Abstract
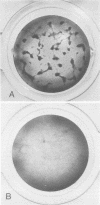
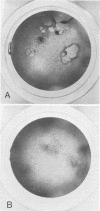
Stocks of Rous sarcoma virus Bryan strain were mutagenized using a bromodeoxyuridine treatment immediately after infection. Thirty temperature-sensitive (ts) mutants defective in transformation (td) were isolated by a replica plating technique. Twenty of these mutants were preliminarily characterized and found to be defective in late functions related to transformation. These mutants were used in experiments of cooperative transformation with four Prague strain td ts mutants of different co-transformation group. A small number of Bryan ts mutants were found to cooperate with some of the Prague mutants in transforming chicken embryo cells at the nonpermissive temperature. However, the amount of co-transformation observed was lower than that observed with cooperating Prague ts mutants and no clear-cut pattern of cotransformation was obtained in Prague and Bryan crosses. Indirect evidence indicates that cooperative transformation is the result of recombination events.
Full text
PDF









Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bader J. P., Brown N. R. Induction of mutations in an RNA tumour virus by an analogue of a DNA precursor. Nat New Biol. 1971 Nov 3;234(44):11–12. doi: 10.1038/newbio234011a0. [DOI] [PubMed] [Google Scholar]
- Balduzzi P. C., Murphy H. Plaque assay of avian sarcoma viruses using casein. J Virol. 1975 Sep;16(3):707–711. doi: 10.1128/jvi.16.3.707-711.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Balduzzi P., Morgan H. R. Mechanism of oncogenic transformation by Rous sarcoma virus. I. Intracellular inactivation of cell-transforming ability of Rous sarcoma virus by 5-bromodeoxyuridine and light. J Virol. 1970 Apr;5(4):470–477. doi: 10.1128/jvi.5.4.470-477.1970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goldberg A. R. Increased protease levels in transformed cells: a casein overlay assay for the detection of plasminogen activator production. Cell. 1974 Jun;2(2):95–102. doi: 10.1016/0092-8674(74)90097-x. [DOI] [PubMed] [Google Scholar]
- HAM R. G. CLONAL GROWTH OF MAMMALIAN CELLS IN A CHEMICALLY DEFINED, SYNTHETIC MEDIUM. Proc Natl Acad Sci U S A. 1965 Feb;53:288–293. doi: 10.1073/pnas.53.2.288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- HANAFUSA H., HANAFUSA T., RUBIN H. ANALYSIS OF THE DEFECTIVENESS OF ROUS SARCOMA VIRUS, II. SPECIFICATION OF RSV ANTIGENICITY BY HELPER VIRUS. Proc Natl Acad Sci U S A. 1964 Jan;51:41–48. doi: 10.1073/pnas.51.1.41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kawai S., Hanafusa H. Genetic recombination with avian tumor virus. Virology. 1972 Jul;49(1):37–44. doi: 10.1016/s0042-6822(72)80005-9. [DOI] [PubMed] [Google Scholar]
- Kawai S., Hanafusa H. The effects of reciprocal changes in temperature on the transformed state of cells infected with a rous sarcoma virus mutant. Virology. 1971 Nov;46(2):470–479. doi: 10.1016/0042-6822(71)90047-x. [DOI] [PubMed] [Google Scholar]
- Kawai S., Metroka C. E., Hanafusa H. Complementation of functions required for cell transformation by double infection with RSV mutants. Virology. 1972 Jul;49(1):302–304. doi: 10.1016/s0042-6822(72)80032-1. [DOI] [PubMed] [Google Scholar]
- Martin G. S. Rous sarcoma virus: a function required for the maintenance of the transformed state. Nature. 1970 Sep 5;227(5262):1021–1023. doi: 10.1038/2271021a0. [DOI] [PubMed] [Google Scholar]
- SIMONS P. J., DOUGHERTY R. M. ANTIGENIC CHARACTERISTICS OF THREE VARIANTS OF ROUS SARCOMA VIRUS. J Natl Cancer Inst. 1963 Nov;31:1275–1283. [PubMed] [Google Scholar]
- TEMIN H. M. The control of cellular morphology in embryonic cells infected with rous sarcoma virus in vitro. Virology. 1960 Feb;10:182–197. doi: 10.1016/0042-6822(60)90038-6. [DOI] [PubMed] [Google Scholar]
- Toyoshima K., Vogt P. K. Temperature sensitive mutants of an avian sarcoma virus. Virology. 1969 Dec;39(4):930–931. doi: 10.1016/0042-6822(69)90030-0. [DOI] [PubMed] [Google Scholar]
- Unkeless J. C., Tobia A., Ossowski L., Quigley J. P., Rifkin D. B., Reich E. An enzymatic function associated with transformation of fibroblasts by oncogenic viruses. I. Chick embryo fibroblast cultures transformed by avian RNA tumor viruses. J Exp Med. 1973 Jan 1;137(1):85–111. doi: 10.1084/jem.137.1.85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vogt P. K. Envelope classification of avian RNA tumor viruses. Bibl Haematol. 1970;(36):153–167. doi: 10.1159/000391704. [DOI] [PubMed] [Google Scholar]
- Vogt P. K. Genetically stable reassortment of markers during mixed infection with avian tumor viruses. Virology. 1971 Dec;46(3):947–952. doi: 10.1016/0042-6822(71)90093-6. [DOI] [PubMed] [Google Scholar]
- Wyke J. A., Bell J. G., Beamand J. A. Genetic recombination among temperature-sensitive mutnats of Rous sarcoma virus. Cold Spring Harb Symp Quant Biol. 1975;39(Pt 2):897–905. doi: 10.1101/sqb.1974.039.01.104. [DOI] [PubMed] [Google Scholar]
- Wyke J. A. Complementation of transforming functions by temperature-sensitive mutants of avian sarcoma virus. Virology. 1973 Jul;54(1):28–36. doi: 10.1016/0042-6822(73)90111-6. [DOI] [PubMed] [Google Scholar]
- Wyke J. A., Linial M. Temperature-sensitive avian sarcoma viruses: a physiological comparison of twenty mutants. Virology. 1973 May;53(1):152–161. doi: 10.1016/0042-6822(73)90474-1. [DOI] [PubMed] [Google Scholar]
- Wyke J. A. The genetics of C-type RNA tumor viruses. Int Rev Cytol. 1974;38(0):67–109. doi: 10.1016/s0074-7696(08)60924-9. [DOI] [PubMed] [Google Scholar]
- Wyke J. A. The selective isolation of temperature-sensitive mutants of Rous sarcoma virus. Virology. 1973 Apr;52(2):587–590. doi: 10.1016/0042-6822(73)90357-7. [DOI] [PubMed] [Google Scholar]