Abstract
Chemosensory stimuli from same species (conspecific) and different species (heterospecific) elicit categorically different immediate-early gene (IEG) response patterns in medial amygdala in male hamsters and mice. All heterospecific stimuli activate anterior medial amygdala (MeA) but only especially salient heterospecific stimuli, such as those from predators activate posterior medial amygdala (MeP). We previously reported that characteristic patterns of response in separate populations of cells in MeA and MeP distinguish between different conspecific stimuli. Both gamma aminobutyric acid (GABA)-immunoreactive (ir) cells and GABA-receptor-ir cells make this distinction. Here, using zinc sulfate lesions of the main olfactory epithelium, we show evidence that main olfactory input does not contribute to the characteristic patterns of response in GABA-ir cells of male hamster amygdala, either for conspecific or heterospecific stimuli. Some GABAergic cells are output neurons carrying information from medial amygdala to behavioral executive regions of basal forebrain. Thus, the differential response to different conspecific signals can lead to differential activation of downstream circuits based on nonolfactory input. Finally, we show that an intact vomeronasal organ is necessary and sufficient to produce the characteristic patterns of response to conspecific and heterospecific chemosensory stimuli in hamster medial amygdala. Although main olfactory input may be critical in species with less prominent vomeronasal input for equivalent medial amygdala responses, work presented here suggests that hamster medial amygdala uses primarily vomeronasal input to discriminate between important unlearned conspecific social signals, to distinguish them from the social signals of other species, and may convey that information to brain circuits eliciting appropriate social behavior.
Key words: chemosignal, circuit, GABA, IEG, lesion, vomeronasal
Introduction
Chemosensory communication regulates reproductive, agonistic, and other social behaviors in rodents and many other species, including courtship and mating behavior, and competitive marking of territory or home range (Halpern 1987; Meredith 1998; Tirindelli et al. 1998; Keverne 1999; Brennan 2010). These conspecific (same species) signals carry different messages and must be discriminated by the chemosensory systems of recipient animals. They also must be distinguished from potentially similar heterospecific signals of other species. In rodents, both conspecific chemical communication signals (including “pheromones”) and heterospecific (including predator derived) chemosensory stimuli can be detected by the vomeronasal system (Scalia and Winans 1975; Johnston 1998; Samuelsen and Meredith 2009b; Papes et al. 2010; Kaur et al. 2014; Dey et al. 2015). Both may be detected by specific vomeronasal receptors, producing potentially identifiable neural activation in the vomeronasal sensory neurons and their centrally projecting sensory pathway. (Leinders-Zufall et al. 2000; Luo et al. 2003; He et al. 2008; Ben-Shaul et al. 2010; Isogai et al. 2011; Kaur et al. 2014). However, volatile main olfactory stimuli are sufficient for discrimination of male from female chemosensory stimuli and may carry other information even in untrained animals (Baum and Cherry 2015). Some social and threat-associated chemosignals with specific unlearned meanings are effective via the main olfactory system (Hudson and Distel 1986; Meredith 1998; Schaal et al. 2003; Baum and Kelliher 2009; Govic and Paolini 2015; Matsuo et al. 2015; Perez-Gomez et al. 2015) as can some specific aversive or attractive stimuli (Kobayakawa et al. 2007; Root et al. 2014; Gore et al. 2015).
Thus, it is not always clear which system(s) contribute when social signals activate brain areas critical for engaging appropriate behaviors. Here, we use peripheral sensory ablation to show that vomeronasal but not olfactory input is an essential contribution to characteristic patterns of immediate-early gene (IEG) activation in medial amygdala gamma aminobutyric acid-immunoreactive (GABA-ir) cells. These cells are presumably GABAergic and have been shown to be important for the generation of discriminable responses to different social conspecific and heterospecific chemosignals (Westberry and Meredith 2016, see also Donato et al. 2010), not only as circuit interneurons but also as amygdala output neurons (Bian et al. 2008; Keshavarzi et al. 2014).
Both vomeronasal and olfactory systems send direct afferent inputs to the amygdala that terminate mainly in separate, but adjacent, nuclei. The vomeronasal system projects via accessory olfactory bulb (AOB) to anterior medial amygdala (MeA) and posterior medial amygdala (MeP) and posteromedial cortical nucleus (Scalia and Winans 1975; Kevetter and Winans 1981a, 1981b; Keshavarzi et al. 2014, 2015). The olfactory system projects via main olfactory bulb (MOB) to the olfactory amygdala, anterior and postero-lateral cortical nuclei, and piriform cortex (PC) (Kevetter and Winans 1981b; Coolen and Wood 1998), with onward connections to medial amygdala (Coolen and Wood 1998; Keshavarzi et al. 2015; Cádiz-Moretti et al. 2016). There are also minor but potentially important projections from MOB to medial amygdala for specialized olfactory receptors (Thompson et al. 2012), the Grueneberg ganglion receptors (Perez-Gomez et al. 2015), and others, at least some of which are direct projections (Kang et al. 2011). All the nasal chemosensory information streams necessary for an appropriate behavioral response to social- and predator-related chemosensory stimuli finally converge in the amygdala. The first brain area where these can be integrated (Matsuo et al. 2015; Perez-Gomez et al. 2015) for relay to basal forebrain regions where appropriate behavioral responses are initiated (Choi et al. 2005; Been and Petrulis 2011, 2012).
We previously reported significantly different patterns of activation by different conspecific and heterospecific chemosensory stimuli in subdivisions and in different cell phenotypes of male hamster medial amygdala (Meredith and Westberry 2004; Westberry and Meredith 2016). These patterns were categorically different for conspecific versus heterospecific stimuli in hamsters and similarly distinguished in mice. In male hamsters, exposure to conspecific stimuli, used as social signals by other male and female hamsters, increased IEG activity in MeA and MeP. In contrast, chemosensory stimuli from mice and other (heterospecific) species, less relevant to hamsters, increased overall expression only in MeA, not MeP, and no similar categorical pattern was seen in the IEG responses upstream, in the AOB (Meredith and Westberry 2004). Heterospecific stimuli also increased Fos-related antigens (FRAs) expression in the caudal main intercalated nucleus (mICNc), a group of small GABA-ir cells adjacent to MeP, which we have suggested may inhibit MeP (Meredith and Westberry 2004; Biggs 2016). Other intercalated cell groups similarly play an important role in other amygdala circuits via regulation of activity in their adjacent main amygdala nuclei (Busti et al. 2011). In mice, the categorical patterns of response in medial amygdala were preserved but with the conspecific pattern now elicited by mouse stimuli (Samuelsen and Meredith 2009a). Olfactory input to MeA was not critical for this overall pattern of activation in hamster medial amygdala (Meredith and Westberry 2004). Here, we examine olfactory contribution to patterns of response in 2 subsets of cells: GABA-ir (GABA+) and non-GABA-ir (GABA−) that respond differently to conspecific chemosignals.
Olfactory lesions produced by intranasal zinc sulfate infusion, produced severe deficits in an olfactory food-finding task, and dramatically decreased activation in primary olfactory cortical areas. However, the same treatment had almost no effect on patterns of response in either MeA or MeP to chemosensory social signals, conspecific or heterospecific. We also show that removal of the vomeronasal organ (VNO), leaving the main olfactory system intact, disrupts the characteristic activation of both MeA and MeP in response to the stimuli tested here (as in male mice; Samuelsen and Meredith 2009a).
Thus, we conclude that main olfactory input appears not to contribute to the pattern of activation of medial amygdala GABA cells by chemosensory social signals, as we have previously shown for medial amygdala cells generally (Meredith and Westberry 2004). These data support the proposal that GABA circuits contribute to vomeronasal-driven characteristic patterns of response to socially relevant stimuli in medial amygdala in hamsters; patterns that would allow medial amygdala to discriminate critical chemical communication signals and engage appropriate behavioral responses.
Experimental procedures
Animal care and housing
All animals used in these experiments were sexually naive adult (2–3 month old) male golden hamsters (Mesocricetus auratus), bred in our laboratory or ordered from Charles River Laboratories, and maintained on a long photoperiod (a partially reversed—14L/10D light cycle). The animals were group housed in clear plastic cages (44cm × 21cm × 18cm) containing bedding with food and water ad libitum. On the day before stimulus exposure, each male hamster was separated from its cage mates and housed alone. All animal use was according to the NIH Guide for the Care and Use of Experimental Animals and was approved by the Institutional Animal Care and Use Committee of Florida State University.
