ABSTRACT
It has been shown that the nucleoporin ALADIN plays a significant role in the redox homeostasis of the cell, but its function in steroidogenesis contributing to adrenal atrophy in triple A syndrome remains largely unknown. In an attempt to identify new interaction partners of ALADIN, co-immunoprecipitation followed by proteome analysis was conducted in different expression models using the human adrenocortical tumour cell line NCI-H295R. Our results suggest an interaction of ALADIN with the microsomal protein PGRMC2. PGRMC2 is shown to be activity regulator of CYP P450 enzymes and, therefore, to be a possible target for adrenal dysregulation in triple A syndrome. We show that there is a sexual dimorphism regarding the expression of Pgrmc2 in adrenals and gonads of wild-type (WT) and Aaas knock-out (KO) mice. Female Aaas KO mice are sterile due to delayed oocyte maturation and meiotic spindle assembly. A participation in meiotic spindle assembly confirms the recently investigated involvement of ALADIN in mitosis and emphasises an interaction with PGRMC2 which is a regulator of the cell cycle. By identification of a novel interaction partner of ALADIN, we provide novel aspects for future research of the function of ALADIN during cell cycle and for new insights into the pathogenesis of triple A syndrome.
KEY WORDS: ALADIN, Cell division, Nuclear pore complex, PGRMC2, Triple A syndrome
Summary: We report the interaction of the microsomal integral membrane protein progesterone receptor membrane component 2 with the nucleoporin ALADIN at the perinuclear ER and nuclear envelope.
INTRODUCTION
Triple A syndrome (MIM#231550) is an autosomal recessive disorder characterised by three distinct symptoms: ACTH-resistant adrenal insufficiency, oesophageal achalasia and absent tear production (alacrima) in combination with progressive neurological impairment (Allgrove et al., 1978). The disorder is caused by mutations in AAAS (achalasia-adrenal insufficiency alacrima syndrome) encoding the protein ALADIN (alacrima-achalasia-adrenal insufficiency neurologic disorder) (Handschug et al., 2001; Tullio-Pelet et al., 2000). ALADIN presents enhanced protein levels in neuroendocrine and gastrointestinal tissue; structures which are predominately affected in triple A patients (Handschug et al., 2001).
ALADIN is a scaffold nucleoporin (NUP) anchored within the nuclear pore complex (NPC) by the transmembrane NUP NDC1 [nuclear division cycle 1 homologue (S. cerevisiae)] (Kind et al., 2009; Yamazumi et al., 2009). It belongs to the group of barely exchangeable NUPs and therefore seems to be involved in building the structural scaffold backbone of the complex at the nuclear membrane (Rabut et al., 2004). Over the last few years it has been shown that NUPs have fundamental functions in cell biology, especially beyond nucleo-cytoplasmic transport (Fahrenkrog, 2014; Nofrini et al., 2016).
Our group has reported that ALADIN is involved in the oxidative stress response of fibroblasts and adrenocortical cells, but the role of ALADIN in adrenal steroidogenesis contributing to the adrenal phenotype in triple A patients is largely unknown (Jühlen et al., 2015; Kind et al., 2010; Koehler et al., 2013; Storr et al., 2009). Recently, we showed that a depletion of ALADIN in adrenocortical carcinoma cells leads to an alteration in glucocorticoid and androgenic steroidogenesis (Jühlen et al., 2015). Our results described in this article propose an interaction of ALADIN with the microsomal integral membrane protein progesterone receptor membrane component 2 (PGRMC2). PGRMC2 belongs to the group of membrane-associated progesterone receptors (MAPRs). These receptors, restricted to the endoplasmic reticulum (ER), are thought to act on mitosis while localising to the somatic spindle apparatus and to regulate the activity of some CYP P450 enzymes (e.g. CYP21A2) (Keator et al., 2012; Peluso et al., 2014; Wendler and Wehling, 2013).
By attempting to identify new interaction partners of ALADIN, we aimed to clarify the cellular functions of ALADIN at the NPC and to explain the mechanisms which contribute to the adrenal insufficiency in triple A syndrome. Our observations give the basis for further research on the association between ALADIN and PGRMC2, and about the function of ALADIN during cell cycle and steroidogenesis.
RESULTS
PGRMC2 precipitates with ALADIN in an exogenous and endogenous ALADIN adrenal cell expression model
Co-IP was conducted in NCI-H295R cells either expressing endogenous ALADIN or additionally exogenous GFP-ALADIN.
We performed mass spectrometry analyses of bound fractions of GFP(-ALADIN) and ALADIN co-IP. In the GFP-ALADIN expression model adequate peptides of ALADIN could be detected (Fig. 1). The analysis of ALADIN co-IP resulted in less detected peptides (one exclusive unique peptide), but had a 100% probability of correct protein identification. Despite several methodological optimisations using different protocols and antibodies, ALADIN co-IP had a low yield and analysis of bound fractions using mass spectrometry was more difficult to process. All proteins identified in mass spectrometry in GFP-ALADIN co-IP and ALADIN co-IP, but which were not found in the specific control pulldown assays, are presented in Tables S1 and S2.
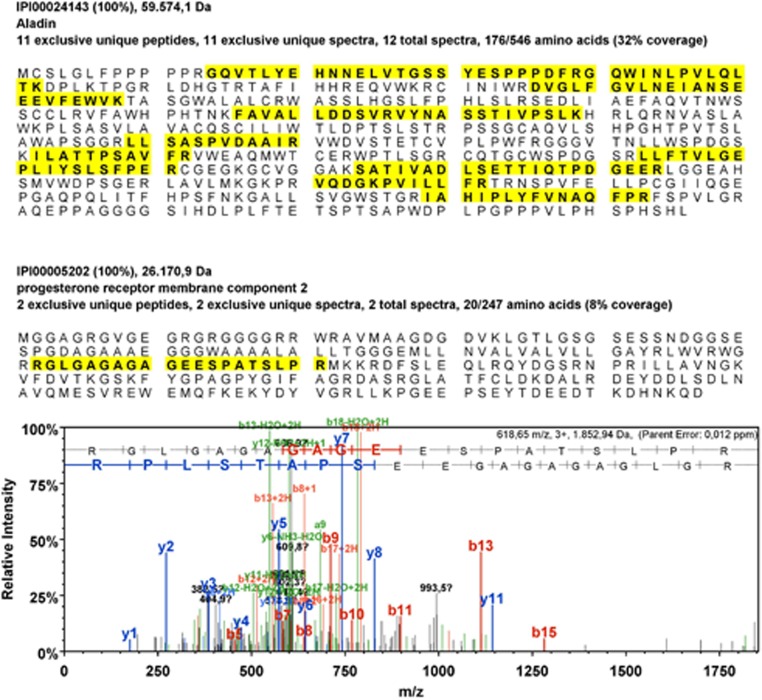
Fig. 1.
