Abstract
T1R2-T1R3 is a heteromeric receptor that binds sugars, high potency sweeteners, and sweet taste blockers. In rodents, T1R2-T1R3 is largely responsible for transducing sweet taste perception. T1R2-T1R3 is also expressed in non-taste tissues, and a growing body of evidence suggests that it helps regulate glucose and lipid metabolism. It was previously shown that clofibric acid, a blood lipid-lowering drug, binds T1R2-T1R3 and inhibits its activity in vitro. The purpose of this study was to determine whether clofibric acid inhibits sweetness perception in humans and is, therefore, a T1R2-T1R3 antagonist in vivo. Fourteen participants rated the sweetness intensity of 4 sweeteners (sucrose, sucralose, Na cyclamate, acesulfame K) across a broad range of concentrations. Each sweetener was prepared in solution neat and in mixture with either clofibric acid or lactisole. Clofibric acid inhibited sweetness of every sweetener. Consistent with competitive binding, inhibition by clofibric acid was diminished with increasing sweetener concentration. This study provides in vivo evidence that the lipid-lowering drug clofibric acid inhibits sweetness perception and is, therefore, a T1R carbohydrate receptor inhibitor. Our results are consistent with previous in vitro findings. Given that T1R2-T1R3 may in part regulate glucose and lipid metabolism, future studies should investigate the metabolic effects of T1R inhibition.
Key words: cholesterol, fibrates, human, lipid lowering, sweet, sweetness inhibition, sweeteners, sugars, taste
Introduction
In vitro functional expression and mouse knock-out data suggest that sweet taste perception is chiefly transduced by T1R2-T1R3, a heteromeric carbohydrate receptor of the 7 transmembrane family, Class C (Nelson et al. 2001; Li et al. 2002). T1R2-T1R3 is activated by several mono- and disaccharides as well as high potency sweeteners (HPSs) (Cui et al. 2006). In humans it is inhibited by sodium lactisole, an inverse agonist which binds the transmembrane domain of human-T1R3 (Jiang et al. 2005; Galindo-Cuspinera and Breslin 2006). A growing body of evidence shows that T1R2-T1R3 is expressed in tissues throughout the body and that it serves physiological roles in glucose metabolism, insulin secretion, lipid metabolism, adipocyte function, and reproductive health (Margolskee et al. 2007; Mosinger et al. 2013; Smith et al. 2016).
Several studies have established a role for T1R2-T1R3 in glucose metabolism. In vitro, HPSs induce the secretion of GLP-1 by intestinal L-cells (Jang et al. 2007; Margolskee et al. 2007). High potency sweeteners upregulate the expression of glucose transporters in the intestine (Mace et al. 2007; Moran et al. 2010). T1R2-T1R3 is also expressed in pancreatic beta cells and in vitro stimulation with a HPS induces insulin release (Nakagawa et al. 2009; Nakagawa et al. 2013). Several clinical studies have shown that a HPS preload alters the plasma insulin, glucose, and incretin responses to an oral glucose load, despite the fact that HPS are not by themselves insulinogenic, neither do they contain glucose nor any calories of note (Brown et al. 2009, 2012; Pepino et al. 2013; Temizkan et al. 2015).
T1R2-T1R3 is also expressed in adipocytes (Masubuchi et al. 2013). Stimulation with HPSs alters adipogenesis and lipolysis in vitro (Masubuchi et al. 2013; Simon et al. 2013). When fed an obesogenic diet, T1R2 KO mice and T1R3 KO mice are protected against fat mass gain (Simon et al. 2014; Smith et al. 2016). This may be due to oral perceptual influences on nutrient utilization and metabolism (Glendinning et al. 2012), or to a reduced ability of adipocytes to sense and transport glucose internally (Smith et al. 2016).