Collection of stimuli
The stimuli described here were collected in the same manner for all of the experiments and by the same methods as used in the previously published work (Fernandez-Fewell and Meredith 1994, 1998; Westberry and Meredith 2003a, 2003b; Meredith and Westberry 2004). Female hamster vaginal fluid (HVF) was collected from several (5–6) naturally cycling females on the day of behavioral estrus. For collection, the female was placed on a plexi-glass lid with holes on the top of a cage containing a stud male. The female hamster was allowed to run freely and sniff toward the holes for approximately 2min. HVF was collected by gently scraping around the edge of the vagina with a blunt metal spatula. After collection, whole HVF was diluted 1:10 w/w with distilled water, centrifuged to remove solids and stored at −20 °C. Flank gland secretion (FGS) was collected from both male and female hamsters. Male and female FGS (mFGS, fFGS) were transferred to clean cotton swabs by gently pinching up the loose skin around the flank gland and rubbing the swab on the secretion soaked fur at least 10 times up and down. FGS source animals were different for each exposure and males were not cage or litter mates of the test animals. Each stimulus swab had FGS from 3 different donors. Female FGS was collected from naturally cycling females on the day of behavioral estrous. Both male and female FGS was collected immediately before each test exposure. Male and female mouse urine (mMU and fMU) was collected from several (5–6) male or female mice placed in a metabolic cage overnight. Female donor mice were naturally cycling, but were not separated according to different estrous stages. Fresh urine was diluted 1:10 with distilled water and stored frozen. Male cat urine (mCU) was collected from 2 castrated domestic cats by lining a clean litter box with absorbent paper and centrifuging out the urine, diluting 10:1 with distilled water and storing frozen. On the day of testing, frozen liquid stimuli were thawed and 200 µL was added to a cotton swab for each presentation. The test males in these experiments had no previous experience with or exposure to any of the heterospecific stimulus materials, no contact with female stimuli since weaning, and no experience or exposure to the individual donors of any hamster stimuli.
Exposure and behavior testing
Five minutes before the addition of a cotton swab containing the chemosensory stimulus, male hamsters were removed from their home cage and placed in a clean cage. Each swab was replaced every 3min for a total of 15min of stimulus (or clean swab; CS) exposure. The animal was then returned to its home cage for an additional 30min. During exposure, we observed and used a keypad, computer, and custom software to record latency, number, and duration of behavior assigned to each key, including sniffing the swab, licking the swab, general investigation of the clean cage, grooming, escape behavior (scrabbling up the wall of the cage), flank marking, and sleep.
Tissue processing for double-labeled immunofluorescence immunocytochemistry
Brains were double labeled for GABA and FRAs immunoreactivity to identify the phenotypes of activated GABA-ir (GABA(+)) and non-GABA-ir (GABA(−)) cells as described and illustrated in Westberry and Meredith (2016). After stimulus exposure for 15min, animals were returned to their home cage for a further 30min, then deeply anesthetized with sodium pentobarbital (90mg/kg; Ovation Pharmaceuticals) and perfused through the heart with 0.1M phosphate-buffered saline (PBS; pH 7.4) followed by 4% paraformaldehyde. Brains were removed and postfixed for 1–2h, cryoprotected in 30% sucrose overnight, and then sectioned serially on a freezing microtome at 25-μm thickness. After sectioning, free-floating coronal sections were washed for 1h in 0.1M PBS (3 washes), incubated in 1% hydrogen peroxide for 30min, then washed at least 3 times in 0.1M PBS. All secondary antibodies were made in donkey, so sections were blocked with 0.1M PBS with 5% Normal Donkey Serum (NDS; Jackson Immunoresearch) for 1h. Primary antibodies were as follows: FRAs (Santa Cruz sc253; 1:10000; Santa Cruz Biotechnology) and a mouse monoclonal anti-GABA (Sigma A0130, clone GB-69; 1:10000; Sigma Aldrich). Both primary antibodies were diluted in NDS solution, and sections were incubated for 24h at room temperature. The following day, sections were washed in 0.1M PBS (5 washes) and incubated in a solution containing NDS and both secondary antibodies for 2h at room temperature. The secondary antibodies, raised in donkey, were conjugated to Alexa 594 (red) for the FRA anti-rabbit secondary (Molecular Probes A-21209; 1:500; Life Technologies Corp.) and Alexa 488 (green) for the GABA anti-mouse secondary (Molecular Probes A-21202; 1:500). After secondary antibody incubation, sections were washed 3 times in 0.1M phosphate buffer and mounted on Superfrost Plus slides (Fisher Brand) using Vectashield Hard Mount (Vector Laboratories).
Counting
Sections were taken throughout the rostral/caudal extent of the medial amygdala. For regions of interest within MeA and MeP, every other section was collected and processed for GABA and FRAs double-labeled immunofluorescence. Within each area, 2 sections (separated by 25 µm) were analyzed and numbers averaged to represent FRAs counts for MeA. For MeP, the 2 sections were located approximately 300 µm caudal to the set of 2 sections in MeA. The sections through MeP were also the sections in which we counted cells of the intercalated nucleus (mICNc). GABA and FRA-labeled cells were counted using Metamorph software (Molecular Devices) (courtesy of Dr M. Freeman) and a monochrome camera with filters of the appropriate wavelength for the 2 fluorophores. All densely FRA-labeled nuclei (red filter) and all densely labeled cell bodies (green filter) were counted to generate numbers of FRA-activated cells and numbers of GABA-ir cells within the outlines of each anatomical nucleus of interest (MeA, MeP, mICNc, etc.). For double-labeled counts, merged (red plus green) images taken with a 20× power objective were closely inspected, and all apparent double-labeled cells were recorded and marked. Each area of the nucleus of interest was then reexamined in the original section, using a 40× objective and focusing up and down to determine whether ambiguous double labeling was actually in the same cell or due to superimposition of more than 1 cell. Erroneous counts were removed from the total.
Zinc sulfate olfactory lesions
Olfactory lesions were produced and their effectiveness tested behaviorally, as in previous publications (Fernandez-Fewell and Meredith 1998; Meredith and Westberry 2004). Zinc sulfate solution was sprayed into each nostril from a modified syringe to ablate the olfactory epithelium (OLFX). An equal volume of 0.9% saline was used in control saline-treated (SAL) animals. The spray needle was made from a 25-gauge hypodermic needle. The tip was blunted and pinched into a narrow slit and the shaft bent into a curve so that liquid forced through the needle emerged in a narrow spray pattern, centered approximately 45° from the angle of the syringe and needle shank. Male hamsters were anesthetized with halothane (Halocarbon Laboratories) and then placed prone on an inclined surface with the head down. Five percent of zinc sulfate solution (0.17M) was drawn into a 1-mL syringe, and 0.1mL of solution was forcibly expelled into each nostril, with the needle slit directed dorsally toward the olfactory area. The animal was then held in a head down position for approximately 1min, with aspiration to remove any solution draining from the nostrils. Animals were allowed to recover for 3–5 days before testing for anosmia and stimulus exposure.
Verification of anosmia: food discrimination test
The effectiveness of olfactory lesions (OLFX) was evaluated by testing for behavioral anosmia rather than histological damage to the epithelium. Three to 5 days after zinc sulfate or saline treatment, animals were tested for their ability to detect food odor (Fernandez-Fewell and Meredith 1998), immediately after exposure to the various chemosensory stimuli. Prior to testing, male hamsters were food deprived for 21–42h, then placed in a 24×16×30cm high Plexiglas test chamber with 2 ports in the floor, 1.3-cm diameter, 8cm apart and 8cm from each of the 3 nearest walls. One port was approximately 2.5cm directly above the contents of a beaker filled with food pellets, whereas the other port was above an identical beaker filled with corks of similar size to the food pellets. In order to avoid a place preference for one side of the chamber, a coin was flipped to determine which port would have the food pellets. The time each animal spent sniffing and biting each port was recorded for 3min. To be sure the animals found both ports, they were required to actively explore both ports, to spend at least 2s at each and at least 9s at the preferred port. If this criterion was not met, the test was continued for another 3min and results for the 2 periods were combined. An animal was considered anosmic if the time spent sniffing and biting at the food port was less than 2 times greater than at the other port. All zinc sulfate-treated animals included in these experiments were anosmic according to these criteria; all VNX animals passed the test. The food discrimination test was conducted 20min following exposure to the stimulus swab, 10–11min before perfusion, so that the test would not affect IEG protein expression. Immediately following the food test, the male hamsters were placed in a cage with a receptive female for a 5-min mating test. In the absence of functional olfactory epithelium, normal mating behavior is an indication that the VNO was not damaged by the zinc sulfate treatment (Fernandez-Fewell and Meredith 1998). In our hands, Fos or FRAs protein expression begins to become detectable after approximately 15min and peaks at 45min after the start of scented-swab stimulation. The food test and mating test were completed with the 15min before animals were euthanized so the neural activity involved would not have time to contribute to observed FRAs protein expression.
VNO-removal surgery (VNX)
For all VNX animals, a midline incision through the palate exposed the bony vomeronasal (VN) capsules, after pentobarbital anesthesia. The natural openings in the palatal bones were extended rostrally with a dental drill, and then a U-shaped groove was made with the drill to connect the 2 openings. Forceps were used to break the medial palatine process of the maxillary bones to disconnect the capsules at the caudal end. The capsules were then separated at the midline suture with a scalpel. The final, anterior, connection with the palate was broken by drilling rostro-dorsally from the midline of the U groove. Each capsule containing 1 VNO was then removed with small forceps separately. The palatal incision was closed with 3–5 sutures and sealed with cyanoacrylate adhesive. Animals were allowed to recover for at least 7 days before testing. The noses of the VNX animals were collected, postfixed separately, then decalcified, and sectioned for verification of complete VNO lesions (Fernandez-Fewell and Meredith 1994).
