Pharmacotherapy has seen remarkable advances in recent decades, but surgery remains a vital tool for treating a wide variety of diseases, often offering the only curative or definitive therapy. However, the decision to have surgery is often not straightforward, as potential long-term benefit comes with upfront risk of morbidity and mortality. Minimizing that upfront risk and ensuring that patients understand the trade-offs are critical components of high-quality preoperative care.
The preoperative process has changed drastically over the past half century. Patients used to be routinely admitted to the hospital at least the day prior to surgery, but the high cost of inpatient care has pushed the preoperative evaluation into the outpatient arena. The outpatient evaluation often includes an array of diagnostic tests and may involve office visits with non-surgeons including primary care providers, medical specialists, and anaesthesiologists, sometimes in dedicated preoperative clinics. Various terms are used to describe the reason for these preoperative medical evaluations, including risk assessment,1 risk stratification,2 medical clearance,3 and optimization.4 But it is not only the terminology that varies, the practice of preoperative medical evaluations varies widely between providers and hospitals.5 6 This imprecision in terminology and variation in practice may reflect conceptual confusion regarding surgical risk, which may contribute to misuse or overuse of preoperative services. In this article we explore the concept of surgical risk and assert that the rationale for performing preoperative medical evaluations follows naturally from an understanding of surgical risk.
Surgical risk
For the purpose of this article we use surgical risk to refer to a probability of morbidity and mortality during and after surgery. Some surgical risk may not be confined to the immediate perioperative period (e.g., adhesions from an abdominal surgery increase the risk of subsequent small bowel obstruction, which can occur years later7), but for simplicity we largely focus on risk in the perioperative period.
When considering the risk associated with surgery, one should also consider the risk associated with not having surgery, which is driven by the underlying disease process.8 For example, in the case of a lacerated spleen, the risk of mortality with surgery may be lower than the risk of mortality from internal haemorrhage without surgery. However, for many patients considering elective surgery, such as the patient considering a knee replacement for osteoarthritis, the short-term risk without surgery may be negligible.
Components of surgical risk
The total risk associated with an operation can be conceptualized as consisting of two mutually exclusive components: intrinsic risk and modifiable risk (Fig. 1). The total risk is simply the risk that can be expected for a given patient undergoing a given operation in actual practice, and can be quantified and predicted based on historical observations of previous patients in similar situations.9 This is precisely what is done with the American College of Surgeons National Surgical Quality Improvement Program (NSQIP) Surgical Risk Calculator.10 This free online calculator allows prediction of mortality and seven other postoperative complications—such as cardiac event, surgical site infection, and venous thromboembolism—based on 21 preoperative factors.
Fig 1.
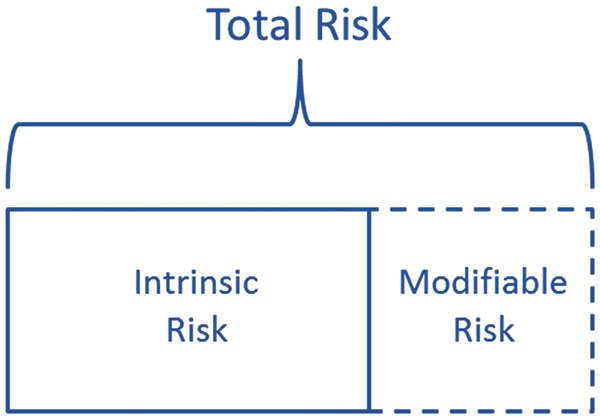
Conceptualization of surgical risk.
Some portion of the total risk—the ‘modifiable’ risk—may be reduced or eliminated. For example, smoking is associated with an increased risk of surgical complications, and smoking cessation interventions have been shown to reduce the risk of complications.11 However, reducing modifiable risk should not be thought of as exclusively confined to preoperative care. For example, one of the main factors responsible for variation in surgical mortality rates among hospitals is variation in ‘rescue’—the ability to deal effectively with postoperative complications—rather than variation in complication rates.12 Successfully managing postoperative complications is clearly not in the realm of preoperative care.