PGRMC2 was identified in mass spectrometry after GFP-(ALADIN) pulldown. Identified exclusive unique peptides (yellow) (number of different amino acid sequences, regardless of any modification, that are associated only with this protein) of ALADIN and progesterone receptor membrane component 2 (PGRMC2) are highlighted. Also for PGRMC2, relative intensities of annotated spectra are shown after GFP(-ALADIN) co-IP of whole cell lysates of GFP-ALADIN expressing NCI-H295R cells.
PGRMC2 was simultaneously identified by mass spectrometry analysis in co-IP of GFP-ALADIN using GFP-Trap_A agarose beads and in co-IP of ALADIN using anti-ALADIN coupled to Protein G UltraLink resin sepharose beads. Exclusive unique peptides of ALADIN and PGRMC2 detected in mass spectrometry after GFP-ALADIN are shown in Fig. 1A. Fewer peptides of PGRMC2 were detected after ALADIN pulldown (one exclusive unique peptide), with 100% protein identification probability.
We additionally confirmed the identification of PGRMC2 in GFP-ALADIN and ALADIN co-IP by western blot. Successful GFP-ALADIN pulldown in lysates of NCI-H295R cells stably expressing GFP-ALADIN (86 kDa) is presented in Fig. 2A. In accordance with our mass spectrometry results, PGRMC2 (24 kDa) could also be detected after GFP-ALADIN pulldown (Fig. 2A, 24 kDa, arrow). The negative control remained empty.
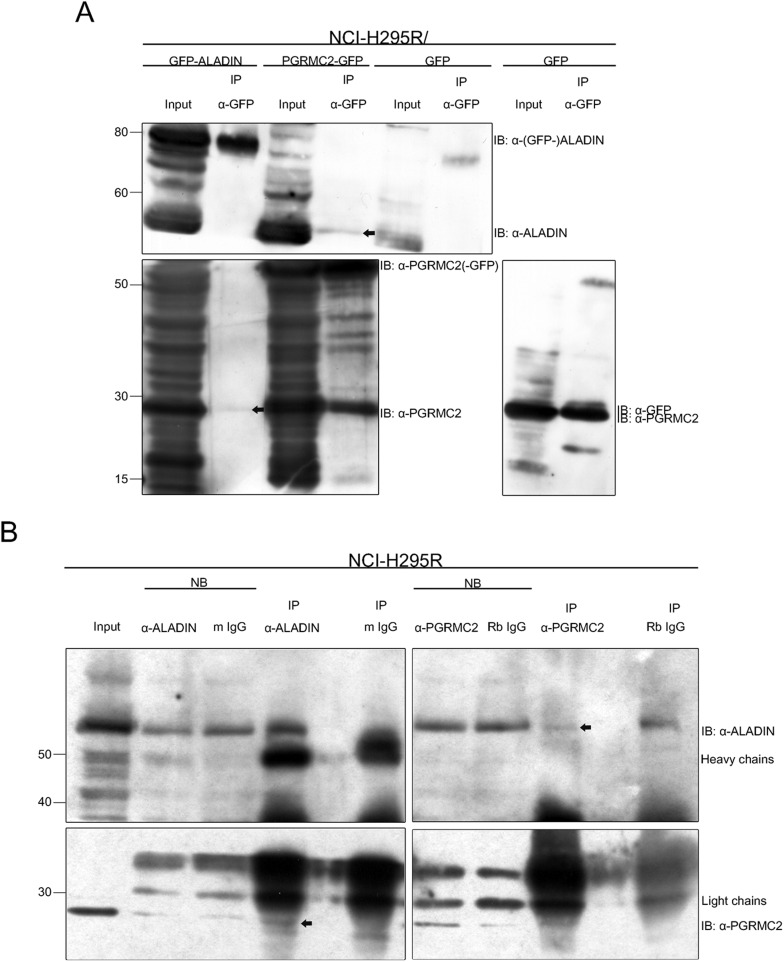
Fig. 2.
PGRMC2 interacts with ALADIN determined by IP-western and reciprocal IP-western assays. (A) Whole cell lysates of GFP-ALADIN, PGRMC2-GFP- and GFP- (as negative control) expressing NCI-H295R cells were used and GFP pulldown performed followed by western blot with indicated antibodies. PGRMC2 (24 kDa, arrow) could be detected after GFP-ALADIN (86 kDa) pulldown. ALADIN (59 kDa, arrow) and PGRMC2 could be both detected after PGRMC2-GFP (51 kDa) pulldown. GFP (27 kDa) was ascertained after GFP control pulldown but the control remained empty for PGRMC2 and ALADIN. (B) Whole cell lysates of NCI-H295R cells were used for ALADIN and PGRMC2 pulldown. Normal mouse (m IgG) and rabbit IgGs (Rb IgG) served as negative control. Western blot was performed with indicated antibodies. Non-bound (NB) IP fractions are also shown for each pulldown and bound IP eluates are separated by one lane each from the specific controls to eliminate false positive detection. PGRMC2 (arrow) precipitated in endogenous ALADIN pulldown. Control m IgG pulldown remained empty. ALADIN (arrow) reciprocally precipitated after PGRMC2 pulldown. Control Rb IgG pulldown remained empty showing a cross-reactive band a few kDa higher than ALADIN.
Successful endogenous ALADIN pulldown is shown in Fig. 2B. ALADIN (59 kDa) was found in the bound fraction of the ALADIN co-IP, but the negative control using normal mouse IgG was shown to be empty. In accordance with our result after GFP-ALADIN pulldown, PGRMC2 precipitated in endogenous ALADIN pulldown with no unspecific interaction in the negative control (Fig. 2B, arrow).
ALADIN precipitates with PGRMC2 in an exogenous and endogenous PGRMC2 adrenal cell expression model
In order to further evaluate a possible interaction between the nucleoporin ALADIN and microsomal PGRMC2 we conducted reciprocal co-IP assays.
Reciprocal pulldowns were done in NCI-H295R cells transiently expressing exogenous PGRMC2-GFP and endogenous PGRMC2.
Efficient pulldown of (PGRMC2-)GFP in lysates of NCI-H295R cells transiently expressing PGRMC2-GFP (51 kDa) is presented in Fig. 2A. Confirming our previous results identifying a possible interaction between ALADIN and PGRMC2, ALADIN could be detected after PGRMC2-GFP pulldown (Fig. 2A, 59 kDa, arrow). Additionally, PGRMC2 could be identified after PGRMC2-GFP pulldown (Fig. 2A). The negative control was empty for both.
PGRMC2 pulldown is shown in Fig. 2B. PGRMC2 was successfully detected in the bound fraction of the PGRMC2 co-IP, and the negative control using rabbit IgG remained empty. According to our results after PGRMC2-GFP pulldown, ALADIN slightly but visibly precipitated after PGRMC2 pulldown with no unspecific interaction in the negative control (Fig. 2B, arrow).