Mating test
Directly following the food discrimination test (5min before perfusion), male hamsters were tested in a 5-min mating test. The male hamster was placed in the cage of a receptive female and various aspects of male sexual behavior including mounts, thrusts, and intromissions were recorded on a keypad. This test was conducted 5min before perfusion so that mating would not contribute to observable IEG expression.
Statistical analysis
Two types of analysis of variance (ANOVA) were used to analyze the data. For analyzing the effect of intranasal saline infusion or zinc sulfate infusion (OLFX) on responses of cells of different phenotype within an area, separate (non-repeated-measures [non-RM]) ANOVAs were used, one for each phenotype (GABA(+) or GABA(−)), comparing response to each stimulus (Exposure) after each type of infusion (Treatment). Similar ANOVAs comparing Exposure and Treatment were used for behavioral data on investigation or mating after intranasal infusion or after VNO removal (VNX). For comparison of responses of cells of different phenotype within an area, a 2-way RM ANOVA compared numbers of cells with dense FRAs-ir of each cell type (Phenotype) for each stimulus (Exposure). RM ANOVAs were used for these analyses because all areas were present in each animal and both phenotypes were present in the same areas, so different phenotypes were not fully independent. Differences in FRAs expression between different brain areas in response to the same stimulus are not meaningful by themselves, because the areas differ in size and cell packing. However, changes in expression with different stimuli and after different treatments indicate information available to discriminate stimuli or conditions. Thus, Exposure was a factor for all analyses because we were primarily interested in differences in responses to the different stimuli—within and across areas, cell phenotypes, or treatments. Tukey post hoc tests were used to reveal significant differences between the response of a cell type in a specific brain area to each stimulus, compared with the response to clean control-swabs in the same phenotype cells in the same brain area of animals with the same treatment (asterisks in Figure 1).
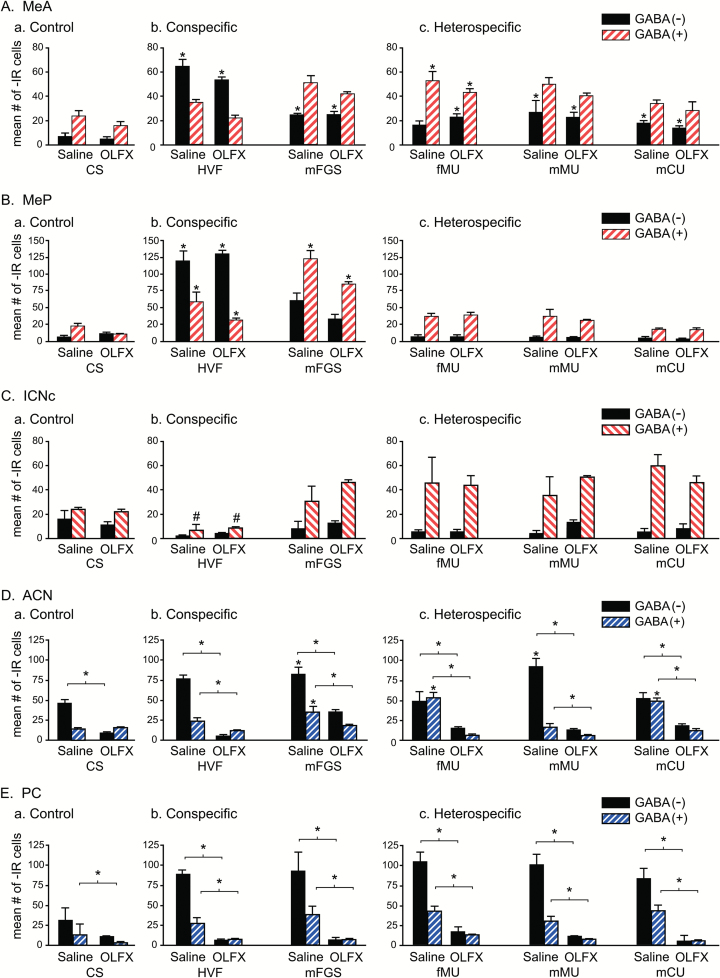
Figure 1.
Olfactory lesions: FRAs expression in GABA(+) and GABA(−) cells. Double-label FRAs(+)/GABA(+) cells in vomeronasal and olfactory projection areas and mICNc for animals treated with intranasal infusion of zinc sulfate to ablate olfactory epithelium (OLFX) or with intranasal saline, as control. Asterisks without brackets indicate a significant difference in FRAs expression compared with animals with the same intranasal treatment but exposed to clean-swab control stimulation. Asterisks with associated brackets indicate significant differences in FRAs expression between saline-treated and OLFX animals exposed to the same stimulus. (A, B) Vomeronasal projection areas: (A) MeA; (B) MeP: the patterns of expression are essentially identical for OLFX and SAL animals and similar to those for intact untreated animals (Westberry and Meredith, 2016), suggesting olfactory input is not necessary to produce the characteristic patterns of response. In MeA, there was a small but significant main effect of treatment such that OLFX animals had slightly lower FRAs expression overall, but no individual stimuli showed a significant difference with treatment. Heterospecific stimuli significantly activated neither GABA(+) nor GABA(−) cells in MeP. (C) mICNc: there were no significant differences between OLFX and saline-treated animals. Almost all cells in mICNc are GABA-ir. HVF significantly suppressed GABA(+) cells in both saline-treated and OLFX animals (#). FRAs expression in mICNc GABA(+) cells was increased in all other groups, that is, except those exposed to HVF, but the increases were not sufficient for statistical significance. (D, E) Olfactory projection areas: (D) ACN; (E) PC; unlike vomeronasal projection area response, there was a significant difference between FRAs expression in OLFX and saline-treated animals for all stimuli, including CS controls. In OLFX animals, there was no activation of FRAs above the (OLFX) control level for either cell phenotype with any stimulus, in either ACN or PC. FRAs expression for saline-treated animals was similar to that for intact untreated animals (Meredith and Westberry 2004), although increases in FRAs expression did not reach significant levels for all stimuli in ACN or for any in PC.
Results
Experiment 1: olfactory lesions (OLFX)
We previously demonstrated that animals treated with intranasal zinc sulfate solution to destroy the olfactory epithelium (OLFX) had the same overall patterns of FRAs expression in medial amygdala and mICNc as saline-treated controls in response to conspecific and heterospecific stimuli (Meredith and Westberry 2004). Here, we looked at responses of GABA(+) and GABA(−) cells after OLFX or intranasal saline treatment, in these same brain regions (Figure 1). These include the primarily vomeronasal projection areas: MeA and MeP, as well as mICNc, and the olfactory projection areas: anterior cortical nucleus (ACN) and PC. Numbers of animals were, for OLFX animals: CS (n = 8), HVF (n = 5), mFGS (n = 7), fMU (n = 5), mMU (n = 7), and mCU (n = 5). For saline-treated animals: CS (n = 6), HVF (n = 5), mFGS (n = 5), fMU (n = 4), mMU (n = 6), and mCU (n = 5). Compared with the previous study of GABAergic amygdala circuit response to chemosignals (Westberry and Meredith 2016), we dropped 1 conspecific stimulus (fFGS) and added 1 heterospecific stimulus (mCU) to extend the range of heterospecific stimuli sampled.
Experiment 1A: GABA + FRAs
First, we wanted to determine if loss of main olfactory input resulting from zinc sulfate lesions of the main olfactory epithelium (MOE) would change the responses to chemosensory stimuli in the different populations of cells (i.e., within different phenotypes) in MeA, MeP, mICNc, ACN, and PC. We compared FRAs expression in saline-treated and OLFX males across exposures separately for GABA(+) cells and for GABA(−) cells. The second goal was to look at differences in the pattern of activation of each phenotype across different exposures for each treatment group independently. For this comparison, saline and OLFX groups were analyzed separately. We conducted 4 statistical tests (described in Methods) to accomplish these 2 goals.
Vomeronasal projection areas
Within MeA, for GABA(+) cells, there was a significant main effect of both OLFX treatment (P = 0.005, F = 8.481, degrees of freedom [df] = 1, 50) and stimulus exposure (P < 0.001, F = 24.364, df = 5, 50), with no significant interaction. Thus, although there was a small net decrease in FRAs activation in GABA(+) cells of OLFX males, this did not vary significantly for the different stimuli. Similarly, for GABA(−) cells, there was no significant main effect of OLFX treatment, but a significant effect of stimulus exposure (P = 0.01, F = 3.379, df = 5, 50), and no significant interaction. Although significant for GABA(+) cells and not for GABA(−), the magnitude of the difference in the effect of main olfactory lesion is minimal. There was also no difference in the pattern of response across the various stimuli between OLFX and saline-treated animals, as indicated by a lack of interaction and by an inspection of Figure 1.
