Abstract
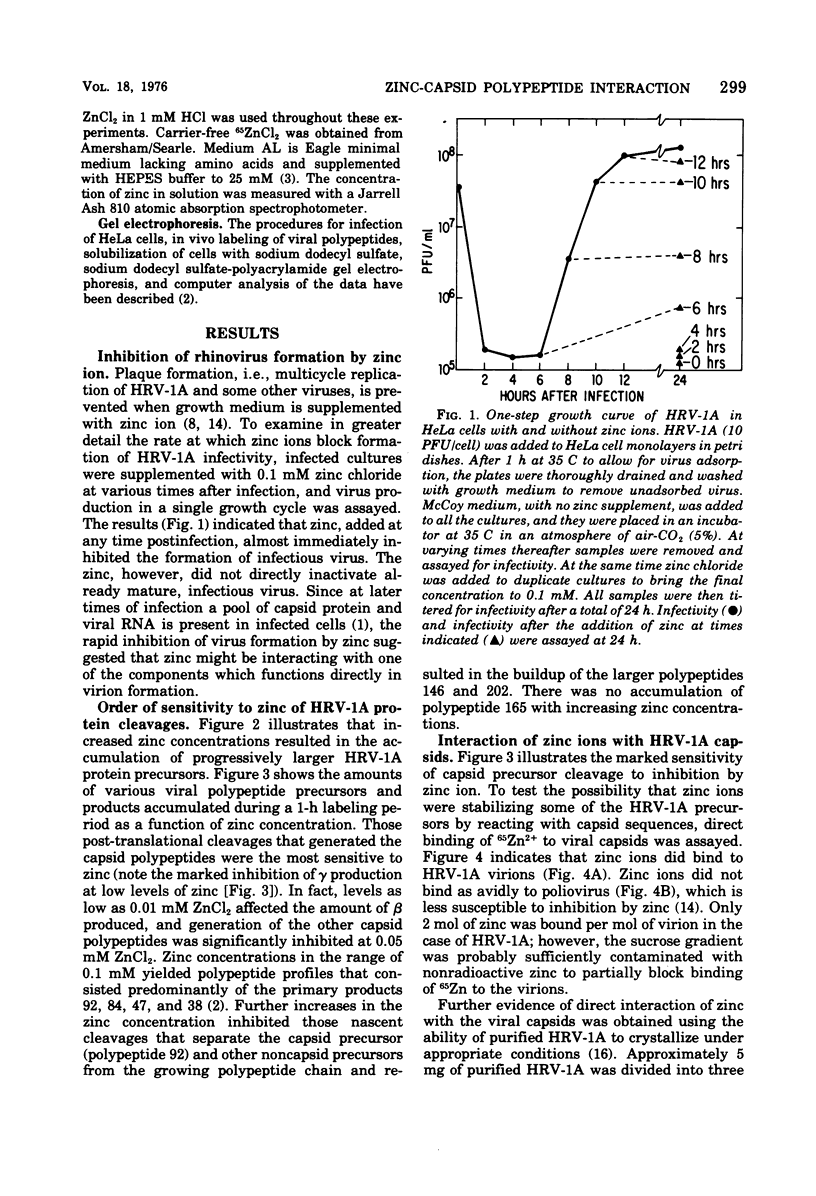
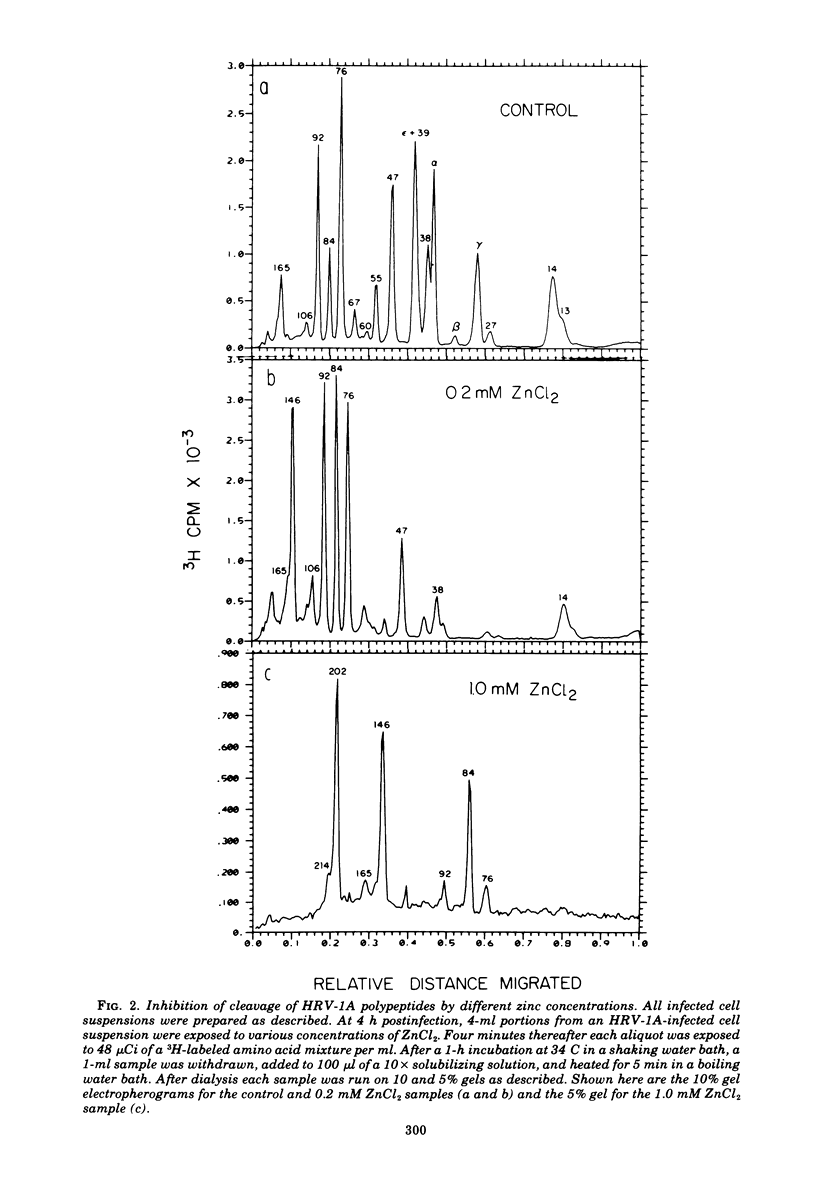
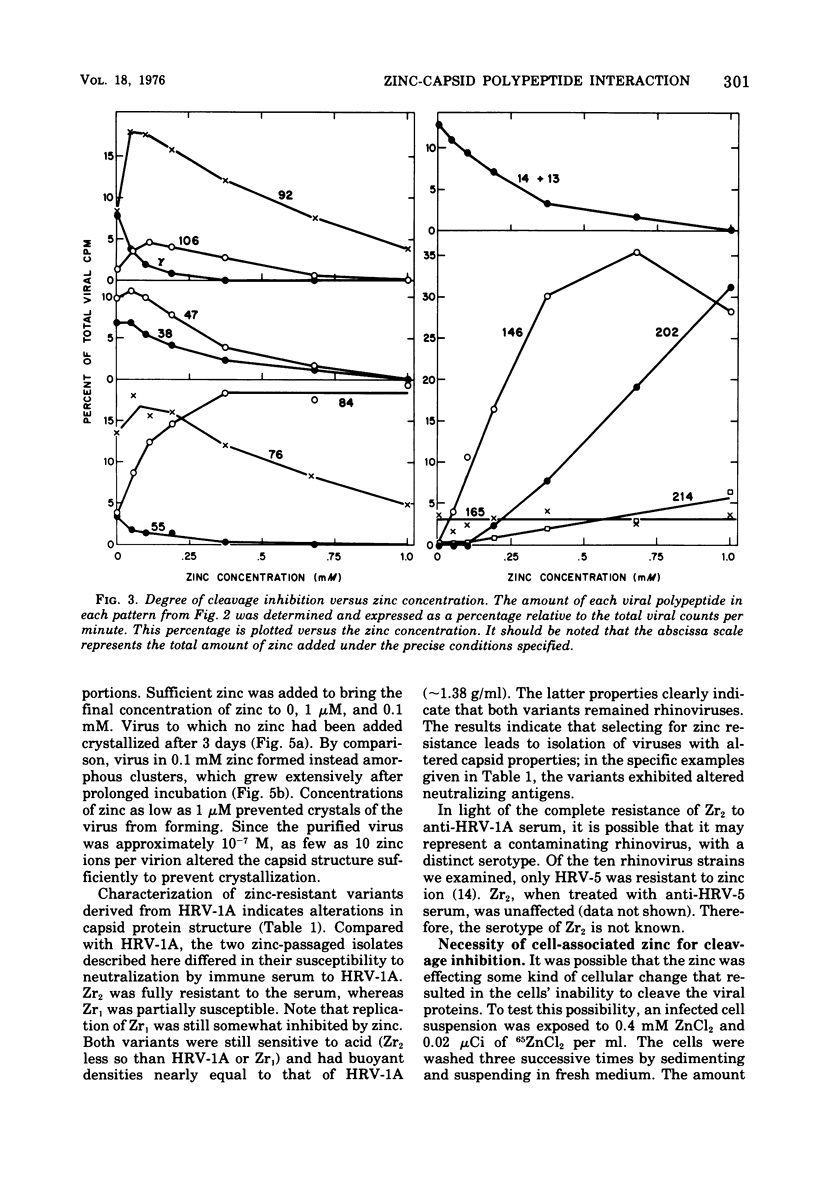
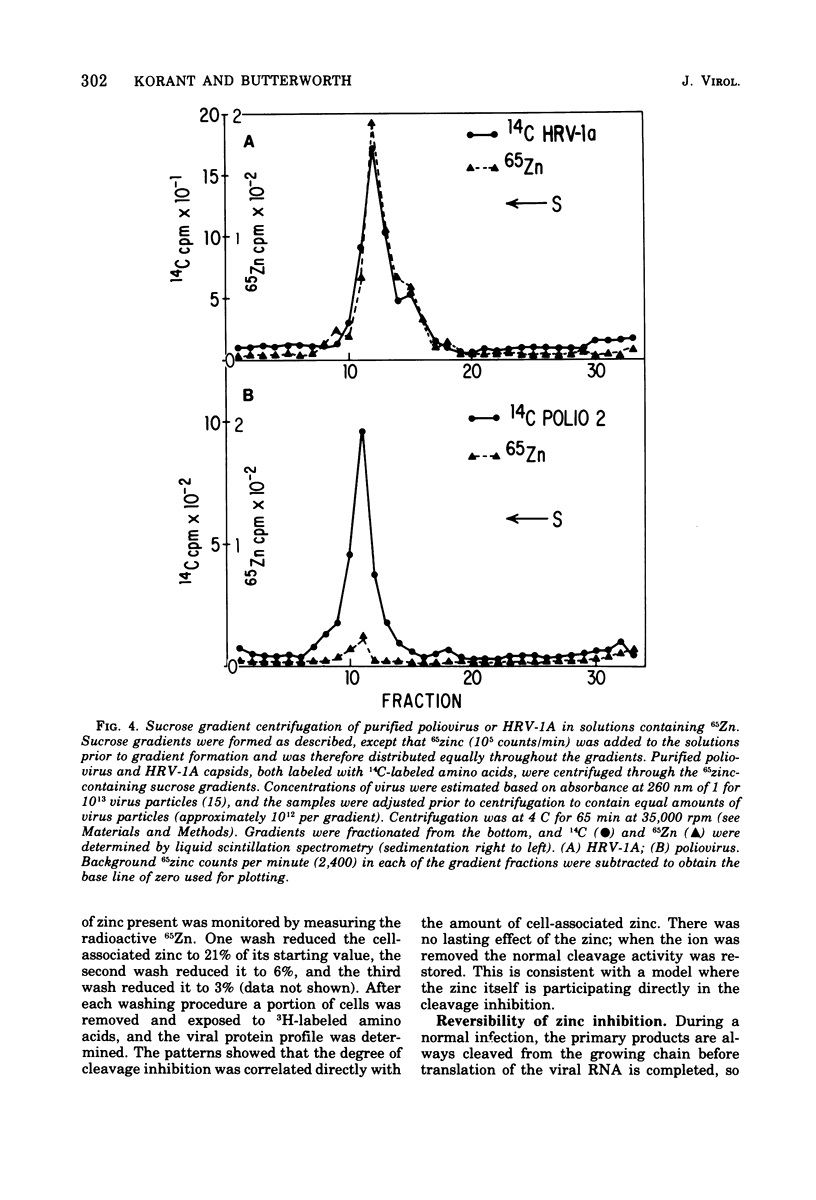
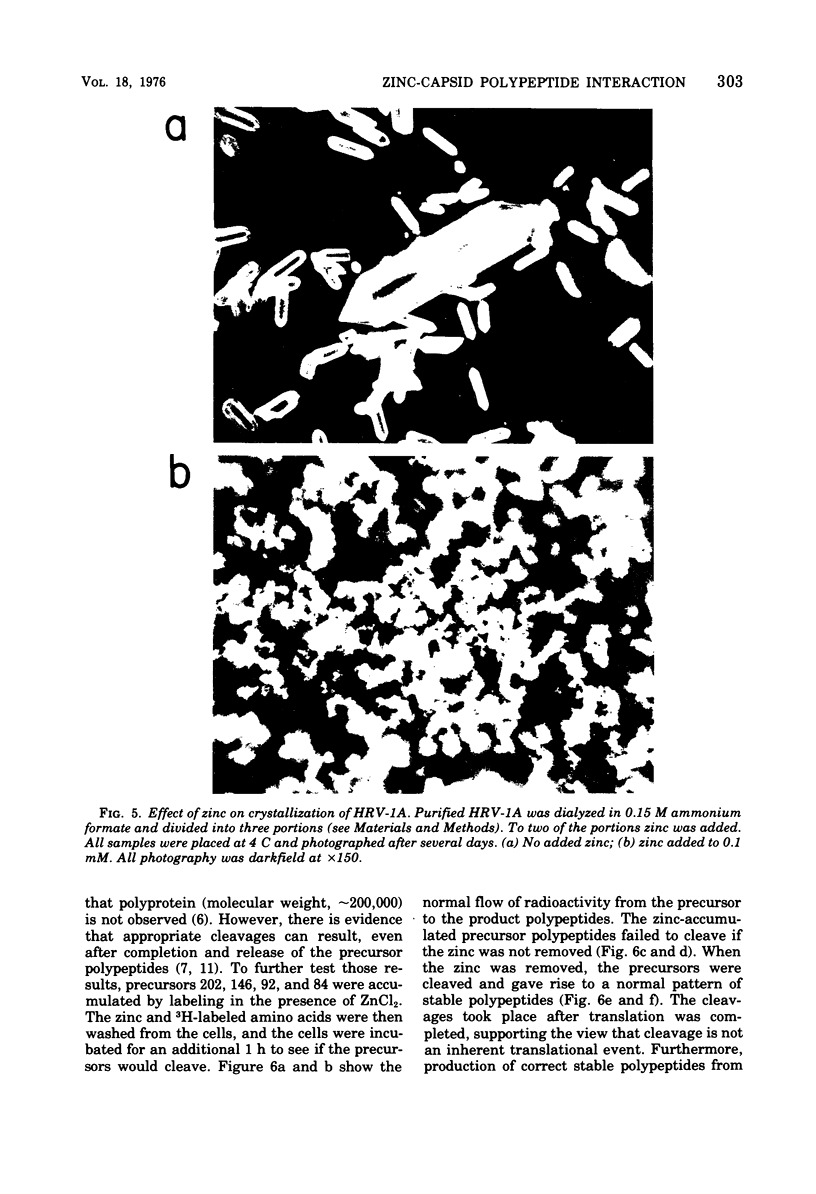
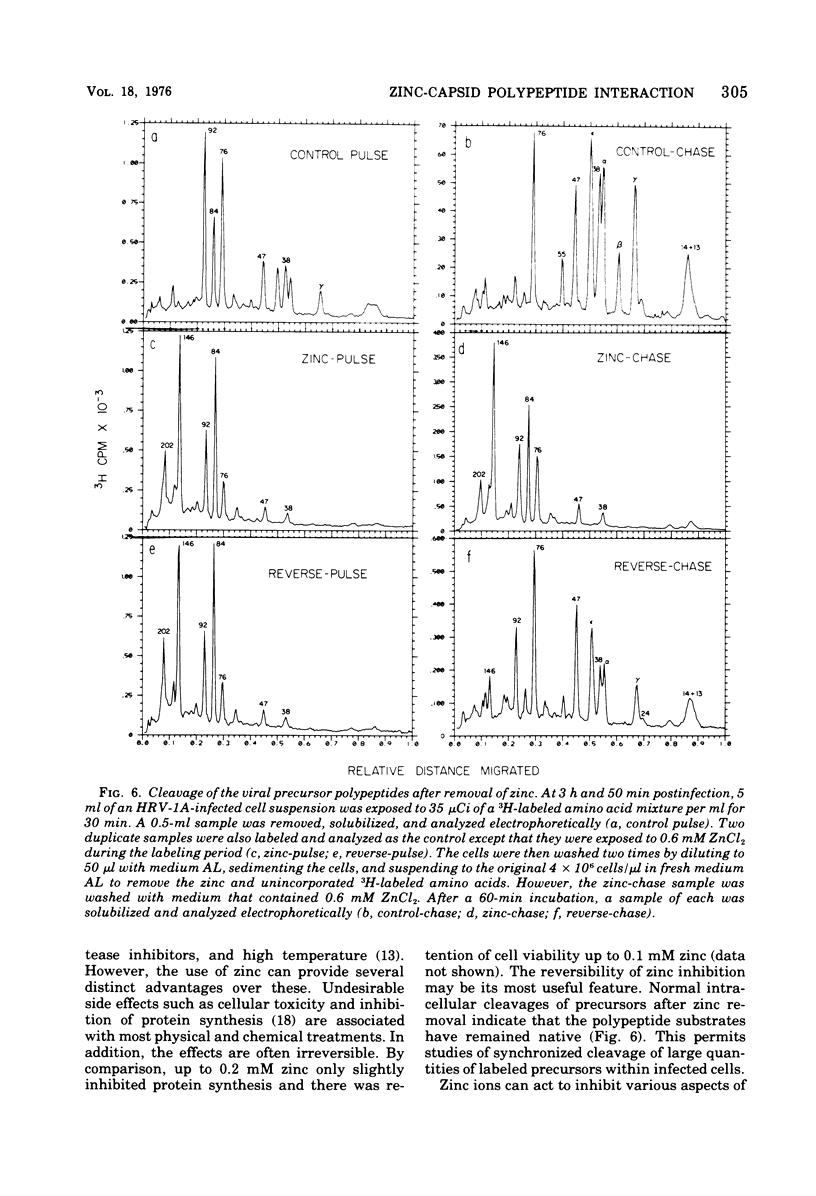
Zinic ions rapidly inhibit virus production in HeLa cells infected with human rhinovirus type 1A and lead to the accumulation of human rhinovirus type 1A precursor polypeptides. The degree to which cleavage of these precursors is inhibited is directly dependent on the quantity of cell-associated zinc. Proteolysis resumes after the removal of zinc-containing medium, and the accumulated viral precursors are cleaved predominantly to stable virus polypeptides. The precursors stabilized at the lowest zinc levels are those that contain capsid protein sequences. Furthermore, added zinc is bound to human rhinovirus type 1A capsids and prevents them from forming crystals. Zinc-resistant mutants display antigenic alterations in coat proteins. These results suggest that zinc complexes with rhinovirus coat proteins and alters them so that they cannot function as substrates for proteases or as reactants in the assembly of the virus particles.