The remaining component of risk is what we call the ‘intrinsic’ risk. Some amount of risk will remain even if all modifiable risk is eliminated. After all, even healthy patients with no risk factors experience adverse events from surgery. We resist calling this component of risk ‘unmodifiable’ or ‘unpreventable’. After all, future interventions may be developed that reduce risk further than is currently possible—in that case, not applying those interventions would represent a modifiable risk.
While total risk can be measured from observation, the contribution of each component cannot be directly measured. In fact, modifiable risk is largely theoretical, and its existence is only indirectly ascertained by evidence that surgical risk can be reduced through some intervention. Conversely, the lack of ability to reduce risk associated with some risk factor suggests that the risk may be intrinsic for that operation. For example, obstructive coronary artery disease is associated with an increased risk of surgical complications, but revascularization has not been shown to reduce that risk.13
Others have similarly categorized the components of other types of risk as we have,14 although surgical risk could be divided in other ways, such as patient-specific and surgery-specific, or intraoperative and postoperative. However, categorizing risk as we have highlights a sound rationale for performing preoperative medical evaluations: to reduce modifiable risk and to estimate total risk to assist with informed decision making.
Rationale for preoperative medical evaluations
Reducing modifiable risk
One primary reason for performing preoperative medical evaluations is to make surgery safer—to reduce modifiable risk. If a diagnostic test is capable of identifying modifiable risk or if a therapeutic intervention is capable of reducing modifiable risk, those services may be appropriate. If those services are beyond the expertise of the surgeon, referral to a non-surgeon to ensure that eligible patients receive them may be appropriate.
For example, consider perioperative beta-blockers. Beginning in the late 1990s, a number of small trials showed that beta-blockers reduced the perioperative risk of cardiac events for patients at high risk. As a result, assessing eligibility for perioperative beta-blockers was a sound rationale for surgeons to refer patients for preoperative medical consults. Indeed, patients who received preoperative medical consults were more likely to be started on beta-blockers preoperatively.15 However, later trials showed that despite the reduction in cardiac events, perioperative beta-blockers also increased the risk of stroke and mortality, and clinical guidelines have been updated to reflect the limited role for perioperative beta-blockers.16 Now, consideration of perioperative beta-blockers is a much less compelling reason for referral.
Unfortunately, most potential preoperative interventions have not had rigorous evaluation. Routine testing with electrocardiograms, chest radiographs, and blood tests have not been shown to reduce surgical risk.17 Routine preoperative medical consults have uncertain benefit and may even be harmful.15 Even a straightforward intervention like treating moderate hypertension has uncertain benefit.18 Ultimately, surgeons looking for evidence-based ways to reduce surgical risk will be disappointed with the current options.
Estimating total risk
In addition to reducing modifiable risk, estimating the total risk and communicating that to patients may be a valuable outcome of the preoperative evaluation. Deciding to have surgery requires a complex consideration of risks, short- and long-term benefits, and potentially a myriad of alternatives; even if risk is not modifiable, knowing the risk will affect the decision of whether to have the surgery.19
The importance of risk estimation may not be the same in all cases. Sometimes, precise risk quantification may not be necessary. The potential for benefit from surgery might be so large that it outweighs the risk over any plausible range (e.g., potentially curative surgery for early stage pancreatic cancer). In these situations, additional tests and office visits prior to surgery for the purpose of more precisely estimating surgical risk would not change the ultimate decision to have surgery, and could delay surgery and even diminish the potential benefit of surgery. In other cases, precise risk quantification is extremely important, such as when viable alternatives exist (e.g., surgery vs radiation for early stage prostate cancer) or the potential for benefit is relatively small (e.g., cosmetic plastic surgery).