Both GABA(+) and GABA(−) cells responded to the various stimuli, with a significant main effect of stimulus exposure (P < 0.001, F = 21.871, df = 5, 16 for saline-treated animals; P < 0.001, F = 17.200, df = 5, 34 for OLFX animals). However, a difference between the cell types in the pattern of activation across the stimuli is indicated by a significant effect of phenotype for both OLFX (P < 0.001, F = 18.208, df = 1, 34) and saline-treated males (P < 0.001, F = 39.808, df = 1, 16). The differences between GABA(+) and GABA(−) responses were consistent for OLFX and saline-treated animals, however, with no significant interaction between the effects of phenotype and stimulus exposure. Significant responses (above control) are indicated by asterisks in Figure 1Aa–c.
Within MeP for both GABA(+) and GABA(−) cells, there was a significant main effect of stimulus exposure (GABA(+): P = 0.01, F = 21.370, df = 5, 50; GABA(−): P < 0.001, F = 24.364, df = 5, 50), but no significant effect of OLFX treatment and no significant interaction. Thus, responses of either cell type show no evidence of main olfactory influence. However, as in intact animals (Meredith and Westberry 2004; Westberry and Meredith 2016), responses do discriminate between the 2 conspecific stimuli tested and taken together, their responses discriminate the conspecific from heterospecific stimuli. Neither cell type has significant response to heterospecific stimuli as seen in Tukey post hoc tests (lack of asterisks in Figure 1B, no significant difference from control) and in the significant interaction between exposure and phenotype for both saline-treated (P < 0.001, F = 45.082, df = 1, 16) and OLFX animals (P < 0.001, F = 18.272, df = 1, 34). There was no overall main effect of phenotype, presumably due to the similar levels of expression for control and for heterospecific stimuli. The 2 conspecific stimuli elicited widely different activation but were only 2 of 7 exposures. Again as in untreated animals and as in MeA, HVF elicited more activation of GABA(−) than of GABA(+) cells. Other stimuli had more expression in GABA(+) cells, but for the heterospecific stimuli, there was no difference from clean-swab control animals.
Within mICNc, for GABA(+) cells, there was a significant main effect of stimulus-exposure (P = 0.01, F = 21.370, df = 5, 50) but not of OLFX treatment and no significant interaction, indicating differential activation of mICNc by the various stimuli but no influence of main olfactory input. The patterns of response were the same as for untreated animals (Westberry and Meredith 2016). FRAs expression was higher than for controls in GABA(+) cells with all heterospecific exposures and for mFGS, in both saline-treated and OLFX males (Figure 1Ca–c), contributing to a main effect of exposure, but these small increases did not reach significance for any individual stimulus. HVF stimulation, however, significantly decreased FRAs expression, compared with controls, in GABA(+) cells in mICNc (saline: P = 0.02; OLFX: P = 0.031; a similar but nonsignificant depression occurred for intact animals with no intranasal infusion; Westberry and Meredith 2016). Within the few GABA(−) cells in mICNc, there were no significant main effects, no differences between treatments or exposures and no interaction. In all cases, FRAs expression in GABA(+) cells was higher than in GABA(−) cells, with a significant main effect of phenotype for both saline-treated (P < 0.001, F = 49.323, df = 1, 50) and OLFX animals (P < 0.001, F = 162.633, df = 1, 34) and a significant interaction (saline: P < 0.001, F = 8.899, df = 5, 50; OLFX: P < 0.001, F = 5.911, df = 1, 34).
Olfactory projection areas
In the olfactory projection areas, ACN and PC, OLFX males had significantly lower FRAs expression in both areas than saline-treated males exposed to the same stimuli; for all stimuli and in both GABA(+) and GABA(−) cells. There were no significant increases in FRAs expression above control levels in either of the olfactory projection areas for any stimulus in OLFX animals, as expected for a successful olfactory deafferentation (see also behavioral data).
The ACN receives input from the MOB and projects to MeA but does not receive chemosensory input directly from the AOB. As expected, there were significant differences in FRAs responses between OLFX and saline-treated males. Zinc sulfate lesions (OLFX) significantly reduced FRAs expression in both GABA(+) and GABA(−) cells for all exposures and eliminated the responses to chemosensory input that were seen in saline-treated males (Figure 1Da–c).
For both GABA(+) and GABA(−) cells within ACN, there were significant main effects of both stimulus-exposure and OLFX treatments and a significant interaction, indicating a strong influence of main olfactory input in this area. Statistics for stimulus exposure were as follows: GABA(+) (P < 0.001, F = 13.669, df = 5, 50); GABA(−) (P < 0.001, F = 12.914, df = 5, 50). Statistics for treatment were as follows: GABA(+) (P < 0.001, F = 312.902, df = 1, 50); GABA(−) (P < 0.001, F = 129.372, df = 1, 50). Statistics for interaction were as follows: GABA(+) (P < 0.001, F = 8.284, df = 5, 50); GABA(−) (P < 0.001, F = 15.370, df = 5, 50). There was significantly less FRAs expression for all stimuli (P < 0.05), including clean-swab controls, in OLFX compared with saline-treated animals (asterisks with bracket lines in Figure 1Da–c).
In saline-treated animals, both GABA(+) and GABA(−) cells showed significant responses to some stimuli (asterisks over individual bars in Figure 1Ca–c) but with a different pattern across stimuli. There was a significant effect of phenotype (P < 0.001, F = 84.834, df = 1, 16) and a significant interaction with stimulus exposure (P < 0.001, F = 10.229, df = 5, 16). There was no significant response to any individual stimulus in either cell type in OLFX animals and no significant effect of phenotype.
The piriform cortex (PC) also receives input from the MOB but not the AOB and has projections to medial amygdala. Here, zinc sulfate lesions of the MOE reduced FRAs expression to near zero in males exposed to any of the stimuli, including clean swabs. Asterisks associated with brackets indicate a significant difference between saline-treated and OLFX animals with exposure to the same stimulus. In saline-treated males, all stimuli increased expression in both cell types but none reached significantly increased levels. However, for both GABA(+) and GABA(−) cells, there were significant main effects of stimulus exposure (P < 0.001, F = 11.618, df = 5, 50; P = 0.01, F = 3.420, df = 5, 50, respectively) as well as the obvious significant effect of OLFX treatment (P < 0.001, F = 401.720, df = 1, 50; P < 0.001, F = 93.708, df = 1, 50, respectively), and a significant interaction (P < 0.001, F = 12.937, df = 5, 50; P < 0.001, F = 5.725, df = 5, 50, respectively). As in can, these results and the graphs in Figure 1Ea–c) show a strong effect of main olfactory input with no clear evidence of activation after OLFX treatment (see also Fernandez-Fewell and Meredith 1994, 1998). For intact untreated animals, only HVF significantly activated total FRAs in the PC above the level in clean swab controls (Meredith and Westberry 2004). All such tests were conducted in clean but not odor-free cages, where the addition of the low-concentration swab-borne stimuli makes little difference in overall response. Here, there were differences between FRAs expression in the 2 phenotypes in saline-treated animals but no significant increase above control level for either phenotype for any stimulus. For saline-treated animals, there was sufficient difference in FRAs expression for a significant main effect of phenotype (P < 0.001, F = 102.72, df = 1, 16), with no significant interaction between phenotype and stimulus exposure. For OLFX animals analyzed separately, there was no significant effect of stimulus exposure or phenotype and no interaction. Thus, although the main olfactory input to the “olfactory” amygdala areas (ACN and PC) may be able to produce distinguishable responses to these stimuli, especially in ACN, any output carrying this information appears to be directed to other areas and appears not to make essential contributions to the patterns in “vomeronasal” amygdala.
Experiment 2: VNO removal (VNX)
In previous, published experiments (Fernandez-Fewell and Meredith 1994; Fewell and Meredith 2002), removal of the VNOs dramatically reduced Fos expression in medial amygdala in response to HVF stimulation. Here, we extended these findings using FRAs expression and additional stimuli. At least 7 days following removal of the VNOs, groups of sexually naive VNX males were exposed to representative test stimuli: clean swabs (n = 4), female HVF (n = 5), mFGS (n = 3), or mMU (n = 4). All VNX animals included here had complete removal of both VNOs and/or complete disconnection of vomeronasal nerves at the level of the organ. All also passed the olfactory detection task and failed to achieve 5 intromissions in the 5-min mating test (except the HVF-exposed group, see Mating test). Tissue from VNX animals was processed as for the other groups reported here, using fluorescent immunocytochemistry for FRAs. The results are shown in Figure 2. In assessing the overall effect of VNX across brain areas, we first used a 2-way RM ANOVA comparing “exposure” (CS, HVF, mFGS, and mMU) and “brain area” (MeA, MeP, and mICNc). There were no significant main effects of exposure or brain area and no significant interaction indicating that at least with this test, there was no significant stimulation of FRAs expression after VNX, in any of these brain areas by any of the stimuli. Because this 2-way RM ANOVA including multiple exposures and brain areas is a conservative test, differences may have been obscured by the increase in variance when all 3 brain regions were taken into account. We, therefore, conducted 1-way ANOVAs for each brain region to determine if any of the exposure groups were significantly different from the group exposed to clean swabs. With this more liberal test, there were significant differences in FRAs expression in MeA and MeP.
