Abstract
Mobility-disability is a common condition in older individuals. Many factors, including the age-related hormonal dysregulation, may concur to the development of disability in the elderly. In fact, during the aging process it is observed an imbalance between anabolic hormones that decrease (testosterone, dehydroepiandrosterone sulphate (DHEAS), estradiol, insulin like growth factor-1 (IGF-1) and Vitamin D) and catabolic hormones (cortisol, thyroid hormones) that increase. We start this review focusing on the mechanisms by which anabolic and catabolic hormones may affect physical performance and mobility. To address the role of the hormonal dysregulation to mobility-disability, we start to discuss the contribution of the single hormonal derangement. The studies used in this review were selected according to the period of time of publication, ranging from 2002 to 2013, and the age of the participants (≥65 years). We devoted particular attention to the effects of anabolic hormones (DHEAS, testosterone, estradiol, Vitamin D and IGF-1) on both skeletal muscle mass and strength, as well as other objective indicators of physical performance. We also analyzed the reasons beyond the inconclusive data coming from RCTs using sex hormones, thyroid hormones, and vitamin D (dosage, duration of treatment, baseline hormonal values and reached hormonal levels). We finally hypothesized that the parallel decline of anabolic hormones has a higher impact than a single hormonal derangement on adverse mobility outcomes in older population. Given the multifactorial origin of low mobility, we underlined the need of future synergistic optional treatments (micronutrients and exercise) to improve the effectiveness of hormonal treatment and to safely ameliorate the anabolic hormonal status and mobility in older individuals.
Keywords: Multiple hormonal derangement, mobility, muscle function, older persons
INTRODUCTION
A progressive decline in physical performance in older people predicts a large number of adverse outcomes such as mobility-disability, cognitive impairment, nursing home admission and mortality. The reduced mobility is directly linked to sarcopenia, which is a multifactorial syndrome featured by a progressive and generalized loss of skeletal muscle mass and strength [1]. As assessed by the recent consensus documents developed by the European (EWGSOP) and North-American taskforces, the diagnosis of sarcopenia must be based on the presence of both low muscle mass and low muscle function (strength or performance) [2,3]. Sarcopenia is linked to functional limitation, including balance problem, higher risk of falling and fractures, depression, obesity, chronic obstructive pulmonary disease, type 2 diabetes, kidney and liver diseases, reduced quality of life and mortality [4,5].
Many mechanisms such as reduced physical activity and nutritional intake, increase of oxidative stress and inflammatory cytokines and changes in hormonal levels are involved in the path-physiology of sarcopenia and share similarities with the frailty phenotype suggested by Fried and coauthors [6].
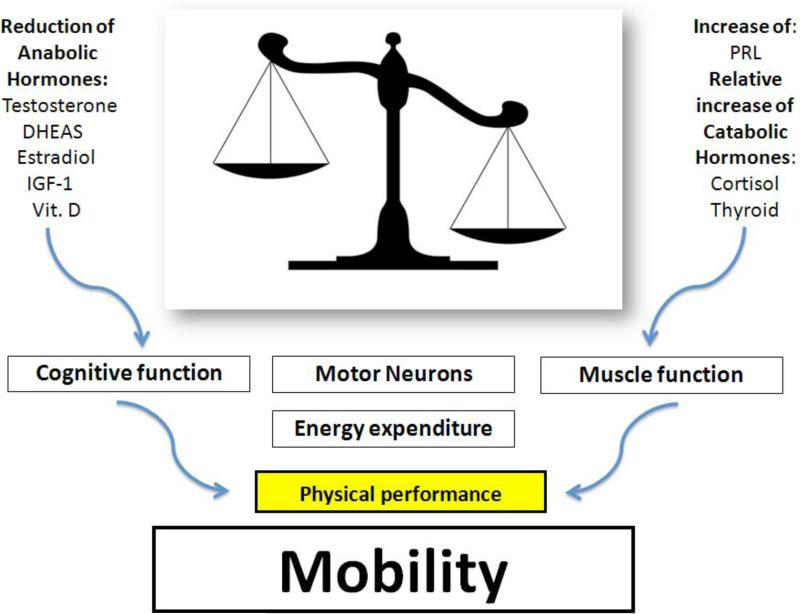
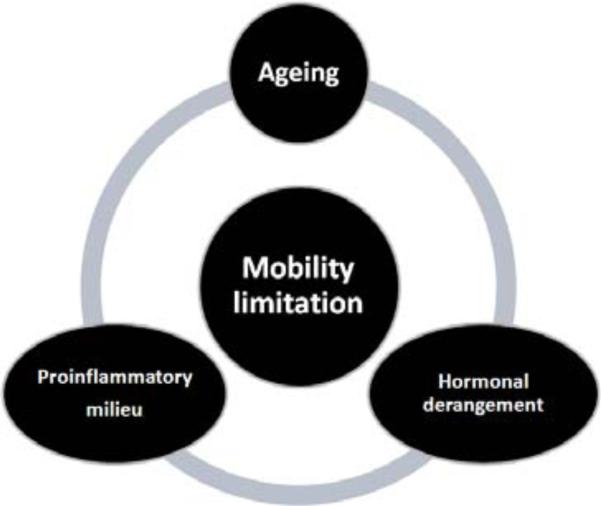
However, the precise contribution of each mechanism to sarcopenia and mobility-limitation is still unknown [7]. In our review we will particularly address the contribution of multiple hormonal dysregulation occurring with advanced age. During this phase of life it is observed an imbalance between anabolic hormones, dehydroepiandrosterone and its sulphate derivative (DHEA/DHEAS), testosterone (T), estrogens, insulin like growth factor-1 (IGF-1), Vitamin D, that decrease and catabolic hormones (thyroid hormones, cortisol) that increase. These parallel hormonal changes seem to play an important role in the development of mobility impairment in the elderly. This hypothesis is supported by many studies where alterations in hormonal axes have been related to reduction of muscle function, cognitive impairment, loss of spinal motor-neurons, and increase of energy expenditure [8,9]. However, the decline in anabolic hormones deserves particular attention, given the profound interrelationship between DHEAS, T, IGF-1, and Vitamin D. DHEAS can be converted into active androgens and estrogens and stimulates IGF-1 which exerts important actions in muscle growth and repair [10]. It is often difficult to distinguish between specific symptoms due to a single anabolic hormonal derangement. In fact, partial androgen deficiency (reduced activity of DHEA and T), somatopause (decreased Growth Hormone (GH)-IGF-1 activity), and Vitamin D deficiency share many clinical aspects. As first goal, this review wants to underline the need of recognizing and using the contribution of anabolic hormonal deficiency, occurring with aging, for identifying elderly subjects at risk of frailty and progressive functional decline. The second step is to hypothesize hormonal cut-offs more likely to be associated with lower functional performance. This is an important premise for reliable preventive and therapeutic strategies, targeting older individuals, at risk of mobility-limitation. Figure 1 shows the potential link between the single hormonal status and the maintenance of mobility in older population. In the left column we have the anabolic hormones and in the right column the catabolic hormones. The purpose of the figure is not just to depict a generic list of hormones. In fact, given the profound interaction between these hormones, the painting of O'Campo might be a perfect reproduction of hormonal homeostasis. Octavio O'Campo is a Mexican artist famous for depicting in his paintings images that are intricately woven together to create larger images. As result, there is an optical illusion fading back and stepping forward. By only studying each piece, it is possible to notice the details, and to recognize the large scale intention. By translating this artistic perspective into clinical practice the correct balance between anabolic and catabolic hormones is the whole figure that contains, as parts of the puzzle, the important contribution of each single hormone, especially of the anabolic ones. We start our painting from DHEA/DHEAS which is a pro-hormone peripherally transformed into T and estrogens but also capable to directly influence physical function.
Fig. (1).
The imbalance between the decrease of anabolic hormones and the relative or net increase of catabolic hormones leads to so called multiple hormonal dysregulation of aging. This dysregulation has an impact of mobility by different mechanisms listed in the figure.
DEHYDROEPIANDROSTERONE
DHEA is secreted by the adrenal glands and its secretory rate changes throughout the human lifespan. When human development is completed and adulthood is reached, DHEA and DHEAS levels start to decline, so that at 70-80 years of age, peak DHEAS concentrations are only 10-20% of those of young adults. Conversely, the adrenal secretion of cortisol remains unchanged. Thus, the hormonal balance with aging moves toward the catabolic status [11]. The age-associated decline in DHEAS levels has been termed ‘adreno-pause’ [11]. The adrenal pro-hormones DHEA and DHEAS are important precursors of the major sex steroids. They undergo peripheral metabolism by a network of enzymes leading to bioconversion into T and estrogens [12]. DHEA and DHEAS are important in adult women because are the most important source of androgens, and in both sexes, because they can affect the development and function of skeletal muscle [12]. In fact, skeletal muscle is one of the sites of conversion of DHEA into active androgens, because of the presence of steroidogenic enzymes capable of converting circulating DHEA or DHEAS into T and dehydrotestosterone [13, 14]. DHEA is also capable to stimulate the liver secretion and the biological activity of IGF-1 another important determinant of adult muscle function [10,15] in physiological status and frailty condition [16,17].
OBSERVATIONAL STUDIES TESTING DHEAS AND MOBILITY IN MEN AND WOMEN
Low DHEA/DHEAS levels alone or as part of multiple anabolic hormone deficiencies have been associated with mobility limitation in older adults.
In 2004 Valenti et al [18], using data from 596 men (age range 20-100 years) from the InCHIANTI Study, an epidemiological study conducted in the Chianti geographic area (Tuscany, Italy), tested the relationship between DHEAS serum levels and muscle mass and strength over the life span. Lower extremity muscle strength was assessed by hand-held dynamometry while calf muscle area was estimated from computerized tomography. The relationship of DHEAS with muscle mass and strength was adjusted for age and other confounders including anthropometric measurements, physical activity, smoking, energy and alcohol intake, albumin, total cholesterol, interleukin-6 (IL-6), comorbidity, depressive symptoms, and disability in activity of daily living (ADL). In age-stratified models, DHEAS was an independent predictor of muscle strength (p<0.02) and mass (p<0.01), but only in men between 60 and 79 years old.
Dehydroepiandrosterone-sulphate was one of the anabolic hormones along with total IGF-1 and Free-T evaluated in 2009 by Cappola et al. [19] to analyze the role of serum levels of anabolic hormones across different stages of frailty status. The study population was composed by 494 women aged 70-79 years enrolled in the Women's Health and Aging Studies I or II.
Frailty status was defined as originally operationalized by Fried and associates [6] in the Cardiovascular Health Study (CHS). Five characteristics of frailty were used: weight loss (body mass index (BMI) <18.5 kg/m2 or lost ≥10% of weight since age 60), weakness (grip strength equivalent to the lowest quartile in CHS, by gender and BMI strata), poor endurance (self-report of exhaustion), slowness (walking speed equivalent to the lowest quartile in CHS, by height), and low activity (activity level in kcal/wk equivalent to the lowest quartile in CHS) [19]. Those with none of the five characteristics were considered to be non-frail, those with one or two were deemed pre-frail, and those with three, four, or five were considered to be frail. Using multivariate analysis adjusted for different confounders (age, race, education, smoking habit, BMI, chronic disease, corticosteroid and estrogen use), the authors calculated the odds ratio of frailty for deficiency in each hormone. The cut-offs of low hormone deficiency were identified by IGF-1 levels <87.8μg/L, DHEAS<0.22 μg/L, Free-T < 0.7pg/mL [19]. The prevalence of DHEA deficiency by frailty status was 18% in non-frail, 28% in pre-frail, and 33% in frail individuals. The prevalence of IGF-1 deficiency by frailty status was 21% in non-frail, 29% in pre-frail, and 33% in frail subjects.
The prevalence of Free-T deficiency by frailty status was estimated as 23% in non-frail, 24% in pre-frail, and 36% in frail individuals, respectively.
Thus, for each hormone, subjects with the deficiency were more likely to be frail than those without the deficiency, although this did not achieve statistical significance (IGF-1: OR 1.82, CI 0.81-4.08; DHEAS: OR 1.68, CI 0.77-3.69; Free-T: OR 2.03, CI 0.89-4.64). Compared with subjects with no hormonal deficiencies, those with one deficiency were not more likely to be frail (OR 1.15, CI 0.49-2.68), whereas those with two or three hormonal deficiencies had a very high likelihood of being frail (OR 2.79, CI 1.06-7.32). These findings suggest that the burden of anabolic hormone deficiencies is a stronger predictor of frailty status than the single hormonal deficiency [19].
Dehydroepiandrosterone-sulphate was also part of the evaluation of relationship of anabolic and catabolic biomarkers and changes in muscle strength [20]. The biomarkers of catabolic status were C-reactive protein (CRP), IL-6, IL-1 receptor antagonist (IL-1RA), tumor necrosis factor-alpha receptor 1 (TNFR1) while those anabolic hormones in addition to DHEAS were IGF-1, and bioavailable-T. The evaluated population was composed of 716 men and women aged 65 years or older from the InCHIANTI Study. The biomarker values were divided into tertiles with subsequent calculation of the numbers of catabolic/anabolic biomarkers in the highest/lowest tertiles. Hand-grip strength was measured at baseline and during 3- and 6-year follow-up period. For each tertile of catabolic/anabolic biomarkers the mean strength decline was also estimated according to the number of dysregulated catabolic/anabolic biomarkers. The analysis was adjusted for covariates and other variables (age, BMI, waist circumference, total energy intake, baseline grip strength, smoking status, physical activity, and chronic diseases). Higher concentration of IL-6 (p=0.02) and IL-1RA (p=0.04) as well as lower levels of DHEAS (p=0.01) predicted the muscle strength decline. After combining all inflammatory markers, the rate of decline in grip strength was progressively greater with the increasing number of dysregulated catabolic biomarkers (p=0.01). Thus, this study demonstrates that cumulated burden of multiple inflammatory markers may worsen the decline in muscle strength due to anabolic hormone reduction. As already discussed, part of the anabolic actions of DHEAS are due to its peripheral conversion into T and E2 [20].
PHYSIOLOGY OF TESTOSTERONE IN MEN AND WOMEN
Testosterone is the major male sex steroid. In men 95% of T production occurs in the Leydig cells of the testes with men having a 25-fold higher circulating T level than women. The remaining 5% is due to peripheral conversion of adrenal androgens [21]. In women plasma T concentration is 25-fold lower than men and comes mainly from the conversion of adrenal androgens. In ovaries, T, which is a precursor of estrogen production, exerts an important role in the maturation process of ovarian follicles [22]. In men, T levels decrease by 1% per year, and bioavailable -T (free plus albumin bound T) by 2% per year, from the age of 35. Therefore, approximately 20% of men over 60 years and 50% of men over 80 years have serum T concentration below the normal range for young men [23]. This might be the result of the reduced testicular response to gonadotropin stimuli with aging, coupled with the incomplete hypothalamic-pituitary compensation for the fall in total and Free-T levels [24]. Similarly, in women, T levels decrease from the age of 40 approaching prior to menopause 50% of T levels present in younger adults. The physiological effects of T, induced by binding to the intracellular androgen receptor, depend on the nuclear transcription of specific genes [25]. Testosterone is involved in many physiological actions including spermatogenesis, testicular function and secondary sexual characteristics in men. This hormone has also a role in the activation and maintenance of Bone Mineral Density (BMD), muscle mass and strength, and libido in both sexes.
MECHANISMS OF ACTION BY WHICH TESTOSTERONE AFFECTS MOBILITY
The maintenance of adult skeletal muscle, primarily depending on satellite cell survival, activation, proliferation and differentiation can be also stimulated by T [15; 26]. Testosterone may improve muscle function by three different mechanisms: anabolic, anti-catabolic, and neurotropic. Indeed, T stimulates muscle anabolism by inducing protein synthesis, reducing muscle catabolism with recycling of intracellular aminoacids, and promoting nerve conduction by stimulating motoneurons [27]. Moreover, T promotes the commitment of pluripotent stem cells to myogenic lineage and inhibits their differentiation into adipocytes via an androgen receptor (AR)- mediated pathway. This mechanism suggests the rationale for T beneficial effects on fat mass, insulin-resistance and lean body mass [28-30]. The increase in hemoglobin levels (0.8 g/dL in average), associated with T treatment, is another potential beneficial effect by which T may improve muscle metabolism especially in older men with mild anemia [31].
The aging process is also characterized by the increased production of inflammatory cytokines in both sexes [32]. The opposite trajectory between inflammation and anabolic hormones might have a link and common biological basis (Fig. 3). Indeed, T might be capable of reducing systemic inflammatory cytokines such as TNF-α, IL-6 and IL-1β [33] and stimulating the anti- inflammatory cytokine IL-10 [34]. This is of importance because high levels of inflammatory markers are known to influence the decline in muscle mass [35] and to be strong independent risk factors for frailty, disability and cardiovascular events [36, 20].
Fig. (3).
The hormonal derangement occurring with aging has a profound interaction with the pro-inflammatory milieu in determining mobility limitations.
OBSERVATIONAL STUDIES IN AGING MEN AND WOMEN LINKING TESTOSTERONE AND MOBILITY
The aging process is characterized by the decrease of BMD, muscle mass and strength, and by the parallel increase of central body fat [37], and shares many similarities with the phenotype of hypogonadism [38,39].
Many epidemiological studies show that the age-related decline of T is associated with higher risk of osteo-metabolic diseases including osteoporosis, falls and fractures, morbidity and global mortality [8, 40-43].
Gonadal status has been associated with objective measures and determinants of physical performance in older men [44]. In the InCHIANTI Study, Maggio and colleagues evaluated 455 men, 65 years old or older with complete data on T levels, hand grip strength, cross-sectional muscle area (CSMA) and short physical performance battery (SPPB). Linear models were used to test the relationship between gonadal status and determinants of physical performance. Three different groups of older men were created: (1) severely hypogonadal (N=23), total T levels ≤230 ng/dL; (2) moderately hypogonadal (N=88), total T >230 and ≤350 ng/dL and (3) eugonadal (N=344), T levels >350 ng/dL. With the increased severity of hypogonadal status, participants were significantly older while their BMI was substantially similar. In the age and BMI adjusted analysis, there was a significant difference in hemoglobin levels, hand grip strength and SPPB score (p for trend < 0.001) among three groups, with severely hypogonadal men having lower values of hemoglobin, muscle strength and physical performance. No association was found between T group assignment and calf muscle mass and 4-m walking speed. In the multivariate analysis grip strength (p for trend = 0.004) and hemoglobin (p for trend < 0.0001), but not SPPB and other determinants of physical performance, were significantly different between the three groups.
In cross-sectional and longitudinal analyses of 1445 community-dwelling older men participating at Framingham Offspring Study examinations 7 and 8, Krasnoff and colleagues [45] showed that Free-T levels were positively associated with SPPB, usual walking speed and lower risk of subjective health. Lower levels of baseline Free-T were also associated with a 57% higher risk of incident and 68% higher odds of mobility limitation.
In older women T levels are about 15% of those present in young women [46]. In old women T deficiency has been associated with impaired sexual, muscle, and cognitive function, and with bone loss and frailty [19; 47]. Thus, the observational studies created the rationale for T replacement among menopausal and post-menopausal women [48]. Preliminary studies suggest that T may counteract the age-related changes of body composition and physical function beyond its direct influence on muscle function and mobility [49-52].
ESTROGENS
Estrogens are classes of female sex steroids secreted primarily by ovaries and placenta in women and, to a lesser extent, by peripheral steroidogenic conversion. The effects of estrogens are mediated by estrogen receptors (ER-a and ER-β), which act as transcription factors. Estrogen receptors are expressed in many tissues including uterus, prostate, ovary, testes, bone, breast, white adipose tissue, liver, muscle, colon, salivary gland, bone marrow and vascular endothelium [53].
MECHANISMS UNDERLYING THE RELATIONSHIP BETWEEN ESTROGENS AND PHYSICAL FUNCTION
Estrogens are primarily involved in the development and the maintenance of normal sexual and reproductive function in women [54], but they have also a wide range of biological effects in both women and men. In fact, a lot of animal and in vitro studies have shown that estrogens affect muscle function through several pathways. Estrogens play a protective role against oxidative stress [55], and mitigate post-injury inflammation in muscle damage by anti-oxidant and membrane-stabilizing properties and the activation of satellite cells [56-58]. Estrogens may also affect myosin function [59], muscle carbohydrate and lipid metabolism and muscle anabolism by interaction with IGF-1 [60,61]. Estrogens positively affect muscle function by also contributing to the development of peripheral nervous system, especially at the neuromuscular junction [62]. Estrogens were also shown to increase muscle anabolism [63] and nitric oxide bioavailability [64]. Data from epidemiological studies in humans reinforce the evidence that estrogens improve muscle function. Estrogens also play an important role in osteomineral metabolism by enhancing the activity of bone-forming osteoblasts and inhibiting the effect of osteoclasts in bone-resorption [41; 65].
THE ROLE OF ESTROGENS IN OLDER MEN
Estrogens in men are produced by T aromatization in different tissues. Aromatase, the enzyme responsible for the conversion of androgens to estrogens, is present in brain, gonads, adipose tissue and bone in both sexes. The importance of estrogens in male health has been studied by using three models: genetic mutation resulting in aromatase deficiency or estrogen resistance in men [66], prostate cancer on androgen deprivation therapy (ADT) [67], and transgenic animal model deficient in estrogen receptor (ERKO) and/or aromatase (ArKO) [68]. Men with mutation of either exon V or IX of the CYP19A1 gene, which encodes aromatase, have undetectable estrogen concentrations [69]. This phenotype is characterized by tall stature due to delayed skeletal maturation and epiphyseal closure, eunuchoidal skeletal proportions, osteoporosis [66], insulin resistance with impaired lipid and carbohydrate metabolism, and signs of precocious atherosclerosis [70]. In patients with prostate cancer on ADT exogenous estrogens can reduce some of the side effects, especially sexual dysfunction and osteoporosis [67]. Male ERKO mice are infertile and show atrophy of the testes resulting in decreased spermatogenesis and inactive sperm [68]. There is evidence that estrogens exert a pivotal role in male skeletal metabolism and the age-related reduction in estradiol levels is associated with an increased risk of fractures [71-72]. Case reports of severe osteoporosis in young men with estrogen resistance or aromatase deficiency suggest a key role of estradiol (E2) in the regulation of bone growth and maintenance [68]. Men with estrogen resistance, due to mutation in the ERα, or with estrogen deficiency, due to mutation in the CYP19A1 gene, have normal or elevated T levels, but severe osteopenia associated with elevated markers of bone remodeling. The aromatase-deficient men respond to exogenous estrogen treatment with a significantly increased bone mass and suppression of bone resorption. On the contrary, the estrogen-resistant men do not respond to estrogen therapy [72]. Lanfranco et al have indicated that estrogen replacement therapy in patients with aromatase deficiency has to target serum E2 level of almost 20 pg/mL in order to complete bone maturation and mineralization [73].
There are several indirect mechanisms by which estrogens can affect mobility including the above and below mentioned positive actions on bone, and the potential effects on brain. However, there is less evidence of direct effects of estrogens on muscle function and body composition.
Estrogens and Mobility Influence of Estrogens on Bone
Several observational studies report that serum E2 is a more powerful determinant of BMD than T [74,75]. Prospective studies have shown that serum E2 is the best predictor of BMD in elderly men [76]. Some studies identified a threshold for bioavailable E2 of 11 pg/mL (40 pmol/L), corresponding to total E2 levels of 31 pg/mL (114 pmol/L), below which there is an increased rate of bone loss at the radius, ulna, femoral neck and lumbar spine in older men [76]. Above this level, there is no evidence of an association between the bone density and bioavailable E2 levels [72].