Common terminology
Given the imprecision in terminology surrounding the preoperative medical evaluation, what are we to make of the terms risk assessment, risk stratification, medical clearance, and optimization? The notion of ‘risk assessment’ largely overlaps with estimating total risk. However, it is unclear which tests, if any, are necessary solely for the purpose of risk estimation. Furthermore, given the sophistication of modern risk estimating tools such as the NSQIP calculator, it is uncertain whether there is any role for preoperative medical consults solely for the purpose of risk estimation. After all, surgeons have written much of the literature on surgical risk and they are best positioned to estimate surgery-specific risks, such as the risk of adhesions from abdominal surgery. Finally—and most importantly—in order for patients to be fully engaged in making informed health decisions, they should ideally consider the potential risk of surgery in the context of the potential benefit. As surgeons are clearly best suited to discuss the potential benefit of surgery, they should also communicate at least a first-order risk estimation to the patient.
The term ‘risk stratification’ connotes placing patients into risk categories, which could be considered a less precise form of a risk estimation. The concept of stratification is codified by clinical guidelines that include management algorithms based on assigned risk categories.16 In some ways this is less than ideal for the purposes of informed decision making, as qualitative risk estimates (i.e., low, intermediate, and high) are less precise than accurate quantitative estimates.
Risk stratification is also sometimes used to imply the refinement of risk estimation. In other words, after an initial estimation of surgical risk, it may be possible to further stratify risk. For example, non-invasive cardiac testing is sometimes used in patients in an intermediate risk category,20 often with the intention of pursing risk reduction strategies for those with positive test results. Even in the absence of potential risk reduction strategies, a precise risk estimate may be sufficiently important for some patients considering elective surgery that additional testing simply to refine the estimation of risk may be appropriate.
Despite recommendations by commentators to abandon use of the term ‘medical clearance’,3 it is still widely used to describe the preoperative medical evaluation. Discomfort with the term arises from ambiguities in the meaning of ‘cleared’. If it is meant to convey that the surgery is safe or free from risk, then its use is misleading, because patients undergoing surgery will always face some risk. However, a consultant could use the term to convey that there are no additional risk reduction strategies to implement before surgery, which surgeons and anaesthesiologists could find helpful. Additionally, simply saying that the patient is cleared does not communicate any sort of risk estimate.
Finally, the term ‘optimization’ has intuitive appeal, because it seems obvious that optimizing a patient’s medical status before an operation would be beneficial. However, this term seems to cloud the fact that there is very little evidence regarding which preoperative interventions actually improve surgical outcomes. For example, it may be optimizing a patient to get their moderate hypertension under better control, but if it does not reduce their surgical risk or improve the outcome, then treating the hypertension has simply delayed surgery. Similar to cleared, optimized could also simply convey that there are no additional risk reduction strategies to implement before surgery, but this term also communicates nothing about the risk estimate.
In conclusion, the practice of preoperative medical evaluation varies widely, as does the terminology to describe it. Terms like risk assessment, risk stratification, medical clearance, and optimization may have some utility in communicating about the preoperative medical evaluation, but they should be used carefully. A clear conceptualization of surgical risk can assist clinicians in efficiently using preoperative services to minimize modifiable risk and to estimate risk for the purpose of assisting patients in informed decision making, as well as assisting in the design of future research to improve the ability to achieve these goals.
Declaration of interest
None declared.
Funding
K.R.R. was funded by NIH grant T32 HL007180. K.B.S. was funded by NIH grant K24 AG049036.