Localisation of ALADIN and PGRMC2 in adrenal cells using different expression models
Further evidence of possible co-localisation of ALADIN and PGRMC2 is given after immunofluorescent staining. Cells expressing GFP-ALADIN and PGRMC2-GFP were used to verify the specificity of anti-ALADIN and anti-PGRMC2 staining in NCI-H295R cells. Staining was done using anti-ALADIN, anti-PGRMC2 and anti-NPC proteins (mAb414).
Immunostaining with mAb414 in all adrenal cell expression models gave a thin circle around the nucleus indicating punctate localisations of NPCs (Fig. 3).
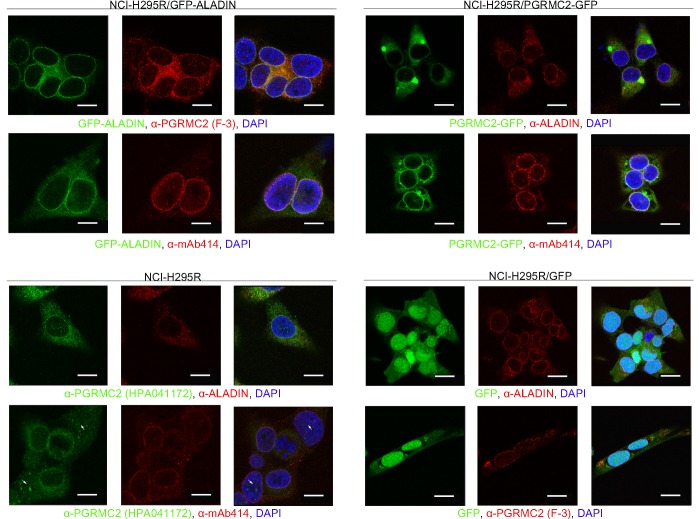
Fig. 3.
ALADIN and PGRMC2 localise to the perinuclear space in human adrenocortical carcinoma cells. NCI-H295R/GFP-ALADIN, NCI-H295R/PGRMC2-GFP, NCI-H295R and NCI-H295R/GFP cells were stained with anti-ALADIN (red), anti-PGRMC2 [red (F-3) and green (HPA041172)], anti-NPC proteins (mAb414) (red) and DAPI (blue). Scale bars: 11 µm (NCI-H295R/GFP-ALADIN, NCI-H295R), 12 µm (NCI-H295R/PGRMC2-GFP) and 16 µm (NCI-H295R/GFP).
Immunofluorescent staining of the nucleoporin ALADIN appeared at the nuclear envelope at the proximity of NPCs in all adrenal cell expression models. In the exogenous GFP-ALADIN cell model, the fusion protein was correctly targeted to the nuclear envelope and did not accumulate to a greater extent in the cytoplasm. We showed that ALADIN almost completely co-localises with anti-NPC proteins (mAb414) immunostaining at the nuclear envelope, substantially verifying the localisation of ALADIN at the nuclear pore (Fig. 3).
In immunofluorescent staining the microsomal protein PGRMC2 localised to the central ER, but also revealed a patchy and punctate staining pattern around the nucleus to the perinuclear space between nuclear envelope and ER in all adrenal cell expression models (Fig. 3). The same PGRMC2 immunostaining pattern was observed in human cervical carcinoma (HeLa) and human fibroblasts (Fig. S1A,B, respectively). In the exogenous adrenal cell model the PGRMC2-GFP fusion protein was still correctly targeted to the central ER and perinuclear space. In the staining with the anti-PGRMC2 in NCI-H295R cells we could also observe nuclear staining in some adrenal cells (Fig. 3, arrow). Nuclear localisation of PGRMC2 was absent in the PGRMC2-GFP adrenal cell expression model. Co-localisation between mAb414 and PGRMC2 or ALADIN and PGRMC2 was not complete but showed positivity in the perinuclear space and in the nuclear membrane in all cell expression models (Fig. 3).
Depletion of ALADIN affects localisation of PGRMC2 in human skin fibroblasts
In order to address whether depletion of ALADIN affects localisation of PGRMC2 at the perinuclear ER we immunostained PGRMC2 and ALADIN in the inducible adrenocortical ALADIN knock-down cell line (NCI-H295R1-TR/AAAS knock-down), and in the specific control cell lines (NCI-H295R1-TR/Scrambled shRNA and NCI-H295R1-TR), recently described by our group (Jühlen et al., 2015). To verify these results we also used skin fibroblasts of a triple A patient (homozygous mutation in Exon 9, c.884G>A, p.Trp295X) and human skin fibroblasts of an anonymised in-house control (Fig. 4).
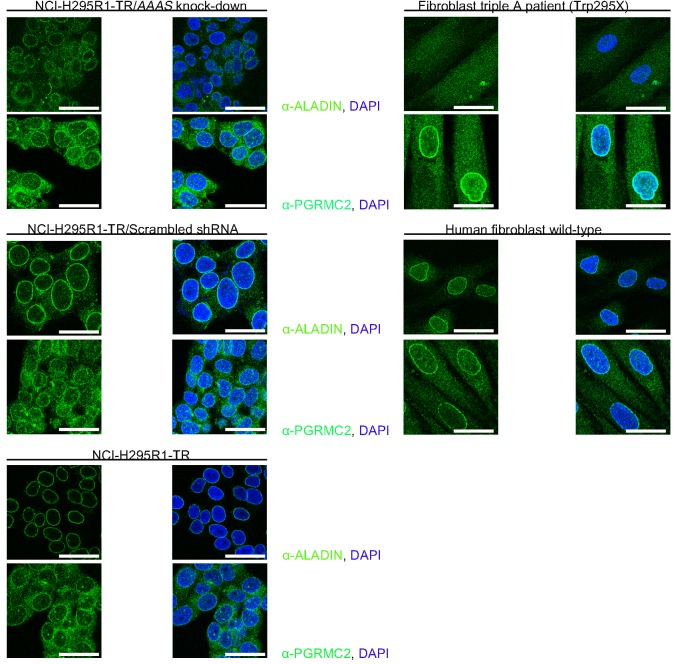
Fig. 4.
Depletion of ALADIN affects PGRMC2 localisation at the nuclear envelope/perinuclear ER in human skin fibroblasts. NCI-H295R1-TR/AAAS knock-down, NCI-H295R1-TR/Scrambled shRNA, NCI-H295R1-TR cells and skin fibroblasts of a triple A patient (homozygous mutation in Exon 9, c.884G>A, p.Trp295X) and of an anonymised control were stained with mouse anti-ALADIN (green), mouse anti-PGRMC2 (green) and DAPI (blue). Scale bars for anti-ALADIN staining (upper panels): 38 µm (NCI-H295R1-TR/AAAS knock-down), 24 µm (NCI-H295R1-TR/Scrambled shRNA), 32 µm (NCI-H295R1-TR cells) and 38 µm (skin fibroblasts). All scale bars for anti-PGRMC2 staining: 24 µm. NCI-H295R1-TR cells with AAAS knock-down or scrambled shRNA were induced as described previously (Jühlen et al., 2015).