As already mentioned, the majority of E2 in elderly men comes from peripheral conversion of androgens [77]. Therefore, the extent of peripheral aromatase activity influences serum E2 levels. Men with a high number of repeats in the aromatase gene have higher E2 levels and decreased rates of bone loss than those with a low number [78]. Moreover, CYP19A1 gene repeats have been significantly associated with BMD change in elderly community-dwelling men [79]. CYP19A1 polymorphism has also been significantly related with BMD and cortical bone size in young adult Swedish men [80]. A recent paper from the European Male Aging Study has reported that CAG repeat length in the CYP19A1 gene correlated with bone calcaneus parameters assessed by ultrasound [81].
Despite the inverse association between serum E2, T and the risk of fracture documented in cross sectional studies [71; 74], the role of low serum E2 as predictor of fracture risk has not been confirmed in prospective analyses [41; 82-84]. In the Osteoporotic Fractures in Men (MrOS) Sweden Study, the largest population-base-study in older men (N = 2902, mean age of 75 years) serum E2 and T were inversely associated with fracture risk [85]. However, when the effects of low E2 and/or low T levels were considered in the same model, subjects with low serum E2 levels had an increased fractures risk, independent of T status. By contrast, subjects with low T levels, but normal E2 levels, were not at higher fracture risk. Moreover, the inverse relationship between serum E2 levels and fracture risk was nonlinear, with a strong relationship for total E2 levels below 16 pg/mL (59 pmol/L). This observation confirms the hypothesis of a threshold E2 level for skeletal health in men [86]. It should be acknowledged that the threshold E2 level identified in the MrOS Sweden Study [85] is slightly lower than E2 cut-off, previously reported in other studies, already associated with bone maturation, BMD and markers of bone resorption [76]. This difference could be due to the fact that E2 serum assessment in the MrOS Sweden Study was based on mass spectrometry, while previous studies used immunoassay-based techniques, with less specificity, especially at lower sex steroid concentrations.
Influence of Estrogens on Brain
In the brain estrogens improve neuronal trophism and plasticity. It is well known that the positive effects of T on cognitive parameters are largely explained by its aromatization into E2. Estradiol levels have been positively associated with cognitive tests score in healthy older women. In men, exogenous estrogens are able to improve verbal memory for a paired associate learning task [9].
However, there are inconclusive data about the relationship between E2 and cognitive function. Some studies suggest that higher E2 levels may preserve brain function [87] while more recent observations showed none or even detrimental effects of endogenous and exogenous estrogens on brain function in both women [88] and men [89]. Muller and co-investigators, in a population-based prospective study of 242 independently living elderly men, found that high serum levels of total and free E2 and estrone were associated with an increased risk of cognitive decline, independent of age, cardiovascular risk factors and ApoE-e4 [89]. Other studies failed to find any association between E2 levels and cognitive performance [90, 91]. Le Blanc et al, in 1602 men from MrOS during 4.5 year follow-up period found no association between E2 levels and cognition [92].
In addition, it is still unclear whether T depletion or changes in E2 levels can contribute to the onset of Alzheimer's disease (AD). Rosario et al analyzed T and E2 levels in brain samples from the mid-frontal gyrus of 45 men during autopsy. They observed that men with AD exhibited significantly lower levels of T, but not of E2, in the brain. This finding suggests that T depletion, more than estrogen changes may contribute to the development of AD [93].
In summary, there are several indirect mechanisms by which estrogens can affect mobility including the positive influence on bone and brain, especially in women. The role of estrogens in older men, as anabolic hormone, is more controversial and will be not discussed in the present review.
GROWTH HORMONE AND INSULIN-LIKE GROWTH FACTOR-1 AXIS AND MOBILITY
Growth Hormone is a pituitary hormone released in a pulsatile fashion from somatotropic cells of the anterior pituitary gland. Growth Hormone secretion is mainly under control of hypothalamic and peripheral peptides, which exert inhibiting and stimulating effects on somatotropic cells including somatostatin, GH releasing hormone, and ghrelin [94]. Growth Hormone acts on the epiphyseal plates of long bones stimulating linear growth in children [95], but it has also other specific anabolic actions in the adults. Most of GH actions are manifested through the stimulation of synthesis of IGF-1 in the liver and its local expression in peripheral tissues [96]. Insulin like growth factor-1 in turn seems to modulate GH secretion through a negative feedback mechanism [97]. Insulin like growth factor-1 is an important regulator of muscle development and growth [98, 99], and could exert beneficial vascular and metabolic actions [100]. Insulin like growth factor-1 also regulates proliferation, differentiation and cellular apoptosis [101]. Insulin like growth factor-1 actions are profoundly interrelated with the global hormonal status and its bioactivity and bioavailability is influenced by six binding proteins (IGFBPs). IGFBPs have also independent biological actions [102]. The most abundant IGFBP in serum is IGFBP-3 which is a circulating IGF-1 reservoir and may also have IGF-1-independent effects on cell survival and proliferation [103,104]. IGF-binding protein-1 has inhibitory and stimulating actions on IGF-1 activity [105]; stress, inflammation, muscle wasting and malnutrition can contribute to increase IGFBP-1 serum levels and to subsequent inhibit IGF-1 biological action. In fact, IGFBP-1 is inversely associated with free-IGF-1 concentrations and IGF biological activity [106]. Growth-Hormone/IGF-1 signaling is a key pathway in the regulation of protein synthesis [106], glucose homeostasis, bone growth and density [99; 107], and erythropoiesis [100].
Aging model is characterized by hyposomatotrophism, which also results in a typical gradual decline and alteration in GH secretion pattern and IGF-1 production. The daily secretion of GH progressively decreases by about 14% per decade after puberty and up to 70% by the age of eighty [108]. In a rather similar fashion, IGF-1 and IGFBPs serum levels also decrease with age. It is estimated that more than 30% of non-obese older men have IGF-1 levels below the lowest serum concentration of 16 nmol/l [109]. In women, a profound reduction in GH levels mainly occurs after menopause [96]. A sharper decline in GH/IGF-1 activity can be also observed when unbalanced diet, excessive alcohol intake and impaired liver function coexist [110-112]. Hoffman et al. [113] termed the clinical consequences of the age-related decline in GH-IGF-1 axis activity as somatopause. This phenomenon has been associated with unfavorable changes in body composition, physical performance [97] and sex hormone levels [114,115].
INSULIN-LIKE GROWTH FACTOR-1 AND PHYSICAL PERFORMANCE
Studies performed in older adults tried to assess the relationship between IGF-1, physical performance tasks and body composition, with evidence of a clear positive association between IGF-1 and these parameters, especially in women [116-126].
Onder et al. [116] tested the relationship between Free-IGF-1, IGFBP-3 and muscle strength and physical performance by collecting data on 349 men and women, enrolled in a prospective cohort study (SIRENTE Study). After stratification of the study sample by BMI groups, subjects with high IGF-1 levels and BMI>30 kg/m2 displayed a significantly better grip strength (+21%; P=0.03), walking speed (+38%; P=0.01), as well as SPPB score (+27%; P=0.01) than those participants with lower IGF-1 levels. No association was observed between IGFBP-3 and the study outcomes, independent of BMI. In the Health, Aging and Body Composition Study including men and women aged 70 to 79, the authors found a cross-sectional positive relationship between circulating IGF-1 levels and poor thigh muscle area and density [117].
Consistently, Kaplan et al. [118] in their observational study of older adults aged 65 or older, suggested for IGFBP-1 levels a role as adverse prognostic factor. These authors assessed the impact of total IGF-1, IGFBP-1 and IGFBP-3 levels, on physical performance and mortality. The risks of death were 30% to 50% higher in those in the highest tertiles of fasting IGFBP-1 levels (436.9 mg/L). High IGFBP-1 level was also associated with poorer handgrip strength (P-trendT1-T3<.01) and slower walking speed (P-trendT1-T3=.03). Low levels of total IGF-1 had a marginal association with handgrip strength (P-trendT1-T3=.06). Total IGF-1 levels did not predict walking speed, decline in functional status or all-cause mortality. IGFBP-3 levels had a U-shaped association with hand-grip strength. A better handgrip performance was observed in individuals in the middle IGFBP-3 tertiles (P=0.03).
In 432 community-dwelling study of men and women, no association was detected between IGF-1, IGFBP-3, or IGF-1/IGFBP-3 ratio and BMI, adipose tissue distribution, and visceral adipose tissue [119].
The role of the gradual decline of IGF-1 production as possible determinant of the age-related reduction in muscle mass, strength and physical performance is more evident in men than in women [120]. In the New Mexico Aging Process Study, IGF-1 levels were correlated with muscle mass in men but not in women [121]. Other studies have shown that higher serum IGF-1 levels were strictly associated with longer exercise time in men, even after adjusting for serum IGFBP-3, confirming that an active lifestyle could represent an effective positive modulator of IGF-1 production [122,123]. The potential role of IGF-1 in the development of physical disability has been confirmed in a cross-sectional study population including frail and healthy older women (70-79 yrs old) [124]. Low IGF-1 levels were associated with poor knee extensor strength and self-reported difficulty in mobility task but not with other strength or anthropometric parameters. A positive relationship between IGF-1 levels and walking speed was found only for IGF-1 levels below 50 μg/L.
In a more recent cross-sectional analysis, Taekema et al. [125] assessed this relationship in two different age-groups composed of middle-aged and oldest-old men and women. IGF-1 and IGFBP-3 levels were positively associated with muscle strength in women but not in men. Furthermore, these investigators found that serum levels of IGF-1 were negatively associated with walking speed in older men and IGFBP-3 serum levels were positively associated with ADL in the oldest-old women (P=0.002).
Payette et al. [126] in the Framingham Heart Study showed in 558 elderly (72-92 years old) that lower IGF-1 levels were predictors in men, but not in women, of the loss of free fatty mass (FFM). Data from the InCHIANTI study [120], a prospective population-based study of older people, whose goal is to identify risk factors for mobility-disability, showed that only in men total IGF-1 levels were important and independent predictors of time to walk 400 m (P<0.0001) and lower extremity performance score. It is noteworthy that, in men, the effect of IGF-1 on physical performance was substantially reduced and no longer statistically significant, after adjustment for knee extension torque. The reason for such sex-related difference is unclear. In the studies considering only healthy older subjects, the association between IGF-1, muscle strength and physical performance is statistically significant only in men. However, when frail subjects are also included, the significant interaction between IGF-1 and physical performance is observed also in women.
INSULIN-LIKE GROWTH FACTOR-1 AND PHYSICAL PERFORMANCE: THE ROLE OF INFLAMMATION
As for other anabolic hormones, the activity of IGF-1 seems also to be affected by inflammatory status (Fig. 3). There are recent data showing an interesting and inverse relationship between IGF-1 and IL-6. IL-6 is a pro-inflammatory cytokine with both immunological and non-immunological effects and its serum concentrations tend to increase with age [127]. IGF-1 and IL-6 may have an aggregate effect to accelerated aging [117, 128]. It is already known that high IL-6 levels have a role in mobility impairment and mortality [129-132]. Recent data suggest that IGF-1 and IL-6 could be considered important targets to prevent or minimize disability. Cappola et al. [133] have shown how the combination of low IGF-1 and high IL-6 levels confers a high risk for progressive disability and death in a cohort of 718 disabled older women enrolled in the Women's Health Study I, a 3- year cohort study with 5-yr follow-up period. Women with IGF-1 levels in the lowest quartile were more likely to have walking limitation (OR 2.54; 95% CI, 1.05-6.11) independently of different covariates. No differences in terms of disability and Instrumental ADL (IADL) were observed across the IGF-1 quartiles. Conversely, participants with IL-6 levels in the top quartiles displayed an increased likelihood to have walking limitation (OR 4.99; CI, 1.95-12.80), disability (OR 1.79; CI, 1.00-3.19) and severe impairment of IADL (OR 2.00; CI, 1.00-4.01) than those in the bottom three quartiles. Interestingly, women with low IGF-1 and high IL-6 levels were at greater risk for functional impairment compared with those with high IGF-1 and low IL-6 levels. Barbieri et al. [134] showed that both biomarkers were independent predictors of total muscle power, whereas IL-6, but not IGF-1, was an independent predictor of handgrip strength. In particular, an association between IGF-1 and handgrip strength and total power was only evident in subjects in the lowest IL-6 tertiles, which enforces the evidence of a joint effect of IL-6 and IGF-1 on muscle function.
All these observations suggest the potential usefulness of a positive modulation of IGF-1 levels in older individuals in order to increase muscle strength and physical function.
THYROID HORMONES AND MOBILITY
Physiological changes in thyroid hormone concentrations are part of the overall changes in the hormonal milieu occurring during aging [135]. Serum thyroid-stimulating hormone (TSH) concentrations decrease in healthy elderly humans, serum total and free triiodothyronine (T3) levels demonstrate a clear, age-related decline, whereas serum total and free thyroxine (T4) levels remain unchanged [135]. These modifications are often associated with a poor health status. Indeed serum reverse T3 (rT3), an inactive metabolite of T4, seems to increase with aging together with the decrease of serum T3 level. These changes may indicate a decreased peripheral hepatic metabolism of T4, by the activation of liver type I deiodinase (D1), due to inflammatory cytokines [136]. These characteristics describe the “low T3 syndrome”, which is particularly observed during the aging process and diseases [136]. The prevalence of overt and subclinical hypothyroidism (Shypot) in the older population is about 20% [137]. Similarly, the prevalence of subclinical hyperthyroidism (Shypert) increases with age, being 1-2%, in iodine-sufficient areas [137,138], and 7-8% in iodine-deficient areas [139]. Subclinical thyroid disease (STD) is defined as circulating concentration of free T4 and free T3 within their respective reference range, in the presence of abnormal circulating concentration of TSH [140]. These conditions are more frequently observed in elderly than adult or young populations [141]. Subclinical thyroid disease in older subjects might influence the physical function. Van den Beld et al. [142] reported data from a cross-sectional study (the Zoetermeer Study) of 403 independently living and outpatient men, aged 73 yr and older. They evaluated the relationship between thyroid hormones and several physical characteristics of aging, including physical performance and muscle strength. Physical performance, or lower extremity function, was assessed by measuring standing balance, walking speed and chair stand ability. A summary of physical performance scale (PPS) was created by summing the category scores. Muscle strength was assessed as isometric grip strength tested by using a handheld dynamometer in the non-dominant hand. Body composition was measured by Dual- Energy X-Ray Absorptiometry (DEXA). 63 men met the criteria for the “low T3 syndrome” (low serum T3 and high serum rT3). This was associated with a lower PPS, independently of diseases. Furthermore, higher serum T4 (within the normal reference range of healthy adults) and rT3 levels (above the normal reference range of healthy adults) were related with lower grip strength and PPS, independent of age and diseases.
44 subjects met the biochemical criteria for Shypert (TSH=0.1-0.45 mIU/L) and 6 subjects had Shypot (TSH=4.5-10.0 mIU/L), with serum T3 and T4 in their normal reference range. The 44 subjects with Shypert had a significantly lower lean body mass (LBM) than euthyroid (Eut) subjects. No other significant differences in physical characteristics were observed between these groups [142]. These data suggest that both overt and Shypert might cause a reduction of muscle mass and strength [143], and this is of importance because subclinical altered thyroid function is the most frequent endocrine abnormality occurring with age [144,145].
Ceresini et al. [146] explored the relationship between mild hyperthyroidism and physical function in the elderly. The first part of the study was a cross-sectional analysis in the InCHIANTI Study, in which several parameters of physical function were compared between 364 Eut men and 28 Shypert men and between 502 Eut and 39 Shypert women. Muscle function was evaluated by measuring CMSA and handgrip strength assessed by a handheld dynamometer. Physical performance was measured by SPPB and nerve conduction velocity (NCV) at the right peroneal nerve. Shypert men, but not women, had a significantly lower SPPB score than Eut controls (P=0.02), but comparable CMSA, hand grip strength and NCV. Thus, men affected by Shypert were more likely to have an impaired mobility, than those Eut (OR=2.97; 95% CI, 1.01-8.71; P < 0.05). The second part of the study was a longitudinal analysis, in which the authors evaluated the relationship between TSH, FT3 and FT4 and 3-year change in SPPB score in 304 men and 409 women who were Eut at enrolment. The analysis showed that in Eut men higher baseline FT4 was a significant independent predictor of steeper decline in SPPB score during 3-year follow-up period (P=0.02) [146].
The findings of this study suggest that Shypert may negatively affect physical function through its detrimental effects on muscle mass and/or muscle strength, especially in older men who already have reduced LBM and mobility [146]. Simonsick et al. [147], in a similar double design study investigated the relationship between Shypot and mobility in older adults. The objective was to compare the functional mobility of 2290 community-dwelling resident aged 70 to 79 categorized by TSH levels as Eut (≥ 0.4 < 4.5 mIU/L), mild Shypot (≥ 4.6 < 7 IU/L) and moderate Shypot (≥ 7 < 20.0 mIU/L with normal free-T4 level) at baseline and after 2 years. The main outcomes included both self- report mobility capacity and limitation (assessed by a questionnaire) and performance-based measures of mobility (usual gait speed). Cardiorespiratory fitness and endurance walking ability were assessed by performance in the Long Distance Corridor Walk, a 2-stage, self-paced endurance walk test performed over 20-m course. These parameters were measured at study baseline and 2 years later. In the age- and sex- adjusted cross-sectional analyses, the mild Shypot group (vs the Eut group) showed better mobility (faster mean usual and rapid gait speed) [1.20 vs 1.15 m/s and 1.65 vs 1.56 m/s, respectively; P < 0.001], good cardiorespiratory fitness and less reported walking difficulty [39.2% vs 28.0% and 44.7% vs 36.5%, respectively; P < 0.001]. After 2 years, the mild Shypot group had a similar physical decline as the Eut group, but maintained the mobility advantage, while the moderate Shypot group had similar mobility decline than the Eut group. Thus, a lower activity of the thyroid hormone axis seems to be beneficial during the aging process [147]. Klubo-Gwiezdzinska and Wartofsky in a comment to this issue arose a couple of key questions. First of all, the data could have been affected by sample bias because of the single TSH measurement for the whole study group [148]. Transient elevation in TSH levels is common and does not necessarily reflect a real condition of Shypot. TSH subsequent reversion to normal levels is estimated in about 5% of the population, when it is apparently due to non-thyroidal cause [149]. Indeed, in the elderly, TSH level may be transiently elevated due to recovery from non-thyroidal illness (low T3 syndrome) or certain medications. Thus, it is suggested to be cautious to easily make a diagnosis of Shypot. Conversely, it would be better to repeat TSH measurement after 6 or 8 weeks. Second, the study focuses on the low impact of Shypot on functional mobility in elderly persons [148]. An association of mild thyroid failure with mortality risk was found in a recent meta-analysis [150] and it should be particularly considered in patients with comorbidity [151]. These authors found that among male participants with baseline euthyroidism, high baseline FT4 concentrations were predictors of a steeper SPPB decline over 3-year follow-up. Although they did not measure rT3 in their samples, they excluded from the analysis those subjects who, at the enrolment, had low T3 levels, because of “low-T3 syndrome”. Therefore, the association between high-normal FT4 at the enrolment and the 3 year decline in SPPB score, is unlikely to be due to a baseline “low-T3 syndrome”. Although in this study subclinical hyperthyroid subjects had a reduced SPPB score without changes in both muscle mass and strength, as compared with Eut subjects, the authors suggested in elderly people that Shypert is linked to reduced physical performance independent of skeletal muscle function. This data enforces the hypothesis that excess thyroid hormone concentration may cause damage in the central control of movement, through its well-known adverse effects on central nervous system physiology [144].
VITAMIN D AND MOBILITY
Vitamin D (also known as 25-hydroxyvitamin D or 25OHD) is a pro-hormone receiving growing attention as a musculoskeletal nutrient. Vitamin D deficiency (also known as rickets) is a major health problem worldwide, which burden seems even greater in elderly populations due to low consumption of 25OHD-enrich food, decline of sunlight exposure and inefficient renal hydroxylation [152]. Low Vitamin D levels influence calcium transport, uptake of inorganic phosphate for generation of energy-rich phosphate compounds and protein synthesis in the muscle, thus resulting in poorer physical performance, lower muscle strength and physical function [153]. In particular, the reduced function of the Vitamin D receptor (VDR), which is frequently observed in older adults with low serum 25OHD, contributes to impair protein synthesis in the muscle cells, decreasing type II fibers and finally inducing sarcopenia [154]. Finally, hypovitaminosis D, especially lower levels of 1,25OHD, is associated with an increase of parathyroid hormone (PTH) levels, which are known to trigger the production of inflammatory cytokines, one of the determinants of the age-related decline of physical function [155].
All these mechanisms explain the plethora of case reports and cross-sectional and longitudinal studies supporting the existence of a potential association between 25OHD deficiency and mobility limitations.
Vitamin D Receptor has been identified in several tissues including the skeletal muscle [156]. The VDR, was discovered for the first time in 1974 by Brumbaugh and Haussler [157]. The structure of the protein is based on three regions: a domain that binds the DNA which is N-terminal dual zinc finger, a C-terminal domain that binds the Vitamin D, and an unstructured region that links these two domains [158]. The bindings between Vitamin D and its receptor, lead to gene transcription mediating the genomic response and playing a central role in the regulation of contractile properties of the skeletal muscle [159]. The role of Vitamin D on muscle function can also be explained by the modulation of calmodulin-binding cytoskeletal proteins. In vitro studies performed in myoblasts treated with 1-25(OH) D2 showed an increase of calmodulin synthesis after Vitamin D exposure [160]. However, recent data questioned the nature of antibodies used for detecting the VDR in the skeletal muscle. Most of the antibodies can generate false-positive data. There is some evidence that VDR is undetectable in skeletal muscle, suggesting the intriguing idea that the effect of Vitamin D on skeletal muscle is independent of VDR [161].
Vitamin D receptor pathway is not the only mechanism explaining the influence of Vitamin D on muscle strength. In vitro studies show that the rapid effect of 1-25(OH) D2 on the calcium uptake and the muscle contractility [162] can be due to non-genomic pathways with involvement of phospholipase C and adenylyl-cyclase [163]. This mechanism leads to the recruitment of intracellular calcium, the activation of calcium-channels with the final raise of the calcium influx.
Another open mechanism linking Vitamin D status and mobility is the potential relationship with musculoskeletal pain, seldom im- properly attributed to arthritis. This is a frequent cause of functional decline and progressive disability in older adults. Leveille et al. [164] investigated physical and psychological mediators of pain-disability relationship in the Women's Health and Aging Study including women aged 65 years old or older who had at least mild disability at baseline (difficulty in stair climbing, N=676; or difficulty in walking, N=510). Lower extremity pain was not associated with walking (OR 1.13; 95% CI, 0.63-2.03), but only with difficulty climbing stairs (OR 1.85; 95% CI, 1.14-2.99). However, this association was attenuated after adjusting for physical impairments and psychological symptoms (OR 1.66; 95% CI, 0.99-2.77). Nevertheless, substantial differences were observed in the likelihood for onset of mobility -difficulty versus inability according to pain category during the follow-up. Women with widespread pain had increased odds of developing stair climbing (adjusted OR 2.86; 95% CI, 1.74-4.68) or walking difficulties (adjusted OR 1.85; 95% CI, 1.08-3.17) as compared with women with none or mild pain at baseline, and independent of several demographic and behavioral characteristics [162]. According to these results, it is thereby interesting to establish whether or not 25OHD metabolism is associated with muscoloskeletal pain. Significant insights into this issue have come from data of the InCHIANTI Study [165]. After adjusting for potential confounders, 25OHD deficiency was not associated with lower extremity pain or dual-region pain, although a significantly higher prevalence of at least moderate back pain without lower extremity pain was observed in women (OR 1.96, 95% CI, 1.01-3.59) but not in men.
In the recent years many studies have investigated the potential links between Vitamin D and skeletal muscle.