References
- 1.Bader AM. Advances in preoperative risk assessment and management. Curr Probl Surg 2012; 49: 11–40 [DOI] [PubMed] [Google Scholar]
- 2.Moonesinghe SR, Mythen MG, Das P, Rowan KM, Grocott MP. Risk stratification tools for predicting morbidity and mortality in adult patients undergoing major surgery: qualitative systematic review. Anesthesiology 2013; 119: 959–81 [DOI] [PubMed] [Google Scholar]
- 3.Choi JJ. An anesthesiologist’s philosophy on ‘medical clearance’ for surgical patients. Arch Intern Med 1987; 147: 2090–2 [PubMed] [Google Scholar]
- 4.Olson RP, Dhakal IB. Day of surgery cancellation rate after preoperative telephone nurse screening or comprehensive optimization visit. Perioper Med (Lond) 2015; 4: 12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Thilen SR, Bryson CL, Reid RJ, Wijeysundera DN, Weaver EM, Treggiari MM. Patterns of preoperative consultation and surgical specialty in an integrated healthcare system. Anesthesiology 2013; 118: 1028–37 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Kirkham KR, Wijeysundera DN, Pendrith C, et al. Preoperative testing before low-risk surgical procedures. CMAJ 2015; 187: E349–58 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Attard JA, MacLean AR. Adhesive small bowel obstruction: epidemiology, biology and prevention. Can J Surg 2007; 50: 291–300 [PMC free article] [PubMed] [Google Scholar]
- 8.Bogardus ST, Jr, Holmboe E, Jekel JF. Perils, pitfalls, and possibilities in talking about medical risk. JAMA 1999; 281: 1037–41 [DOI] [PubMed] [Google Scholar]
- 9.Wilson R, Crouch EA. Risk assessment and comparisons: an introduction. Science 1987; 236: 267–70 [DOI] [PubMed] [Google Scholar]
- 10.Bilimoria KY, Liu Y, Paruch JL, et al. Development and evaluation of the universal ACS NSQIP surgical risk calculator: a decision aid and informed consent tool for patients and surgeons. J Am Coll Surg 2013; 217: 833–42 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Moller AM, Villebro N, Pedersen T, Tonnesen H. Effect of preoperative smoking intervention on postoperative complications: a randomised clinical trial. Lancet 2002; 359: 114–7 [DOI] [PubMed] [Google Scholar]
- 12.Ghaferi AA, Birkmeyer JD, Dimick JB. Complications, failure to rescue, and mortality with major inpatient surgery in Medicare patients. Ann Surg 2009; 250: 1029–34 [DOI] [PubMed] [Google Scholar]
- 13.McFalls EO, Ward HB, Moritz TE, et al. Coronary-artery revascularization before elective major vascular surgery. N Engl J Med 2004; 351: 2795–804 [DOI] [PubMed] [Google Scholar]
- 14.Calman KC. Cancer: science and society and the communication of risk. BMJ 1996; 313: 799–802 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Wijeysundera DN, Austin PC, Beattie W, Hux JE, Laupacis A. Outcomes and processes of care related to preoperative medical consultation. Arch Intern Med 2010; 170: 1365–74 [DOI] [PubMed] [Google Scholar]
- 16.Fleisher LA, Fleischmann KE, Auerbach AD, et al. 2014. ACC/AHA guideline on perioperative cardiovascular evaluation and management of patients undergoing noncardiac surgery: a report of the American College of Cardiology/American Heart Association task force on practice guidelines. J Am Coll Cardiol 2014; 64: e77–e137 [DOI] [PubMed] [Google Scholar]
- 17.Balk EM, Earley A, Hadar N, Shah N, Trikalinos TA. Benefits and Harms of Routine Preoperative Testing: Comparative Effectiveness. Rockville, MD: Agency for Healthcare Research and Quality, 2014 [PubMed] [Google Scholar]
- 18.Howell SJ, Sear JW, Foex P. Hypertension, hypertensive heart disease and perioperative cardiac risk. Br J Anaesth 2004; 92: 570–83 [DOI] [PubMed] [Google Scholar]
- 19.Mulsow JJ, Feeley TM, Tierney S. Beyond consent—improving understanding in surgical patients. Am J Surg 2012; 203: 112–20 [DOI] [PubMed] [Google Scholar]
- 20.Ahn JH, Park JR, Min JH, et al. Risk stratification using computed tomography coronary angiography in patients undergoing intermediate-risk noncardiac surgery. J Am Coll Cardiol 2013; 61: 661–8 [DOI] [PubMed] [Google Scholar]