In adrenocortical cells we could not detect an alteration of PGRMC2 localisation after ALADIN knock-down. However, immunostaining of PGRMC2 in NCI-H295R1-TR/Scrambled shRNA and NCI-H295R1-TR compared to NCI-H295R1-TR/AAAS knock-down was more cytoplasmic and nuclear (Fig. 4).
In human skin fibroblasts, ALADIN depletion in the triple A patient lead to an increased staining of PGRMC2 at the perinuclear ER compared to human control skin fibroblasts (Fig. 4).
The expression of PGRMC2 is not affected after AAAS knock-down in human adrenal cells
To test if PGRMC2 expression is affected when ALADIN is down-regulated we used the inducible NCI-H295R1-TR cells with AAAS knock-down shRNA or scrambled shRNA as negative control (Jühlen et al., 2015).
We could not find an alteration on PGRMC2 mRNA level after induction of ALADIN depletion by doxycycline in NCI-H295R1-TR cells in at least ten triplicate experiments (Fig. S2).
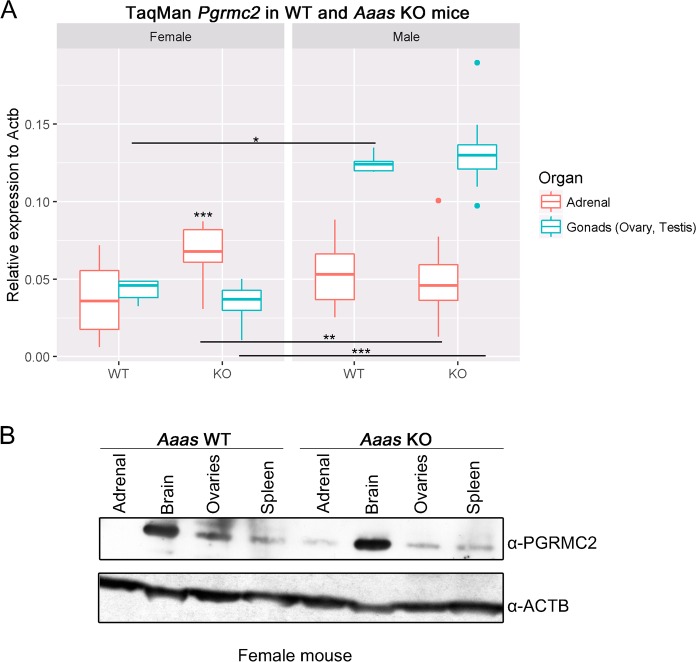
Pgrmc2 exhibits a sexual dimorphism in adrenals and gonads of WT and Aaas KO mice
In order to examine the expression of Pgrmc2 in WT and Aaas KO mice we looked at the adrenals and gonads using TaqMan analysis and western blot (Fig. 5A).
Fig. 5.
Pgrmc2 has a sexual dimorphic role in mice and ALADIN KO in female mice leads to an alteration in PGRMC2. (A) Total RNA was isolated from dissected adrenals and gonads of WT and Aaas KO mice. *P<0.05, **P<0.01, ***P<0.001. Significant differences were measured with unpaired Wilcoxon–Mann–Whitney U-test. Boxplot widths are proportional to the square root of the samples sizes. Whiskers indicate the range outside 1.5 times the inter-quartile range (IQR) above the upper quartile and below the lower quartile. Outliers were plotted as dots. (B) Total protein was isolated from dissected adrenals, brain, ovaries and spleen of female WT and Aaas KO mice followed by western blot with indicated antibodies.
Indeed, we found that Pgrmc2 displayed a sexual dimorphism in female and male WT and Aaas KO mice: the expression in testes was significantly higher independent of genotype compared to female ovaries, in female KO adrenals the expression was significantly higher compared to male KO adrenals (Fig. 5A).
Interestingly, in female adrenals the depletion of ALADIN leads to a significant increase in Pgrmc2 expression, whereas in female ovaries a decrease compared to WT ovaries was observed (Fig. 5A). The expression of Pgrmc2 was not altered in male Aaas KO adrenals or testes compared to male WT organs in at least four triplicate experiments (Fig. 5A).
To examine our findings in female WT and Aaas KO mice on PGRMC2 RNA level, we conducted western blot of several female murine WT and Aaas KO tissues, i.e. adrenals, brain, ovaries and spleen (Fig. 5B). We could confirm our results on PGRMC2 RNA level and show an increase in PGRMC2 protein in female adrenals of Aaas KO mice compared to female adrenals of WT mice (Fig. 5B). Furthermore, in ovaries of Aaas KO mice PGRMC2 protein was diminished compared to ovaries of WT mice.
DISCUSSION
The exact role of the nucleoporin ALADIN at the NPC and its involvement in steroidogenesis leading to the characteristic adrenal atrophy in triple A syndrome remains largely unknown. We and others have provided evidence of the involvement of ALADIN in the oxidative stress response of the cell (Jühlen et al., 2015; Kind et al., 2010; Koehler et al., 2013; Prasad et al., 2013; Storr et al., 2009). Recently, we have shown that a depletion of ALADIN in adrenocortical carcinoma cells leads to an alteration in glucocorticoid and androgenic steroidogenesis (Jühlen et al., 2015).
Despite the reported interaction between ALADIN and ferritin heavy chain 1, no other interaction partner which would lead to the identification of a plausible function and signal transduction of ALADIN in the cell is known so far (Storr et al., 2009).
In an attempt to identify new interaction partners of ALADIN, co-IP analyses showed that PGRMC2 precipitated with ALADIN. To verify the identified association between ALADIN and PGRMC2, reciprocal IP was conducted. Our results showed co-IP of ALADIN with PGRMC2. Different co-IP approaches using exogenous and endogenous expression systems in human adrenal cells have shown for the first time that the nucleoporin ALADIN associates in a complex with the microsomal protein PGRMC2.
PGRMC2 belongs to the MAPR family. MAPRs are proteins found at the membrane of the ER and are thought to be regulators of CYP P450 enzymes. The first identified MAPR, PGRMC1, gained wide-spread attention and is extensively investigated compared to its homologue PGRMC2 (Falkenstein et al., 1996). PGRMC1 is a cytochrome-related protein with several implications in cancer (Clark et al., 2016; Falkenstein et al., 1996; Kabe et al., 2016).