Although the relationships between T1R2-T1R3 agonists and human physiology have come under scrutiny, the physiological effects of T1R2-T1R3 antagonists are less clearly understood. Genetic knock-out studies represent the ultimate loss of function of the T1R2-T1R3 receptors, but pharmacological inhibition may have similar, albeit less potent, effects on metabolism. It has been shown that the T1R3 inhibitor sodium lactisole increases plasma glucose ‘area under the curve’ (AUC) response to a glucose load (Gerspach et al. 2011). This effect is consistent with reduced stimulation of insulin by T1Rs (Jiang et al. 2005; Galindo-Cuspinera and Breslin 2006; Hamano et al. 2015). Whether other sweet taste inhibitors have similar physiological effects on human physiology is unclear.
Lactisole is structurally a phenoxy propionic acid, and so has molecular similarity to other members of this class, including the phenoxy herbicides and the metabolic fibrate drugs. The fibrates are a class of “plasma lipid lowering” drug which are presumed to exert their effects by binding and inhibiting the nuclear receptor proteins, the peroxisome proliferator-activated receptors (PPARs), especially PPAR-α (Lalloyer and Staels 2010). The PPARs form heterodimers with the retinoid X receptors (RXR) to regulate gene expression and modulate metabolism, among many other physiological functions (Staels et al. 1998; Lalloyer and Staels 2010). These heterodimers are the presumed mechanism for how fibrate drugs are able to lower plasma lipids in patients. In addition to binding PPARs, fibrate drugs behave pharmacologically in vitro like the T1R2-T1R3 inhibitor lactisole and bind the transmembrane domain of human T1R3 (Maillet et al. 2009). Curiously, fibrates bind PPARs and T1R3 with a similar affinity (Maillet et al. 2009).
Fibrates have clinical physiological effects on metabolism and are known to bind the sweet taste receptor T1R2-T1R3. This observation raises the question of whether fibrates might exert some of their physiological effects through their actions on T1R2-T1R3. Thus, we wished to determine whether fibrates inhibit T1R2-T1R3 in vivo in humans. As one measure of this effect, we sought in the present study to determine whether the fibrate drug, clofibric acid, inhibits perception of sweetness in humans and is, therefore, a T1R2-T1R3 receptor antagonist in conscious behaving humans. We tested 4 sweeteners (sucrose, sucralose, acesulfame-K, and Na cyclamate) in mixture with either clofibric acid or the positive control sweet taste inhibitor, sodium lactisole.
Methods
Subjects
Fourteen adult subjects (5 males, 9 females) aged between 18 and 29 years were paid to participate after providing their informed consent on Rutgers University’s Institutional Review Board (IRB) approved forms. All participants were from Rutgers University and the surrounding community. Each subject participated in 6 sessions. They were asked not to eat, drink, or smoke 1 h prior to each session. This protocol complies with the Declaration of Helsinki for Medical Research involving Human Subjects and the study was approved by the Institutional Review Board at Rutgers University.
Training
Subjects were trained in the use of a general Labeled Magnitude Scale (gLMS) following standard published procedures (Green et al. 1993). The top of the scale was described as the strongest imaginable sensation of any kind (Bartoshuk et al. 2004). This gLMS required participants to rate the perceived intensity along a vertical axis lined with the following adjectives: barely detectable, weak, moderate, strong, very strong, and strongest imaginable. The adjectives are spaced semi-logarithmically, based upon experimentally determined intervals to yield ratio quality data. The subjects were shown both adjectives and numbers on the scale.
Stimuli
The sweet taste stimuli were sucrose (ranging in concentration from 0.0292 to 1.64 M), sucralose (7.95 × 10−6 to 7.95 × 10−2 M), acesulfame potassium (1.57 × 10−5 to 0.15 M), and sodium cyclamate (4.97 × 10−4 to 0.496 M). Concentrations increased in quarter- and semi-logarithmic increments and captured asymptotic sweetness intensity. The sweet taste inhibitors used were 1.37 mM sodium lactisole and 1.37 mM clofibric acid. Each sweet compound was presented neat and in combination with each inhibitor. Clofibric acid was neutralized with sodium hydroxide to match the pH of the neat solution.
