluate the means and to judge whether reduction in concentration of antibiotics in combination is significant or not. P < 0.05 was considered statistically significant.
Ethical Declaration
The study was granted approval by the Ethical Committee for surgeons of Rawalpindi Medical College, University of Health Sciences and found no ethical issues in this study.
Results
Antibacterial Effect of Tested Triterpenoids
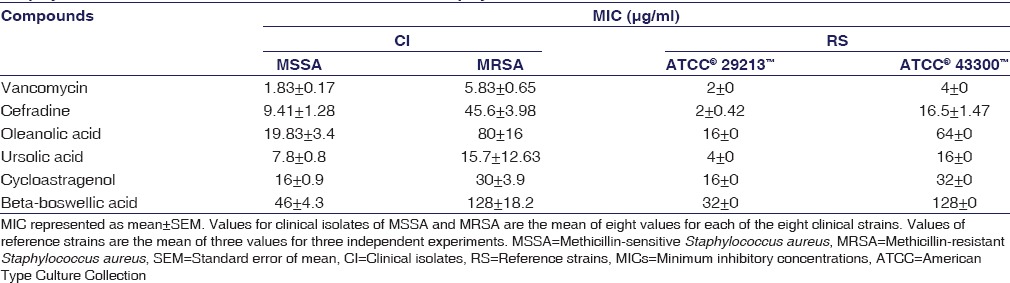
MIC values are listed in Table 1, MSSA for both clinical and reference strains. Against MSSA, all the triterpenoids showed moderate to poor antistaphylococcal properties. Only ursolic acid had an activity comparable to that of cefradine [Table 1 MSSA]. Cycloastragenol and oleanolic acid both showed potency against S. aureus. Against MRSA, the individual activity for every triterpenoid worsened, and none of the four triterpenoids had an activity comparable to vancomycin as is evident by their increased inhibitory concentrations [Table 1 MRSA].
Table 1.
Minimum inhibitory concentration values for tested compounds in methicillin-sensitive Staphylococcus aureus and methicillin-resistant Staphylococcus aureus
Antibacterial Effect of Combination of Triterpenoids and Antibiotics
Effective combinations are listed in Table 2. Many of the combinations of triterpenoids and antibiotics were effective against S. aureus except for six combinations [Table 2]. According to the FICS for different combinations of triterpenoids and antibiotics [Table 2], the most synergistic action was between ursolic acid and vancomycin in clinical strain MSSA (FICs = 0.17), whereas the least effective combination was between beta-boswellic acid and vancomycin in reference MRSA (FICs = 1). The triterpenoids decreased the concentration of antibiotics by 95%–46% [Table 3] (P < 0.05).
Table 2.
Combination testing of antibiotics and compounds against methicillin-sensitive Staphylococcus aureus and methicillin-resistant Staphylococcus aureus
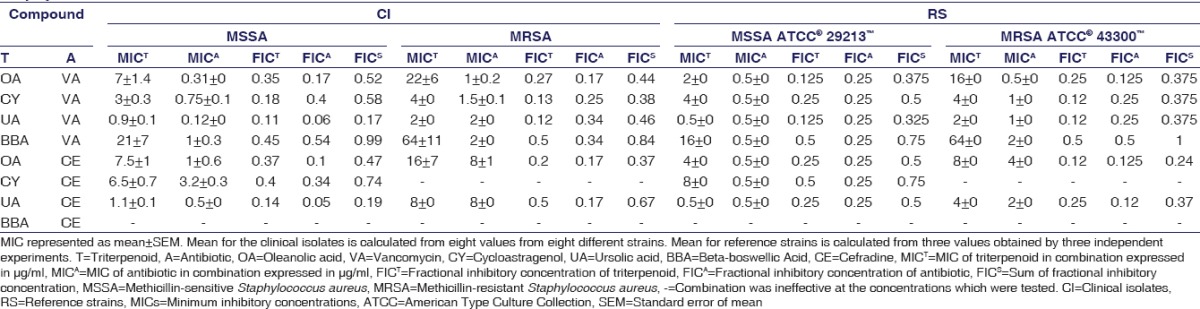
Table 3.