In this work, PGRMC2 was found to interact with the nucleoporin ALADIN. We could additionally show that PGRMC2 is detected after PGRMC2-GFP pulldown on western blot suggesting a homodimeric role of PGRMC2. It has been reported that PGRMC2 interacts with PGRMC1 as a heterodimer in an exogenous expression system in human ovarian carcinoma cells (Peluso et al., 2014), but homodimerisation of PGRMC2 has not been shown yet.
Visualising PGRMC2 in the cell using immunofluorescence and confocal microscopy has shown the presence of PGRMC2 at the central ER, and, interestingly, at the nuclear envelope and the perinuclear ER. We detected that PGRMC2 co-localises with ALADIN and with different FG-repeat NUPs [stained with anti-NPC proteins (mAb414)] to the nuclear envelope and the perinuclear ER.
Microsomal CYP P450 systems are haemoproteins and are localised to the membrane of the ER (Pandey and Flück, 2013). PGRMC2 is also restricted to the ER and is thought to be an electron donor for some CYP P450 enzymes, most likely through its cytochrome b5-similar haem-binding domain (Albrecht et al., 2012; Wendler and Wehling, 2013). Most recently, both PGRMC1 and PGRMC2 were identified as putative interacting partners of ferrochelatase, an enzyme catalysing the terminal step in the haem biosynthetic pathway, thereby possibly controlling haem release as chaperone or sensor (Piel et al., 2016). The mixed-function oxidase system of microsomal CYP P450 enzymes requires a donor transferring electrons from NADPH to reduce the prosthetic haem group (Pandey and Flück, 2013). Interaction of ALADIN with PGRMC2 at the perinuclear ER could influence CYP P450 enzyme activity through electron transfer from NADPH and/or control haem synthesis. In triple A syndrome, altered CYP P450 enzyme activity would consecutively contribute to adrenal atrophy.
In order to address if ALADIN anchors PGRMC2 at the perinuclear ER, we conducted immunostaining in adrenocortical cells after inducible knock-down of ALADIN and in skin fibroblasts of a triple A patient. The knock-down model has been used in our lab as an in vitro model for triple A syndrome (Jühlen et al., 2015). In immunostaining we could not detect an alteration of PGRMC2 protein intensity and localisation after ALADIN knock-down; however, PGRMC2 immunostaining was more nuclear and cytoplasmic in control cell lines compared to ALADIN knock-down cells. Additionally, we showed that there is no alteration in PGRMC2 expression after ALADIN knock-down in adrenocortical cells.
Localisation of PGRMC2 in triple A patient skin fibroblasts was not changed compared to human control skin fibroblasts in immunostaining analyses. Astonishingly, patient cells presented an increased staining of PGRMC2 at the perinuclear ER and nuclear envelope compared to control cells. Cytoplasmic and nuclear staining was not altered compared to control cells.
Taken together, our results in immunofluorescence microscopy using different ALADIN and PGRMC2 adrenal cell expression systems provide a basis for future research of how ALADIN and PGRMC2 possibly associate in a complex close to the nuclear envelope, and what the effects on steroidogenesis of this association would be. Additionally, ALADIN depletion affects PGRMC2 localisation at the perinuclear ER in triple A patient cells, suggesting a regulatory role of ALADIN for PGRMC2 protein localisation. Probably because ALADIN is not fully depleted in the adrenocortical knock-down model compared to patient fibroblasts we could not detect such effect in the in vitro model.
In summary, our work identifies microsomal PGRMC2 as novel interactor for ALADIN and provides new insights into the molecular function of the nucleoporin in the pathogenesis of triple A syndrome. We found that Pgrmc2 has a sexual dimorphic role in adrenals and gonads of WT and Aaas KO mice, and, of note, ALADIN depletion leads to an alteration in PGRMC2 RNA and protein level in adrenals and ovaries of female Aaas KO mice. Our group reported that female mice homozygous deficient for Aaas are infertile (Huebner et al., 2006). Carvalhal et al. recently presented that ALADIN is involved in mitotic and meiotic spindle assembly, chromosome segregation and production of fertile mouse oocytes (Carvalhal et al., 2015, 2016 preprint). Interestingly, both PGRMC1 and PGRMC2 seem to be involved in regulation of ovarian follicle development and therefore seem to have neuroendocrine functions (Wendler and Wehling, 2013). PGRMC1 and PGRMC2 locate to the mitotic spindle and are shown to exploit a specific role during metaphase of mitosis (Griffin et al., 2014; Peluso et al., 2014; Sueldo et al., 2015). Additionally, ALADIN and PGRMC2 have been identified to interact with the human centrosome-cilium interface (Gupta et al., 2015; Hanson et al., 2014; Yan et al., 2014). The centrosome is a fundamental organelle which participates in cell cycle progression and mitotic spindle assembly.
Future work needs to address how ALADIN associates with PGRMC2 at the perinuclear ER and influences PGRMC2 localisation. It needs to be explored which molecular mechanisms are altered by the interaction between ALADIN and PGRMC2, allowing yet another important task of ALADIN to be elucidated.
MATERIALS AND METHODS
Cell culture
NCI-H295R cells stably expressing GFP-ALADIN fusion protein or GFP were generated as described previously using the gamma-retroviral transfer vectors pcz-CFG5.1-GFP-AAAS and pcz-CFG5.1-GFP (Kind et al., 2009).
NCI-H295R cells transiently expressing PGRMC2-GFP fusion protein were generated as follows. Cells were transfected with pCMV6-AC-PGRMC2-GFP vector (RG204682) (OriGene Technologies, Rockville MD, USA) using X-tremeGENE HP DNA transfection reagent (Roche Diagnostics, Mannheim, Germany) following the manufacturer's protocols. Cells were harvested or fixed after 48 h.
In all exogenous expression models clones were selected by moderate expression of the desired fusion protein and true cellular localisation in order to exclude the possibility of false positive protein interactions.
NCI-H295R cells were cultured in DMEM/F12 medium (Lonza, Cologne, Germany) supplemented with 1 mM L-glutamine (Lonza, Cologne, Germany), 5% Nu-serum (BD Biosciences, Heidelberg, Germany), 1% insulin-transferrin-selenium (Gibco, Life Technologies, Darmstadt, Germany) and 1% antibiotic-antimycotic solution (PAA, GE Healthcare GmbH, Little Chalfont, UK).
NCI-H295R1-TR cells with AAAS knock-down or scrambled shRNA were generated, selected and cultured as described previously (Jühlen et al., 2015).
Triple A patient skin fibroblasts and human anonymised control skin fibroblasts were obtained and cultured as described earlier by our group (Kind et al., 2010). Informed consent was obtained from all subjects and experiments were approved by the local ethics review board (Medical Faculty, Technische Universitaät Dresden, EK820897). HeLa cells were cultured as described previously (Kind et al., 2009).