Aqueous solutions were prepared every other day with Millipore filtered water and stored in amber glass at 4 °C. All solutions were removed from refrigerator and allowed to rise to room temperature for at least 1 h prior to tasting. All solutions were fully dissolved and there were no visible signs of undissolved solids or precipitation from solutions.
Stimulus delivery
Sample presentation was randomized using a random integer generator (random.org) and 10 ml of each solution was presented in 30 ml polyethylene medicine cups (Dynarex) on a numbered tray. Each session consisted of 2 trials with an interstimulus interval of 30 s and a 5-min interval between trials. For each sample, subjects held 10 ml of solution in the mouth for 5 s and rated the taste qualities (sweet, bitter, salty, sour, savory) and intensity on a gLMS before expectorating. Subjects separately rated each taste quality on a gLMS. After expectorating, subjects rinsed with Millipore water 4 times during the interstimulus interval.
Statistical analysis
For each of the 4 sweeteners studied, 2-way repeated measures analysis of variance (ANOVA) was used to analyze the effects of stimulus (sweetener neat, sweetener + lactisole, sweetener + clofibric acid). Post hoc Tukey’s honestly significant difference (HSD) tests were used to analyze differences among responses.
Results
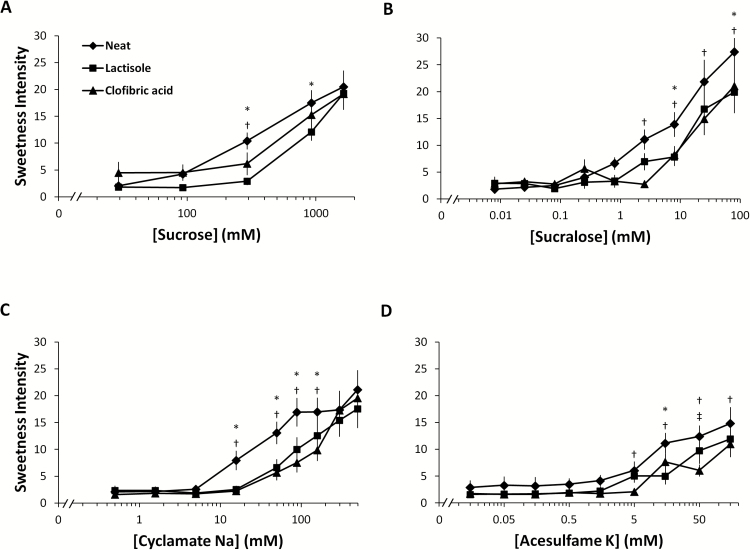
Clofibric acid inhibited the sweetness elicited by all of the sweeteners studied (Figure 1). The main effect of stimulus was significant for sucrose (F 2,26 = 6.4; P < 0.01), sucralose (F 2,26 = 8.4; P < 0.01), acesulfame K (F 2,26 = 9.1; P < 0.01), and Na cyclamate (F 2,26 = 50.2; P < 0.01). There was a significant interaction of stimulus and concentration for each sweetener, indicating that the efficacy of inhibitor was affected by concentration. Consistent with competitive binding, the inhibition by clofibrate was diminished with increasing concentration of sucrose and cyclamate. However, in the case of sucralose and acesulfame K, inhibition was not completely diminished at maximum sweetener concentration.
Figure 1.
Effect of clofibric acid and lactisole on the perceived sweetness elicited by (A) sucrose, (B) sucralose, (C) Na cyclamate, and (D) acesulfame K. Concentration-intensity functions were determined for each sweetener neat, in admixture with lactisole, and in admixture with clofibric acid. The main effect of stimulus was significant for every sweetener studied. Main effects of stimuli (sweetener neat, sweetener + lactisole, sweetener + clofibric acid) were determined using 2-way repeated measures analysis of variance (ANOVA). Pairwise differences were determined using post hoc Tukey HSD tests (n = 14). Each subject was tested in sextuplicate. Letters denote significant differences (P < 0.05) between stimuli. * denotes significant difference between neat and lactisole. † denotes significant difference between neat and clofibric acid. ‡ denotes significant differences between lactisole and clofibric acid.