Percentage reduction in minimum inhibitory concentrations of antibiotics when used with triterpenoids
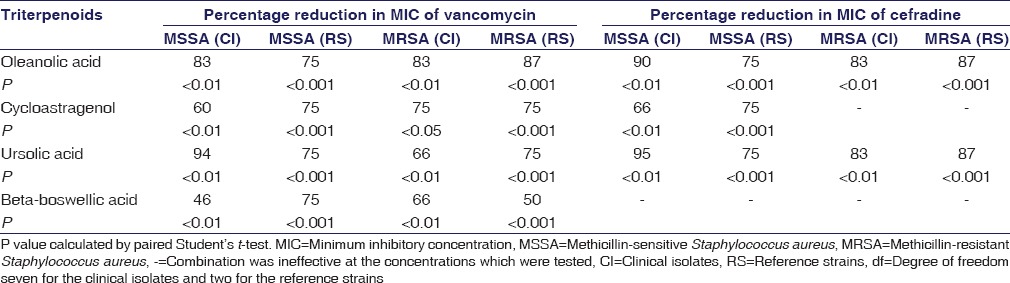
Cefradine is a drug not suitable to treat MRSA infections as its MIC is >32 μg/ml, but in combination with oleanolic acid or ursolic acid, its MIC decreased to 83%–87% (P < 0.01). Similarly, against MSSA, cefradine concentration is reduced by 90%–75% with oleanolic acid (P < 0.01) [Table 3].
Vancomycin is suitable for both MSSA and MRSA, but its combination with triterpenoids helps to reduce its concentration by 46%–94% against MSSA and MRSA (P < 0.05) in both clinical and reference strains [Table 3].
Discussion
The results of this study suggest a possible use of these triterpenoids as an active antistaphylococcal agent. Individually, these compounds are weaker than the prevalent antibiotics, but their combination with vancomycin and cefradine reduces the MIC of both the triterpenoids as well as the antibiotics, which might in turn reduce resistance in S. aureus and toxicity in the body as demonstrated by other researchers.[4,6,9]
The experiments performed in this study were in vitro, and hence it would take in vivo studies to determine the full extent of the importance of these triterpenoids. The cytotoxicity of these compounds is a concern since these compounds are more cytotoxic than antibiotics,[9,10,11,12] but oleanolic acid and ursolic acid are safe in dosages which are antistaphylococcal.[13,14,15] Moreover, as this study indicates that the use of oleanolic acid or ursolic acid in combination with antibiotics reduces their MIC to 7–1 μg/ml from 16 to 128 μg/ml [Tables 2 and 3 P < 0.01] and hence might decrease the risk of cytotoxicity. This study shows that using triterpenoids in combination with antibiotics can help to reduce their toxicity and would provide a unique and efficient way to combat emerging staphylococcal resistance.
The exact mechanism of antibacterial action of the triterpenoids under study has not been fully understood. Research on zeylasterone and demethylzeylasterone (also a triterpenoid) shows that it acts by disrupting the cytoplasmic membrane.[16] Vancomycin is responsible for disrupting the cell wall by inhibiting peptidation of the growing peptidoglycan chain while cefradine is a beta-lactam antibiotic, inhibiting enzymes for cell wall synthesis.[17] The triterpenoids can theoretically act in a way that makes bacteria more susceptible to these antibiotics by destroying biofilms,[18] or they can adopt some other pathways to exert antibacterial effect. Oleanolic acid and ursolic acid take up a variety of different mechanisms to exert their effects. They could inhibit peptidoglycan synthesis and thus affect cell wall, or they can induce stress conditions in bacteria,[19] but their mechanism in S. aureus is not properly understood.[6] The MIC of these triterpenoids was greater in MRSA than MSSA [Table 1], and this finding correlates with other researchers as well.[6,9] This phenomenon too, however, remains unexplained.
Conclusion
The four triterpenoids such as oleanolic acid, ursolic acid, beta-boswellic acid, and cycloastragenol showed moderate antistaphylococcal properties in MSSA. The MIC of ursolic acid was the lowest among the four and was considerable to cefradine in MSSA. In MRSA, all the triterpenoids showed poor antistaphylococcal effect. Synergism was observed in many of the triterpenoid-antibiotic combinations. The best combination was between ursolic acid and vancomycin in MSSA. The triterpenoids reduced the MIC of antibiotics by 95%–46% (P < 0.01). This highlights their importance in dealing with bacterial resistance. These compounds are fairly toxic, but their synergistic action with antibiotics may bring their concentration below toxic levels.
Financial Support and Sponsorship
Nil.
Conflicts of Interest
There are no conflicts of interest.