Animals
All procedures were approved by the Regional Board for Veterinarian Affairs, Dresden, Germany (AZ 24-9168.21-1-2002-1) in accordance with the institutional guidelines for the care and use of laboratory animals. C57BL/6J mice were obtained from Janvier Labs (Le Genest-Saint-Isle, France). Aaas KO mice were generated as described previously (Huebner et al., 2006).
RNA extraction, cDNA synthesis and quantitative real-time PCR using TaqMan
Total RNA from cultured cells (n=10) and from frozen murine organs (at least four animals per genotype and sex) was isolated using the NucleoSpin RNA (Macherey-Nagel, Düren, Germany) according to the protocol from the manufacturer. Purity of the RNA was assessed using Nanodrop Spectrophotometer (ND-1000) (NanoDrop Technologies, Wilmington DE, USA). 500 ng of total RNA was reverse transcribed using the GoScript Reverse Transcription System (Promega, Mannheim, Germany) following the protocols from the manufacturer. Primers for the amplification of the target sequence were designed using Primer Express 3.0 (Applied Biosystems, Life Technologies, Darmstadt, Germany) and compared to the human or murine genome database for unique binding using BLAST search (https://blast.ncbi.nlm.nih.gov/Blast.cgi). The primer sequences are listed in Table S3.
The qPCR amplifications were performed in triplicates using the GoTaq Probe qPCR Master Mix (Promega) according to the manufacturer's reaction parameter on an ABI 7300 Fast Real-Time PCR System (Applied Biosystems). In all results repeatability was assessed by standard deviation of triplicate Cts and reproducibility was verified by normalizing all real-time RT-PCR experiments by the Ct of each positive control per run.
Immunoblots
After SDS-PAGE separation onto 4-12% PAGE (150 V for 1.5 h) and electroblotting (30 V for 1.5 h) (Invitrogen, Life Technologies, Darmstadt, Germany) onto Amersham hybond-ECL nitrocellulose membrane (0.45 µm) (GE Healthcare GmbH, Little Chalfont, UK) non-specific binding of proteins to the membrane was blocked by incubation in PBS containing 3% BSA (Sigma-Aldrich, Munich, Germany) at room-temperature.
The membrane was then probed with primary antibodies either anti-ALADIN (mouse, B-11: sc-374073; 1:100 in 3% PBS/BSA) (Santa Cruz Biotechnology, Inc., Heidelberg, Germany), anti-PGRMC2 (rabbit, HPA041172; 1:200 in 5% PBS/milk powder) (Sigma-Aldrich, Munich, Germany) or anti-PGRMC2 (mouse, F-3: sc-374624; 1:100 in 3% PBS/BSA) (Santa Cruz Biotechnology, Inc.) overnight at 4°C. Secondary antibodies goat anti-mouse IgG conjugated to horseradish peroxidase (1:2000 in 3% PBS/BSA) (Invitrogen, Life Technologies, Darmstadt, Germany) or goat anti-rabbit IgG conjugated to horseradish peroxidase (1:3000 in 5% PBS/milk powder) (Cell Signalling Technology Europe B.V., Leiden, Netherlands) were incubated for 1 h at room-temperature.
Co-immunoprecipitation
For GFP co-IP lysates from NCI-H295R expressing GFP-ALADIN or PGRMC2-GFP were used. Lysates from cells expressing GFP were used as negative control. Cell lysates (500 µg protein) were added to the pre-equilibrated GFP-Trap_A agarose beads (ChromoTek GmbH, Planegg-Martinsried, Germany), gently re-suspended by flipping the tube and bound overnight at constant mixing at 4°C. After washing steps, the beads were gently re-suspended in 60 µl NUPAGE 2X LDS sample buffer and in order to dissociate the captured immunocomplexes from the beads, boiled at 95°C for 10 min and western blot analysis was conducted with 20 µl of the eluate. The leftover 40 µl of the eluate using the lysates of NCI-H295R expressing GFP-ALADIN or GFP was further processed for proteomic profiling using mass spectrometry. These experiments following mass spectrometry analysis were repeated three times.
For co-IP of ALADIN or PGRMC2 lysates from NCI-H295R cells and Protein G UltraLink resin sepharose beads (Pierce, Thermo Scientific, Fischer Scientific, Schwerte, Germany) were used. Beads were gently re-suspended in anti-ALADIN (2 µg/ml) or anti-PGRMC2 (HPA041172) (2 µg/ml) and as negative controls normal mouse or rabbit IgG (2 µg/ml) (Invitrogen, Life Technologies, Darmstadt, Germany). All antibodies were bound to the beads over-night at 4°C in a rotation chamber. After washing cell lysates (500 µg protein) were added to the beads, gently re-suspended by flipping the tube and bound overnight as described before. After washing the beads were gently re-suspended in 60 µl sample buffer containing dilution buffer, NUPAGE 1X LDS Sample Buffer and 1× reducing agent. The captured immunocomplexes were dissociated and the eluates were collected and processed by western blot as described previously. These experiments were repeated three times. The left 40 µl of the eluate after ALADIN co-IP and negative control was further processed for proteomic profiling using mass spectrometry. Mass spectrometry analysis was conducted once.
Proteomic profiling using tandem mass spectrometry
Entire gel lanes were cut into 40 slabs, each of which was in-gel digested with trypsin (Shevchenko et al., 2006). Gel analyses were performed at the Mass Spectrometry Facility at the Max Planck Institute for Molecular Cell Biology and Genetics (Dresden) on a nano high-performance liquid chromatograph (UltiMate) interfaced on-line to a LTQ Orbitrap Velos hybrid tandem mass spectrometer as described previously (Vasilj et al., 2012).
A database search was performed against the IPI human database (Kersey et al., 2004) (downloaded in July 2010) and NCBI protein collection without species restriction (https://www.ncbi.nlm.nih.gov/protein, updated in June 2014) using MASCOT software v.2.2. Scaffold software v.4.3.2 was used to validate MS/MS-based protein identifications. Protein probabilities were assigned by the Protein Prophet algorithm (Nesvizhskii et al., 2003).
Immunofluorescence microscopy
Cells grown onto glass cover slips were fixed for 5 min with 4% PFA (SAV LP, Flinsbach, Germany) in PBS, permeabilised for 5 min with 0.5% Triton-X-100 in PBS and fixed again for 5 min. Blocking was performed for 30 min with 2% BSA/0.1% Triton-X-100 in PBS at room temperature.