Clofibric acid and lactisole were similar in terms of inhibitory potency. For sucrose, however, the effect of lactisole was greater than the effect of clofibric acid (P < 0.05). There were no other significant differences in main effects between clofibric acid and lactisole, although clofibric acid tended to be a more potent inhibitor of sweet taste elicited by acesulfame K (Figure 1D).
There were no significant differences in bitter, salty, sour, or savory qualities between neat and inhibited conditions (not shown). Sucralose and acesulfame K elicited weak bitterness at high concentrations in the neat and inhibited conditions in some subjects. There were no differences in bitterness (P > 0.05). Sucrose elicited only sweetness in the neat and inhibited conditions.
Discussion
These data show that clofibric acid inhibits sweet taste elicited by four different T1R2-T1R3 agonists: a sugar and 3 HPSs. This is consistent with previous findings that clofibric acid binds a transmembrane domain of T1R2-T1R3 (Maillet et al. 2009). These data also further support the hypothesis that T1R2-T1R3 is largely responsible for transducing sweet taste in humans.
Although clofibric acid and lactisole inhibited sweet taste, neither compound abolished sweet taste completely. Also, sweetness inhibition was overcome at higher concentrations of sucrose and cyclamate. This observation is consistent with competitive inhibition, similar to previously studied sweet taste inhibitors (Schiffman et al. 1999; Winnig et al. 2007). The highest molar concentrations of sucrose and cyclamate used in this study were greater than those of acesulfame K and sucralose. It is unclear whether inhibition of acesulfame K and sucralose sweetness would continue at higher levels of these sweeteners or whether sweetness would return to uninhibited high levels.
As previously reported (Antenucci and Hayes 2014), high levels of sucralose (25 and 79 mM) and acesulfame K (50 and 150 mM) elicited bitter taste in some participants. In mixtures of sweet and bitter compounds, inhibition of sweetness has been shown to enhance bitterness (Lawless 1979). However, despite reducing sweetness from sucralose and acesulfame K, neither inhibitor affected ratings for bitter, sour, salty, or savory taste qualities. It is possible that some participants conflated bitterness with metallic tastes or other off tastes that we did not measure.
Clofibric acid only modestly inhibited sweet taste from sucrose relative to the effects of lactisole. This finding could be explained by non-T1R3 mediated sweet taste (Damak et al. 2003). KATP channels are part of a metabolic signaling pathway and are expressed in many taste cells and may contribute to depolarization of these cells and activation of downstream signaling events (Merigo et al. 2011; Yee et al. 2011). In T1R3 knockout mice, nerve responses to glucose were diminished but not abolished (Damak et al. 2003), indicating residual signaling from sugars in mice.
Subjects in this study reported sweet water taste after expectorating either lactisole or clofibric acid (not shown). Previous studies have shown that lactisole elicits a sweet water-taste, most likely because it is a T1R2-T1R3 inverse agonist (Galindo-Cuspinera et al. 2006). Our finding that clofibric acid elicits a similar rebound effect suggests that, like lactisole, it too is an inverse agonist of T1R2-T1R3.
Clofibric acid is used pharmacologically to lower blood cholesterol and triglycerides. Treatment with clofibric acid reduces hepatic lipid deposition (Ye et al. 2001), as well as fasting and postprandial glycemia (Enger et al. 1977; Ferrari et al. 1977; Ratzmann et al. 1983). Clofibric acid is hypothesized to act through peroxisome proliferator-activated receptor α (PPARα) activation (Staels et al. 1998). PPARα is a transcription factor that upregulates genes responsible for fatty acid uptake, beta oxidation, lipolysis, and lipoprotein synthesis (Lalloyer and Staels 2010). Although clofibric acid is known to bind PPARα, it is not known whether all of its physiological effects are due exclusively to PPARα agonism.