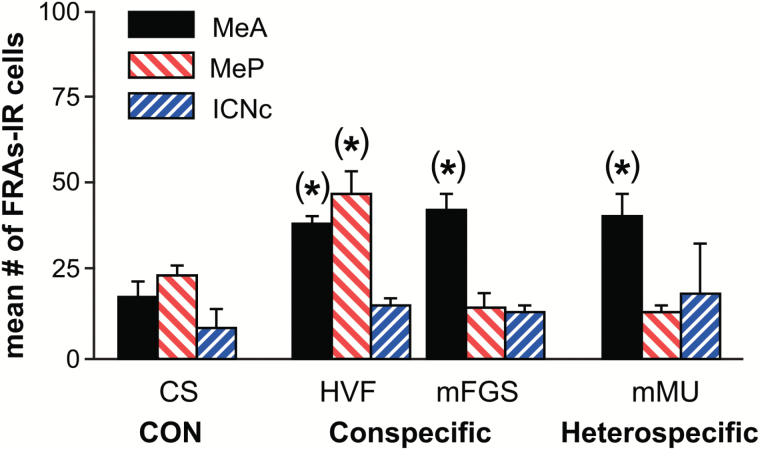
Figure 2.
Vomeronasal organ removal (VNX). There was no overall response to tested stimuli after VNX. When all 3 areas (MeA, MeP, and mICN) and all stimuli (CS, HVF, mFGS, and mMU) were analyzed in a common 2-way RM ANOVA, there were no significant main effects or interactions. However, 1-way ANOVAs for each brain area indicated a possible small differential response not evident in the overall analysis, indicated by asterisks in parentheses. In intact or OLFX animals, all conspecific stimuli activate MeP, but after VNX, only HVF did so—suggesting the categorical pattern seen in intact (and OLFX) animals was disrupted after VNX in naive males.
For MeA, a 1-way ANOVA indicated that the conspecific stimuli HVF (P = 0.001) and mFGS (P < 0.001) and the heterospecific stimulus mMU (P = 0.03) significantly activated MeA above clean swab controls. For MeP, a 1-way ANOVA indicated significant activation for only 1 conspecific stimulus, HVF (P < 0.001). The other conspecific stimulus only significantly activated MeA and no heterospecific stimuli activated MeP. For ICN, there was no significant activation for any stimuli in these VNX males by any analysis. All stimuli were equally ineffective. These data suggest some small residual response in medial amygdala, perhaps due to main olfactory input. Such input may be strengthened in sexually experienced VNX males, which have no deficits in mating behavior and have strong MeA/P responses, at least to HVF (Fewell and Meredith 2002).
Experiment 3: behavior
Investigation time
For the analysis of behavior, we compared the animals with different treatments described here and also included the intact untreated animals described in a previous article (Westberry and Meredith 2016). There were no significant differences in time spent investigating the stimulus swabs between any of the treatment groups: intact untreated males, zinc sulfate-treated OLFX males, saline-treated control males, and VNX males. A 2-way ANOVA comparing the factors “surgery/treatment” and “stimulus exposure” revealed no overall effect of surgery/treatment, but there was an overall main effect of stimulus exposure (P < 0.001, F = 34.743, df = 4, 36). Tukey post hoc tests revealed that all groups of male hamsters regardless of VNX, saline, or OLFX treatment spent significantly (P < 0.01) more time investigating each of the stimuli presented compared with time spent investigating clean swabs. The sole exception was investigation of mFGS by VNX males. These data are shown in Figure 3.
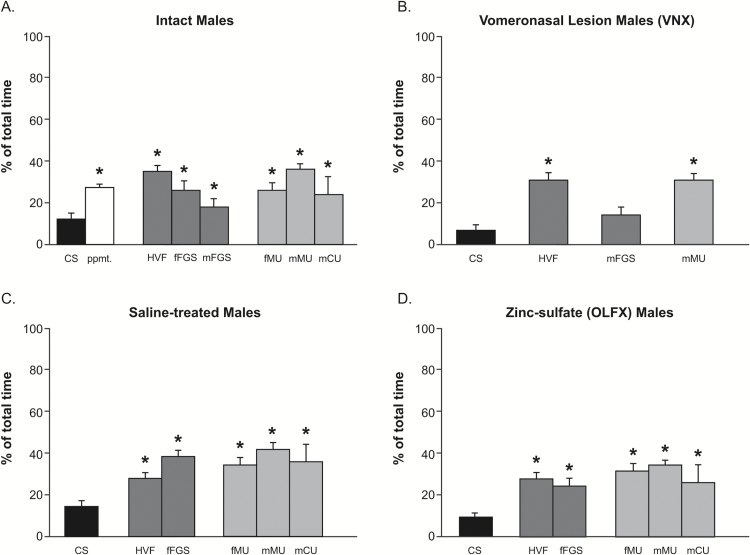
Figure 3.
Investigation time for stimulus swabs. (A) Intact animals investigate scented-swab stimuli significantly more than unscented CS control swabs but not significantly differently between individual stimuli. Thus, differential FRAs response to different stimuli cannot generally be attributed to different investigation times. (B) VNX animals also investigate scented swabs. Investigation time for mFGS was not significantly different from control swabs or from other stimuli tested. (C, D) Investigation of scented swabs in saline-treated and OLFX animals was similar, and similar to that in intact animals, despite behavioral anosmia in OLFX animals. Asterisks = different from CS.
Food discrimination test
Saline-treated and OLFX males were tested after 24–48h of food deprivation. Male hamsters could investigate 2 ports, one over a container of food pellets and the other over a container of corks of approximately the same size and color. We measured the time spent at each port and males were considered anosmic if they spent less than twice the time at the food port compared with the cork port. All of the OLFX males included in the FRAs expression data and behavioral data described above were considered anosmic based on this criterion.
Mating test
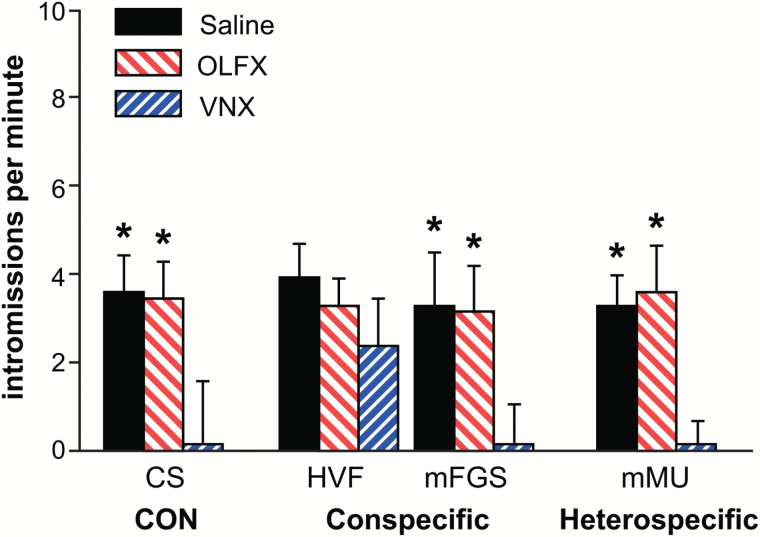
We also recorded mating behavior in a 5-min test for OLFX, SAL, and VNX males. The male hamster was allowed to mate until it reached 5 correct intromissions or for a total of 5min. The test was conducted 5min before perfusion so IEG expression would not be affected (see Methods, Discussion). We used intromissions per minute over this brief period as a general indicator of the male hamster’s willingness and capability to mate. All of the saline-treated males mated normally. One of 32 OLFX males did not reach 5 intromissions in 5min and was excluded from the experiment. Of the naive VNX males, 3 of the 5 males that were exposed to HVF 25min prior to the mating test mated normally. The other 2 males had 4 intromissions in 5min. Pre-exposure to HVF before the mating test rescued mating behavior in these naive VNX males as previously demonstrated (Westberry and Meredith 2003b). None of the 11 VNX males exposed to other stimuli before the mating test reached 5 intromissions in 5min. In fact, most did not have even one correct intromission. These data are shown in Figure 4. A 2-way ANOVA comparing “treatment” (SAL, OLFX, and VNX) and “stimulus exposure” (CS, HVF, mFGS, and mMU) revealed an overall main effect of treatment on mating (P < 0.001, F = 44.645, df = 3, 36), an overall main effect of stimulus exposure (P < 0.001, F = 34.743, df = 4, 36), and a significant interaction (P < 0.001, F = 134.743, df = 4, 36). Within each stimulus-exposure group, saline and OLFX males were not significantly different from each other. Mating performance in both saline and OLFX groups was significantly greater than in VNX males for all of the exposure groups except for HVF. In VNX males exposed to HVF, intromissions per minute were not significantly different from OLFX or SAL-treated males that were exposed to HVF. Asterisks on the graph in Figure 4 indicate significant differences from VNX males (within each exposure group).
Figure 4.