All antibodies used for immunofluorescence were diluted in blocking solution. Primary antibodies anti-ALADIN (1:25), or anti-PGRMC2 (HPA041172; 1:50) or anti-PGRMC2 (F-3: sc-374624; 1:25) and anti-NPC proteins (mAb414; 1:800; Covance, Berkley CA, USA) were incubated at 4°C overnight in a humidified chamber. Secondary antibodies goat anti-mouse IgG Cy3 (1:800; Amersham Biosciences, Freiburg, Germany), Alexa Fluor 488 and 555 goat anti-rabbit IgG (1:500; Molecular Probes, Life Technologies) were incubated one hour at room temperature in the dark.
Fluorescence was visualised using the confocal laser microscope TCS SP2 (Leica Microsystems, Mannheim, Germany). The experiments were repeated at least three times.
Statistics of TaqMan analyses
Statistical analyses were made using the open-source software R version 3.3.0 and R Studio version 0.99.902 (R Core Team, 2015). Unpaired Wilcoxon–Mann–Whitney U-test was performed. During evaluation of the results a confidence interval alpha of 95% and P-values lower than 0.05 were considered as statistically significant. Results are shown as box plots which give a fast and efficient overview of median, first and third quartile (25th and 75th percentile, respectively), interquartile range (IQR), minimal and maximal values and outliers.
Acknowledgements
We thank Waldemar Kanczkowski (Technische Universität Dresden, Dresden, Germany) for providing the NCI-H295R cells. Barbara Kind (Technische Universität Dresden, Dresden, Germany) generously generated pseudo retroviruses containing pcz-CFG5.1-GFP-ALADIN and pcz-CFG5.1-GFP. We thank the Mass Spectrometry Facility at the Max Planck Institute for Molecular Cell Biology and Genetics Dresden for MS-based peptide analyses. Some of the work of this article has been previously published in a PhD thesis (Ramona Jühlen, 2015, Role of ALADIN for Oxidative Stress Response and Microsomal Steroidogenesis in Human Adrenocortical Cells, Technische Universität Dresden, Dresden, Germany).
Footnotes
Competing interests
The authors declare no competing or financial interests.
Author contributions
R.J., A.H. and K.K. conceived and designed the experiments. R.J. performed all experiments. D.L. assisted with immunofluorescence staining and K.K. with confocal microscopy. R.J. analysed the data and wrote the paper. A.H. and K.K. helped improving the manuscript. All authors read the final version of the manuscript and gave their permission for publication.
Funding
This work was supported by Deutsche Forschungsgemeinschaft (grant HU 895/5-2) (Clinical Research Unit 252) to A.H. The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
Supplementary information
Supplementary information available online at http://bio.biologists.org/lookup/doi/10.1242/bio.021162.supplemental
References
- Albrecht C., Huck V., Wehling M. and Wendler A. (2012). In vitro inhibition of SKOV-3 cell migration as a distinctive feature of progesterone receptor membrane component type 2 versus type 1. Steroids 77, 1543-1550. 10.1016/j.steroids.2012.09.006 [DOI] [PubMed] [Google Scholar]
- Allgrove J., Clayden G. S., Grant D. B. and Macaulay J. C. (1978). Familial glucocorticoid deficiency with achalasia of the cardia and deficient tear production. Lancet 311, 1284-1286. 10.1016/S0140-6736(78)91268-0 [DOI] [PubMed] [Google Scholar]
- Carvalhal S., Ribeiro S. A., Arocena M., Kasciukovic T., Temme A., Koehler K., Huebner A. and Griffis E. R. (2015). The nucleoporin ALADIN regulates Aurora A localization to ensure robust mitotic spindle formation. Mol. Biol. Cell 26, 3424-3438. 10.1091/mbc.E15-02-0113 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carvalhal S., Stevense M., Koehler K., Naumann R., Huebner A., Jessberger R. and Griffis E. (2016). ALADIN is required for the production of fertile mouse oocytes. bioRxiv 43307 10.1101/043307 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clark N. C., Friel A. M., Pru C. A., Zhang L., Shioda T., Rueda B. R., Peluso J. J. and Pru J. K. (2016). Progesterone receptor membrane component 1 promotes survival of human breast cancer cells and the growth of xenograft tumors. Cancer Biol. Ther. 17, 262-271. 10.1080/15384047.2016.1139240 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fahrenkrog B. (2014). Nucleoporin Gene Fusions and Hematopoietic Malignancies. New J. Sci. 2014, 468306 10.1155/2014/468306 [DOI] [Google Scholar]
- Falkenstein E., Meyer C., Eisen C., Scriba P. C. and Wehling M. (1996). Full-length cDNA sequence of a progesterone membrane-binding protein from porcine vascular smooth muscle cells. Biochem. Biophys. Res. Commun. 229, 86-89. 10.1006/bbrc.1996.1761 [DOI] [PubMed] [Google Scholar]
- Griffin D., Liu X., Pru C., Pru J. K. and Peluso J. J. (2014). Expression of progesterone receptor membrane component-2 within the immature rat ovary and its role in regulating mitosis and apoptosis of spontaneously immortalized granulosa cells. Biol. Reprod. 91, 36 10.1095/biolreprod.114.117481 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gupta G. D., Coyaud É., Gonçalves J., Mojarad B. A., Liu Y., Wu Q., Gheiratmand L., Comartin D., Tkach J. M., Cheung S. W. T. et al. (2015). A dynamic protein interaction landscape of the human centrosome-cilium interface. Cell 163, 1484-1499. 10.1016/j.cell.2015.10.065 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Handschug K., Sperling S., Yoon S.-J. K., Hennig S., Clark A. J. L. and Huebner A. (2001). Triple A syndrome is caused by mutations in AAAS, a new WD-repeat protein gene. Hum. Mol. Genet. 10, 283-290. 10.1093/hmg/10.3.283 [DOI] [PubMed] [Google Scholar]