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Butterworth B. E. A comparison of the virus-specific polypeptides of encephalomyocarditis virus, human rhinovirus-1A, and poliovirus. Virology. 1973 Dec;56(2):439–453. doi: 10.1016/0042-6822(73)90048-2. [DOI] [PubMed] [Google Scholar]
- Butterworth B. E., Hall L., Stoltzfus C. M., Rueckert R. R. Virus-specific proteins synthesized in encephalomyocarditis virus-infected HeLa cells. Proc Natl Acad Sci U S A. 1971 Dec;68(12):3083–3087. doi: 10.1073/pnas.68.12.3083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Butterworth B. E., Korant B. D. Characterization of the large picornaviral polypeptides produced in the presence of zinc ion. J Virol. 1974 Aug;14(2):282–291. doi: 10.1128/jvi.14.2.282-291.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Butterworth B. E., Rueckert R. R. Gene order of encephalomyocarditis virus as determined by studies with pactamycin. J Virol. 1972 May;9(5):823–828. doi: 10.1128/jvi.9.5.823-828.1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Butterworth B. E., Rueckert R. R. Kinetics of synthesis and cleavage of encephalomyocarditis virus-specific proteins. Virology. 1972 Nov;50(2):535–549. doi: 10.1016/0042-6822(72)90405-9. [DOI] [PubMed] [Google Scholar]
- Garfinkle B. D., Tershak D. R. Effect of temperature on the cleavage of polypeptides during growth of LSc poliovirus. J Mol Biol. 1971 Aug 14;59(3):537–541. doi: 10.1016/0022-2836(71)90318-4. [DOI] [PubMed] [Google Scholar]
- Gordon Y. J., Asher Y., Becker Y. Irreversible inhibition of herpes simplex virus replication in BSC-1 cells by zinc ions. Antimicrob Agents Chemother. 1975 Sep;8(3):377–380. doi: 10.1128/aac.8.3.377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jacobson M. F., Asso J., Baltimore D. Further evidence on the formation of poliovirus proteins. J Mol Biol. 1970 May 14;49(3):657–669. doi: 10.1016/0022-2836(70)90289-5. [DOI] [PubMed] [Google Scholar]
- Jacobson M. F., Baltimore D. Polypeptide cleavages in the formation of poliovirus proteins. Proc Natl Acad Sci U S A. 1968 Sep;61(1):77–84. doi: 10.1073/pnas.61.1.77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Korant B. D. Cleavage of poliovirus-specific polypeptide aggregates. J Virol. 1973 Sep;12(3):556–563. doi: 10.1128/jvi.12.3.556-563.1973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Korant B. D. Cleavage of viral precursor proteins in vivo and in vitro. J Virol. 1972 Oct;10(4):751–759. doi: 10.1128/jvi.10.4.751-759.1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Korant B. D., Kauer J. C., Butterworth B. E. Zinc ions inhibit replication of rhinoviruses. Nature. 1974 Apr 12;248(449):588–590. doi: 10.1038/248588a0. [DOI] [PubMed] [Google Scholar]
- Korant B. D., Lonberg-Holm K., Noble J., Stasny J. T. Naturally occurring and artificially produced components of three rhinoviruses. Virology. 1972 Apr;48(1):71–86. doi: 10.1016/0042-6822(72)90115-8. [DOI] [PubMed] [Google Scholar]
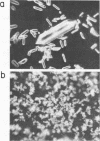
- Korant B. D., Stasny J. T. Crystallization of human rhinovirus 1A. Virology. 1973 Oct;55(2):410–417. doi: 10.1016/0042-6822(73)90182-7. [DOI] [PubMed] [Google Scholar]
- Lawrence C., Thach R. E. Identification of a viral protein involved in post-translational maturation of the encephalomyocarditis virus capsid precursor. J Virol. 1975 Apr;15(4):918–928. doi: 10.1128/jvi.15.4.918-928.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pong S. S., Nuss D. L., Koch G. Inhibition of initiation of protein synthesis in mammalian tissue culture cells by L-1-tosylamido-2-phenylethyl chloromethyl ketone. J Biol Chem. 1975 Jan 10;250(1):240–245. [PubMed] [Google Scholar]
- Taber R., Rekosh D., Baltimore D. Effect of pactamycin on synthesis of poliovirus proteins: a method for genetic mapping. J Virol. 1971 Oct;8(4):395–401. doi: 10.1128/jvi.8.4.395-401.1971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vogt V. M., Eisenman R., Diggelmann H. Generation of avian myeloblastosis virus structural proteins by proteolytic cleavage of a precursor polypeptide. J Mol Biol. 1975 Aug 15;96(3):471–493. doi: 10.1016/0022-2836(75)90174-6. [DOI] [PubMed] [Google Scholar]
- Yin F. H., Lonberg-Holm K., Chan S. P. Lack of close relationship between three strains of human rhinoviruses as determined by their RNA sequences. J Virol. 1973 Jul;12(1):108–113. doi: 10.1128/jvi.12.1.108-113.1973. [DOI] [PMC free article] [PubMed] [Google Scholar]