In 2009 Annweiler et al. [166] performed a systematic review of all published articles in English and French Medline from January 2004 to November 2008 testing the impact of low serum 25OHD concentration on muscle function, balance and gait performance among people aged 65 years and older. By using the Medical Subject Heading (MeSH) terms “aged OR aged, 80 and over” AND “Vitamin D OR vitamin D deficiency” combined with the terms “Gait” OR “Gait Apraxia” OR “Gait Disorders, Neurologic” OR “Walking” OR “Mobility Limitation” OR “Polyneuropathy” OR “Proprioception” OR “Ataxia” OR “Accidental Falls”, the authors identified 102 studies, 8 of which met the selection criteria and were included in the final analysis. Five studies showed a significant positive association between physical performance and serum 25OHD concentration, while 2 showed no significant association, whereas one study failed to prove any association with 25OHD. A significant association between low performance assessed by Timed Up & Go test and serum 1,25OHD concentration has been also found. The authors of this review concluded that the association between 25OHD status and physical performance remains a controversial issue.
In a subsequent investigation conducted in 70 female older patients >65 years of age with low serum 25OHD concentrations, Janssen et al. [167] found an inverse significant association between Vitamin D and knee extension and handgrip strength, leg extension power, Timed Up & Go test and Modified Cooper test. More recently, Houston et al. [168] assessed serum 25OHD, SPPB, grip and knee extensor strength, mobility disability (i.e., difficulty walking half a mile or up 10 steps) and ADL at baseline and every 6 months over 3 years of follow-up period in 988 community-dwelling adults aged 77 to 100 yrs. The SPPB scores were found to be lower in those with 25OHD deficiency after adjustment for sociodemographic characteristics, season, health behaviors, and chronic conditions. The grip strength was also positively associated with serum 25OHD. Elderly patients with 25OHD deficiency were also more likely to have ADL disability (OR 1.51; 95% CI, 1.01-2.25), and also greater risk of incident mobility disability over 3 years of follow-up (HR 1.56; 95% CI, 1.06-2.30). To assess whether serum levels of 25OHD may be associated with transitions between the states of robustness, prefrailty, and frailty and with mortality in older adults, as a part of the large prospective cohort InCHIANTI Study, Shardell et al. [169] followed 1,155 subjects aged 65 and older for up to 6 years after enrollment. Prefrail participants with 25OHD levels <20 ng/mL had a greater risk of mortality (8.9; 95% CI, 2.5-15.2), becoming frail (3.0; 95% CI, 5.6-14.6), and a lower chance to become robust (7.7; 95% CI, from 3.5-18.7) than prefrail participants with serum levels of 25OHD ≥20 ng/mL. Moreover, each 5 ng/mL decrement of 25OHD in prefrail participants was associated with a nearly 1.5-time higher risk of mortality (95% CI, 1.18-2.07). In participants without weakness at the baseline, each 5 ng/mL decrement in the serum level of 25OHD was associated with greater risk of developing weakness.
Toffanello et al. [170] also investigated the role of Vitamin D in muscle-skeletal function in 2694 community dwelling-elderly people from the Pro.V.A. (Progetto Veneto Anziani). Physical Performance was assessed through several tests including Tandem Test based on three different positions (a side-by-side, a semi tandem, and a full-tandem), 5- Timed Chair Stands (TCS), Gait Speed, six Minutes Walking Test (6MWT), Strength Handgrip and Quadriceps strength. They initially observed a linear association with 25OHD quintiles levels and TCS, Gait Speed, Strength and Hand-grip Strength, but not with Tandem Test and Quadriceps strength. After adjusting for potential confounders such as age, BMI, smoking habit, regular physical activity, season, depression, cognitive status, glomerular filtration rate (according to the MDRD formula), cardiovascular and osteoarticular diseases, chronic obstructive pulmonary disease (COPD) and visual impairment, the linear association was still evident only for the 6MWT in both sexes, TCS in women, gait speed and handgrip strength in men. Based on these data, the authors suggested, for optimal physical performance, a serum concentration of Vitamin D close to 100 nmol/L in order to keep Vitamin D levels as nearest as possible to the desired threshold and to obtain benefits for muscle-skeletal function in elderly people.
These data were replicated by Snijder et al. [171] in a prospective cohort study including 1231 men and women of the Longitudinal Aging Study Amsterdam (LASA) investigating the association between 25OHD levels and risk of falling.
They found that 25OHD levels <10ng/mL, were associated with an increased risk of falling independent of age, sex, education level, region, season, physical activity, smoking, and alcohol intake. The odds ratios were 1.78 for subjects who had two or more falls compared with those who did not fall or that fell once, and 2.23 for those who fell three or more times compared with those who fell two times or less. The authors concluded that low levels of Vitamin D are independently related with an increased risk of falling in the elderly.
Similarly, Witchers et al [172] in cross-sectional and longitudinal analyses within the LASA found that serum 25OHD levels below 20 ng/mL were a cut-off associated with poor physical performance measured by three performance tests (chair stand, walking time and tandem stand). After adjustment for confounders, subjects with 25-OHD less than 10 ng/mL and those with 25OHD between 10 and 20 ng/mL had significantly higher odds ratios for decline in physical performance after 3 years, compared with participants with 25OHD of at least 30 ng/mL.
Recent trials also support the hypothesis of an independent positive effect of Vitamin D on mobility limitation in older population.
In the last paragraph we will list the intervention studies testing the effects of single hormonal replacement therapy on indirect and direct measures of physical function. Although there is the rationale to start some hormonal approaches to improve mobility in older persons, very few RCTs have investigated this topic.
HORMONAL THERAPEUTIC STRATEGIES TO IMPROVE MOBILITY
RCTs on DHEAS and Sarcopenia in Men and Women
In the last decade many studies evaluated the usefulness of DHEA replacement therapy on physical function of older individuals. Baker et al. [173], in a recent interesting review describes the characteristics of five selected double-blind RCT intervention studies in women and men of at least 50 years and older. The studies differed in terms of patients included, duration of follow-up, and interventions (supplementation alone or plus exercise). Study sizes ranged from 19 to 280 participants, with duration of follow-up ranging from 3 to 24 months. The dose of oral DHEA ranged from 50 [174] to 75 mg/day [175]. Three studies used concomitant interventions in addition to DHEA supplementation, two using exercise training [176,177] and the other gentle exercise (endurance and resistance training) [174].
All the studies examined different measures of muscle strength. Two studies showed an improvement in leg press [174; 177], but in both DHEA treatment was combined with exercise. Nevertheless, similar number of studies had negative results for this endpoint [175,176]. Four studies also examined measures of physical function and performance [178; 174-177]. Only one study showed an improvement in a composite score measuring physical performance [174]. All together these trials suggest an improvement in muscle strength or function, especially in women and only when DHEA treatment is combined with exercise.
In elderly people physical performance cannot be separated from BMD. DHEA, similarly to T and estrogens, increases BMD. The DAWN Trial (DHEA And Well-Ness) [179] is an interesting, double-blind, placebo-controlled randomized trial designed to examine the effects of 1-year 50 mg daily oral DHEA supplementation on BMD, bone metabolism and body composition in 225 healthy adults (110 men and 115 women) aged 55 to 85 years. DHEA treatment increased serum DHEA and DHEAS levels to concentrations seen in young adults. Testosterone, E2 and IGF-1 levels increased in women (all P<0.001), but not in men, on DHEA treatment. After 12 months, there was a positive effect of DHEA on lumbar spine BMD in women (P=0.03), but no effect in hip, femoral neck or total body BMD. No significant changes were observed at any site in men. In women, the authors observed beneficial changes in BMD that may reflect the combined effects from the peripheral conversion of DHEA into sex steroids and the stimulation of IGF-1 [179]. Of particular interest is one RCT including subjects of both sexes with low androgen levels and aged 60 years of age or older [175]. Eligibility criteria of this trial were, for men, DHEAS levels less than 157 μg/dL (4.3 μmol/L), and for women, DHEAS levels less than 95 μg/dL (2.6 μmol/L). These cut-off values, represented the 15th percentile of levels for normal young men and women. Oral DHEA treatment (75 and 50 mg of DHEA per day to older men and women, respectively, for about 23 months) increased DHEAS levels to high-normal range for young people. This therapy slightly increased levels of T and E2 in women and levels of E2 in men. DHEA treatment had no effect on fat-free mass in men or women when the groups were analyzed separately according to sex. The lack of a significant effect on thigh-muscle area, strength, or fitness largely discounts the relevance of the change in fat-free mass. Among the five sites measured for BMD, the women in the DHEA group had a small but significant increase in BMD of the ultradistal radius; men in the DHEA group had an increase in BMD in the femoral neck. On the other hand, treatment with DHEA caused no detectable harm [175].
RCTs on Testosterone Supplementation and Mobility
Ottenbacher [180] in a meta-analysis published in 2006 evaluated for the first time the most important RCTs addressing the effects of T on muscle function. The findings from 11 RCTs were examined by using the methods of meta-analysis to determine whether androgen treatment (T/dihydrotestosterone, DHT) increases strength in men aged 65 and older [180]. A moderate increase in muscle strength was found in subjects on T/DHT therapy versus placebo group. The average subjects assigned to T performed approximately 19.3% better than the placebo group [180].
Nair et al conducted a 2-year, placebo-controlled, randomized, double-blind study involving 87 elderly men (median age=66.2 years) with low levels of DHEAS and bioavailable T (less than 103 ng/dL). 27 subjects received T, and 31 received placebo [175]. Participants received transdermal T (5 mg/day). Primary outcomes were physical performance, peak aerobic capacity and BMD. Men in the T group had a slight increase in fat free mass but not in muscle strength and other parameters of physical performance.
Kenny et al in 2010 [181] investigated in a double-blind RCT the effects of T supplementation on body composition, muscle, physical function and BMD in 131 men (mean age 77.1) with low T, history of fracture, or BMD with T-score ≤ −2.0 and frailty. Participants received transdermal T supplementation 50 mg/day or placebo for 12 to 24 months; all participants also received calcium (1500 mg/day diet and supplement) and Vitamin D (1000 IU/day). In T group there was an increase in LBM and a decrease in fat mass, and a modest increase in axial BMD, but no differences were observed in terms of strength or physical performance.
The achieved mean total and bioavailable T levels were 583 and 157 ng/dL, respectively. These values can be considered quite modest. The Endocrine Society Clinical Task Force recommends to achieve T levels mild to normal range and to adjust the dose of T treatment if T levels are lower than 350 ng/dL. Twenty percent of men in the T group had T levels less than 350 ng/dL at 12 months and the dosage of T was not adjusted for T levels in this study. The largest changes in BMD, body composition, and physical performance in older men were achieved in a study in which T levels were 734 ng/dL for T alone and 942 ng/dL for T plus finasteride [182].
Srinivas-Shankar U et al. [183], in the same year, tested the effects of 6 month T treatment in intermediate frail population of 24 healthy, community-dwelling older men (60-85 year) with T ≤12 nmol/L or Free T ≤250 pmol/L. Transdermal hydro-alcoholic T gel (Testogel 1%) at a dose of 50 mg/d for 6 months improved lower limb muscle strength, body composition, quality of life, and physical function. More convincing data come from Testosterone in Older Men with Mobility Limitations (TOM) Trial [184]. The aim of this placebo-controlled randomized trial was to determine whether T therapy in 209 community-dwelling older men, 65 years or older, affected by severe mobility limitation and low T levels (100 to 350 ng/dL [3.5 to 12.1 nmol/L]), could improve lower extremity muscle strength and physical function. Participants were randomized to placebo or 100 mg T gel therapy daily for 6 months. Primary outcome was leg-press strength, while the secondary outcomes included chest-press strength, stair-climb, 40-m walk, muscle mass and physical activity. Compared with placebo, a significantly greater proportion of men receiving T improved their leg-press and chest-press strengths (43% vs 18%, P = 0.01) and stair-climbing power (28% vs 10%, P = 0.03). Additionally, the increase in leg-press strength and stair-climbing power was associated with changes in T levels [184].
More convincing and definitive data are expected at the end of this year when the ongoing Testosterone trial (T trial) will be completed. T trial is a multicenter study to test the effects of T in elderly men with low T levels on physical function, vitality, sexual function, cognitive function, anemia, and cardiovascular risk. Eight hundred men ≥65 years whose serum T <250 ng/dL were double-blindly randomized to receive T or placebo for one year. The primary end points for each trial were 6MWT, fatigue-vitality, sexual activity, delayed verbal memory, hemoglobin, and coronary artery plaque burden.
Testosterone therapy in women has been less investigated than in men. Sheffield-More at al. [185], showed that skeletal muscle of women is anabolic responsive to androgens, but the full physical impact of this effect has not been studied. More research is needed to investigate whether low T levels in women could lead to mobility impairment and whether T replacement therapy can reduce the negative health consequences of age-related disability. Thus, especially in individuals with impaired mobility, the possibility of increasing muscle strength via androgen administration should be kept in consideration.
RCTs on Estrogen Supplementation and Mobility in Older Women
There is no agreement on the opportunity to start Hormone Replacement Therapy (HRT) in postmenopausal women to improve physical performance.
In their meta-analysis Greising and colleagues [186] addressed the effects of HRT (without concomitant exercise) on muscle strength in RCTs with the following criteria: (1) post- menopausal women at baseline, (2) HRT based on the use of estrogens, (3) objective assessment of muscle strength, (4) explanation of muscle strength assessment, (5) age of the patients, (6) presence in the single studies of inclusion and exclusion criteria, (7) results published in English. By using this research methodology, 23 studies conducted between 1987 and 2007 were considered in the meta-analysis.
The results revealed a great variability with regard to the effect of estrogens on muscle strength with an effect size (ES) between −0.56 and 1.25. Overall, the meta- analysis showed a statistically small positive effect (ES =0.23, P=0.003) of estrogens on skeletal muscle strength. This ES was roughly equivalent to 5% of muscle strength in post-menopausal women taking estrogens than women who did not use it.
Only 5 of the 23 studies reported muscle strength normalized for muscle mass, which provides a measure of muscle quality. In these studies, the size of the muscle was measured by computed tomography or through an equivalent methodology. The results of these studies indicated that HRT may have a moderate effect on the muscle strength normalized for muscle mass, but the ES (0.45) was not statistically significant, in view of the few studies taken into consideration [187-191].
Of the all 23 studies considered in the meta-analysis, there was a high variability around the muscle-groups that were analyzed. The results of the meta-analysis showed that the assessment of the adductor muscles of the thumb was more positively affected by estrogens with an ES significantly higher than the scores of other muscle sizes (P<0.001). The global ES of the studies examining the adductor muscles of the thumb was expression of approximately 17% greater muscle strength for women receiving HRT than control.
One of the limitations of this meta-analysis was the small number of the studies considering participants on specific hormone preparation (N=9).
Nevertheless, it was possible to evaluate two aspects of HRT that might have influenced the ES.
First, the previous use of hormones as factor affecting ES. In only 6 of the 23 studies examined, women did not take HRT, while in the other 17 studies, patients were taking HRT. There was no statistical difference in ES between studies that did and did not allow previous HRT use. However, there was a more favorable trend toward HRT in comparison to no prior use (P=0.12). Five of 23 RCTs included strict control of hormonal treatment. The ES combining these 5 studies, where HRT was closely monitored, was three times higher than ES of those 18 studies with less controlled treatment (ES 0.46 vs. 0.16). However, the difference was not statistically significant (P=0.10) [186].
Another limitation of the meta-analysis was the exclusion of the studies considering the concomitant physical activity.
Taaffe et al. [192] enlightened this specific issue in a recent study testing the effects of HRT with or without exercise on muscle composition. In this study 80 women with an age range between 50 and 57 years were enrolled. Subjects were randomized to receive (a) HRT (20 subjects), (b) exercise (Ex, 20 subjects), (c) HRT in combination with exercise (ExHRT, 20 subjects) and a control group (control, 20 subjects) for one year. The study was double-blinded with subjects randomly assigned to E2 and norethisterone acetate or placebo. Exercise included progressive high-impact training for the lower limbs. Skeletal muscle attenuation in Hounsfield (HU) was determined by computed tomography of the mid-thigh. The areas examined were: the quadriceps compartment (including inter-muscular adipose tissue), quadriceps muscles, the posterior compartment and posterior muscles. Muscle performance was determined by knee extensor strength, vertical jump height, and running speed over 20 m. Fifty-one women completed the study. The authors found a statistically significant difference between the groups on HRT, compared to ExHRT control with regard to the vertical jump and running speed. No statistical difference among HRT, Ex and ExHRT was observed. For the posterior compartment, HU for the HRT and ExHRT was significantly increased compared with controls, while for posterior muscles, the HU in ExHRT group was significantly greater than controls. The HU was significantly higher in HRT, Ex and ExHRT groups at quadriceps compartment and quadriceps muscles, compared to control group.
The main limitations of these studies were the inclusion of early post-menopausal women and the limited information on specific tests of physical function and mobility.
In 2005 Greenspan et al. [193] conducted a RCT, aimed at comparing the effects of HRT or alendronate using a 2 × 2 factorial design. The authors failed to show any significant effect of HRT on rising time, usual walking speed and fast walking. No statistically significant differences in IADL, Physical Activity Scales of the Elderly (PASE) and Folstein Mini-Mental State Examination score were also observed between the two study-groups.
The population under investigation was composed of 373 women, 130 with an history of hysterectomy, treated with conjugated equine estrogens (0.625 mg/day) with or without medroxyprogesterone (2.5 mg/day) or placebo. Throughout the trial, all women received calcium and Vitamin D and multivitamin supplements to ensure a calcium intake greater than 1000 mg/day and Vitamin D intake between 400 and 800 IU/day. Participants underwent physical examination every 6 months, while physical function and mental status were assessed every 12 months. Information on falls was collected every 6 months. The walking time at a normal pace increased by 28±38% in women on HRT and 30±44% of women treated with placebo, while the time walk rapidly increased 33±44% in women receiving HRT and 29±49% in women treated with placebo. This difference was not statistically significant. Furthermore there was no significant difference between women on estrogen plus progesterone and unopposed estrogens. The physical performance assessment consisted of a modified group of previously measures of balance and mobility. The score declined by an average of 25±54 points in women on HRT versus 22±59 points in women on placebo and the difference did not reach the statistical significance [193].
RCTs on Growth Hormone Supplementation in the Elderly
Most of the evidence, available in the literature, confines GH treatment to its specific effects on body composition in the selected population of healthy older adults.
Muscle strength and physical performance, but not the frail phenotype, were also secondary outcomes of the studies testing the effects of GH treatment alone, or in combination with physical exercise, in older persons. The anabolic effects of GH appear to be dose-dependent with older men more sensitive to GH than younger individuals [92]. One potential mechanism underlying the anabolic effect of GH is the dose-dependent increase in IGF-1 levels in both sexes. This phenomenon is more pronounced in men and reaches a plateau after 1-week treatment [195].
The first study aimed at investigating the effects of GH treatment in elderly population was conducted by Rudman et al. in 1990 [196]. Six months of high dose-GH administration (0.03 mg/kg/day) three times a week in 12 older men resulted in a significant improvement in IGF-1 levels, muscle mass and BMD. However, the design of study was not placebo-controlled.
Interestingly, aging is often associated with GH resistance and the direct administration of IGF-1 could represent an alternative or therapeutic option.
Thompson et al. [197] have for the first time evaluated in 16 elderly women the combined effects of recombinant human GH administration (rhGH; 0.025 mg/kg/day) and one of two doses of recombinant IGF-1 (rh IGF-1; 0.015 and 0.060 mg/kg, twice daily) on body composition. As expected, all groups experienced a significant increase in serum IGF-1 and IGFBP-3 levels over the 4-week treatment period, accompanied by a significant decrease in fat mass. An increase in LBM and nitrogen retention was only observed in the high dose IGF-1 and GH groups.
Conversely, Huang et al. observed that low-dose of GH administration increases protein synthesis in healthy aged women and men [198].
Similarly, Blackman and colleagues reported in healthy, aged women and men that 26-week GH administration (starting dose, 30 μg/kg, reduced to 20 μg/kg, subcutaneously 3 times/week) with or without sex steroids was able to increase LBM and to decrease fat mass [199].
After single injection or short-term rhGH treatment, the proportion of type 2 muscle fiber and fiber size, the breakdown and synthesis of myofibrillar protein were not significantly affected [200-202]. Lange et al. [203] did not observe in healthy older men undergoing 12-week GH treatment alone any increase in isokinetic quadriceps muscle strength, power and CSMA.
In older patients with GH deficiency (GHD) baseline IGF-1 levels seem to be not sufficient predictors of the improvement of physical performance.
In fact, when baseline IGF-1 levels are low, but the functional ability is well-preserved, short-term low dose GH treatment fails to improve the functional abilities [204].
We cannot also ignore that even short-term GH administration has been frequently associated with numerous dose-dependent side effects including peripheral edema, paresthesia, carpal tunnel syndrome, arthralgia and alteration in glucose metabolism [96, 197, 205]. The high frequency of adverse events and the relative insufficient evidence for a clear therapeutic role of GH alone during somatopause, have restricted the usefulness of GH therapy in this age- group. Moreover, all the effects of GH treatment have been ob- served in small series of patients using a GH dose calculated on subject's body weight. In healthy adults GH secretion is inversely related to BMI and fat mass, and should be optimized based on these parameters [206]. There is also scarce information about long- term safety of GH treatment in non-GHD elderly patients.
GH treatment has been shown to determine more beneficial effects in older patients with GHD or with ADL dependency. In the group of adult onset GHD with low baseline muscle strength, GH replacement therapy seems to be more effective in comparison to younger GHD group [207]. In particular, the positive effects on quality of life, body composition, metabolism and cardiovascular risk in adult GHD are maintained in patients taking long-term continuous GH replacement [207].
Götherström, et al [208] in their prospective, open-label, single-center study evaluated the effects of 5 year GH replacement therapy on muscle strength and function in elderly adults with adult onset GHD. Their data showed that GH replacement therapy normalized knee flexor strength and improved, but did not fully normalize, knee extensor strength and handgrip strength. The same authors show that ten year-GH replacement therapy in elderly GHD adults resulted in an improved normal age-related decline in muscle performance and neuromuscular function [209].
GH therapy has been also shown to induce a sustained increase in overall bone remodeling activity and in a net gain of BMD in adults with adult-onset GHD with a low pretreatment z-score [210]. Elbornsson M et al. [211] recently supported this data by showing an increase in lumbar (L2-L4) spine and femur neck BMD and bone mass cells after long-term GH replacement. Benefits of GH treatment are also evident in other clinical conditions. In a double blind placebo controlled trial Weissberger et al. [212] have demonstrated in older patients undergoing total hip replacement and 18 week GH treatment (14 pre-operative and 4 post-operative), a significant improvement in LBM and skeletal muscle mass, a preservation or an improvement in muscle strength and a better postoperative 4-min walking ability. White et al. [213] in healthy older adults at risk for functional decline have shown a positive effect of oral GH secretagogue capromorelin administration on physical function. Guebre-Egziabher et al. [214] examined the potential additive anabolic effects of rhGH + rhIGF-1 compared with rhIGF-1 in eight well-nourished hemodialysis patients with severe chronic kidney disease. Recombinant human IGF-1 administration at a moderate dose of 40 μg/kg per 12 h had no effect on protein metabolism. Conversely, moderate dose of rhGH (50 μg/kg) combined with rhIGF-1 was followed by a significant increase of whole-body protein net balance.
All these data suggest that GH treatment alone is more effective in subjects with mobility limitation or diseases affecting physical performance. New hormonal cut-offs for IGF-1, related to physical impairment, are needed to correctly identify those future “responders” to GH treatment. However, the actual normal range of IGF-1 sufficiency or deficiency applies to adult and young subjects rather than old populations with mobility limitation.