- Hanson D., Stevens A., Murray P. G., Black G. C. M. and Clayton P. E. (2014). Identifying biological pathways that underlie primordial short stature using network analysis. J. Mol. Endocrinol. 52, 333-344. 10.1530/JME-14-0029 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huebner A., Mann P., Rohde E., Kaindl A. M., Witt M., Verkade P., Jakubiczka S., Menschikowski M., Stoltenburg-Didinger G. and Koehler K. (2006). Mice lacking the nuclear pore complex protein ALADIN show female infertility but fail to develop a phenotype resembling human triple A syndrome. Mol. Cell. Biol. 26, 1879-1887. 10.1128/MCB.26.5.1879-1887.2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jühlen R., Idkowiak J., Taylor A. E., Kind B., Arlt W., Huebner A. and Koehler K. (2015). Role of ALADIN in human adrenocortical cells for oxidative stress response and steroidogenesis. PLoS ONE 10, e0124582 10.1371/journal.pone.0124582 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kabe Y., Nakane T., Koike I., Yamamoto T., Sugiura Y., Harada E., Sugase K., Shimamura T., Ohmura M., Muraoka K. et al. (2016). Haem-dependent dimerization of PGRMC1/Sigma-2 receptor facilitates cancer proliferation and chemoresistance. Nat. Commun. 7, 11030 10.1038/ncomms11030 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Keator C. S., Mah K. and Slayden O. D. (2012). Alterations in progesterone receptor membrane component 2 (PGRMC2) in the endometrium of macaques afflicted with advanced endometriosis. Mol. Hum. Reprod. 18, 308-319. 10.1093/molehr/gas006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kersey P. J., Duarte J., Williams A., Karavidopoulou Y., Birney E., Apweiler R. (2004). The International Protein Index: An integrated database for proteomics experiments. Proteomics 4, 1985-1988. 10.1002/pmic.200300721 [DOI] [PubMed] [Google Scholar]
- Kind B., Koehler K., Lorenz M. and Huebner A. (2009). The nuclear pore complex protein ALADIN is anchored via NDC1 but not via POM121 and GP210 in the nuclear envelope. Biochem. Biophys. Res. Commun. 390, 205-210. 10.1016/j.bbrc.2009.09.080 [DOI] [PubMed] [Google Scholar]
- Kind B., Koehler K., Krumbholz M., Landgraf D. and Huebner A. (2010). Intracellular ROS level is increased in fibroblasts of triple A syndrome patients. J. Mol. Med. 88, 1233-1242. 10.1007/s00109-010-0661-y [DOI] [PubMed] [Google Scholar]
- Koehler K., End K., Kind B., Landgraf D., Mitzscherling P. and Huebner A. (2013). Changes in differential gene expression in fibroblast cells from patients with triple A syndrome under oxidative stress. Horm. Metab. Res. 45, 102-108. 10.1055/s-0032-1331196 [DOI] [PubMed] [Google Scholar]
- Nesvizhskii A. I., Keller A., Kolker E. and Aebersold R. (2003). A statistical model for identifying proteins by tandem mass spectrometry. Anal. Chem. 75, 4646-4658. 10.1021/ac0341261 [DOI] [PubMed] [Google Scholar]
- Nofrini V., Di Giacomo D. and Mecucci C. (2016). Nucleoporin genes in human diseases. Eur. J. Hum. Genet. 24, 1388-1395. 10.1038/ejhg.2016.25 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pandey A. V. and Flück C. E. (2013). NADPH P450 oxidoreductase: structure, function, and pathology of diseases. Pharmacol. Ther. 138, 229-254. 10.1016/j.pharmthera.2013.01.010 [DOI] [PubMed] [Google Scholar]
- Peluso J. J., Griffin D., Liu X. and Horne M. (2014). Progesterone receptor membrane component-1 (PGRMC1) and PGRMC-2 interact to suppress entry into the cell cycle in spontaneously immortalized rat granulosa cells. Biol. Reprod. 91, 104 10.1095/biolreprod.114.122986 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Piel R. B., Shiferaw M. T., Vashisht A. A., Marcero J. R., Praissman J. L., Phillips J. D., Wohlschlegel J. A. and Medlock A. E. (2016). A Novel Role for Progesterone Receptor Membrane Component 1 (PGRMC1): a partner and regulator of ferrochelatase. Biochemistry 55, 5204-5217. 10.1021/acs.biochem.6b00756 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prasad R., Metherell L. A., Clark A. J. and Storr H. L. (2013). Deficiency of ALADIN impairs redox homeostasis in human adrenal cells and inhibits steroidogenesis. Endocrinology 154, 3209-3218. 10.1210/en.2013-1241 [DOI] [PMC free article] [PubMed] [Google Scholar]
- R Core Team (2015). R: A Language and Environment for Statistical Computing. Vienna, Austria: R Foundation for Statistical Computing. [Google Scholar]
- Rabut G., Doye V. and Ellenberg J. (2004). Mapping the dynamic organization of the nuclear pore complex inside single living cells. Nat. Cell Biol. 6, 1114-1121. 10.1038/ncb1184 [DOI] [PubMed] [Google Scholar]
- Shevchenko A., Tomas H., Havlis J., Olsen J. V. and Mann M. (2006). In-gel digestion for mass spectrometric characterization of proteins and proteomes. Nat. Protoc. 1, 2856-2860. 10.1038/nprot.2006.468 [DOI] [PubMed] [Google Scholar]
- Storr H. L., Kind B., Parfitt D. A., Chapple J. P., Lorenz M., Koehler K., Huebner A. and Clark A. J. L. (2009). Deficiency of ferritin heavy-chain nuclear import in triple a syndrome implies nuclear oxidative damage as the primary disease mechanism. Mol. Endocrinol. 23, 2086-2094. 10.1210/me.2009-0056 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sueldo C., Liu X. and Peluso J. J. (2015). Progestin and AdipoQ Receptor 7, Progesterone Membrane Receptor Component 1 (PGRMC1), and PGRMC2 and their role in regulating progesterone's ability to suppress human granulosa/luteal cells from entering into the cell cycle. Biol. Reprod. 93, 63 10.1095/biolreprod.115.131508 [DOI] [PubMed] [Google Scholar]
- Tullio-Pelet A., Salomon R., Hadj-Rabia S., Mugnier C., de Laet M.-H., Chaouachi B., Bakiri F., Brottier P., Cattolico L., Penet C. et al. (2000). Mutant WD-repeat protein in triple-A syndrome. Nat. Genet. 26, 332-335. 10.1038/81642 [DOI] [PubMed] [Google Scholar]
- Vasilj A., Gentzel M., Ueberham E., Gebhardt R. and Shevchenko A. (2012). Tissue proteomics by one-dimensional gel electrophoresis combined with label-free protein quantification. J. Proteome Res. 11, 3680-3689. 10.1021/pr300147z [DOI] [PubMed] [Google Scholar]
- Wendler A. and Wehling M. (2013). PGRMC2, a yet uncharacterized protein with potential as tumor suppressor, migration inhibitor, and regulator of cytochrome P450 enzyme activity. Steroids 78, 555-558. 10.1016/j.steroids.2012.12.002 [DOI] [PubMed] [Google Scholar]
- Yamazumi Y., Kamiya A., Nishida A., Nishihara A., Iemura S.-I., Natsume T. and Akiyama T. (2009). The transmembrane nucleoporin NDC1 is required for targeting of ALADIN to nuclear pore complexes. Biochem. Biophys. Res. Commun. 389, 100-104. 10.1016/j.bbrc.2009.08.096 [DOI] [PubMed] [Google Scholar]
- Yan J., Yan F., Li Z., Sinnott B., Cappell K. M., Yu Y., Mo J., Duncan J. A., Chen X., Cormier-Daire V. et al. (2014). The 3M complex maintains microtubule and genome integrity. Mol. Cell 54, 791-804. 10.1016/j.molcel.2014.03.047 [DOI] [PMC free article] [PubMed] [Google Scholar]