Mating test. Animals from OLFX and saline-treated groups were unimpaired in a 5-min test for mating behavior, indicating (for OLFX animals) a functional vomeronasal organ. VNX animals were significantly impaired in mating behavior except when pre-exposed to HVF (see Results). Asterisks indicate a significant difference from VNX. Mating tests were given at a too short latency to result in changes in FRAs expression.
Discussion
Summary
As previously reported, medial amygdala in male hamsters distinguished categorically between conspecific social chemosensory signals and heterospecific signals used by other species for similar types of communication. Both GABA-ir and non-GABA-ir cells in medial amygdala produced characteristically different patterns of response (IEG expression) to different stimuli. Here, we show that the reduction or elimination of main olfactory input sufficient to produce severe functional anosmia did not significantly alter the patterns of response in MeA and MeP of either phenotype of cell to either category of stimuli. When GABA-ir and non-GABA-ir cells are counted separately, conspecific stimuli activated both MeA and MeP with discriminably different patterns of activity for different stimuli. Heterospecific stimuli significantly activated either population of cells only in MeA (and mICNc for GABA-ir cells) but not MeP. We conclude that main olfactory input, at least in these laboratory conditions, does not appear to contribute significantly to the generation of the patterns of response in these cell populations, which are sufficient to distinguish different chemosensory social stimuli. However, removal of vomeronasal sensory input by surgical removal of the VNOs eliminated the categorical distinction in overall patterns of amygdala response despite intact main olfactory input and functional olfactory discrimination of food odors. It is clear that main olfactory information is critical for many rodent behaviors including social behavior, but the particular capacity tested here, which we believe contributes directly to the selection of appropriate behaviors in male hamsters, does not seem to require that input.
We used double-labeled immunocytochemistry to show coexpression of IEG (FRAs) together with either GABA-ir (GABA+) or non-GABA-ir (GABA−) in animals with intranasal infusions to destroy olfactory epithelium (or control saline infusion). The results reveal, as we have shown before for animals with no intranasal treatment, that subpopulations of medial amygdala cells distinguish in their responses between different ecologically relevant conspecific signals carrying potentially different social messages. These differential responses could engage downstream circuits responsible for an appropriately differential behavioral response to different conspecific signals. Additionally, MeP failed to show increased IEG responses in GABA(+) or GABA(−) cells to any heterospecific stimulus, whereas all heterospecific stimuli activated GABA(+) cells of the adjacent mICNc, as predicted by our hypothesis that an overall MeP response is suppressed by heterospecific stimuli via GABA inhibition from mICNc. We have previously shown (Westberry and Meredith 2016) that MeP cells expressing GABA receptor (some of which also express GABA) are selectively suppressed by heterospecific stimuli—but GABA(+) cells per se do not show a significant suppression compared with control. The elimination of a MeP response means that heterospecific stimuli as a group can be distinguished categorically from conspecific stimuli. All of which activate MeP. Thus, amygdala processing may be sufficient for the selection of appropriate behavioral response or to avoid inappropriate response to both conspecific and heterospecific stimuli.
Here, in males treated with intranasal zinc sulfate (OLFX), the patterns of activation of GABA(+/−) cells in MeA and MeP (and in mICNc) were essentially identical to those in saline-treated animals or in animals with no intranasal treatment, suggesting olfactory input is not necessary for the characteristic response patterns of MeA and MeP or for the putative inhibitory influence from mICNc. In VNX males, the remaining intact olfactory input was not sufficient to generate the characteristic responses to different categories of stimuli in MeA and MeP.
Main olfactory input is not necessary for categorical response: OLFX males
Lesions of the MOE (OLFX) by intranasal zinc sulfate infusion can produce behavioral anosmia with VNO sparing. In these animals, there is very little or no change in IEG response in VN projection areas (MeA and MeP) to chemosignals such as HVF (Fernandez-Fewell and Meredith 1998) and more varied stimuli (Meredith and Westberry 2004). The activation of the mICNc, which we have proposed contributes to suppression of MeP by heterospecific stimuli, was also not altered by olfactory lesions (Meredith and Westberry 2004; Biggs 2016). Here, as in the previous study, there was a slight decrease in overall response in OLFX males, but no significant differences in the FRAs responses of GABA(+/−) phenotypes compared with saline-treated controls for any of the individual stimuli. We conclude that the characteristic responses to conspecific and heterospecific stimuli in medial amygdala and the “categorical” differences between responses to these 2 types of stimuli (Meredith and Westberry 2004; Westberry and Meredith 2016) are not dependent on olfactory input for the GABA(+) and GABA(−) populations at large. By contrast, in main olfactory areas ACN and PC, FRAs expression was dramatically reduced in OLFX animals compared with saline-treated controls, with no significant FRAs response to any of the tested stimuli or in the overall main effect. All OLFX animals included here were also anosmic in behavioral tests so we see no evidence of significant residual main olfactory capacity. These same animals mated normally, suggesting that VNOs were not damaged, as an intact VNO is critical for mating in sexually naive male hamsters (Meredith 1986; Fernandez-Fewell and Meredith 1994, 1998; Fewell and Meredith 2002; Westberry and Meredith 2003a).
VNO input is necessary for normal categorical response: VNX males
Removal of the VNOs (VNX) in sexually naive male hamsters given no prior exposure to female stimuli severely impairs or eliminates mating behavior in our 5-min mating tests (Meredith 1986). Naive VNX males show no significant IEG response in medial amygdala on first exposure to HVF (Fernandez-Fewell and Meredith 1994). Here, naive VNX males also had decreased FRAs responses in MeA and MeP, compared with intact males, when exposed to the wider range of chemosensory stimuli. When brain areas were analyzed together in a 2-way ANOVA, there were no significant responses to any stimuli in the VNX animals. A more liberal, area-by-area analysis suggested a small activation in some areas for some of the stimuli. However, lesions of the vomeronasal system appear to disrupt the mechanisms for categorical MeP response to conspecific and heterospecific stimuli as the characteristic patterns seen in intact animals did not appear.
These findings suggest that olfactory input provides little contribution to the categorical analysis of unlearned male and female conspecific and heterospecific signals by the medial amygdala in intact sexually naive male hamsters. Without the VNO, there was no evidence of engagement of the inhibitory circuit via mICNc, which showed no increase or decrease in FRAs expression with exposure of VNX animals to any of the stimuli. The evidence for significant main olfactory input to medial amygdala in mice is greater than in hamsters (Lehman and Winans 1982; Kang et al. 2011; Thompson et al. 2012; Perez-Gomez et al. 2015; Keshavarzi et al. 2015), but we also found no significant chemosensory-driven FRAs expression in MeA and MeP after VNX in mice (Samuelsen and Meredith 2009b). Bergan et al. (2014) also saw little electrophysiological response in medial amygdala neurons in anesthetized mice with conspecific or heterospecific (predator) chemosensory stimuli unless the vomeronasal pump (Meredith and O’Connell 1979) was activated to deliver stimuli to the VNO. On the other hand, electrical stimulation of the MOB in hamsters can drive single neurons in medial amygdala (Case G, Meredith M, unpublished data) and posteromedial cortical amygdala (Licht and Meredith 1987), both parts of the “vomeronasal amygdala” (Kevetter and Winans 1981a, 1981b).
There is a possibility of a special pathway for chemosensory input to male hamster medial amygdala under some circumstances. As we have previously reported (Westberry and Meredith 2003b) VNX males exposed to HVF do have a small increase in IEG expression in MeP and show little impairment in a subsequent 5-min mating test. Exposure to other stimuli does not result in the IEG expression in MeP that one would see in intact animals (or OLFX animals) and has no facilitating effect on mating, even though mating itself involves exposure to a female’s HVF. Sexually experienced male hamsters also have MeP activated by HVF and mate normally (Fewell and Meredith 2002). It is tempting to speculate that HVF pre-exposure, or sexual experience, may produce an alteration in access of chemosensory information to medial amygdala. If this were a main olfactory information pathway, it would appear to be limited to HVF—and perhaps activate the same sets of neurons as the normal vomeronasal input, so there would be no significant reduction of expression after OLFX.