Effects of treatment for subclinical hypothyroidism and hyperthyroidism on functional mobility
While in overt hyperthyroidism and hypothyroidism there is no doubt on the need for treatment, it is not such clear whether or not the correction of subclinical thyroid dysfunctions in older persons has to be pursued. The inclusion of mobility as target might influence the clinical decision to treat the subclinical thyroid dysfunctions in this age-group. However, data on this topic are very scant. In 1999, Norrelund et al [215] demonstrated in a very small group of subjects that treatment of thyrotoxicosis leads to an increased thigh muscle area and strength of the biceps brachialis and quadriceps muscles. In 2006, Brennan and co-workers [216] measured both thigh cross-sectional area as well as lower-extremity muscle strength in thirty adult patients with overt hyperthyroidism and 24 age-matched subjects with Shypert. The subjects were evaluated before treatment of hyperthyroidism and 6-9 months after the restoration of Eut state. Forthy-eight euthyroid controls were studied at similar time intervals. Mid-thigh muscle cross-sectional area was reduced in both the overt hyperthyroidism and Shypert groups at baseline compared to controls and increased significantly after treatment. Prior to treatment, both knee flexor and extensor muscle strength was reduced in both patients with overt hyperthyroidism and Shypert compared to controls. After treatment, all strength measurements improved in patients affected by overt hyperthyroidism. The majority of muscle strength measurements also improved in the subclinical hyperthyroid group. However, in spite of the improved muscle strength observed after the treatment of thyroid hormone excess, the impact on functional mobility in subclinical hyper- thyroid subjects, remains to be addressed. This particular aspect is of particular importance in older adults in whom subclinical thyroid dysfunction represents one of the most frequently observed endocrine disorders in the clinical practice [144].
Preliminary data suggest that elderly people with Shypot have a similar, even better, functional mobility of those with normal thyroid function. However, it is unknown whether older individuals with Shypot who start replacement therapy would benefit in terms of mobility. Simonsick and co-workers [147], in a small sample of patients found no difference in gait speed decline between patients with Shypot undergoing replacement therapy or without any treatment. Recently, Reuters and colleagues [217] performed a randomized double-blind study, in which adults with Shypot were assigned either to treatment (n=35) or placebo (n=36). The Authors showed an improvement of inspiratory muscle strength in the treatment arm, along with an improved quality of life.
Altogether, these data do not give definitive answers on the opportunity to treat subclinical thyroid disorders in older persons for improving functional mobility. Intervention studies, especially in elderly populations, with a representative number of subjects, are still needed to clarify this issue.
RCTs on Vitamin D and mobility
Because of its expected effects on bone and muscle, Vitamin D has been used to improve mobility limitation in older persons. The main outcomes of Vitamin D treatment considered in RCTs were muscle strength and physical performance.
Lagari et al. [218] investigated the effects of two different doses of oral cholecalciferol (400 UI, and 2000 UI per day) on Vitamin D serum levels, during 6-month follow-up period on parameters of muscle strength and physical performance in 86 community-dwelling elderly Vitamin D deficient subjects aged 65 to 95 years.
In participants with slow gait speed, the authors observed an improved ability to perform repeated chair-stand tests after both lower and higher dosages of Vitamin D. This effect was independent of potential confounders suggesting that Vitamin D supplementation can be effective in older subjects with low baseline physical functioning and at higher risk of frailty and falls. However, the lack of placebo control group represented a significant limitation of this study.
Stockton et al. [219] in a meta-analysis of several studies investigated the effects of Vitamin D supplementation at different daily doses on physical performance. Even in this case, the results were quite controversial. The meta-analysis of these authors found no significant change in either handgrip strength or knee extension strength. A small but significant increase in knee extension strength and in Timed Up & Go test was detected in the group taking 800 IU.
Sato et al [220] performed 2-year RCT in a group of 96 Japanese elderly women affected by post-stroke hemiplegia. Subjects were randomly assigned to receive 1000 UI Vitamin D daily or placebo. The number of falls and hip fractures were compared between the two groups. Starting from baseline levels, both groups had serum 25OHD levels <10 ng/mL. After Vitamin D treatment, the authors observed a 59% reduction of falls and hip fractures. The fractures occurred in 4/48 subjects of placebo group compared to 0/48 subjects of Vitamin D group. This data enforced the idea that Vitamin D may reduce the risk of falls and hip fractures by improving muscle strength.
In 2003 Latham et al [221], realized a multicenter RCT to investigate the effects of 25OHD treatment and quadriceps resistance exercise on reducing falls in frail older people after hospitalization. They selected 243 participants who were randomized to single administration of 25OHD (300000 UI) or placebo tablets and 10 weeks of intense exercises. Physical health, at 3 months, and falls, over 6 months, were the primary endpoints, whereas physical performance and self-rated function were the secondary endpoints. They observed no effects in both intervention groups on physical health and falls.
Based on the hypothesis that Vitamin D supplementation combined with calcium would improve muscle skeletal and decrease the incidence of falls, Bischoff-Ferrari et al. [222] performed a 12-week double-blind RCT in 122 elderly women with a mean age 85 and mean baseline value of 25OHD of 30 nmol/L. The subjects of this study were assigned to 1200 mg of calcium supplementation plus 800 IU of cholecalciferol (62 patients) and only 1200 mg of calcium (60 patients). The number of falls was compared between the two groups and changes in muscle-skeletal performance were measured by using summed score from knee flexor and extensor strength, grip strength, and Timed Up & Go test. Among calcium + Vitamin D group, there was also a significant increase in serum 25OHD levels which reached 65.5 nmol/L. Before treatment, mean number of falls per person per week was 0.059 in the calcium + Vitamin D group and 0.056 in the calcium-group. After 12-week treatment period, the mean number of falls per person per week was 0.034 in the calcium + Vitamin D group and 0.076 in the calcium group. After adjustment, calcium + Vitamin D- treatment accounted for a 49% reduction of falls. The muscle- skeletal performance also improved significantly in calcium + Vitamin D group. These data suggested that recurrent fallers could benefit in terms of muscle-skeletal function from the combined treatment of calcium and Vitamin D.
Similarly, Janssen et al. [167] tested the effect of Vitamin D, in combination with calcium, or calcium alone, on muscle strength and mobility in female geriatric patients with Vitamin D insufficiency. These authors selected 70 women aged > 65 years, with serum baseline levels of 25OHD between 20 and 50 nmol/L. Subjects received either cholecalciferol 400 IU plus calcium 500 mg daily or placebo plus 500 mg calcium daily for 6 months.
Muscle strength and mobility were tested at baseline and after 6 months using knee extension strength, handgrip strength, leg extension power, Timed Up & Go and Modified Cooper test. At baseline low levels of 25OHD were significantly associated with muscle strength and mobility tests. Vitamin D levels significantly increased in Vitamin D + calcium group compared to placebo+calcium group, but this increment did not result in any significant difference in terms of muscle strength or functional mobility between the two groups. The authors speculated that 400 UI of vitamin D plus 500 mg of calcium daily could be effective to improve strength and mobility in older women with vitamin D insufficiency. These data have been confirmed in younger population of female subjects where oral cholecalciferol/calcium supplementation for 6 months (Vitamin D 60,000 IU/week for 8 week followed by 60,000 IU/fortnight, elemental calcium 500 mg twice per day) did not lead to any improvement of handgrip strength, and 6MWT [223]
These results corroborate the recent hypothesis of Wang et al [224], which doubts the real presence of the VDR in muscle suggesting a different mechanism underlying the effects of vitamin D on skeletal muscle. Vitamin D supplementation has also been shown to increase muscle function in weakest and slowest patients [225].
Although a potential pathophysiological link between hypovitaminosis D and mobility limitations emerges from RCTs, most of which performed in adult postmenopausal women, no definitive evidence can be brought to justify the routinely use of Vitamin D replacement therapy in older persons with mobility limitations. Most of the controversy is due to the variability between studies in participants’ characteristics, baseline serum 25OHD levels, and baseline physical functioning.
HORMONAL CUT-OFFS ASSOCIATED WITH MOBILITY IN OLDER POPULATION
In Tables 1 and 2 we summarized the cut-offs emerging from observational and intervention studies associated with low mobility in older persons.
Table 1.
Potential hormonal cut-off values associated with low physical performance in older persons in observational studies.
| ALL | WOMEN | MEN | ||||
|---|---|---|---|---|---|---|
| HORMONAL CUT-OFF | OUTCOMES | HORMONAL CUT-OFF | OUTCOMES | HORMONAL CUT-OFF | OUTCOMES | |
| TESTOSTERONE |
Cappola et al (ref 19) Free T<0.7 pg/mL N. 494 Mean age: 70±7 |
More vulnerability to frailty than those without the deficiency although did not achieve statistical significance |
Maggio M et al (ref 44) Severely hypogonadal: total T levels ≤ 230 ng/dL; Moderately hypogonadal: total T >230 and < 350 ng/dL Eugonadal, T levels ≥ 350 ng/dl. N. 455 Mean age: 67.5±90 Krasnoff, et al ref (45) Free T < 70pg/mL n.1445 mean age 61.0±9.5 y |
Hand Grip strength SPPB Scores Muscle strength Significantly higher in eugo-nadal group No association with grip strength Predictor of lower SPPB Scores and decline in Walking speed |
||
| IGF-1 |
Onder G et al (ref 116) IGF-1 level 0.74, [0.42-1.17] BMI ≥ 30 kg/m2 N. 56 mean age 84.3±4.0 |
significantly better grip strength [35.2 +/− 1.6 vs. 29.2 +/− 2.0 (SE) kg, P = 0.03], walking speed (0.55 +/− 0.04 vs. 0.40 +/− 0.04 m/s, P = 0.01), and SPPB score (1.9 +/− 0.1 vs. 1.5 +/− 0.1 m/s, P = 0.01) | ||||
|
Colbert LH (ref 117) <77 ng/l in women <94 ng/l in men n.158 mean age:70±9 |
poor thigh muscle area and density | |||||
|
Kaplan et al. (ref 118) IGF-1 <121.1 ng/mL |
Borderline significant association with worse handgrip strength and not significantly relationship with walking speed, functional status or mortality |
Cappola et al (ref 133) <50 ng/mL N.617 Mean age: 70±9 |
poor knee extensor muscle strength, slow walking speed, and self-reported difficulty with mobility tasks | |||
|
Cappola et al (Ref 19) < 87.8 μg/L N. 494 Mean age: 70±7 |
More vulnerability to frailty than those without the deficiency although did not achieve statistical significance | |||||
| VITAMIN D |
Witcherts et al (Ref 172) N=1234 Age= 75.3± 6.5 25OHCategories: 1) <10ng/mL (N=134) 2)10-20 ng/mL (N=453) 3)20-30 ng/mL (N=420) 4) > 30 ng/mL (reference group N=227) |
Declyne in Physical Performace (Odds Ratio): 1)2.21 2)2.01 3)1.56 4) 1 |
Houston et al (Ref 168) N = 541 Age= 74.8 (mean) 25 OH Categories: 1) <25nmol/L (N=156) 2) 25 to<50nmol/L(N=249) 3) >50nmol/L(N=136) |
SPPB Scores significantly lower in group with serum 25OHD < 25.0 nmol/L compared to those with serum 25OHD >25.0 nmol/L Handgrip strength significantly lower in group with serum 25OHD<50.0 nmol/L compared to those with serum 25OHD >50 nmol/L |
Houston et al (Ref 168) N = 435 Age= 74.8 (mean) 25 OH Categories: 1) <25nmol/L (N=59) 2) 25 to<50nmol/L(N=163) 3) >50nmol/L(N=213) |
SPPB Scores significantly lower in group with serum 25OHD <25.0 nmol/L compared to those with serum 25OHD >25.0 nmol/L Handgrip strength significantly lower in group with serum 25OHD<50.0 nmol/L compared to those with serum 25OHD >50 nmol/L |
|
Toffanello et al (Ref 170) N = 1597 Age= >75 (mean) 25 OHD Levels: 1) <32nmol/L 2)32 – 49 noml/L 3)49 - 68 nmol/L 4)68 – 93 nmol/L 5) >93 nmol/L |
Observed Linear Associations Between Physical Performance and 25OHD levels (after adjusting of potential confounders) Tandem Test:No 5 Timed Chair Stands:Yes Gait speed:No 6 min walking test: Yes Handgrip strength :No Quadriceps strength:No |
Toffanello et al (Ref 170) N = 1097 Age= >75 (mean) 25 OHD Levels : 1) <53nmol/L 2)53 – 79 noml/L 3)79 - 103 nmol/L 4) 103 – 143 nmol/L 5) >143 nmol/L |
Observed Linear Associations Between Physical Performance and 25OHD levels (after adjusting of potential confounders) Tandem Test:No 5Timed Chair Stands:No Gait speed:Yes 6min walking test: Yes Handgrip strength : Yes Ouadriceps strength:No i |
|||
|
Snijder et al. (Ref 171) 25OHD <10ng/mL |
Increased risk of falling | |||||
| DHEAS |
Cappola et al (Ref 19) DHEAS < 0.22 g/L N. 494 Mean age: 70±7 |
More vulnerability to frailty than those without the deficiency although did not achieve statistical significance |
Valenti, et al (Ref 18) 50.5-64,5 μg/dL N=558 mean age 20-95 |
muscle strength and calf muscle area | ||
|
Stenholm, et al (Ref 20) DHEAS< 42.7 μg/dL N. 406 Mean age: 74.2±6.6 |
Muscle strength decline |
Stenholm et al (Ref 20) DHEAS< 53.9 μg/dL n.310 Mean age: 73±6.4 |
Muscle strength decline | |||
| TSH |
Van den Beld, et al (Ref 142) N. 403 men age 73 and older < 0.46 mUI/L FT3 - FT4 in normal range |
⇓ lean body mass | ||||
|
Ceresini, et al (Ref 146) N. 392 men mean age 74 < 0.46 mUI/L FT3 - FT4 in normal range |
⇓ SPPB score | |||||
Table 2.
Hormonal baseline and post-intervention hormonal cut-off that have been associated with low mobility in older persons, categorized as all, women and men.
| ALL | ||||
|---|---|---|---|---|
| Baseline values | Intervention | Values after treatment | Study outcomes | |
| IGF-1 |
Huang et al (Ref 198) n. 23 Mean age: 71±4.4 years < 160 ng/mL |
3 times/week, 6 months | 240 | Increased protein synthesis |
|
Blackman et al (Ref 199) N.30 Mean age: 71± 1.1 years < 160 ng/mL |
GH starting dose 30 reduced to 20 μg/kg, 3 times/week, 26 weeks | 244 ng/mL | Increased LBM and decreased total and sc fat; no changes in muscle function | |
| VITAMIN D |
Lagari et al (Ref 218) N. 86 Mean Age 73.4 ± 6.4 25 OHD= 33± 10 ng/mL 25 OHD < 20ng/mL= 8% 25 OHD < 30ng/mL= 35% Gait Speed : 0.94± 0.2 m/sec Single-leg bal. : 18± 19.8 sec Chair stands: 15.3± 4.5 n/30sec Grip Strength: 23.8± 6.7 kg Gallon jug test: 10.7± 2.4 sec |
GROUP 1: 400 IU D3 daily GROUP 2: 2000 IU D3 daily 6 months |
2.4 ± 12 ng/mL | Gait Speed : −0.02±0.11 m/sec Single-leg bal. : −1.7±.11.9sec Chair stands: ---0.17±11.9 sec Grip Strength: −0.9±2.9 sec Gallon jug test: −0.34± 1.3i sec |
|
Latham et al (Ref 221) n.243 mean age: 79 ± 2 Baseline 25(OH)D: Median 16 mcg/L |
Active dose: 300000UI in a single dose Control group: Placebo Length of study= 6 months |
Falls over 6 months, strength (hand-held dynamometer), physical performance (Berg balance test and timed walk), and self- reported function (SF-36 and Barthel Index) Findings: No difference between groups for any outcomes |
||
| WOMEN | ||||
|---|---|---|---|---|
| Baseline values | Intervention | Values after treatment | Study outcomes | |
|
Lagari et al (Ref 218) N.71 6 months GROUP 1: N= 25; Age: 72 ±5.4 25 OHD= 33.5± 8.4 ng/mL 25 OHD < 20ng/mL= 3% 25 OHD < 30ng/mL= 11% Gait Speed : 0.93± 0.1 m/sec Single-leg bal. : 19.3± 19.9 sec Chair stands: 13.7±4 n/30sec Grip Strength: 22.4± 4.2 kg Gallon jug test: 11± 2 sec GROUP 2: n=46; Age: 74 ± 6.7 25 OHD= 33.0± 9.9 ng/mL 25 OHD < 20ng/mL= 4% 25 OHD < 30ng/mL= 23% Gait Speed : 0.91± 0.2 m/sec Single-leg bal. : 16.8± 18.5 sec Chair stands:15.1± 3.8 n/30 s Grip Strength: 22.3± 6.3 kg Gallon jug test: 11.1± 2.5 sec |
GROUP 1: 400 IU D3 daily GROUP 2: 2000 IU D3 daily |
GROUP 1: 25(OH)D −3.4 ± 10.8 ng/ml GROUP 2: 25(OH)D 5.3 ± 12.3ng/ml |
GROUP 1: Gait Speed : 0.05±0.1 m/sec Single-leg bal. : 0.62±.1.14sec Chair stands: 0.26±3.1 n/30sec Grip Strength: −1.3±2.5kg Gallon jug test: −0.62± 1.1 sec GROUP 2: Gait Speed : 0.03± 0.09 m/sec Single-leg bal. : −2.7±.12.4 sec Chair stands: 0.50±3.7 n/30sec Grip Strength: −1.1±2.8kg Gallon jug test: −0.28± 1.4 sec |
|
|
Sato et al (Ref 220) n.96 Mean age: 74.2 Length of study:2 years 25OHVit D baseline: <10ng/mL |
Treatment group: 1,000 IU D2 daily Control Group: Placebo |
Treatment group: 33.4± 3.3ng/mL Control Group: 5.3± 1.1ng/mL |
Muscle strength improving: Treatment group: 6.9± 1.1 Control Group: 3.5± 1.3 |
|
| Baseline values | Intervention | Values after treatment | Study outcomes | |
|
Bischoff et al (Ref 222) N. 122 Mean age:85.3 25OHD baseline:30 nmol/L (mean value) Length of study: 12 week |
Treatment group: 800 IU D3 and 1200 mg calcium Control Group: Calcium |
Treatment group: 65.5 nmol/L Control group: 28.5 nmol/L |
Treatment group: Knee flexor strength: +3.7% (percent change) Knee extensor strength: +8.6% Grip strength: +5.5% Control group: Knee flexor strength: −3.9% Knee extensor strength: +1.4% Grip strength: 0% |
|
|
Janssen et al (Ref 167) N.70 Mean age: 80.5 25OHVit D baseline: 33.5 nmol/L (mean) |
Treatment group 400 IU D3 and 500 mg calcium daily Control Group: Placebo and 500mg calcium |
Treatment group: 77.2 nmol/L (mean) Control Group 41.6 nmol/L (mean) |
No significant difference in strength or functional mobility between the two groups | |
| DHEAS |
Nair et al (Ref 175) N. 57 0,4 (0,3-0,5) mcg/mL mean age: 69 Length of study: 2 years |
50 mg/day | Increase by median of 3.8 μg/mL | No increase in muscle strength Increase in BMD ultradistal radius |
| MEN | ||||
|---|---|---|---|---|
| Baseline values | Intervention | Values after treatment | Study outcomes | |
| IGF-1 |
Giannoulis et al (Ref 238) N.18 mean age: 70.7 ± 0.7 102±5.3 ng/dl |
GH starting dose 0.1 mg/d; increased gradually to a mean of 0.54 mg/d, 6 month | 193.4 ± 10.6 ng/mL | LBM and whole protein turnover increased |
| TESTOSTERONE |
Lange et al (Ref 203) N.8 mean age: 74 ± 2 145±14 ng/mL |
GH increased gradually over 3 wk to 12 μg/kg * d, 12 wk | 247 ± 30 ng/mL | Positive Changes in body composition. No significant effect on isokinetic quadriceps muscle strength, power, CSA or fiber size. |
|
Nair et al (ref 175) N.30 median age: 66.2 median 357.3 ng/dL Kenny et al (Ref 181) N. 131 mean age 77.1 < 350 ng/dL |
Transdermal T 5 mg per day (23 months) transdermal T 5 mg/day (12 months) |
total T 461 ng/dL Increase by median 104.5 total T 583 ng/dL |
slight increase in fat-free mass, and increase in BMD at the femoral neck ⇑ lean mass ⇓ fat mass no changes in muscle strength |
|
|
Srinivas-Shankar et al (Ref 183) N. 274 mean age 72.5 < 350 ng/dL |
transdermal T 50 mg/day (6 months | total T 547ng/dL | ⇑ lower limb muscle strength, body composition, physical function | |
|
Travison et al (Ref 184) N. 209 mean age 74 100-350 ng/dL |
transdermal T 10 mg/day (6 months) | total T 361 ng/dL | ⇑ muscle strength and stair-climbing power | |
| VITAMIN D |
Lagari et al (Ref 218) N.15 Length of study: 6 months GROUP 1:n= 6;Age: 74.8 ± 7.9 25 OHD= 31.3 ± 9.1 ng/mL 25 OHD < 20ng/mL= 0 25 OHD < 30ng/mL= 13% Gait Speed : 1± 0.22 m/sec Single-leg bal. : 9± 8.9 sec Chair stands: 15.3± 6.1 n/30sec Grip Strength: 30± 9.9 kg Gallon jug test: 9.5± 1.5 sec |
GROUP 1: 400 IU D3 daily |
GROUP 1: 25OHD −1.2± 5.9 ng/mL |
GROUP 1: Gait Speed : −0.1± 0.18 m/sec Single-leg bal. : 3.8± .8.2sec Chair stands: −5± 1.5 n/30sec Grip Strength: −1 ±4.5 kg Gallon jug test: −0.35± 1.1 sec |
|
GROUP 2 n= 9;Age: 73.3 ± 25 OHD= 32.6± 15.5 ng/mL 25 OHD < 20ng/mL= 13% 25 OHD < 30ng/mL= 16% Gait Speed : 1.1± 0.16m/sec Single-leg bal. : 36.5± 25.6 sec Chair stands:20.1± 4 n/30s Grip Strength: 30.6± 6.6 kg Gallon jug test: 8.4± 1.6 sec |
GROUP 2: 2000 IU D3 daily |
GROUP 2: 25 OHD 6.1 ± 12.6ng/mL |
GROUP 2: Gait Speed : −0.01± 0.1 m/sec Single-leg bal. : −8.6± .13.3 sec Chair stands: 0.67± 3.6 n/30sec Grip Strength: 1.3±2.4 kg Gallon jug test: −0.52± 0.74sec |
|
| DHEAS |
Nair et al (Ref 175) N. 87 0,4 (0,3-0,5) mcg/mL mean age 68 |
0,6 (0,4-1,0) μg/mL 2 years |
Increase by median of 3.8 μg/mL | No effect on muscle mass and strength |
The main outcomes considered in both observational and RCTs ranged from muscle protein synthesis, muscle strength and muscle mass, to walking distance and physical performance.
Hormonal values before and after treatment were depicted in intervention studies having complete information on dosage of hormones used, duration of treatment period and outcomes.
We listed a number of factors that may concur to explain why the actual results coming from RCTs are largely unsatisfactory. They include: inclusion criteria (healthy older persons with preserved functional ability), insufficient duration of treatment, inadequate adherence to treatment, different hormonal levels reached at the end of study, poor techniques used to measure hormones, inconsistent assessment of objective measure of physical performance.
After the analysis of all these studies, we hypothesize that frail older persons with lower baseline hormonal levels and at risk of mobility limitation could be more sensitive to all hormonal treatment. However, the available data on this specific older population are very limited.
THE CONCEPT OF MULTIPLE HORMONAL DYSREGULATION
We have described the relationship between single hormonal derangement and mobility and the impact of single hormone therapy on mobility. However, especially in older persons, this painting is unlikely to be real because the single mild hormonal alteration occurs rarely isolated, and the symptoms associated with the single hormonal derangement are usually not specific.