The effects of chronic treatment with clofibric acid share some overlap with the effects of T1R2 and T1R3 ablation. Similar to clofibric acid treatment, T1R ablation alters glucose and lipid metabolism (Margolskee et al. 2007; Glendinning et al. 2012; Simon et al. 2014; Smith et al. 2016). T1R2 knockout animals are protected against diet induced obesity, hepatic lipid deposition, and hypersinsulinemia (Simon et al. 2014; Smith et al. 2016). Like the T1R2 knockout, T1R3 knockout animals are protected against diet induced weight gain and fat mass gain, independent of energy intake (Glendinning et al. 2012).
T1R knockouts may be protected against lipid accumulation because of impaired assimilation of dietary carbohydrate. T1R2-T1R3 is expressed in many tissues throughout the body, including the intestine (Margolskee et al. 2007), liver (Taniguchi 2004), pancreas (Taniguchi 2004), adipocytes (Masubuchi et al. 2013), and brain (Ren et al. 2009). T1R2-T1R3 agonists enhance intestinal glucose absorption (Dyer et al. 2007), incretin response (Brown et al. 2009), insulin secretion (Pepino et al. 2013), and adipocyte differentiation (Masubuchi et al. 2013). Sodium lactisole, a T1R2-T1R3 inhibitor, has been shown to lower insulin secretion and GLP-1 secretion in vitro (Jang et al. 2007; Nakagawa 2011) and in vivo (Gerspach et al. 2011). Given that clofibric acid inhibits T1R2-T1R3, it is possible that its metabolic effects may be due, in part, to T1R inhibition.
These data show that clofibric acid inhibits sweet taste perception and is thus not only a T1R2-T1R3 receptor inhibitor in vitro but appears to inhibit it in vivo as well. These perceptual data are supported by previous findings that clofibric acid binds selectively and inhibits T1R3 in vitro. Future studies should investigate the metabolic effects of T1R inhibition to determine the degree to which fibrates and other T1R inhibitors influence dyslipidemias and cholesterol.
Funding
The study was supported in part by NIH DC 014286 (P.A.S.B.).
Conflict of interest statement
The authors claim no conflict of interest.
Acknowledgements
The study was designed by P.A.S.B. and M.K., the study was executed by M.K., data analyses and writing were performed by P.A.S.B. and M.K. Paul Breslin is the guarantor of this work.
References
- Antenucci RG, Hayes JE. 2015. Nonnutritive sweeteners are not supernormal stimuli. Int J Obes (Lond). 39(2):254–259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bartoshuk LM, Duffy VB, Green BG, Hoffman HJ, Ko CW, Lucchina LA, Marks LE, Snyder DJ, Weiffenbach JM. 2004. Valid across-group comparisons with labeled scales: the gLMS versus magnitude matching. Physiol Behav. 82(1):109–114. [DOI] [PubMed] [Google Scholar]
- Brown RJ, Walter M, Rother KI. 2009. Ingestion of diet soda before a glucose load augments glucagon-like peptide-1 secretion. Diabetes Care. 32(12):2184–2186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown RJ, Walter M, Rother KI. 2012. Effects of diet soda on gut hormones in youths with diabetes. Diabetes Care. 35(5):959–964. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cui M, Jiang P, Maillet E, Max M, Margolskee RF, Osman R. 2006. The heterodimeric sweet taste receptor has multiple potential ligand binding sites. Curr Pharm Des. 12(35):4591–4600. [DOI] [PubMed] [Google Scholar]
- Damak S, Rong M, Yasumatsu K, Kokrashvili Z, Varadarajan V, Zou S, Jiang P, Ninomiya Y, Margolskee RF. 2003. Detection of sweet and umami taste in the absence of taste receptor T1r3. Science. 301(5634):850–853. [DOI] [PubMed] [Google Scholar]