Nonvomeronasal-dependent communication
Although we find no evidence for main olfactory contributions here, main olfactory signals are clearly essential for some social communication responses, notably in suckling responses in newborn rabbit pups (Hudson and Distel 1986), female responses to male signals in pigs (Dorries et al. 1997), social communication in sheep (Kéller and Levy 2012), and several social behaviors in mice (Matsuo et al. 2015). Among rodents, noncontact erection response in male rats exposed to female odors does not occur after medial amygdala lesions (Kondo and Sachs 2002) possibly indicating a main olfactory pathway for these volatile air-borne stimuli that is convergent with the vomeronasal sensory pathway demonstrated in rodents and investigated here. Socially communicated food-safety signals also appear to be dependent on main olfactory transmission (Galef et al. 1988). In this case, the “social odor” appears to be a food metabolite (carbon disulfide; Munger et al. 2010) that activates a specialized subset of olfactory sensory neurons projecting to specialized (necklace) glomeruli in the caudal olfactory bulb. Whether mitral cells in these glomeruli project to medial amygdala is unclear. Information from selective responses of specialized sensory cells in the MOE and Grueneberg ganglion to various predator stimuli is also processed in the MOB in mice and reaches medial amygdala along with input from the VNO (Perez-Gomez et al. 2015). Whether this is true for hamsters is also unclear. Rodents and other mammals can clearly distinguish male and female odors using main olfactory signals, and many nonrodent species appear not to use vomeronasal signals for some aspects of social communication, even when the VNO is present and appears functional (sheep: Kéller and Levy 2012; ferret: Baum and Cherry 2015). If males have experience with chemosensory signals while both vomeronasal and main olfactory systems are intact, the main olfactory system can take over the discriminatory task. For example, male hamsters, given sexual experience before VNX (Meredith 1986; Fewell and Meredith 2002), have no deficits in mating behavior and have substantial responses in medial amygdala to female chemosignals. We assume that the association of main and accessory olfactory input leads to learning of the significance of main olfactory signals—and may also alter access of olfactory information to the amygdala (Blake and Meredith 2010). There are other examples where learning of main olfactory signals is dependent on vomeronasal input. Female mice learn the odor signature of individual dominant males from the male’s scent marks—when a particular major urinary protein (MUP 20 or Darcin) is present in the mark (Roberts et al. 2010). On the other hand, the vomeronasal system can also be used for learning chemosensory signatures, as seen with male mouse learning of competitors’ scent marks (Kaur et al. 2014). However, both intact and vomeronasal-ablated male mice can discriminate male from female odors without experience (Pankevich et al. 2004) using only volatile odors. In those experiments, intact mice but not those with VNO lesions preferred to investigate estrous female over male odors suggesting additional salience is contributed by vomeronasal input. The experiments reported here document an additional discriminative capacity in the response of medial amygdala cells of different phenotype, which may contribute to such behavioral responses.
Conclusions
Overall, we show that the categorically different response patterns elicited in the medial amygdala, including its GABA-ir(+) and GABA-ir(−) cells, by conspecific and heterospecific stimuli (Meredith and Westberry 2004; Samuelsen and Meredith 2009a), and the discriminable differences in patterns of response to different conspecific stimuli (Westberry and Meredith 2016) are not altered by ablation of main olfactory input. The differential response of the mICNc, which appears to contribute to medial amygdala response patterns, was also unchanged. These separate patterns of response in medial amygdala cells of different phenotype discriminate between different stimuli within the conspecific category and distinguish conspecific from heterospecific stimuli but appear not to be dependent on main olfactory input. Vomeronasal input, however, appears essential for these patterns. The response of different amygdala cell populations to different stimuli could activate different downstream circuits in hypothalamic regions, leading to appropriate behavioral responses (Been and Petrulis 2011; Lin et al. 2011; Been and Petrulis 2012).
Funding
This work was supported by National Institute on Deafness and Other Communication Disorders grants DC005813 (to M.M.), F31DC05725 (to J.W.), and T32 DC000044 (to M.M.) and by Florida State University.
Author contribution
J.M.W. and M.M. designed the research, J.M.W. performed the research, and J.M.W. and M.M. analyzed the data and wrote the article.
Conflict of interest
The authors declare no competing financial or other interests.
Acknowledgments
We thank Dr G. Howard for technical help with confirmation of completeness of VNX surgery and Dr C. Blake for help with olfactory lesion experiments. Thanks to Dr M. Freeman for use of his microscope and image analysis system for fluorescence double-labeled sections and to Dr M. Sellix for his help with image analysis in the initial experiments. Thanks to C. Badland for expert assistance with the preparation of the figures.
References
- Baum MJ, Cherry JA. 2015. Processing by the main olfactory system of chemosignals that facilitate mammalian reproduction. Horm Behav. 68:53–64. [DOI] [PubMed] [Google Scholar]
- Baum MJ, Kelliher KR. 2009. Complementary roles of the main and accessory olfactory systems in mammalian mate recognition. Annu Rev Physiol. 71:141–160. [DOI] [PubMed] [Google Scholar]
- Been LE, Petrulis A. 2011. Chemosensory and hormone information are relayed directly between the medial amygdala, posterior bed nucleus of the stria terminalis, and medial preoptic area in male Syrian hamsters. Horm Behav. 59(4):536–548. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Been LE, Petrulis A. 2012. Dissociated functional pathways for appetitive and consummatory reproductive behaviors in male Syrian hamsters. Horm Behav. 61(2):204–211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bergan JF, Ben-Shaul Y, Dulac C. 2014. Sex-specific processing of social cues in the medial amygdala. Elife. 3:e02743. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ben-Shaul Y, Katz LC, Mooney R, Dulac C. 2010. In vivo vomeronasal stimulation reveals sensory encoding of conspecific and allospecific cues by the mouse accessory olfactory bulb. Proc Natl Acad Sci USA. 107(11):5172–5177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bian X, Yanagawa Y, Chen WR, Luo M. 2008. Cortical-like functional organization of the pheromone-processing circuits in the medial amygdala. J Neurophysiol. 99(1):77–86. [DOI] [PubMed] [Google Scholar]
- Biggs LM. 2016. A medial-amygdala/intercalated-nucleus circuit for modulation of amygdala response to chemosensory signals and its subsequent output. AChemS Abstract #031 Chem Senses. 41 : e12. [Google Scholar]
- Blake CB, Meredith M. 2010. Selective enhancement of main olfactory input to the medial amygdala by GnRH. Brain Res. 1317:46–59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brennan PA. 2010. Pheromones and animal behavior. In: Menini A, editor. The neurobiology of olfaction. Chap. 6. Boca Raton (FL): CRC Press; p. 157 – 180. [Google Scholar]
- Busti D, Geracitano R, Whittle N, Dalezios Y, Mańko M, Kaufmann W, Sätzler K, Singewald N, Capogna M, Ferraguti F. 2011. Different fear states engage distinct networks within the intercalated cell clusters of the amygdala. J Neurosci. 31(13):5131–5144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cádiz-Moretti B, Otero-García M, Martínez-García F, Lanuza E. 2016. Afferent projections to the different medial amygdala subdivisions: a retrograde tracing study in the mouse. Brain Struct Funct. 221(2):1033–1065. [DOI] [PubMed] [Google Scholar]
- Choi GB, Dong HW, Murphy AJ, Valenzuela DM, Yancopoulos GD, Swanson LW, Anderson DJ. 2005. Lhx6 delineates a pathway mediating innate reproductive behaviors from the amygdala to the hypothalamus. Neuron. 46(4):647–660. [DOI] [PubMed] [Google Scholar]
- Coolen LM, Wood RI. 1998. Bidirectional connections of the medial amygdaloid nucleus in the Syrian hamster brain: simultaneous anterograde and retrograde tract tracing. J Comp Neurol. 399(2):189–209. [DOI] [PubMed] [Google Scholar]
- Dey S, Chamero P, Pru JK, Chien MS, Ibarra-Soria X, Spencer KR, Logan DW, Matsunami H, Peluso JJ, Stowers L. 2015. Cyclic regulation of sensory perception by a female hormone alters behavior. Cell. 161(6):1334–1344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Donato J, Jr, Cavalcante JC, Silva RJ, Teixeira AS, Bittencourt JC, Elias CF. 2010. Male and female odors induce Fos expression in chemically defined neuronal population. Physiol Behav. 99(1):67–77. [DOI] [PubMed] [Google Scholar]
- Dorries KM, Adkins-Regan E, Halpern BP. 1997. Sensitivity and behavioral responses to the pheromone androstenone are not mediated by the vomeronasal organ in domestic pigs. Brain Behav Evol. 49(1):53–62. [DOI] [PubMed] [Google Scholar]
- Fernandez-Fewell GD, Meredith M. 1994. c-fos expression in vomeronasal pathways of mated or pheromone-stimulated male golden hamsters: contributions from vomeronasal sensory input and expression related to mating performance. J Neurosci. 14(6):3643–3654. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fernandez-Fewell GD, Meredith M. 1998. Olfactory contribution to Fos expression during mating in inexperienced male hamsters. Chem Senses. 23(3):257–267. [DOI] [PubMed] [Google Scholar]