The simultaneous anabolic hormonal deficiency (T, DHEA, E2, GH-IGF-1, Vitamin D) with “multiple hormonal dysregulation” is a more frequent phenomenon than single hormonal derangement in older individuals. Thus, the correct clinical and research approach should account for all the anabolic hormones, and the way the anabolic hormones interact becomes of fundamental importance.
This concept is essential to explain why a partial failure of a single hormone might be partially or fully compensated by one or more parallel systems without producing a significant detrimental clinical outcome.
There is recent evidence that anabolic hormones do not operate independent of each other and might have synergistic effects (Fig. 2).
Fig. (2).
The figure depicts the combined effects of Vitamin D, Testosterone and Insulin-like Growth Factor 1 (IGF-1) on mobility based on the current evidence of Clinical Trials. Each rectangle represents an anabolic hormone. The arrows in the opposite direction between the rectangles indicate the profound interrelationship of these 3 anabolic hormones. Each horizontal arrow describes the impact of a single anabolic hormone and the additive effects of multiple hormones on different objective measures of physical performance.
SPPB: Short Physical Performance Battery; ADL: Activities of Daily Living; PASE: Physical Activity Scale for the Elderly; LBM=lean Body Mass; 30 MWT: 30- minute- walking test; ASMM: appendicular skeletal muscle mass.
It is very well known that T has mainly anabolic effects while GH exerts anti-catabolic action [226]. IGF-1 can be also considered the cross-road of many stimuli other than GH and including DHEAS and T.
All together the anabolic hormones influence the anabolic nutrition status, the satellite cell activation and together with exercise and other mechanical stimuli have a strong influence on muscle hypertrophy.
Inside the anabolic hormones it is appreciable a profound interrelationship especially between the GH-IGF-1 system and T.
In 1995, Urban and colleagues [227] concluded that muscular T effects may be realized by the stimulation of the intramuscular IGF-1 system. 4-week T administration to six healthy middle-older men with serum T concentrations less than 480 ng/dL resulted in an increased skeletal muscle protein synthesis (stable-isotope infusion) and strength (isokinetic dynamometer). Interestingly, after ribonuclease protection assays on total RNA from muscle, they found increased mRNA concentrations of IGF-1 and decreased mRNA concentrations of IGFBP-4.
Sattler and associates [228] investigated one hundred and twelve men aged 65-90 years receiving T gel (5 g/day vs 10 g/d via Leydig cell clamp) and rhGH (0 vs 3 vs 5 μg/kg/day) in a double-masked 2 × 3 factorial design for 16 weeks. Outcomes included LBM assessed by DEXA, one-repetition maximum strength, Margaria stair power, and activity questionnaires. Pathway analysis was also used to determine the relationship between changes in hormone levels, muscle mass, strength, and function.
In these older men, the increase in T and IGF-1 concentrations was robustly associated with gains in total lean body and appendicular skeletal muscle mass but was not directly related to clear improvements in muscle performance or physical function. By contrast, the global enhancements in maximal voluntary strength of the major muscle-groups of the upper and lower body along with significant increases in Margaria stair climbing power were directly related to increased LBM. Changes in T levels were directly related to change in PASE score, but the pathway analysis indicated that the latter was not related to changes in muscle mass or performance per se. The authors hypothesized that the improved well-being or the ameliorated mood and blood flow of different brain areas might be the determinants of improved physical activity.
The advantage to use GH and T together is not a novel concept. One of the first studies was published in 2002 [229]. Single and combined effects of GH and T administration on measures of body composition, physical performance, mood, sexual function, bone turnover, and muscle gene expression in healthy older men. Ten men [mean (SEM) age, 68 (2.5) year)] completed each of the following 1-month, double-blind interventions after a baseline study in randomized order with a 3-month washout: transdermal T patch (5.0 mg/day); recombinant human GH (6.25 μg/kg sc daily) and combined hormones (GH-T). Integrated serum GH concentrations were elevated comparably by GH and GHT: [B = 363 (55), GH = 1107 (120), T = 459 (131), and GHT = 1189 (46) μg /L.min; P < 0.0001]. Serum IGF-I concentrations also increased after GH and GHT: [B = 168 (14), GH = 285 (16), T = 192 (25), and GHT = 294 (25) μg /L; P < 0.0001]. GHT administration increased total E2 : [B =110 (20), GH = 106 (13), T = 129 (13), and GHT = 153 (17) pmol/L; P < 0.02], and both T and GHT elevated free T: [B = 12 (2.1), GH = 11 (1.5), T = 22 (2.8), and GHT = 24 (2.5) pg/mL; P <0.0001]. No significant changes occurred in terms <0.0001]. No significant changes occurred in terms of strength, flexibility, percentage body fat, or sexual function and mood. However, fat- free mass increased under combined GHT exposure: [B = 55 (1.3), GH = 56 (1.1), T = 55 (1.5), GHT = 57 (1.7) kg; P < 0.03]. Balance improved in response to GH intervention (P < 0.05), as did 30-m walk time during T and GHT interventions [B = 6.6 (0.3), GH = 6.2 (0.7), T = 5.9 (0.3), GHT = 5.5 (0.3) sec; P = 0.04] and stair climb time for all three interventions [B = 32.2 (1.4), GH = 29.8 (1.2), T =30.5 (1.4), and GHT = 29.9 (1.2) sec (P = 0.0034), wherein the effects of GH, T, and GHT were different from that of B]. Muscle IGF-1 gene expression increased by 1.9 fold during GH administration and by 2.3 fold during GHT administration (P<0.05, compared with B). There were no significant adverse events during 30 months of intervention. The authors concluded that 1 month of GH and/or T administration is effective to improve certain measures of balance and physical performance in older men and leads to an increased muscle IGF-1 gene expression.
These data have been recently confirmed in a study conducted in twelve older male patients (mean age 74±6 years) on long-term pharmacological glucocorticoid therapy and with skeletal muscle atrophy and weakness [230]. In this prospective, open-label, randomized, crossover study, the authors investigated the effects of 2 week- treatment with GH, T alone and the combination of two treatments on LBM, appendicular skeletal muscle mass (ASMM), extracellular water (ECW), body cell mass (BCM) and plasma glucose concentrations. LBM increased significantly after GH (Δ1.7±1.4 kg; P=0.007) and GH+T (Δ2.4±1.1 kg; P=0.003), but not after T alone. ASMM increased after all three treatment periods; by 1.0±0.8 kg after GH (P=0.005), 1.7±0.4 kg after GH+T (P=0.002) and 0.8±1.0 kg after T (P=0.018). The increase in ASMM was larger with combined treatment than either GH or T alone (P<0.05). ECW increased significantly after GH+T by 1.5±2.6 l (P=0.038) but not after GH or T alone. Body cell mass increased slightly after single and combined treatments, but the changes were not statistically significant. Fasting glucose increased significantly after GH (Δ0.4±0.4 mmol/L, P=0.006) while both fasting (Δ 0.2±0.3 mmol/L, P=0.045) and post glucose-load (Δ 1.8±2.3 mmol/l, P=0.023) plasma glucose concentrations increased after GH plus T. Growth Hormone and T induced favorable and additive body compositional changes in men on chronic, low-dose GC treatment. In the doses used, the combination therapy increased fasting and postprandial glucose concentration.
Several studies have reported that T administration improves LBM and maximal voluntary strength in healthy older men. On the other hand, most studies have shown that GH treatment alone failed to improve muscle strength despite amelioration of the detrimental somatic changes of aging. Both GH and T are anabolic agents that promote muscle protein synthesis and hypertrophy but work through separate mechanisms. The combined administration of GH and T, albeit in only a few studies, has resulted in greater efficacy than either hormone alone. However, further studies are needed to establish the long-term efficacy and safety of combined HRT in older men to treat sarcopenia of aging [94].
VITAMIN D AND ANABOLIC HORMONES
Another intriguing issue of the potential multiple hormonal combined treatment is the interaction between Vitamin D and the other anabolic hormones, particularly T. Recent studies show that Vitamin D levels are positively associated with T suggesting that Vitamin D might increase T levels. Nimptsch et al. [231] investigated the association of Vitamin D and total and Free-T levels in 1362 male participants of Health Professional Follow-up Study who were recruited for a nested case-control study on prostate cancer. Using cut-off for vitamin D, 24% of participants revealed vitamin D deficiency (<50 nmol/L), 44% Vitamin D insufficiency (between 50 and 74.9 nmol/L), and 31% Vitamin D sufficiency (>75 nmol/L). In their study these authors, after observing the shapes of the dose-response curves, found a stronger association between 25OHD and total and Free-T at lower levels of 25OHD (below approximately 75-85 nmol/l), with a plateau at higher levels. This is an interesting association since VDR and Vitamin D metabolizing enzymes have been described in human Leydig cells. Thomas Wang et al. [232] studied genetic determinants of Vitamin D metabolism and risk of its insufficiency. They found that the polymorphism of the gene CYP2R1 which encodes hepatic microsomial enzyme implicated in the 25-hydroxilation of Vitamin D, is related to Vitamin D insufficiency. However, Bièche and collaborators [233], using reverse transcriptase PCR, quantified mRNA levels from cytocrome families in human adult and fetal tissues, showing that CYP2R1 mRNA is also mainly expressed in the testis. There is also recent evidence of a relationship between testiculopathy and Vitamin D deficiency. Foresta and collaborators [234] found that at 3-5 year follow-up controls, 15 male patients orchiectomized for bilateral testicular cancer without chemotherapy or radiotherapy had significantly lower 25OHD levels. They investigated 41 healthy controls (30.2 nmol/L [SD 16.3] vs 74.9 nmol/L [38.0], P<0.0001), with no nutritional derangements, divi-ding into two groups similar in age (34.8 years [6.4] vs 35.8 years [6.2], P=0.554), season of measurement. All these patients, without any evidence of disease, and receiving T-replacement therapy, maintained in the physiological range both serum luteinizing hormone and T concentrations. All these data support the hypothesis that the microsomal enzyme CYP2R1 in the testis seems to have an important role in Vitamin D metabolism, resulting in a relative reduction of almost 60% in 25OHD in orchiectomized patients [235].
Furthermore, recent data concerning the role of CYP2R1 and testis in the activation of Vitamin D, showed a progressive decrease of the expression of CYP2R1 in relation to the severity of testiculopathy suggesting that Leydig cell could play a very important role in this mechanism [235]. If this hypothesis will be confirmed, testiculopathy of any cause could have a role in the pathogenesis of Vitamin D insufficiency. The mechanism underlying the impaired 25-hydroxylase activity, and osteopenia-osteoporosis, is independent of androgen and estrogen levels and other cause of Vitamin D reduction. In a recent study performed in older men who were referred for coronary angiography, Lerchbaum E et al. [236], by using a multivariate adjusted analysis, found an increased risk of cardiovascular and non-cardiovascular mortality for men in the lowest Free-T and Vitamin D quartiles, compared with men in higher Free-T and Vitamin D quartiles. These result suggest that the combination of low Free-T and Vitamin D could be an independent predictor of all-cause mortality.
Finally, there is initial evidence of the ability of Vitamin D supplementation to increase T levels. Preliminary data from a small clinical trial including 165 adult participants (54 men) showed that 1 year daily treatment of Vitamin D 83 μg/day (3332 IU) (N=31) determines a significant increase in total T (from 10.7 ± 3.9 nmol/L to 13.4 ± 4.7 nmol/L; P<0.001), bioavailable T (from 5.21 ± 1.87 nmol/L to 6.25 ± 2.01 nmol/L; P=0.001), and Free-T levels (from 0.222 ± 0.080 nmol/L to 0.267±0.087 nmol/L; P=0.001) without significant change in any T measure in the placebo group (N=23). Importantly, the initial 25OHD concentrations were in the deficiency range (<50 nmol/L) and the T levels were low in both groups [237].
Figure 2 shows the interaction of interventions based on Vitamin D, T and GH-IGF-1 on different indicators of physical performance. The big black arrows indicate whether one hormonal treatment is able to increase the circulating levels of the other anabolic hormones or to improve nutritional status, assessed by IGF-1 levels. Based on the current literature, GH and T have for instance different effects on LBM. These hormones when are separately administered have small or not significant effects on the above mentioned parameters. However, the effect is greater where the combined therapy is administered [238]. In summary, by this multi-anabolic approach, a new therapeutic combined treatment using lower doses of anabolic hormones can be hypothesized.
The combined anabolic hormonal treatment is an interesting future therapeutic option, that needs to be validated in future larger RCTs in older populations. However, the multifactorial nature of mobility limitation in older persons cannot be ignored.
Nutrition and physical exercise are two other promising strategies potentially useful to optimize the effectiveness of anabolic hormones on physical function. We list below some preliminary data available in the literature on the synergistic effects of hormones, nutrition and exercise.
TESTOSTERONE SUPPLEMENTATION PLUS MICRONUTRIENTS
The potential usefulness of the combination of anabolic hormones such as T, exercise and nutritional supplementation emerges from observational and pivotal intervention trials. Micronutrients such as magnesium have been shown to be determinants of muscle strength and function and the use of nutritional supplement is very frequent in adult well trained individuals to improve physical performance [239]. Our group has recently shown in older men of the InCHIANTI population that magnesium serum levels well and positively correlate with anabolic hormones including T [239]. Preliminary intervention studies provide evidence to this concept. Two recent studies in undernourished older men and women showed that combined treatment with T and nutritional supplementation reduce the number of hospitalization and the duration of hospital admissions [240-241]. These preliminary data suggest that targeting muscle and function should be one of the main goals of daily pharmacological approaches in older patients with mobility limitation.
HORMONAL REPLACEMENT THERAPY (GH OR T) PLUS EXERCISE
Exercise, both aerobic and resistance training, are important strategies to prevent or treat low mobility in older persons. Studies conducted in healthy elderly subjects receiving GH treatment in combination with exercise showed favorable changes in body composition, without significant improvements in physical function [242-243]. Similarly [244], short-term GH treatment combined with 16-week (12.5 or 18 μg/kg/day) resistance exercise training program (75-90% maximum strength, 5-10 repetitions/set, 4 sets/day, 4 days/week) was not able to determine any significant increase in whole body or regional BMD in healthy older subjects with normal BMD at baseline.
More numerous data come from the combination of resistance training and T.
Resistance training has been shown to increase the T necessary for muscle anabolism [245]. Testosterone stimulates skeletal muscle protein synthesis (anabolic effect) and inhibits protein degradation (anti- catabolic effect). The endocrine response to resistance training persists during the following days [246]. Nevertheless resistance exercise itself causes contraction-induced stimulation of muscle protein synthesis, independently of T action [246,247]. The magnitude of T elevation due to resistance exercise is generally smaller in older in comparison to younger men [249]. The combination of T supplementation with resistance exercise may synergistically work to improve muscle function and physical performance in elderly men. In women, there are contrasting data on T response to resistance exercise, with both increase [250] or no changes observed [251]. Nevertheless, heavy exercise is a form of acute stress that increases adrenocorticotropic hormone level [252], which also stimulates the production and release of cortisol and androgens by adrenal cortex in women [253]. Thus, if findings about T response to resistance exercise in women are such confuse by now, data in older men support the role of physical exercise as an additional strategy to preserve muscle mass with aging. Because the majority of the body composition changes (loss in LBM and BMD and increase in fat mass) and physical performance are due to this age-related endocrine dysregulation, the use of androgens or estrogens in combination with exercise may contrast the mobility decline occurring with age [48]. Animal studies show that T supplementation administered late in life, in the setting of low-intensity physical training, improves spontaneous physical activity, metabolic rate, and grip strength [254]. Importantly, the authors found that T supplementation plus low-intensity physical training in very old mice is capable to increase mitochondrial biogenesis in the skeletal muscle, with concurrent increase in the expression of markers of mitochondrial fission, fusion, and mitophagy, and resulting in a significant reduction in tissue markers of oxidative stress. These results have been confirmed in male patients with chronic heart failure (CHF) and low T status underwent a 12-week program of exercise, with and without intramuscular T supplementation [255]. Male patients with CHF (N=41, age 67.2 years, range 51-84 years) with mean ± SD T levels of 10.7 ± 2.6 nmol/L (309±76 ng/dL) were randomly allocated to exercise with T or placebo groups. Feasibility was assessed in terms of recruitment, intervention compliance, and attrition. Outcomes included an incremental shuttle walk test, peak oxygen uptake, muscle strength, echocardiographic measures, N-terminal pro-brain natriuretic peptide, inflammatory markers, depression (Beck Depression Inventory), and health-related quality of life (Minnesota Living with Heart Failure Questionnaire and Medical Outcomes Study Short-Form). Attrition was 30% but with 100% compliance to exercise and injections in patients who completed the study. Similar improvements in shuttle walk test (18% vs 19%), body weight (−1.3 kg vs −1.0 kg), and hand grip strength (2.1 kg vs 2.5 kg) from baseline were observed in both groups. The exercise with T group showed improvements from baseline in peak oxygen uptake (P< 0.01), Beck Depression Inventory (P<0.05), leg strength (P< 0.05), and several Medical Outcomes Study Short-Form quality of life domains (P<0.05), which were generally not evident in the exercise with placebo group. Echocardiographic measures, N-terminal pro-brain natriuretic peptide, and inflammatory markers were mostly unchanged. This study showed for the first time that T supplementation during a program of exercise rehabilitation is feasible and can positively affect the key health outcomes of elderly male patients with CHF who have a low T status.
Recently, Hildreth and collaborators tested in highly functioning healthy community-dwelling older men with low-normal baseline total T levels (200-350 ng/dL) the effects of 12-month transdermal T gel and exercise. T supplementation [2.5 g packets daily (2 placebo packets in the placebo group, 1 T gel and 1 placebo packet in the lower-range T group, and 5.0 g gel packets in the higher-range T group)] combined with progressive resistance training, determined an improvement in body composition but no changes in functional performance and strength [256]. Conversely, non-exerciser T-treated groups experienced an improvement in upper body strength compared with placebo.
Despite the potential effects of combined treatment of hormones and exercise, most of the results are inconclusive. The potential explanation is that most of these studies included participants relatively healthy and with preserved ability in physical function. New studies with different inclusion criteria in terms of hormonal baseline levels and low-moderate grade of mobility are desirable.
The combination of hormonal treatment with nutritional supplementation and exercise remains one of the potential strategies to attenuate the detrimental effects of hormonal treatment. Other strategies aimed to fight mobility limitation in older individuals include the selection of route of administration and the dosage, the ideal population to target, the intermittent interventions and the use of selective compounds such as androgen receptor modulators (SARMS) [7].
PERSPECTIVE
Alternative approaches aimed at improving the effectiveness of anabolic hormones treatment in mobility in older persons are object of current studies that include the combination of hormones, exercise, and nutritional supplementation. Epidemiological studies have shown that micronutrients such as magnesium, zinc and selenium are determinants of muscle strength and function and the use of nutritional supplement is very well known among young-adult athletes to improve physical performance [239; 257-258]. Moreover in older men of the InCHIANTI population magnesium and selenium serum levels have been positively associated with anabolic hormones including T and IGF-1 [259] supporting their potential clinical use of adjuvant anabolic support in older population. These concepts also apply to older patients undergoing major surgeries, especially cardiac surgery with extracorporeal circulation, where assessment of body composition and muscle function [260] should become a cornerstone for future strategies, including multiple hormonal treatment, to improve the recovery and length of stay [261].
CONCLUSIONS
There is profound need for additional, large, carefully trials of anabolic hormone administration in well-characterized groups of older subjects to more clearly outline benefits (muscle strength, avoidance of falls, physical function) and risks of multiple hormonal treatment.
Since mobility limitation is a multifactorial process, new clinical trials in older individuals should adopt a multi-intervention approach including selected anabolic hormones, and targeting specific populations more sensitive to multiple hormonal replacement ther apy.
ACKNOWLEDGEMENTS
Declared none.
Footnotes
CONFLICT OF INTEREST
The authors confirm that this article content has no conflicts of interest.