- Dyer J, Daly K, Salmon KS, Arora DK, Kokrashvili Z, Margolskee RF, Shirazi-Beechey SP. 2007. Intestinal glucose sensing and regulation of intestinal glucose absorption. Biochem Soc Trans. 35(Pt 5):1191–1194. [DOI] [PubMed] [Google Scholar]
- Enger SC, Johnsen V, Samuelsen A, Laws EA. 1977. The effect of clofibrate on glucose tolerance, insulin secretion, triglycerides and fibrinogen in patients with coronary heart disease. Acta Med Scand. 201(6):563–566. [DOI] [PubMed] [Google Scholar]
- Ferrari C, Frezzati S, Romussi M, Bertazzoni A, Testori GP, Antonini S, Paracchi A. 1977. Effects of short-term clofibrate administration on glucose tolerance and insulin secretion in patients with chemical diabetes or hypertriglyceridemia. Metabolism. 26(2):129–139. [DOI] [PubMed] [Google Scholar]
- Galindo-Cuspinera V, Breslin PA. 2006. The liaison of sweet and savory. Chem Senses. 31:221–225. [DOI] [PubMed] [Google Scholar]
- Galindo-Cuspinera V, Winnig M, Bufe B, Meyerhof W, Breslin PA. 2006. A TAS1R receptor-based explanation of sweet ‘water-taste’. Nature. 441:354–357. [DOI] [PubMed] [Google Scholar]
- Gerspach AC, Steinert RE, Schönenberger L, Graber-Maier A, Beglinger C. 2011. The role of the gut sweet taste receptor in regulating GLP-1, PYY, and CCK release in humans. Am J Physiol Endocrinol Metab. 301(2):E317–E325. [DOI] [PubMed] [Google Scholar]
- Glendinning JI, Gillman J, Zamer H, Margolskee RF, Sclafani A. 2012. The role of T1r3 and Trpm5 in carbohydrate-induced obesity in mice. Physiol Behav. 107(1):50–58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Green BG, Shaffer GS, Gilmore MM. 1993. Derivation and evaluation of a semantic scale of oral sensation magnitude with apparent ratio properties. Chem Senses. 18:683–702. [Google Scholar]
- Hamano K, Nakagawa Y, Ohtsu Y, Li L, Medina J, Tanaka Y, Masuda K, Komatsu M, Kojima I. 2015. Lactisole inhibits the glucose-sensing receptor T1R3 expressed in mouse pancreatic β-cells. J Endocrinol. 226(1): 57–66. [DOI] [PubMed] [Google Scholar]
- Jang HJ, Kokrashvili Z, Theodorakis MJ, Carlson OD, Kim BJ, Zhou J, Kim HH, Xu X, Chan SL, Juhaszova M, et al. 2007. Gut-expressed gustducin and taste receptors regulate secretion of glucagon-like peptide-1. Proc Natl Acad Sci USA. 104:15069–15074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jiang P, Cui M, Zhao B, Liu Z, Snyder LA, Benard LM, Osman R, Margolskee RF, Max M. 2005. Lactisole interacts with the transmembrane domains of human T1R3 to inhibit sweet taste. J Biol Chem. 280(15):15238–15246. [DOI] [PubMed] [Google Scholar]
- Lalloyer F, Staels B. 2010. Fibrates, glitazones, and peroxisome proliferator-activated receptors. Arterioscler Thromb Vasc Biol. 30(5):894–899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lawless HT. 1979. Evidence for neural inhibition in bittersweet taste mixtures. J Comp Physiol Psychol. 93(3):538–547. [DOI] [PubMed] [Google Scholar]