- Fewell GD, Meredith M. 2002. Experience facilitates vomeronasal and olfactory influence on Fos expression in medial preoptic area during pheromone exposure or mating in male hamsters. Brain Res. 941(1–2):91–106. [DOI] [PubMed] [Google Scholar]
- Galef BG, Jr, Mason JR, Preti G, Bean NJ. 1988. Carbon disulfide: a semiochemical mediating socially-induced diet choice in rats. Physiol Behav. 42(2):119–124. [DOI] [PubMed] [Google Scholar]
- Gore F, Schwartz EC, Brangers BC, Aladi S, Stujenske JM, Likhtik E, Russo MJ, Gordon JA, Salzman CD, Axel R. 2015. Neural representations of unconditioned stimuli in basolateral amygdala mediate innate and learned responses. Cell. 162(1):134–145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Govic A, Paolini AG. 2015. In vivo electrophysiological recordings in amygdala subnuclei reveal selective and distinct responses to a behaviorally identified predator odor. J Neurophysiol. 113(5):1423–1436. [DOI] [PubMed] [Google Scholar]
- Halpern M. 1987. The organization and function of the vomeronasal system. Annu Rev Neurosci. 10:325–362. [DOI] [PubMed] [Google Scholar]
- He J, Ma L, Kim S, Nakai J, Yu CR. 2008. Encoding gender and individual information in the mouse vomeronasal organ. Science. 320(5875):535–538. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hudson R, Distel H. 1986. Pheromonal release of suckling in rabbits does not depend on the vomeronasal organ. Physiol Behav. 37(1):123–128. [DOI] [PubMed] [Google Scholar]
- Isogai Y, Si S, Pont-Lezica L, Tan T, Kapoor V, Murthy VN, Dulac C. 2011. Molecular organization of vomeronasal chemoreception. Nature. 478(7368):241–245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnston RE. 1998. Pheromones, the vomeronasal system, and communication. From hormonal responses to individual recognition. Ann NY Acad Sci. 855:333–348. [DOI] [PubMed] [Google Scholar]
- Kang N, McCarthy EA, Cherry JA, Baum MJ. 2011. A sex comparison of the anatomy and function of the main olfactory bulb-medial amygdala projection in mice. Neuroscience. 172:196–204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaur AW, Ackels T, Kuo TH, Cichy A, Dey S, Hays C, Kateri M, Logan DW, Marton TF, Spehr M, et al. 2014. Murine pheromone proteins constitute a context-dependent combinatorial code governing multiple social behaviors. Cell. 157(3):676–688. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Keller M, Lévy F. 2012. The main but not the accessory olfactory system is involved in the processing of socially relevant chemosignals in ungulates. Front Neuroanat. 6:39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Keshavarzi S, Power JM, Albers EH, Sullivan RK, Sah P. 2015. Dendritic organization of olfactory inputs to medial amygdala neurons. J Neurosci. 35(38):13020–13028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Keshavarzi S, Sullivan RK, Ianno DJ, Sah P. 2014. Functional properties and projections of neurons in the medial amygdala. J Neurosci. 34(26):8699–8715. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Keverne EB. 1999. The vomeronasal organ. Science. 286(5440):716–720. [DOI] [PubMed] [Google Scholar]
- Kevetter GA, Winans SS. 1981. a. Connections of the corticomedial amygdala in the golden hamster. I. Efferents of the “vomeronasal amygdala”. J Comp Neurol. 197(1):81–98. [DOI] [PubMed] [Google Scholar]
- Kevetter GA, Winans SS. 1981. b. Connections of the corticomedial amygdala in the golden hamster. II. Efferents of the “olfactory amygdala”. J Comp Neurol. 197(1):99–111. [DOI] [PubMed] [Google Scholar]
- Kobayakawa K, Kobayakawa R, Matsumoto H, Oka Y, Imai T, Ikawa M, Okabe M, Ikeda T, Itohara S, Kikusui T, et al. 2007. Innate versus learned odor processing in the mouse olfactory bulb. Nature. 450:503–508. [DOI] [PubMed] [Google Scholar]
- Kondo Y, Sachs BD. 2002. Disparate effects of small medial amygdala lesions on noncontact erection, copulation, and partner preference. Physiol Behav. 76(4–5):443–447. [DOI] [PubMed] [Google Scholar]
- Lehman MN, Winans SS. 1982. Vomeronasal and olfactory pathways to the amygdala controlling male hamster sexual behavior: autoradiographic and behavioral analyses. Brain Res. 240(1):27–41. [DOI] [PubMed] [Google Scholar]
- Leinders-Zufall T, Lane AP, Puche AC, Ma W, Novotny MV, Shipley MT, Zufall F. 2000. Ultrasensitive pheromone detection by mammalian vomeronasal neurons. Nature. 405(6788):792–796. [DOI] [PubMed] [Google Scholar]
- Licht G, Meredith M. 1987. Convergence of main and accessory olfactory pathways onto single neurons in the hamster amygdala. Exp Brain Res. 69(1):7–18. [DOI] [PubMed] [Google Scholar]
- Lin D, Boyle MP, Dollar P, Lee H, Lein ES, Perona P, Anderson DJ. 2011. Functional identification of an aggression locus in the mouse hypothalamus. Nature. 470(7333):221–226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luo M, Fee MS, Katz LC. 2003. Encoding pheromonal signals in the accessory olfactory bulb of behaving mice. Science. 299(5610):1196–1201. [DOI] [PubMed] [Google Scholar]
- Matsuo T, Hattori T, Asaba A, Inoue N, Kanomata N, Kikusui T, Kobayakawa R, Kobayakawa K. 2015. Genetic dissection of pheromone processing reveals main olfactory system-mediated social behaviors in mice. Proc Natl Acad Sci USA. 112(3):E311–E320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meredith M. 1986. Vomeronasal organ removal before sexual experience impairs male hamster mating behavior. Physiol Behav. 36(4):737–743. [DOI] [PubMed] [Google Scholar]
- Meredith M. 1998. Vomeronasal, olfactory, hormonal convergence in the brain. Cooperation or coincidence? Ann NY Acad Sci. 855:349–361. [DOI] [PubMed] [Google Scholar]
- Meredith M, O’Connell RJ. 1979. Efferent control of stimulus access to the hamster vomeronasal organ. J Physiol. 286:301–316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meredith M, Westberry JM. 2004. Distinctive responses in the medial amygdala to same-species and different-species pheromones. J Neurosci. 24(25):5719–5725. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Munger SD, Leinders-Zufall T, McDougall LM, Cockerham RE, Schmid A, Wandernoth P, Wennemuth G, Biel M, Zufall F, Kelliher KR. 2010. An olfactory subsystem that detects carbon disulfide and mediates food-related social learning. Curr Biol. 20(16):1438–1444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pankevich DE, Baum MJ, Cherry JA. 2004. Olfactory sex discrimination persists, whereas the preference for urinary odorants from estrous females disappears in male mice after vomeronasal organ removal. J Neurosci. 24(42):9451–9457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Papes F, Logan DW, Stowers L. 2010. The vomeronasal organ mediates interspecies defensive behaviors through detection of protein pheromone homologs. Cell. 141(4):692–703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perez-Gomez A, Bleymehl K, Stein BM, Pyrski M, Birnbaumer L, Munger SD, Leinders-Zufall T, Zufall F, Chamero P. 2015. Innate predator odor aversion driven by parallel olfactory subsystems that converge in the ventromedial hypothalamus. Curr Biol. 25(10):1340–1346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roberts SA, Simpson DM, Armstrong SD, Davidson AJ, Robertson DH, McLean L, Beynon RJ, Hurst JL. 2010. Darcin: a male pheromone that stimulates female memory and sexual attraction to an individual male’s odor. BMC Biol. 8:75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Root CM, Denny CA, Hen R, Axel R. 2014. The participation of cortical amygdala in innate, odour-driven behaviour. Nature. 515(7526):269–273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Samuelsen CL, Meredith M. 2009. a. Categorization of biologically relevant chemical signals in the medial amygdala. Brain Res. 1263:33–42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Samuelsen CL, Meredith M. 2009. b. The vomeronasal organ is required for the male mouse medial amygdala response to chemical-communication signals, as assessed by immediate early gene expression. Neuroscience. 164(4):1468–1476. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scalia F, Winans SS. 1975. The differential projections of the olfactory bulb and accessory olfactory bulb in mammals. J Comp Neurol. 161(1):31–55. [DOI] [PubMed] [Google Scholar]
- Schaal B, Coureaud G, Langlois D, Giniès C, Sémon E, Perrier G. 2003. Chemical and behavioural characterization of the rabbit mammary pheromone. Nature. 424(6944):68–72. [DOI] [PubMed] [Google Scholar]
- Thompson JA, Salcedo E, Restrepo D, Finger TE. 2012. Second-order input to the medial amygdala from olfactory sensory neurons expressing the transduction channel TRPM5. J Comp Neurol. 520(8):1819–1830. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tirindelli R, Mucignat-Caretta C, Ryba NJ. 1998. Molecular aspects of pheromonal communication via the vomeronasal organ of mammals. Trends Neurosci. 21(11):482–486. [DOI] [PubMed] [Google Scholar]
- Westberry J, Meredith M. 2003. a. The influence of chemosensory input and gonadotropin releasing hormone on mating behavior circuits in male hamsters. Brain Res. 974(1–2):1–16. [DOI] [PubMed] [Google Scholar]
- Westberry JM, Meredith M. 2003. b. Pre-exposure to female chemosignals or intracerebral GnRH restores mating behavior in naive male hamsters with vomeronasal organ lesions. Chem Senses. 28(3):191–196. [DOI] [PubMed] [Google Scholar]
- Westberry JM, Meredith M. 2016. GABAergic mechanisms contributing to categorical amygdala responses to chemosensory signals. Neuroscience. 331:186–196. [DOI] [PMC free article] [PubMed] [Google Scholar]