REFERENCES
- 1.Morley JE, Baumgartner RN, Roubenoff R, et al. Sarcopenia. J Lab Clin Med. 2001;137:231–43. doi: 10.1067/mlc.2001.113504. [DOI] [PubMed] [Google Scholar]
- 2.Cruz-Jentoft AJ, Baeyens JP, Bauuer JM, et al. Sarcopenia: European consensus o definition and diagnosisReport of the European Working Group on Sarcopenia in Older People. Age Ageing. 2010;39(4):412–423. doi: 10.1093/ageing/afq034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Rosenberg IH. Sarcopenia: origins and clinical relevance. J Nutr. 1997;127:990S–91S. doi: 10.1093/jn/127.5.990S. [DOI] [PubMed] [Google Scholar]
- 4.Delmonico MJ, Harris TB, Lee JS, et al. Alternative definitions of sarcopenia, lower extremity performance, and functional impairment with aging in older men and women. J Am Geriatr Soc. 2007;55:769–74. doi: 10.1111/j.1532-5415.2007.01140.x. [DOI] [PubMed] [Google Scholar]
- 5.Goodpaster BH, Park SW, Harris TB, et al. The loss of skeletal muscle strength, mass, and quality in older adults: The health, aging and body composition study. J Gerontol A Biol Sci Med Sci. 2006;61:1059–64. doi: 10.1093/gerona/61.10.1059. [DOI] [PubMed] [Google Scholar]
- 6.Fried LP, Tangen CM, Walston J, et al. Frailty in older adults: evidence for a phenotype. J Gerontol A Biol Sci Med Sci. 2001;56(3):M146–56. doi: 10.1093/gerona/56.3.m146. [DOI] [PubMed] [Google Scholar]
- 7.Maggio M, Lauretani F, Ceda GP. Sex hormones and sarcopenia in older persons. Curr Opin Clin Nutr Metab Care. 2013;16(1):3–13. doi: 10.1097/MCO.0b013e32835b6044. [DOI] [PubMed] [Google Scholar]
- 8.Maggio M, Lauretani F, Ceda GP, et al. Relationship between low levels of anabolic hormones and 6-year mortality in older men: the aging in the Chianti Area (InCHIANTI) study. Arch Intern Med. 2007;167(20):2249–54. doi: 10.1001/archinte.167.20.2249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Maggio M, Dall'Aglio E, Lauretani F, et al. The hormonal pathway to cognitive impairment in older men. J Nutr Health Aging. 2012;16(1):40–54. doi: 10.1007/s12603-012-0002-7. [DOI] [PubMed] [Google Scholar]
- 10.Stewart CE, Pell JM. Point: Counterpoint: IGF is/is not the major physiological regulator of muscle mass. Point: IGF is the major physiological regulator of muscle mass. J Appl Physiol. 2010;108:1820–21. doi: 10.1152/japplphysiol.01246.2009. [DOI] [PubMed] [Google Scholar]
- 11.Valenti G. Frailty as a dysruption of steroid “syncrinology” in elderly man. Acta Biomed. 2007;78(1):222–4. [PubMed] [Google Scholar]
- 12.Labrie F. DHEA, important source of sex steroids in men and even more in women. Prog Brain Res. 2010;182:97–148. doi: 10.1016/S0079-6123(10)82004-7. [DOI] [PubMed] [Google Scholar]
- 13.Sato K, Iemitsu M, Aizawa K, et al. Testosterone and DHEA activate the glucose metabolism-related signaling pathway in skeletal muscle. Am J Physiol Endocrinol Metab. 2008;294:E961–E968. doi: 10.1152/ajpendo.00678.2007. [DOI] [PubMed] [Google Scholar]
- 14.Aizawa K, Iemitsu M, Meeda S, et al. Acute exercise activates local bioactive androgen metabolism in skeletal muscle. Steroids. 2010;75:219–223. doi: 10.1016/j.steroids.2009.12.002. [DOI] [PubMed] [Google Scholar]
- 15.Wagers AJ, Conboy IM. Cellular and molecular signatures of muscle regeneration: Current concepts and controversies in adult myo-genesis. Cell. 2005;122:659–667. doi: 10.1016/j.cell.2005.08.021. [DOI] [PubMed] [Google Scholar]
- 16.Kenny AM, Boxer RS, Kleppinger A, et al. Dehydroepiandrosterone (DHEA) improves muscle strength and physical function but not bone mineral density in frail older women. J Am Geriatr Soc. 2010;58:1707–14. doi: 10.1111/j.1532-5415.2010.03019.x. [DOI] [PubMed] [Google Scholar]
- 17.Mills P, Lafreniere JF, Benabdallah BF, et al. A new pro-migratory activity on human myogenic precursor cells for a synthetic peptide within the E domain of the mechano growth factor. Exp Cell Res. 2007;313:527–537. doi: 10.1016/j.yexcr.2006.10.032. [DOI] [PubMed] [Google Scholar]
- 18.Valenti G, Denti L, Maggio M, et al. Effect of DHEAS on skeletal muscle over the life span: the InCHIANTI study. J Gerontol A Biol Sci Med Sci. 2004;59(5):466–72. doi: 10.1093/gerona/59.5.m466. [DOI] [PubMed] [Google Scholar]
- 19.Cappola AR, Xue QL, Fried LP. Multiple hormonal deficiencies in anabolic hormones are found in frail older women: the Women's Health and Aging studies. J Gerontol A Biol Sci Med Sci. 2009;64(2):243–8. doi: 10.1093/gerona/gln026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Stenholm S, Maggio M, Lauretani F, et al. Anabolic and catabolic biomarkers as predictors of muscle strength decline: the InCHI-ANTI study. Rejuvenation Res. 2010;13(1):3–11. doi: 10.1089/rej.2009.0891. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Arianayagam R, Arianayagam M, McGrath S, et al. Androgen deficiency in the aging man. Aust Fam Physician. 2010;39(10):752–55. [PubMed] [Google Scholar]
- 22.Goodman-Gruen D, Barrett-Connor E. Sex differences in the association of endogenous sex hormone levels and glucose tolerance status in older men and women. Diabetes Care. 2000;23(7):912–18. doi: 10.2337/diacare.23.7.912. [DOI] [PubMed] [Google Scholar]
- 23.Feldman HA, Longcope C, Derby CA, et al. Age trends in the level of serum testosterone and other hormones in middle-aged men: longitudinal results from the Massachusetts male aging study. J Clin Endocrinol Metab. 2002;87(2):589–98. doi: 10.1210/jcem.87.2.8201. [DOI] [PubMed] [Google Scholar]
- 24.Veldhuis JD. Aging and hormones of the hypothalamo-pituitary axis: gonadotropic axis in men and somatotropic axes in men and women. Ageing Res Rev. 2008;7(3):189–208. doi: 10.1016/j.arr.2007.12.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Gobinet J, Poujol N, Sultan C. Molecular action of androgens. Mol Cell Endocrinol. 2002;198(1-2):15–24. doi: 10.1016/s0303-7207(02)00364-7. [DOI] [PubMed] [Google Scholar]
- 26.Serra C, Tangherlini F, Rudy S, et al. Testosterone Improves the Regeneration of Old and Young Mouse Skeletal Muscle. J Gerontol A Biol Sci Med Sci. 2012 doi: 10.1093/gerona/gls083. Epub ahead of print. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Dubois V, Laurent M, Boonen S, et al. Androgens and skeletal muscle: cellular and molecular action mechanisms underlying the anabolic actions. Cell Mol Life Sci. 2012;69:1651–67. doi: 10.1007/s00018-011-0883-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Grossmann MJ. Low testosterone in men with type 2 diabetes: significance and treatment. J Clin Endocrinol Metab. 2011;96:2341–53. doi: 10.1210/jc.2011-0118. [DOI] [PubMed] [Google Scholar]
- 29.Saad F. The role of testosterone in type 2 diabetes and metabolic syndrome in men. Arq Bras Endocrinol Metabol. 2009;53(8):901–907. doi: 10.1590/s0004-27302009000800002. [DOI] [PubMed] [Google Scholar]
- 30.Saad F, Gooren LJ. The role of testosterone in the etiology and treatment of obesity, the metabolic syndrome, and diabetes mellitus type 2. J Obes. 2011;2011:471584. doi: 10.1155/2011/471584. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Fernández-Balsells MM, Murad MH, Lane M, et al. Clinical review 1: Adverse effects of testosterone therapy in adult men: a systematic review and meta-analysis. J Clin Endocrinol Metab. 2010;95:2560–75. doi: 10.1210/jc.2009-2575. [DOI] [PubMed] [Google Scholar]
- 32.Ferrucci L, Corsi A, Lauretani F, et al. The origins of age-related proinflammatory state. Blood. 2005;105(6):2294–99. doi: 10.1182/blood-2004-07-2599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Malkin CJ, Pugh PJ, Jones RD, et al. The effect of testosterone replacement on endogenous inflammatory cytokines and lipid profiles in hypogonadal men. J Clin Endocrinol Metab. 2004;89(7):3313–3318. doi: 10.1210/jc.2003-031069. [DOI] [PubMed] [Google Scholar]
- 34.Liva SM, Voskuhl RR. Testosterone acts directly on CD4+ T lymphocytes to increase IL-10 production. J Immunol. 2001;167(4):2060–67. doi: 10.4049/jimmunol.167.4.2060. [DOI] [PubMed] [Google Scholar]
- 35.Schaap LA, Pluijm SM, Deeg DJ, et al. Inflammatory markers and loss of muscle mass (sarcopenia) and strength. Am J Med. 2006;119(6):526.e9–526.e17. doi: 10.1016/j.amjmed.2005.10.049. [DOI] [PubMed] [Google Scholar]
- 36.Maggio M, Guralnik JM, Longo DL, et al. Interleukin-6 in aging and chronic disease: a magnificent pathway. J Gerontol A Biol Sci Med Sci. 2006;61(6):575–84. doi: 10.1093/gerona/61.6.575. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Maggio M, Cattabiani C, Lauretani F, et al. The concept of multiple hormonal dysregulation. Acta Biomed. 2010;81(1):19–29. [PMC free article] [PubMed] [Google Scholar]
- 38.Wu FC, Tajar A, Beynon JM, et al. Identification of late-onset hypogonadism in middle-aged and elderly men. N Engl J Med. 2010;363:123–135. doi: 10.1056/NEJMoa0911101. [DOI] [PubMed] [Google Scholar]
- 39.Maggio M, Ceda GP, Lauretani F, et al. Gonadal status and physical performance in older men. Aging Male. 2011;14:42–47. doi: 10.3109/13685538.2010.518179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Fink HA, Ewing SK, Ensrud KE, et al. Association of testosterone and estradiol deficiency with osteoporosis and rapid bone loss in older men. J Clin Endocrinol Metab. 2006;91(10):3908–15. doi: 10.1210/jc.2006-0173. [DOI] [PubMed] [Google Scholar]
- 41.Meier C, Nguyen TV, Handelsman DJ, et al. Endogenous sex hormones and incident fracture risk in older men: the Dubbo Osteoporosis Epidemiology Study. Arch Intern Med. 2008;168(1):47–54. doi: 10.1001/archinternmed.2007.2. [DOI] [PubMed] [Google Scholar]
- 42.Khaw KT, Dowsett M, Folkerd E, et al. Endogenous testosterone and mortality due to all causes, cardiovascular disease, and cancer in men: European prospective investigation into cancer in Norfolk (EPIC-Norfolk) Prospective Population Study. Circulation. 2007;116(23):2694–2701. doi: 10.1161/CIRCULATIONAHA.107.719005. [DOI] [PubMed] [Google Scholar]
- 43.Shores MM, Matsumoto AM, Sloan KL, et al. Low serum testosterone and mortality in male veterans. Arch Intern Med. 2006;166(15):1660–1665. doi: 10.1001/archinte.166.15.1660. [DOI] [PubMed] [Google Scholar]
- 44.Maggio M, Ceda GP, Lauretani F, et al. Gonadal status and physical performance in older men. Aging Male. 2011;14(1):42–7. doi: 10.3109/13685538.2010.518179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Krasnoff JB, Basaria S, Pencina MJ, et al. Free testosterone levels are associated with mobility limitation and physical performance in community-dwelling men: the Framingham Offspring Study. J Clin Endocrinol Metab. 2010;95(6):2790–9. doi: 10.1210/jc.2009-2680. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Van Geel TA, Geusens PP, Winkens B, et al. Measures of bioavailable serum testosterone and estradiol and their relationships with muscle mass, muscle strength and bone mineral density in postmenopausal women: a cross-sectional study. Eur J Endocrinol. 2009;160(4):681–87. doi: 10.1530/EJE-08-0702. [DOI] [PubMed] [Google Scholar]
- 47.Van der Made F, Bloemers J, Yassem WE, et al. The influence of testosterone combined with a PDE5-inhibitor on cognitive, affective, and physiological sexual functioning in women suffering from sexual dysfunction. J Sex Med. 2009;6(3):777–90. doi: 10.1111/j.1743-6109.2008.01142.x. [DOI] [PubMed] [Google Scholar]
- 48.Horstman AM, Dillon EL, Urban RJ, et al. The role of androgens and estrogens on healthy aging and longevity. J Gerontol A Biol Sci Med Sci. 2012;67(11):1140–52. doi: 10.1093/gerona/gls068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Sheffield-Moore M, Dillon EL, Casperson SL, et al. A randomized pilot study of monthly cycled testosterone replacement or continuous testosterone replacement versus placebo in older men. J Clin Endocrinol Metab. 2011;96(11):E1831–E1837. doi: 10.1210/jc.2011-1262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Bhasin S, Calof OM, Storer TW, et al. Drug insight: testosterone and selective androgen receptor modulators as anabolic therapies for chronic illness and aging. Nature Clinical Practice Endocrinology and Metabolism. 2006;2:146–159. doi: 10.1038/ncpendmet0120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Bhasin S, Cunningham GR, Hayes FJ, et al. Testosterone therapy in men with androgen deficiency syndromes: an Endocrine Society clinical practice guideline. J Clin Endocrinol Metab. 2010;95:2536–59. doi: 10.1210/jc.2009-2354. [DOI] [PubMed] [Google Scholar]
- 52.Bhasin S, Travison TG, Storer TW, et al. Effect of testosterone supplementation with and without a dual 5 -reductase inhibitor on fat-free mass in men with suppressed testosterone production: a randomized controlled trial. JAMA. 2012;307:931–39. doi: 10.1001/jama.2012.227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Dahlman-Wright K, Cavailles V, Fuqua SA, et al. International Union of Pharmacology. LXIV. Estrogen receptors. Pharmacol Rev. 2006;58(4):773–81. doi: 10.1124/pr.58.4.8. [DOI] [PubMed] [Google Scholar]
- 54.Heldring N, Pike A, Andersson S, et al. Estrogen receptors: how do they signal and what are their targets. Physiol Rev. 2007;87(3):905–931. doi: 10.1152/physrev.00026.2006. [DOI] [PubMed] [Google Scholar]
- 55.Vina J, Sastre J, Pallardo FV, et al. Role of mito- chondrial oxidative stress to explain the different longevity between genders: protective effect of estrogens. Free Radic Res. 2006;40(12):1359–1365. doi: 10.1080/10715760600952851. [DOI] [PubMed] [Google Scholar]
- 56.MacNeil LG, Baker SK, Stevic I, et al. 17beta-estradiol attenuates exercise-induced neutrophil infiltration in men. Am J Physiol Regul Integr Comp Physiol. 2011;300(6):R1443–R1451. doi: 10.1152/ajpregu.00689.2009. [DOI] [PubMed] [Google Scholar]
- 57.Greising SM, Baltgalvis KA, Kosir AM, et al. Estradiol's beneficial effect on murine muscle function is independent of muscle activity. J Appl Physiol. 2011;110(1):109–15. doi: 10.1152/japplphysiol.00852.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Baltgalvis KA, Greising SM, Warren GL, et al. Estrogen regulates estrogen receptors and antioxidant gene expression in mouse skeletal muscle. PLoS One. 2010;5(4):e10164. doi: 10.1371/journal.pone.0010164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Enns DL, Tiidus PM. The influence of estrogen on skeletal muscle: sex matters. Sports Med. 2010;40(1):41–58. doi: 10.2165/11319760-000000000-00000. [DOI] [PubMed] [Google Scholar]
- 60.Wiik A, Ekman M, Johansson O, et al. Expression of both oestrogen receptor alpha and beta in human skeletal muscle tissue. Histochem Cell Biol. 2009;131(2):181–89. doi: 10.1007/s00418-008-0512-x. [DOI] [PubMed] [Google Scholar]
- 61.Kamanga-Sollo E, White ME, Hathaway MR, et al. Effect of Estradiol-17beta on protein synthesis and degradation rates in fused bovine satellite cell cultures. Domest Anim Endocrinol. 2010;39(1):54–62. doi: 10.1016/j.domaniend.2010.02.002. [DOI] [PubMed] [Google Scholar]
- 62.Houser A, McNair C, Piccinini R, et al. Effects of estrogen on the neuromuscular system in the embryonic zebrafish (Danio rerio). Brain Res. 2011;1381:106–116. doi: 10.1016/j.brainres.2011.01.033. [DOI] [PubMed] [Google Scholar]
- 63.Kamanga-Sollo E, White ME, Hathaway MR, et al. Effect of Estradiol-17beta on protein synthesis and degradation rates in fused bovine satellite cell cultures. Domest Anim Endocrinol. 2010;39(1):54–62. doi: 10.1016/j.domaniend.2010.02.002. [DOI] [PubMed] [Google Scholar]
- 64.Trachootham D, Lu W, Ogasawara MA, et al. Redox regulation of cell survival. Antioxid Redox Signal. 2008;10(8):1343–74. doi: 10.1089/ars.2007.1957. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Imai Y, Youn MY, Kondoh S, et al. Estrogens maintain bone mass by regulating expression of genes controlling function and life span in mature osteoclasts. Ann N Y Acad Sci. 2009;1173(1):E31–E39. doi: 10.1111/j.1749-6632.2009.04954.x. [DOI] [PubMed] [Google Scholar]
- 66.Rochira V, Carani C. Aromatase deficiency in men: a clinical perspective. Nat Rev Endocrinol. 2009;5(10):559–68. doi: 10.1038/nrendo.2009.176. [DOI] [PubMed] [Google Scholar]
- 67.Wibowo E, Schellhammer P, Wassersug RJ. Role of estrogen in normal male function: clinical implications for patients with prostate cancer on androgen deprivation therapy. J Urol. 2011;185(1):17–23. doi: 10.1016/j.juro.2010.08.094. [DOI] [PubMed] [Google Scholar]
- 68.Carani C, Fabbi M, Zirilli L, et al. Estr ogen resistance and aromatase deficiency in humans. J Soc Biol. 2002;196(3):245–8. [PubMed] [Google Scholar]
- 69.Jones ME, Boon WC, Proietto J, et al. Of mice and men: the evolving phenotype of aromatase deficiency. Trends Endocrinol Metab. 2006;17(2):55–64. doi: 10.1016/j.tem.2006.01.004. [DOI] [PubMed] [Google Scholar]
- 70.Maffei L, Murata Y, Rochira V, et al. Dysmetabolic syndrome in a man with a novel mutation of the aromatase gene: effects of testosterone, alendronate, and estradiol treatment. J Clin Endocrinol Metab. 2004;89(1):61–70. doi: 10.1210/jc.2003-030313. [DOI] [PubMed] [Google Scholar]
- 71.Vandenput L, Ohlsson C. Estrogens as regulators of bone health in men. Nat Rev Endocrinol. 2009;5(8):437–43. doi: 10.1038/nrendo.2009.112. [DOI] [PubMed] [Google Scholar]
- 72.Ohlsson C, Vandenput L. The role of estrogens for male bone health. Eur J Endocrinol. 2009;160(6):883–889. doi: 10.1530/EJE-09-0118. [DOI] [PubMed] [Google Scholar]
- 73.Lanfranco F, Zirilli L, Baldi M, et al. A novel mutation in the human aromatase gene: insights on the relationship among serum estradiol, longitudinal growth and bone mineral density in an adult man under estrogen replacement treatment. Bone. 2008;43:628–35. doi: 10.1016/j.bone.2008.05.011. [DOI] [PubMed] [Google Scholar]
- 74.Mellstrom D, Johnell O, Ljunggren O, et al. Free testosterone is an independent predictor of BMD and prevalent fractures in elderly men: MrOS Sweden. Journal of Bone and Mineral Research. 2006;21:529–35. doi: 10.1359/jbmr.060110. [DOI] [PubMed] [Google Scholar]
- 75.Araujo AB, Travison TG, Leder BZ, et al. Correlations between serum testosterone, estradiol, and sex hormone-binding globulin and bone mineral density in a diverse sample of men. Journal of Clinical Endocrinology and Metabolism. 2008;93:2135–41. doi: 10.1210/jc.2007-1469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Gennari L, Merlotti D, Martini G, et al. Longitudinal association between sex hormone levels, bone loss, and bone turnover in elderly men. Journal of Clinical Endocrinology and Metabolism. 2003;88:5327–33. doi: 10.1210/jc.2003-030736. [DOI] [PubMed] [Google Scholar]
- 77.Kaufman JM, Vermeulen A. The decline of androgen levels in elderly men and its clinical and therapeutic implications. Endocrine Reviews. 2005;26:833–76. doi: 10.1210/er.2004-0013. [DOI] [PubMed] [Google Scholar]
- 78.Gennari L, Masi L, Merlotti D, et al. A polymorphic CYP19 TTTA repeat influences aromatase activity and estrogen levels in elderly men: effects on bone metabolism. Journal of Clinical Endocrinology and Metabolism. 2004;89:2803–10. doi: 10.1210/jc.2003-031342. [DOI] [PubMed] [Google Scholar]
- 79.Van Pottelbergh I, Goemaere S, Kaufman JM. Bioavailable estradiol and an aromatase gene polymorphism are determinants of bone mineral density changes in men over 70 years of age. Journal of Clinical Endocrinology and Metabolism. 2003;88:3075–81. doi: 10.1210/jc.2002-021691. [DOI] [PubMed] [Google Scholar]
- 80.Lorentzon M, Swanson C, Eriksson AL, et al. Polymorphisms in the aromatase gene predict area BMD as a result of affected cortical bone size: the GOOD study. Journal of Bone and Mineral Research. 2006;21:332–39. doi: 10.1359/JBMR.051026. [DOI] [PubMed] [Google Scholar]
- 81.Huhtaniemi IT, Pye SR, Limer KL, et al. Increased estrogen rather than decreased androgen action is associated with longer androgen receptor CAG repeats. Journal of Clinical Endocrinology and Metabolism. 2009;94:277–84. doi: 10.1210/jc.2008-0848. [DOI] [PubMed] [Google Scholar]
- 82.Amin S, Zhang Y, Felson DT, et al. Estradiol, testosterone, and the risk for hip fractures in elderly men from the Framingham Study. American Journal of Medicine. 2006;119:426–33. doi: 10.1016/j.amjmed.2005.10.048. [DOI] [PubMed] [Google Scholar]
- 83.Bjornerem A, Ahmed LA, Joakimsen RM, et al. A prospective study of sex steroids, sex hormone-binding globulin, and nonvertebral fractures in women and men: the Tromso Study. European Journal of Endocrinology. 2007;157:119–125. doi: 10.1530/EJE-07-0032. [DOI] [PubMed] [Google Scholar]
- 84.Goderie-Plomp HW, van der Klift M, de Ronde W, et al. Endogenous sex hormones, sex hormone- binding globulin, and the risk of incident vertebral fractures in elderly men and women: the Rotterdam Study. Journal of Clinical Endocrinology and Metabolism. 2004;89:3261–69. doi: 10.1210/jc.2002-022041. [DOI] [PubMed] [Google Scholar]
- 85.Mellstrom D, Vandenput L, Mallmin H, et al. Older men with low serum estradiol and high serum SHBG have an increased risk of fractures. Journal of Bone and Mineral Research. 2008;23:1552–60. doi: 10.1359/jbmr.080518. [DOI] [PubMed] [Google Scholar]
- 86.Khosla S, Melton LJ III, Riggs B L. Estrogen and the male skeleton. Journal of Clinical Endocrinology and Metabolism. 2002;87:1443–50. doi: 10.1210/jcem.87.4.8417. [DOI] [PubMed] [Google Scholar]
- 87.Muller M, Aleman A, Grobbee DE, et al. Endogenous sex hormone levels and cognitive function in aging men: is there an optimal level? Neurology. 2005;64:866–71. doi: 10.1212/01.WNL.0000153072.54068.E3. [DOI] [PubMed] [Google Scholar]
- 88.Shumaker SA, Legault C, Rapp SR, et al. Estrogen plus progestin and the incidence of dementia and mild cognitive impairment in postmenopausal women: the Women's Health Initiative Memory Study: a randomized controlled trial. JAMA. 2003;289:2651–62. doi: 10.1001/jama.289.20.2651. [DOI] [PubMed] [Google Scholar]
- 89.Muller M, van den Beld AW, Grobbee DE, et al. Sex hormones and cognitive decline in elderly men. Psychoneuroendocrinology. 2009;34:27–31. doi: 10.1016/j.psyneuen.2008.08.008. [DOI] [PubMed] [Google Scholar]
- 90.Yaffe K, Lui LY, Zmuda J, et al. Sex hormones and cognitive function in older men. J Am Geriatr Soc. 2002;50:707–12. doi: 10.1046/j.1532-5415.2002.50166.x. [DOI] [PubMed] [Google Scholar]
- 91.Wolf OT, Kirschbaum C. Endogenous estradiol and testosterone levels are associated with cognitive performance in older women and men. Horm and Behavior. 2002;41:259–66. doi: 10.1006/hbeh.2002.1770. [DOI] [PubMed] [Google Scholar]
- 92.Le Blanc ES, Wang PY, Janowsky JS, et al. AssociationAssociation between sex steroids and cognition in elderly men. Clin Endocrinol (Oxf) 2010;72(3):393–03. doi: 10.1111/j.1365-2265.2009.03692.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Rosario ER, Chang L, Stanczyk FZ, et al. Age-related testosterone depletion and the development of Alzheimer disease. JAMA. 2004;292(12):1431–32. doi: 10.1001/jama.292.12.1431-b. [DOI] [PubMed] [Google Scholar]
- 94.Giannoulis MG, Martin FC, Nair KS. Hormone replacement therapy and physical function in healthy older men. Time to talk hormones? Endocr Rev. 2012;33(3):314–77. doi: 10.1210/er.2012-1002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Lindahl A, Isgaard J, Nilsson A, et al. Growth hormone potentiates colony formation of epiphyseal chondrocytes in suspension culture. Endocrinology. 1986;118(5):1843–8. doi: 10.1210/endo-118-5-1843. [DOI] [PubMed] [Google Scholar]
- 96.Sherlock M, Toogood AA. Aging and the growth hormone/insulin like growth factor-I axis. Pituitary. 2007;10(2):189–203. doi: 10.1007/s11102-007-0039-5. [DOI] [PubMed] [Google Scholar]
- 97.Le Roith D, Bondy C, Yakar S. The somatomedin hypothesis . Endocr Rev. 2001;22(1):53–74. doi: 10.1210/edrv.22.1.0419. [DOI] [PubMed] [Google Scholar]
- 98.Borselli C, Storrie H, Benesch-Lee F, et al. Functional muscle regeneration with combined delivery of angiogenesis and myo-genesis factors. Proc Natl Acad Sci USA. 2010;107(8):3287–92. doi: 10.1073/pnas.0903875106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Yakar S, Rosen CJ, Beamer WG, et al. Circulating levels of IGF-1 directly regulate bone growth and density. J Clin Invest. 2002;110(6):771–81. doi: 10.1172/JCI15463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Kling PJ, Taing KM, Dvorak B, et al. Insulin-like growth factor-I stimulates erythropoiesis when administered enterally. Growth Factors. 2006;24(3):218–23. doi: 10.1080/08977190600783162. [DOI] [PubMed] [Google Scholar]
- 101.Arvat E, Broglio F, Ghigo E. Insulin-Like growth factor I: implications in aging. Drugs Aging. 2000;16(1):29–40. doi: 10.2165/00002512-200016010-00003. [DOI] [PubMed] [Google Scholar]
- 102.Lee PD, Giudice LC, Conover CA, et al. Insulin-like growth factor binding protein -1: recent findings and new directions. Proc Soc Exp Biol Med. 1997;216:319–57. doi: 10.3181/00379727-216-44182. [DOI] [PubMed] [Google Scholar]
- 103.Franklin SL, Ferry RJ, Jr, Cohen P. Rapid insulin-like growth factor (IGF)-independent effects of IGF binding protein-3 on endothelial cell survival. J Clin Endocrinol Metab. 2003;88(2):900–7. doi: 10.1210/jc.2002-020472. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Rajah R, Lee KW, Cohen P. Insulin-like growth factor binding protein-3 mediates tumor necrosis factor-alpha-induced apoptosis: role of Bcl-2 phosphorylation. Cell Growth Differ. 2002;13(4):163–71. [PubMed] [Google Scholar]
- 105.Lang CH, Vary TC, Frost RA. Acute in vivo elevation of insulin-like growth factor (IGF) binding protein-1 decreases plasma free IGF-I and muscle protein synthesis. Endocrinology. 2003;144(9):3922–33. doi: 10.1210/en.2002-0192. [DOI] [PubMed] [Google Scholar]
- 106.Salminen A, Kaarniranta K. Insulin/IGF-1 paradox of aging: regulation via AKT/IKK/NF-kappaB signaling. Cell Signal. 2010;22(4):573–7. doi: 10.1016/j.cellsig.2009.10.006. [DOI] [PubMed] [Google Scholar]
- 107.Tahimic CG, Wang Y, Bikle DD. Anabolic effects of IGF-1 signaling on the skeleton. Front Endocrinol (Lausanne) 2013;4:6. doi: 10.3389/fendo.2013.00006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Iranmanesh A, Lizarralde G, Veldhuis JD. Age and relative adiposity are specific negative determinants of the frequency and amplitude of growth hormone (GH) secretory bursts and the half-life of endogenous GH in healthy men. J Clin Endocrinol Metab. 1991;73(5):1081. doi: 10.1210/jcem-73-5-1081. [DOI] [PubMed] [Google Scholar]
- 109.Leifke E, Wichers C, Von Zur Mühlen A, et al. Age-related changes of serum sex hormones, insulin-like growth factor-1 and sex-hormone binding globulin levels in men: cross-sectional data from a healthy male cohort. Clin Endocrinol (Oxf) 2000;53(6):689–95. doi: 10.1046/j.1365-2265.2000.01159.x. [DOI] [PubMed] [Google Scholar]
- 110.Sattler FR, Castaneda-Sceppa C, Binder EF, et al. Testosterone and growth hormone improve body composition and muscle performance in older men. J Clin Endocrinol Metab. 2009;94(6):1991–2001. doi: 10.1210/jc.2008-2338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Goodman-Gruen D, Barrett-Connor E. Epidemiology of insulin-like growth factor-I in elderly men and women. The Rancho Bernardo Study. Am J Epidemiol. 1997;145(11):970–6. doi: 10.1093/oxfordjournals.aje.a009065. Erratum in: Am J Epidemiol 1997; 146(4): 357.