References
- 1.Cowan MM. Plant products as antimicrobial agents. Clin Microbiol Rev. 1999;12:564–82. doi: 10.1128/cmr.12.4.564. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Kim SG, Kim MJ, Jin D, Park S, Cho E, Freire MO, et al. Antimicrobial effect of ursolic acid and oleanolic acid against methicillin-resistant Staphylococcus aureus. Korean J Microbiol. 2012;48:212–5. [Google Scholar]
- 3.Raja AF, Ali F, Khan IA, Shawl AS, Arora DS, Shah BA, et al. Antistaphylococcal and biofilm inhibitory activities of acetyl-11-keto-ß-boswellic acid from Boswellia serrata. BMC Microbiol. 2011;11:54. doi: 10.1186/1471-2180-11-54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Rani A, Jain S, Dureja P, Kumar R, Kumar A. Synergistic interaction between synthetic and natural products: A promising tool for the development of environmentally safe potent antimicrobial agents. World Appl Sci J. 2009;5:59–63. [Google Scholar]
- 5.Odds FC. Synergy, antagonism, and what the chequerboard puts between them. J Antimicrob Chemother. 2003;52:1. doi: 10.1093/jac/dkg301. [DOI] [PubMed] [Google Scholar]
- 6.Chung PY, Navaratnam P, Chung LY. Synergistic antimicrobial activity between pentacyclic triterpenoids and antibiotics against Staphylococcus aureus strains. Ann Clin Microbiol Antimicrob. 2011;10:25. doi: 10.1186/1476-0711-10-25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.CLSI. Clinical and Laboratory Standards Institute. Performance Standards for Antimicrobial Susceptibility Testing; Twenty-Fourth Informational Supplement – M100-S24. Wayne, Pennsylvania (PA), USA: CLSI; 2014. [Google Scholar]
- 8.Wadhwani T, Desai K, Patel D, Lawani D, Bahaley P, Joshi P, et al. Effect of various solvents on bacterial growth in context of determining MIC of various antimicrobials. Internet J Microbiol. 2008;7:145–51. [Google Scholar]
- 9.Fontanay S, Grare M, Mayer J, Finance C, Duval RE. Ursolic, oleanolic and betulinic acids: Antibacterial spectra and selectivity indexes. J Ethnopharmacol. 2008;120:272–6. doi: 10.1016/j.jep.2008.09.001. [DOI] [PubMed] [Google Scholar]
- 10.Yoeruek E, Spitzer MS, Saygili O, Tatar O, Biedermann T, Yoeruek E, et al. Comparison of in vitro safety profiles of vancomycin and cefuroxime on human corneal endothelial cells for intracameral use. J Cataract Refract Surg. 2008;34:2139–45. doi: 10.1016/j.jcrs.2008.08.022. [DOI] [PubMed] [Google Scholar]
- 11.Hsu HY, Yang JJ, Lin CC. Effects of oleanolic acid and ursolic acid on inhibiting tumor growth and enhancing the recovery of hematopoietic system postirradiation in mice. Cancer Lett. 1997;111:7–13. doi: 10.1016/s0304-3835(96)04481-3. [DOI] [PubMed] [Google Scholar]
- 12.Park YS, Lee JH, Harwalkar JA, Bondar J, Safayhi H, Golubic M. Acetyl-11-keto-beta-boswellic acid (AKBA) is cytotoxic for meningioma cells and inhibits phosphorylation of the extracellular-signal regulated kinase 1 and 2. Adv Exp Med Biol. 2002;507:387–93. doi: 10.1007/978-1-4615-0193-0_60. [DOI] [PubMed] [Google Scholar]
- 13.Lu YF, Wan XL, Xu Y, Liu J. Repeated oral administration of oleanolic acid produces cholestatic liver injury in mice. Molecules. 2013;18:3060–71. doi: 10.3390/molecules18033060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Colla AR, Rosa JM, Cunha MP, Rodrigues AL. Anxiolytic-like effects of ursolic acid in mice. Eur J Pharmacol. 2015;758:171–6. doi: 10.1016/j.ejphar.2015.03.077. [DOI] [PubMed] [Google Scholar]
- 15.Szabo NJ. Dietary safety of cycloastragenol from Astragalus spp.: Subchronic toxicity and genotoxicity studies. Food Chem Toxicol. 2014;64:322–34. doi: 10.1016/j.fct.2013.11.041. [DOI] [PubMed] [Google Scholar]
- 16.de León L, López MR, Moujir L. Antibacterial properties of zeylasterone, a triterpenoid isolated from Maytenus blepharodes, against Staphylococcus aureus. Microbiol Res. 2010;165:617–26. doi: 10.1016/j.micres.2009.12.004. [DOI] [PubMed] [Google Scholar]
- 17.McDermott PF, Walker RD, White DG. Antimicrobials: Modes of action and mechanisms of resistance. Int J Toxicol. 2003;22:135–43. doi: 10.1080/10915810305089. [DOI] [PubMed] [Google Scholar]
- 18.Zhou L, Ding Y, Chen W, Zhang P, Chen Y, Lv X. The in vitro study of ursolic acid and oleanolic acid inhibiting cariogenic microorganisms as well as biofilm. Oral Dis. 2013;19:494–500. doi: 10.1111/odi.12031. [DOI] [PubMed] [Google Scholar]
- 19.Jesus JA, Lago JH, Laurenti MD, Yamamoto ES, Passero LF. Antimicrobial activity of oleanolic and ursolic acids: An update. Evid Based Complement Alternat Med. 2015;2015:620472. doi: 10.1155/2015/620472. [DOI] [PMC free article] [PubMed] [Google Scholar]