- 112.Rosen CJ. Growth hormone and aging. Endocrine. 2000;12(2):197–201. doi: 10.1385/ENDO:12:2:197. [DOI] [PubMed] [Google Scholar]
- 113.Hoffman AR, Pyka G, Liecerman SA, et al. The somatopause. In: muller EE, Cocchi D, Locatelli V, editors. Growth hormone and somatomedins during lifespan. springer Verlag; Berlin: [Google Scholar]
- 114.Maggio M, Cappola AR, Ceda GP, et al. The hormonal pathway to frailty in older men. J Endocrinol Invest. 2005;28(11):15–9. [PubMed] [Google Scholar]
- 115.Ho KY, Evans WS, Blizzard RM, et al. Effects of sex and age on the 24-hour profile of growth hormone secretion in man: importance of endogenous estradiol concentrations. J Clin Endocrinol Metab. 1987;64(1):51–8. doi: 10.1210/jcem-64-1-51. [DOI] [PubMed] [Google Scholar]
- 116.Onder G, Liperoti R, Russo A, et al. Body mass index, free insulin-like growth factor I, and physical function among older adults: results from the ilSIRENTE study. Am J Physiol Endocrinol Metab. 2006;291(4):E829–34. doi: 10.1152/ajpendo.00138.2006. [DOI] [PubMed] [Google Scholar]
- 117.Colbert LH, Rosen CJ, Goodpaster BH, et al. Insulin-like growth factor-1. J Am Geriatr Soc. 2004;52(11):1962–3. doi: 10.1111/j.1532-5415.2004.52529_1.x. [DOI] [PubMed] [Google Scholar]
- 118.Kaplan RC, McGinn AP, Pollak MN, et al. Total insulinlike growth factor 1 and insulinlike growth factor binding protein levels, functional status, and mortality in older adults. J Am Geriatr Soc. 2008;56(4):652–60. doi: 10.1111/j.1532-5415.2007.01637.x. [DOI] [PubMed] [Google Scholar]
- 119.Harris TB, Kiel D, Roubenoff R, Langlois J, et al. Association of insulin-like growth factor-I with body composition, weight history, and past health behaviors in the very old: the Framingham Heart Study. J Am Geriatr Soc. 1997;45(2):133–9. doi: 10.1111/j.1532-5415.1997.tb04497.x. [DOI] [PubMed] [Google Scholar]
- 120.Ceda GP, Dall'Aglio E, Maggio M, et al. Clinical implications of the reduced activity of the GH-IGF-I axis in older men. J Endocrinol Invest. 2005;28(11):96–100. [PubMed] [Google Scholar]
- 121.Baumgartner RN, Waters DL, Gallagher D, et al. Predictors of skeletal muscle mass in elderly men and women. Mech Ageing Dev. 1999;107(2):123–36. doi: 10.1016/s0047-6374(98)00130-4. [DOI] [PubMed] [Google Scholar]
- 122.Gläser S, Friedrich N, Ewert R, Schäper C, et al. Association of circulating IGF-I and IGFBP-3 concentrations and exercise capacity in healthy volunteers: results of the Study of Health in Pomerania. Growth Horm IGF Res. 2010;20(6):404–10. doi: 10.1016/j.ghir.2010.09.002. [DOI] [PubMed] [Google Scholar]
- 123.Wakai K, Suzuki K, Ito Y, et al. Time spent walking or exercising and blood levels of insulin-like growth factor-I (IGF-I) and IGF-binding protein-3 (IGFBP-3): A large-scale cross-sectional study in the Japan Collaborative Cohort study. Asian Pac J Cancer Prev. 2009;10:23–7. [PubMed] [Google Scholar]
- 124.Cappola AR, Bandeen-Roche K, Wand GS, et al. Association of IGF-I levels with muscle strength and mobility in older women. J Clin Endocrinol Metab. 2001;86(9):4139–46. doi: 10.1210/jcem.86.9.7868. [DOI] [PubMed] [Google Scholar]
- 125.Taekema DG, Ling CH, Blauw GJ, et al. Circulating levels of IGF1 are associated with muscle strength in middle-aged- and oldest-old women. Eur J Endocrinol. 2011;164(2):189–96. doi: 10.1530/EJE-10-0703. [DOI] [PubMed] [Google Scholar]
- 126.Payette H, Roubenoff R, Jacques PF, et al. Insulin-like growth factor-1 and interleukin 6 predict sarcopenia in very old community-living men and women: the Framingham Heart Study. J Am Geriatr Soc. 2003;51(9):1237–43. doi: 10.1046/j.1532-5415.2003.51407.x. [DOI] [PubMed] [Google Scholar]
- 127.Ershler WB, Keller ET. Age-associated increased interleukin-6 gene expression, late-life diseases, and frailty. Annu Rev Med. 2000;51:245–70. doi: 10.1146/annurev.med.51.1.245. [DOI] [PubMed] [Google Scholar]
- 128.Janssen JA, Stolk RP, Pols HA, et al. Serum free and total insulin-like growth factor-I, insulin-like growth factor binding protein-1 and insulin-like growth factor binding protein-3 Levels in healthy elderly individuals. Relation to self-reported quality of health and disability. Gerontology. 1998;44(5):277–80. doi: 10.1159/000022026. [DOI] [PubMed] [Google Scholar]
- 129.Ferrucci L, Harris TB, Guralnik JM, et al. Serum IL-6 level and the development of disability in older persons. J Am Geriatr Soc. 1999;47(6):639–46. doi: 10.1111/j.1532-5415.1999.tb01583.x. [DOI] [PubMed] [Google Scholar]
- 130.Taaffe DR, Harris TB, Ferrucci L, et al. Cross-sectional and prospective relationships of interleukin-6 and C-reactive protein with physical performance in elderly persons: MacArthur studies of suc cessful aging. J Gerontol A Biol Sci Med Sci. 2000;55(12):M709–15. doi: 10.1093/gerona/55.12.m709. [DOI] [PubMed] [Google Scholar]
- 131.Cohen HJ, Pieper CF, Harris T, et al. The association of plasma IL-6 levels with functional disability in community-dwelling elderly. J Gerontol A Biol Sci Med Sci. 1997;52(4):M201–8. doi: 10.1093/gerona/52a.4.m201. [DOI] [PubMed] [Google Scholar]
- 132.Harris TB, Ferrucci L, Tracy RP,C, et al. Associations of elevated interleukin-6 and C-reactive protein levels with mortality in the elderly. Am J Med. 1999;106(5):506–1. doi: 10.1016/s0002-9343(99)00066-2. [DOI] [PubMed] [Google Scholar]
- 133.Cappola AR, Xue QL, Ferrucci L, et al. Insulin-like growth factor I and interleukin-6 contribute synergistically to disability and mortality in older women. J Clin Endocrinol Metab. 2003;88(5):2019–25. doi: 10.1210/jc.2002-021694. [DOI] [PubMed] [Google Scholar]
- 134.Barbieri M, Ferrucci L, Ragno E, et al. Chronic inflammation and the effect of IGF-I on muscle strength and power in older persons. Am J Physiol Endocrinol Metab. 2003;284(3):E481–7. doi: 10.1152/ajpendo.00319.2002. [DOI] [PubMed] [Google Scholar]
- 135.Mariotti S, Franceschi C, Cossarizza A, et al. The aging thyroid. Endocrine Rev. 1995;16:686–715. doi: 10.1210/edrv-16-6-686. [DOI] [PubMed] [Google Scholar]
- 136.Magri F, Cravello L, Fioravanti M, et al. Thyroid function in old and very old healthy subjects. J Endocrinol Invest. 2002;25:60–63. [PubMed] [Google Scholar]
- 137.Roberts LM, Pattison H, Roalfe A, et al. Is subclinical thyroid dys-function in the elderly associated with depression or cognitive dys-function? Ann Intern Med. 2006;145:573–81. doi: 10.7326/0003-4819-145-8-200610170-00006. [DOI] [PubMed] [Google Scholar]
- 138.Wilson S, Parle JV, Roberts LM, et al. Prevalence of subclinical thyroid dys- function and its relation to socioeconomic deprivation in the elderly: A com- munity-based cross-sectional survey. J Clin Endocrinol Metab. 2006;91:4809–16. doi: 10.1210/jc.2006-1557. [DOI] [PubMed] [Google Scholar]
- 139.Aghini-Lombardi F, Antonangeli L, Martino E, et al. The spectrum of thyroid disorders in an iodine-deficient community: The Pescopagano survey. J Clin Endocrinol Metab. 1999;84:561–66. doi: 10.1210/jcem.84.2.5508. [DOI] [PubMed] [Google Scholar]
- 140.Surks MI, Ortiz E, Daniels GH, et al. Subclinical thyroid disease: scientific review and guidelines for diagnosis and management. JAMA. 2004 Jan 14;291(2):228–38. doi: 10.1001/jama.291.2.228. [DOI] [PubMed] [Google Scholar]
- 141.Ceresini G, Morganti S, Maggio M, et al. Subclinical thyroid disease in elderly subjects. Acta Biomed. 2010;81(1):31–6. [PubMed] [Google Scholar]
- 142.Van den Beld AW, Visser TJ, Feelders RA, et al. Thyroid hormone concentrations, disease, physical function, and mortality in elderly men. J Clin Endocrinol Metab. 2005;90(12):6403–9. doi: 10.1210/jc.2005-0872. [DOI] [PubMed] [Google Scholar]
- 143.Brennan MD, Powell C, Kaufman KR, et al. The impact of overt and subclinical hyperthyroidism on skeletal muscle. Thyroid. 2006;16:375–80. doi: 10.1089/thy.2006.16.375. [DOI] [PubMed] [Google Scholar]
- 144.Ceresini G, Lauretani F, Maggio M, et al. Thyroid function abnormalities and cognitive impair- ment in elderly people: results of the Invecchiare in Chianti study. J Am Geriatr Soc. 2009;57:89–93. doi: 10.1111/j.1532-5415.2008.02080.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Biondi B, Cooper DS. The clinical significance of subclinical thyroid dysfunction. Endocr Rev. 2008;29:76–131. doi: 10.1210/er.2006-0043. [DOI] [PubMed] [Google Scholar]
- 146.Ceresini G, Ceda GP, Lauretani F, et al. Mild thyroid hormone excess is associated with a decreased physical function in elderly men. Aging Male. 2011;14(4):213–9. doi: 10.3109/13685538.2011.606514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147.Simonsick EM, Newman AB, Ferrucci L, et al. Subclinical hypothyroidism and functional mobility in older adults. Arch Intern Med. 2009;169(21):2011–7. doi: 10.1001/archinternmed.2009.392. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148.Klubo-Gwiezdzinska J, Wartofsky L. Thyrotropin blood levels, subclinical hypothyroidism, and the elderly patient. Arch Intern Med. 2009;23169(21):1949–51. doi: 10.1001/archinternmed.2009.415. [DOI] [PubMed] [Google Scholar]
- 149.Parle JV, Franklyn JA, Cross KW, et al. Prevalence and follow-up of abnormal thyrotrophin (TSH) concentrations in the elderly in the United Kingdom. Clin Endocrinol. (Oxf) 1991;34(1):77–83. doi: 10.1111/j.1365-2265.1991.tb01739.x. [DOI] [PubMed] [Google Scholar]
- 150.Ochs N, Auer R, Bauer DC, et al. Meta-analysis: subclinical thyroid dysfunction and the risk for coronary heart disease and mortality. Ann Intern Med. 2008;148(11):832–45. doi: 10.7326/0003-4819-148-11-200806030-00225. [DOI] [PubMed] [Google Scholar]
- 151.Haentjens P, Van Meerhaeghe A, Poppe K, et al. Subclinicalthyroiddys- function and mortality: an estimate of relative and absolute excess all-cause mor- tality based on time-to-event data from cohort studies. Eur J Endocrinol. 2008;159(3):329–41. doi: 10.1530/EJE-08-0110. [DOI] [PubMed] [Google Scholar]
- 152.Lippi G, Montagnana M, Meschi T, et al. Vitamin D concentration and deficiency across different ages and genders. Aging Clin Exp Res. 2012 doi: 10.3275/8263. [DOI] [PubMed] [Google Scholar]
- 153.Ceglia L. Vitamin D and its role in skeletal muscle. Curr Opin Clin Nutr Metab Care. 2009;12:628–33. doi: 10.1097/MCO.0b013e328331c707. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154.Boland R. Role of vitamin D in skeletal muscle function. Endocr Rev. 1986;7:434–48. doi: 10.1210/edrv-7-4-434. [DOI] [PubMed] [Google Scholar]
- 155.Thacher TD, Clarke BL. Vitamin D insufficiency. Mayo Clin Proc. 2011;86:50–60. doi: 10.4065/mcp.2010.0567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 156.Simpson RU, Thomas GA, Arnold AJ. Identification of 1,25- dihydroxyvitamin D3 receptors and activities in muscle. J Biol Chem. 1985;260:8882–91. [PubMed] [Google Scholar]
- 157.Brumbaugh PF, Haussler MR. 1,25-Dihydroxy- cholecalciferol receptors in intestine. Association of 1,25-dihydroxycholecalciferol with intestinal mucosa chromatin. J Biol Chem. 1974;249:1251–57. [PubMed] [Google Scholar]
- 158.Girgis CM, Clifton-Bligh RJ, Hamrick MW, et al. The Roles of Vitamin D in Skeletal Muscle: Form, Function, and Metabolism. Endocrine Reviews. 2013;34(1):0000–0000. doi: 10.1210/er.2012-1012. [DOI] [PubMed] [Google Scholar]
- 159.Lauretani F, Maggio M, Valenti G, et al. Vitamin D in older population: new roles for this ‘classic actor’? The Aging Male. 2010;13(4):215–32. doi: 10.3109/13685538.2010.487551. [DOI] [PubMed] [Google Scholar]
- 160.Drittanti L, de Boland AR, Boland R. Stimulation of calmodulin synthesis in proliferating myoblasts by 1,25-dihy- droxy-vitamin D3. Mol Cell Endocrinol. 1990;74:143–53. doi: 10.1016/0303-7207(90)90116-p. [DOI] [PubMed] [Google Scholar]
- 161.Wang Y, DeLuca HF. Is the vitamin D receptor found in muscle? Endocrinology. 2011;152:354–63. doi: 10.1210/en.2010-1109. [DOI] [PubMed] [Google Scholar]
- 162.Selles J, Boland R. Rapid stimulation of calcium uptake and protein phosphorylation in isolated cardiac muscle by 1,25- dihydroxyvitamin D3. Mol Cell Endocrinol. 1991;77:67–73. doi: 10.1016/0303-7207(91)90059-2. [DOI] [PubMed] [Google Scholar]
- 163.Morelli S, Boland R, de Boland AR. 1,25(OH)2-vitamin D3 stimulation of phospholipases C and D in muscle cells involves extracellular calcium and a pertussis-sensitive G pro- tein. Mol Cell Endocrinol. 1996;122:207–11. doi: 10.1016/0303-7207(96)03886-5. [DOI] [PubMed] [Google Scholar]
- 164.Leveille SG, Bean J, Ngo L, et al. The pathway from musculoskeletal pain to mobility difficulty in older disabled women. Pain. 2007;128:69–77. doi: 10.1016/j.pain.2006.08.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 165.Hicks GE, Shardell M, Miller RR, et al. Associations between vitamin D status and pain in older adults: the Invecchiare in Chianti study. J Am Geriatr Soc. 2008;56:785–91. doi: 10.1111/j.1532-5415.2008.01644.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 166.Annweiler C, Schott AM, Berrut G, et al. Vitamin D-related changes in physical performance: a systematic review. J Nutr Health Aging. 2009;13:893–8. doi: 10.1007/s12603-009-0248-x. [DOI] [PubMed] [Google Scholar]
- 167.Janssen HC, Samson MM, Verhaar HJ. Muscle strength and mobility in vitamin D-insufficient female geriatric patients: a randomized controlled trial on vitamin D and calcium supplementation. Aging Clin Exp Res. 2010;22:78–84. doi: 10.1007/BF03324819. [DOI] [PubMed] [Google Scholar]
- 168.Houston DK, Tooze JA, Davis CC, et al. Serum 25-hydroxyvitamin D and physical function in older adults: the Cardiovascular Health Study All Stars. J Am Geriatr Soc. 2011;59:1793–801. doi: 10.1111/j.1532-5415.2011.03601.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 169.Shardell M, D'Adamo C, Alley DE, et al. Serum 25-hydroxyvitamin D, transitions between frailty states, and mortality in older adults: the Invecchiare in Chianti Study. J Am Geriatr Soc. 2012;60:256–64. doi: 10.1111/j.1532-5415.2011.03830.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 170.Toffanello ED, Perissinotto E, Sergi G. Vitamin D and physical performance in elderly subjects: the Pro.V.A study. 2012;7(4):e34950. doi: 10.1371/journal.pone.0034950. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 171.Snijder MB, van Schoor NM, Pluijm SM, et al. Vitamin D status in relation to one-year risk of recurrent falling in older men and women. Clin Endocrinol Metab. 2006;91(8):2980–5. doi: 10.1210/jc.2006-0510. [DOI] [PubMed] [Google Scholar]
- 172.Wicherts IS, van Schoor NM, Boeke AJ. Vitamin D status predicts physical performance and its decline in older persons. J Clin Endocrinol Metab. 2007;92(6):2058–65. doi: 10.1210/jc.2006-1525. [DOI] [PubMed] [Google Scholar]
- 173.Baker WL, Karan S, Kenny AM. Effect of dehydroepiandrosterone on muscle strength and physical function in older adults: a systematic review. J Am Geriatr Soc. 2011;59:997–1002. doi: 10.1111/j.1532-5415.2011.03410.x. [DOI] [PubMed] [Google Scholar]
- 174.Kenny AM, Boxer RS, Kleppinger A, et al. Dehydroepiandrosterone (DHEA) improves muscle strength and physical function but not bone mineral density in frail older women. J Am Geriatr Soc. 2010;58:1707–14. doi: 10.1111/j.1532-5415.2010.03019.x. [DOI] [PubMed] [Google Scholar]
- 175.Nair KS, Rizza RA, O'Brien P, et al. DHEA in elderly women and DHEA or testosterone in elderly men. N Engl J Med. 2006;355:1647–59. doi: 10.1056/NEJMoa054629. [DOI] [PubMed] [Google Scholar]
- 176.Igwebuike A, Irving BA, Bigelow ML, et al. Lack of dehydroepiandrosterone effect on a combined endurance and resistance exercise program in post- menopausal women. J Clin Endocrinol Metab. 2008;93:534–38. doi: 10.1210/jc.2007-1027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 177.Villareal DT, Holloszy JO. DHEA enhances effects of weight training on muscle mass and strength in elderly women and men. Am J Physiol Endocrinol Metab. 2006;291:E1003–E1008. doi: 10.1152/ajpendo.00100.2006. [DOI] [PubMed] [Google Scholar]
- 178.Muller M, van den Beld AW, van der Schouw YT, et al. Effects of dehydroepiandrosterone and atemestane supplementation on frailty in elderly men. J Clin Endocrinol Metab. 2006;91:3988–91. doi: 10.1210/jc.2005-2433. [DOI] [PubMed] [Google Scholar]
- 179.von Mühlen D, Laughlin GA, Kritz-Silverstein D, et al. Effect of dehydroepiandrosterone supplementation on bone mineral density, bone markers, and body composition in older adults: the DAWN trial. Osteoporos Int. 2008;19(5):699–07. doi: 10.1007/s00198-007-0520-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 180.Ottenbacher KJ, Ottenbacher ME, Ottenbacher AJ, et al. Androgen treatment and muscle strength in elderly men: A meta-analysis. J Am Geriatr Soc. 2006;54:1666–73. doi: 10.1111/j.1532-5415.2006.00938.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 181.Kenny AM, Kleppinger A, Annis K, et al. Effects of transdermal testosterone on bone and muscle in older men with low bioavailable testosterone levels, low bone mass, and physical frailty. J Am Geriatr Soc. 2010;58:1134–43. doi: 10.1111/j.1532-5415.2010.02865.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 182.Amory JK, Watts NB, Easley KA, et al. Exogenous testosterone or testosterone with finasteride increases bone mineral density in older men with low serum testosterone. J Clin Endocrinol Metab. 2004;89:503–5. doi: 10.1210/jc.2003-031110. [DOI] [PubMed] [Google Scholar]
- 183.Srinivas-Shankar U, Roberts SA, Connolly MJ, et al. Effects of testosterone on muscle strength, physical function, body composition, and quality of life in intermediate-frail and frail elderly men: a randomized, double-blind, placebo-controlled study. J Clin Endocrinol Metab. 2010;95:639–50. doi: 10.1210/jc.2009-1251. [DOI] [PubMed] [Google Scholar]
- 184.Travison TG, Basaria S, Storer TW, et al. Clinical meaningfulness of the changes in muscle performance and physical function associated with testosterone administration in older men with mobility limitation. J Gerontol A Biol Sci Med Sci. 2011;66(10):1090–9. doi: 10.1093/gerona/glr100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 185.Sheffield-Moore M, Paddon-Jones D, Casperson SL, et al. Androgen therapy induces muscle protein anabolism in older women. J Clin Endocrinol Metab. 2006;91(10):3844–49. doi: 10.1210/jc.2006-0588. [DOI] [PubMed] [Google Scholar]
- 186.Greising SM, Baltgalvis KA, Lowe DA. Hormone therapy and skeletal muscle strength: a meta-analysis. J Gerontol A Biol Sci Med Sci. 2009;64(10):1071–81. doi: 10.1093/gerona/glp082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 187.Taaffe DR, Newman AB, Haggerty CL, et al. Estrogen replacement, muscle composition, and physical function: The health ABC study. Med Sci Sports Exerc. 2005;37(10):1741–47. doi: 10.1249/01.mss.0000181678.28092.31. [DOI] [PubMed] [Google Scholar]
- 188.Sipila S, Taaffe DR, Cheng S, et al. Effects of hormone replacement therapy and high-impact physical exercise on skeletal muscle in post-menopausal women: a randomized placebo-controlled study. Clin Sci (Lond) 2001;101(2):14–157. [PubMed] [Google Scholar]
- 189.Onambele GNL, Bruce SA, Woledge RC. Oestrogen status in relation to the early training responses in human thumb adductor muscles. Acta Physiol (Oxf) 2006;188:41–52. doi: 10.1111/j.1748-1716.2006.01597.x. [DOI] [PubMed] [Google Scholar]
- 190.Skelton DA, Phillips SK, Bruce SA, et al. Hormone replacement therapy increases isometric muscle strength of adductor pollicis in post-menopausal women. Clin Sci (Lond) 1999;96(4):357–64. [PubMed] [Google Scholar]
- 191.Phillips SK, Rook KM, Siddle NC, et al. Muscle weakness in women occurs at an earlier age than in men, but strength is preserved by hormone replacement therapy . Clin Sci (Lond) 1993;84(1):95–98. doi: 10.1042/cs0840095. [DOI] [PubMed] [Google Scholar]
- 192.Taaffe DR, Sipilä S, Cheng S, et al. The effect of hormone replacement therapy and/or exercise on skeletal muscle attenuation in postmenopausal women: a yearlong intervention. Clin Physiol Funct Imaging. 2005;25(5):297–304. doi: 10.1111/j.1475-097X.2005.00628.x. [DOI] [PubMed] [Google Scholar]
- 193.Greenspan SL, Resnick NM, Parker RA. The effect of hormone replacement on physical performance in community-dwelling elderly women. Am J Med. 2005;118(11):1232–9. doi: 10.1016/j.amjmed.2005.03.004. [DOI] [PubMed] [Google Scholar]
- 194.Utian WH, Archer DF, Bachmann GA, et al. Estrogen and progestogen use in postmenopausal women: July 2008 position statement of The North American Menopause Society. Menopause. 2008;15(4):584–602. doi: 10.1097/gme.0b013e31817b076a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 195.Marcus R, Butterfield G, Holloway L, et al. Effects of short term administration of recombinant human growth hormone to elderly people. J Clin Endocrinol Metab. 1990;70(2):519–27. doi: 10.1210/jcem-70-2-519. [DOI] [PubMed] [Google Scholar]
- 196.Rudman D, Feller AG, Nagraj HS, et al. Effects of human growth hormone in men over 60 years old. N Engl J Med. 1990;323(1):1. doi: 10.1056/NEJM199007053230101. [DOI] [PubMed] [Google Scholar]
- 197.Thompson JL, Butterfield GE, Marcus R, et al. The effects of recombinant human insulin-like growth factor-I and growth hormone on body composition in elderly women. J Clin Endocrinol Metab. 1995;80(6):1845–5. doi: 10.1210/jcem.80.6.7539817. [DOI] [PubMed] [Google Scholar]
- 198.Huang X, Blackman MR, Herreman K, et al. Effects of growth hormone and/or sex steroid administration on whole-body protein turnover in healthy aged women and men. Metabolism. 2005;54(9):1162–7. doi: 10.1016/j.metabol.2005.03.023. [DOI] [PubMed] [Google Scholar]
- 199.Blackman MR, Sorkin JD, Münzer T, et al. Growth hormone and sex steroid administration in healthy aged women and men: a randomized controlled trial. JAMA. 2002;288(18):2282–92. doi: 10.1001/jama.288.18.2282. [DOI] [PubMed] [Google Scholar]
- 200.Welle S, Thornton C, Statt M, et al. Growth hormone increases muscle mass and strength but does not rejuvenate myofibrillar protein synthesis in healthy subjects over 60 years old. J Clin Endocrinol Metab. 1996;81(9):3239–43. doi: 10.1210/jcem.81.9.8784075. [DOI] [PubMed] [Google Scholar]
- 201.Taaffe DR, Jin IH, Vu TH, et al. Lack of effect of recombinant human growth hormone (GH) on muscle morphology and GH-insulin-like growth factor expression in resistance-trained elderly men. J Clin Endocrinol Metab. 1996;81(1):421–5. doi: 10.1210/jcem.81.1.8550787. [DOI] [PubMed] [Google Scholar]
- 202.Hennessey JV, Chromiak JA, DellaVentura S, et al. Growth hormone administration and exercise effects on muscle fiber type and diameter in moderately frail older people. J Am Geriatr Soc. 2001;49(7):852–8. doi: 10.1046/j.1532-5415.2001.49173.x. [DOI] [PubMed] [Google Scholar]
- 203.Lange KH, Andersen JL, Beyer N, et al. GH administration changes myosin heavy chain isoforms in skeletal muscle but does not augment muscle strength or hypertrophy, either alone or combined with resistance exercise training in healthy elderly men. J Clin Endocrinol Metab. 2002;87(2):513–23. doi: 10.1210/jcem.87.2.8206. [DOI] [PubMed] [Google Scholar]
- 204.Papadakis MA, Grady D, Black D, et al. Growth hormone replacement in healthy older men improves body composition but not functional ability. Ann Intern Med. 1996;124(8):70816. doi: 10.7326/0003-4819-124-8-199604150-00002. [DOI] [PubMed] [Google Scholar]
- 205.Holloway L, Butterfield G, Hintz RL, et al. Effects of recombinant human growth hormone on metabolic indices, body composition, and bone turnover in healthy elderly women. J Clin Endocrinol Metab. 1994;79(2):470–9. doi: 10.1210/jcem.79.2.7519191. [DOI] [PubMed] [Google Scholar]
- 206.Drake WM, Howell SJ, Monson JP, et al. Optimizing gh therapy in adults and children. Rev. 2001;22(4):425–50. doi: 10.1210/edrv.22.4.0438. [DOI] [PubMed] [Google Scholar]
- 207.Filipsson Nyström H, Barbosa EJ, Nilsson AG, et al. Discontinuing long-term GH replacement therapy--a randomized, placebo-controlled crossover trial in adult GH deficiency. J Clin Endocrinol Metab. 2012;97(9):3185–95. doi: 10.1210/jc.2012-2006. [DOI] [PubMed] [Google Scholar]
- 208.Götherström G, Bengtsson BA, Sunnerhagen KS, et al. The effects of five-year growth hormone replacement therapy on muscle strength in elderly hypopituitary patients. Clin Endocrinol (Oxf) 2005;62(1):105–13. doi: 10.1111/j.1365-2265.2004.02181.x. [DOI] [PubMed] [Google Scholar]
- 209.Götherström G, Elbornsson M, Stibrant-Sunnerhagen K, et al. Muscle strength in elderly adults with GH deficiency after 10 years of GH replacement. Eur J Endocrinol. 2010;163(2):207–15. doi: 10.1530/EJE-10-0009. [DOI] [PubMed] [Google Scholar]
- 210.Johannsson G, Rosén T, Bosaeus I, et al. Two years of growth hormone (GH) treatment increases bone mineral content and density in hypopituitary patients with adult-onset GH deficiency. J Clin Endocrinol Metab. 1996;81(8):2865–73. doi: 10.1210/jcem.81.8.8768843. [DOI] [PubMed] [Google Scholar]
- 211.Elbornsson M, Götherström G, Franco C, et al. Effects of 3-year GH replacement therapy on bone mineral density in younger and elderly adults with adult-onsetGH deficiency. Eur J Endocrinol. 2012;166(2):181–9. doi: 10.1530/EJE-11-0886. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 212.Weissberger AJ, Anastasiadis AD, Sturgess I, et al. Recombinant human growth hormone treatment in elderly patients undergoing elective total hip replacement. Clin Endocrinol (Oxf) 2003;58(1):99–107. doi: 10.1046/j.1365-2265.2003.01700.x. [DOI] [PubMed] [Google Scholar]
- 213.White HK, Petrie CD, Landschulz W, et al. Effects of an oral growth hormone secretagogue in older adults. J Clin Endocrinol Metab. 2009;94(4):1198–206. doi: 10.1210/jc.2008-0632. [DOI] [PubMed] [Google Scholar]
- 214.Guebre-Egziabher F, Juillard L, et al. Short-term administration of a combination of recombinant growth hormone and insulin-like growth factor-Iinduces anabolism in maintenance hemodialysis. J Clin Endocrinol Metab. 2009;94(7):2299–305. doi: 10.1210/jc.2008-2262. [DOI] [PubMed] [Google Scholar]
- 215.Nørrelund H, Hove KY, Brems-Dalgaard E, et al. Muscle mass and function in thyrotoxic patients before and during medical treatment. Clin Endocrinol (Oxf) 1999;51(6):693–9. doi: 10.1046/j.1365-2265.1999.00861.x. [DOI] [PubMed] [Google Scholar]
- 216.Brennan MD, Powell C, Kaufman KR, et al. The impact of overt and subclinical hyperthyroidism on skeletal muscle. Thyroid. 2006;16(4):375–80. doi: 10.1089/thy.2006.16.375. [DOI] [PubMed] [Google Scholar]
- 217.Reuters VS, Almeida Cde P, Teixeira Pde F, et al. Effects of sub-clinical hypothyroidism treatment on psychiatric symptoms, muscular complaints, and quality of life. Arq Bras Endocrinol Metabol. 2012;56(2):128–36. doi: 10.1590/s0004-27302012000200006. [DOI] [PubMed] [Google Scholar]
- 218.Lagari V, Gómez-Marín O, et al. The role of vitamin D in improving physical performance in the elderly. J Bone Miner Res. 2013 doi: 10.1002/jbmr.1949. [DOI] [PubMed] [Google Scholar]
- 219.Stockton KA, Mengersen K, Paratz JD, et al. Effect of vitamin D supplementation on muscle strength: a system- atic review and meta-analysis. Osteoporos Int. 2011;22:859–71. doi: 10.1007/s00198-010-1407-y. [DOI] [PubMed] [Google Scholar]
- 220.Sato Y, Iwamoto J, Kanoko T, et al. Low-dose vitamin D prevents muscular atrophy and reduces falls and hip fractures in women after stroke: a randomized controlled trial. Cerebrovasc Dis. 2005;20(3):187–92. doi: 10.1159/000087203. [DOI] [PubMed] [Google Scholar]
- 221.Latham NK, Anderson CS, Lee A, et al. A randomized, controlled trial of quadriceps resistance exercise and vitamin D in frail older people: the Frailty Interventions Trial in Elderly Subjects (FITNESS). J Am Geriatr Soc. 2003;51(3):291–9. doi: 10.1046/j.1532-5415.2003.51101.x. [DOI] [PubMed] [Google Scholar]
- 222.Bischoff HA, Stähelin HB, Dick W, et al. Effects of vitamin D and calcium supplementation on falls: a randomized controlled trial. J Bone Miner Res. 2003;18(2):343–51. doi: 10.1359/jbmr.2003.18.2.343. [DOI] [PubMed] [Google Scholar]
- 223.Goswami R, Vatsa M, Sreenivas V,S, et al. Skeletal muscle strength in young Asian Indian females after vitamin D and calcium supplementation: a double-blind randomized controlled clinical trial. J Clin Endocrinol Metab. 2012;97:4709–16. doi: 10.1210/jc.2012-2340. [DOI] [PubMed] [Google Scholar]
- 224.Wang TJ, Zhang F, Richards JB, et al. Common genetic determinants of vitamin D insufficiency: a genome-wide association study. Lancet. 2010;17376(9736):180–88. doi: 10.1016/S0140-6736(10)60588-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 225.Zhu K, Austin N, Devine A, et al. A randomized controlled trial of the effects of vitamin D on muscle strength and mobility in older women with vitamin D insufficiency. J Am Geriatr Soc. 2010;58:2063–8. doi: 10.1111/j.1532-5415.2010.03142.x. [DOI] [PubMed] [Google Scholar]
- 226.Urban RJ. Growth hormone and testosterone: anabolic effects on muscle. Horm Res Paediatr. 2011;76(1):81–3. doi: 10.1159/000329184. [DOI] [PubMed] [Google Scholar]
- 227.Urban RJ, Bodenburg YH, Gilkison C, et al. Testosterone administration to elderly men increases skeletal muscle strength and protein synthesis. Am J Physiol. 1995;269:E820–6. doi: 10.1152/ajpendo.1995.269.5.E820. [DOI] [PubMed] [Google Scholar]
- 228.Sattler F, Bhasin S, He J, et al. Testosterone threshold levels and lean tissue mass targets needed to enhance skeletal muscle strength and function: the HORMA trial. J Gerontol A Biol Sci Med Sci. 2011;66(1):122–9. doi: 10.1093/gerona/glq183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 229.Brill KT, Weltman AL, Gentili A, et al. Single and combined effects of growth hormone and testosterone administration on measures of body composition, physical performance, mood, sexual function, bone turnover, and muscle gene expression in healthy older men. Clin Endocrinol Metab. 2002;87(12):5649–57. doi: 10.1210/jc.2002-020098. [DOI] [PubMed] [Google Scholar]
- 230.Ragnarsson O, Burt MG, Ho KK, et al. Effect of short-term GH and testosterone administration on body composition and glucose homoeostasis in men receiving chronic glucocorticoid therapy. Eur J Endocrinol. 2013;168(2):243–51. doi: 10.1530/EJE-12-0873. [DOI] [PubMed] [Google Scholar]
- 231.Nimptsch K, Platz EA, Willett WC, et al. Association between plasma 25-OH vitamin D and testosterone levels in men. Clin Endocrinol (Oxf) 2012;77(1):106–12. doi: 10.1111/j.1365-2265.2012.04332.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 232.Wang TJ, Zhang F, Richards JB, et al. Common genetic determinants of vitamin D insufficiency: a genome-wide association study. Lancet. 2010;376(9736):180–188. doi: 10.1016/S0140-6736(10)60588-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 233.Bièche I, Narjoz C, Asselah T, et al. Reverse transcriptase-PCR quantification of mRNA levels from cytochrome (CYP)1, CYP2 and CYP3 families in 22 different human tissues. Pharmacogenet Genomics. 2007;17(9):731–42. doi: 10.1097/FPC.0b013e32810f2e58. [DOI] [PubMed] [Google Scholar]
- 234.Foresta C, Selice R, Di Mambro A, et al. Testiculopathy and vita-min D insufficiency. Lancet. 2010.16;376(9749):1301. doi: 10.1016/S0140-6736(10)61916-2. [DOI] [PubMed] [Google Scholar]
- 235.Foresta C, Strapazzon G, De Toni L, et al. Bone mineral density and testicular failure: evidence for a role of vitamin D 25-hydroxylase in human testis. J Clin Endocrinol Metab. 2011;96(4):E646–52. doi: 10.1210/jc.2010-1628. [DOI] [PubMed] [Google Scholar]
- 236.Lerchbaum E, Pilz S, Boehm BO, et al. Combination of low free testosterone and low vitamin D predicts mortality in older men referred for coronary angiography. Clin Endocrinol (Oxf) 2012;77(3):475–83. doi: 10.1111/j.1365-2265.2012.04371.x. [DOI] [PubMed] [Google Scholar]
- 237.Pilz S, Frisch S, Koertke H, et al. Effect of vitamin D supplementation on testosterone levels in men. Hormone And Metabolic Research. 2011;43:223–225. doi: 10.1055/s-0030-1269854. [DOI] [PubMed] [Google Scholar]
- 238.Giannoulis MG, Sonksen PH, Umpleby M, et al. The effects of growth hormone and/or testosterone in healthy elderly men: a randomized controlled trial. J Clin Endocrinol Metab. 2006;91(2):477–84. doi: 10.1210/jc.2005-0957. [DOI] [PubMed] [Google Scholar]
- 239.Maggio M, Ceda GP, Lauretani F, et al. Magnesium and anabolic hormones in older men. Int J Androl. 2011;34:e594–e600. doi: 10.1111/j.1365-2605.2011.01193.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 240.Chapman IM, Visvanathan R, Hammond AJ, et al. Effect of testosterone and a nutritional supplement, alone and in combination, on hospital admissions in undernourished older men and women. Am J Clin Nutr. 2009;89:880–89. doi: 10.3945/ajcn.2008.26538. [DOI] [PubMed] [Google Scholar]
- 241.Piantadosi C, Visvanathan R, Naganathan V, et al. The effect of testosterone and a nutritional supplement on hospital admissions in under-nourished, older people. BMC Geriatr. 2011;11:66. doi: 10.1186/1471-2318-11-66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 242.Taaffe DR, Pruitt L, Reim J, et al. Effect of recombinant human growth hormone on the muscle strength response to resistance exercise in elderly men. J Clin Endocrinol Metab. 1994;79(5):1361–6. doi: 10.1210/jcem.79.5.7525633. [DOI] [PubMed] [Google Scholar]
- 243.Lange KH, Isaksson F, Juul A, et al. Growth hormone enhances effects of endurance training on oxidative muscle metabolism in elderly women. Am J Physiol Endocrinol Metab. 2000;279(5):E989–96. doi: 10.1152/ajpendo.2000.279.5.E989. [DOI] [PubMed] [Google Scholar]
- 244.Yarasheski KE, Campbell JA, Kohrt WM. Effect of resistance exercise and growth hormone on bone density in older men. Clin Endocrinol (Oxf) 1997;47(2):223–9. doi: 10.1046/j.1365-2265.1997.2461060.x. [DOI] [PubMed] [Google Scholar]
- 245.Vingren JL, Kraemer WJ, Ratamess NA, et al. Testosterone physiology in resistance exercise and training: the up-stream regulatory elements. Sports Med. 2010;40(12):1037–53. doi: 10.2165/11536910-000000000-00000. [DOI] [PubMed] [Google Scholar]
- 246.Aizawa K, Iemitsu M, Maeda S, et al. Endurance exercise training enhances local sex steroidogenesis in skeletal muscle. Med Sci Sports Exerc. 2011;43(11):2072–80. doi: 10.1249/MSS.0b013e31821e9d74. [DOI] [PubMed] [Google Scholar]
- 247.Dreyer HC, Fujita S, Glynn EL, et al. Resistance exercise increases leg muscle protein synthesis and mTOR signalling independent of sex. Acta Physiol (Oxf) 2010;199(1):71–81. doi: 10.1111/j.1748-1716.2010.02074.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 248.Gasier HG, Fluckey JD, Previs SF, et al. Acute resistance exercise augments integrative myofibrillar protein synthesis. Metabolism. 2012;61(2):153–56. doi: 10.1016/j.metabol.2011.07.001. [DOI] [PubMed] [Google Scholar]
- 249.Aker JR, Bemben MG, Anderson MA, et al. Effects of age on testosterone responses to resistance exercise and musculoskeletal variables in men. J Strength Cond Res. 2006;20(4):874–81. doi: 10.1519/R-18885.1. [DOI] [PubMed] [Google Scholar]
- 250.Vingren JL, Kraemer WJ, Hatfield DL, et al. Effect of resistance exercise on muscle steroid receptor protein content in strength-trained men and women. Steroids. 2009;74(1314):1033–1039. doi: 10.1016/j.steroids.2009.08.002. [DOI] [PubMed] [Google Scholar]
- 251.Linnamo V, Pakarinen A, Komi PV, et al. Acute hormonal responses to submaximal and maximal heavy resistance and explosive exercises in men and women. J Strength Cond Res. 2005;19(3):566–71. doi: 10.1519/R-15404.1. [DOI] [PubMed] [Google Scholar]
- 252.Kraemer WJ, French DN, Spiering BA, et al. Cortitrol supplementation reduces serum cortisol responses to physical stress. Metabolism. 2005;54(5):657–68. doi: 10.1016/j.metabol.2004.12.010. [DOI] [PubMed] [Google Scholar]
- 253.Nakamura Y, Hornsby PJ, Casson P, et al. Type 5 17beta-hydroxysteroid dehydrogenase (AKR1C3) contributes to testosterone production in the adrenal reticularis. J Clin Endocrinol Metab. 2009;94(6):2192–98. doi: 10.1210/jc.2008-2374. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 254.Guo W, Wong S, Li M, et al. Testosterone plus low-intensity physical training in late life improves functional performance, skeletal muscle mitochondrial biogenesis, and mitochondrial quality control in male mice. PLoS One. 2012;7(12):e51180. doi: 10.1371/journal.pone.0051180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 255.Stout M, Tew GA, Doll H, et al. Testosterone therapy during exercise rehabilitation in male patients with chronic heart failure who have low testosterone status: a double-blind randomized controlled feasibility study. Am Heart J. 2012;164(6):893–01. doi: 10.1016/j.ahj.2012.09.016. [DOI] [PubMed] [Google Scholar]
- 256.Hildreth KL, Barry DW, Moreau KL, et al. Effects of testosterone and progressive resistance exercise in healthy, highly functioning older men with low-normal testosterone levels. J Clin Endocrinol Metab. 2013;98(5):1891–900. doi: 10.1210/jc.2012-3695. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 257.Maggio M, De Vita F, Lauretani F, et al. IGF-1, the cross road of the nutritional, inflammatory and hormonal pathways to frailty. Nutrients. 2013;5(10):4184–205. doi: 10.3390/nu5104184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 258.Maggio M, De Vita F, Lauretani F, et al. The interplay between magnesium and testosterone in men. Int J Endocrinol. 2014 doi: 10.1155/2014/525249. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 259.Maggio M, Ceda GP, Lauretani F, et al. Association of plasma selenium concentrations with total IGF-1 among older community-dwelling adults: the InCHIANTI study. Clin Nutr. 2010;29(5):674–7. doi: 10.1016/j.clnu.2010.03.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 260.Afilalo J, Eisenberg MJ, Morin JF, et al. Gait speed as an incremental predictor of mortality and major morbidity in elderly patients undergoing cardiac surgery. J Am Coll Cardiol. 2010;56:1668–76. doi: 10.1016/j.jacc.2010.06.039. [DOI] [PubMed] [Google Scholar]
- 261.Maggio M, Nicolini F, Cattabiani C, et al. Effects of testosterone supplementation on clinical and rehabilitative outcomes in older men undergoing on-pump CABG. Contemp Clin Trials. 2012;33(4):730–8. doi: 10.1016/j.cct.2012.02.019. [DOI] [PubMed] [Google Scholar]