- Li X, Staszewski L, Xu H, Durick K, Zoller M, Adler E. 2002. Human receptors for sweet and umami taste. Proc Natl Acad Sci USA. 99(7):4692–4696. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mace OJ, Affleck J, Patel N, Kellett GL. 2007. Sweet taste receptors in rat small intestine stimulate glucose absorption through apical GLUT2. J Physiol. 582(Pt 1):379–392. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maillet EL, Margolskee RF, Mosinger B. 2009. Phenoxy herbicides and fibrates potently inhibit the human chemosensory receptor subunit T1R3. J Med Chem. 52(21):6931–6935. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Margolskee RF, Dyer J, Kokrashvili Z, Salmon KS, Ilegems E, Daly K, Maillet EL, Ninomiya Y, Mosinger B, Shirazi-Beechey SP. 2007. T1R3 and gustducin in gut sense sugars to regulate expression of Na+-glucose cotransporter 1. Proc Natl Acad Sci USA. 104(38):15075–15080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Masubuchi Y, Nakagawa Y, Ma J, Sasaki T, Kitamura T, Yamamoto Y, Kurose H, Kojima I, Shibata H. 2013. A novel regulatory function of sweet taste-sensing receptor in adipogenic differentiation of 3T3-L1 cells. PLoS One. 8(1):e54500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Merigo F, Benati D, Cristofoletti M, Osculati F, Sbarbati A. 2011. Glucose transporters are expressed in taste receptor cells. J Anat. 219(2):243–252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moran AW, Al-Rammahi MA, Arora DK, Batchelor DJ, Coulter EA, Daly K, Ionescu C, Bravo D, Shirazi-Beechey SP. 2010. Expression of Na+/glucose co-transporter 1 (SGLT1) is enhanced by supplementation of the diet of weaning piglets with artificial sweeteners. Br J Nutr. 104(5):637–646. [DOI] [PubMed] [Google Scholar]
- Mosinger B, Redding KM, Parker MR, Yevshayeva V, Yee KK, Dyomina K, Li Y, Margolskee RF. 2013. Genetic loss or pharmacological blockade of testes-expressed taste genes causes male sterility. Proc Natl Acad Sci. 110(30):12319–12324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakagawa Y, Nagasawa M, Yamada S, Hara A, Mogami H, Nikolaev VO, Lohse MJ, Shigemura N, Ninomiya Y, Kojima I. 2009. Sweet taste receptor expressed in pancreatic beta-cells activates the calcium and cyclic AMP signaling systems and stimulates insulin secretion. PLoS One. 4(4):e5106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakagawa Y. 2011. Function of sweet taste receptor in pancreatic beta-cells. Seikagaku. 83(7):647–651. [PubMed] [Google Scholar]
- Nakagawa Y, Nagasawa M, Mogami H, Lohse M, Ninomiya Y, Kojima I. 2013. Multimodal function of the sweet taste receptor expressed in pancreatic β-cells: generation of diverse patterns of intracellular signals by sweet agonists. Endocr J. 60(10):1191–1206. [DOI] [PubMed] [Google Scholar]
- Nelson G, Hoon MA, Chandrashekar J, Zhang Y, Ryba NJ, Zuker CS. 2001. Mammalian sweet taste receptors. Cell. 106(3):381–390. [DOI] [PubMed] [Google Scholar]
- Pepino MY, Tiemann CD, Patterson BW, Wice BM, Klein S. 2013. Sucralose affects glycemic and hormonal responses to an oral glucose load. Diabetes Care. 36(9):2530–2535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ratzmann ML, Rjasanowski I, Bruns W, Ratzmann KP. 1983. Effects of clofibrate therapy on glucose tolerance, insulin secretion and serum lipids in subjects with hyperlipoproteinemia and impaired glucose tolerance. A follow-up study over a five-year period. Exp Clin Endocrinol. 82(2):216–221. [DOI] [PubMed] [Google Scholar]
- Ren X, Zhou L, Terwilliger R, Newton SS, de Araujo IE. 2009. Sweet taste signaling functions as a hypothalamic glucose sensor. Front Integr Neurosci. 3:12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schiffman SS, Booth BJ, Sattely-Miller EA, Graham BG, Gibes KM. 1999. Selective inhibition of sweetness by the sodium salt of +/-2-(4-methoxyphenoxy)propanoic acid. Chem Senses. 24(4):439–447. [DOI] [PubMed] [Google Scholar]
- Simon BR, Parlee SD, Learman BS, Mori H, Scheller EL, Cawthorn WP, Ning X, Gallagher K, Tyrberg B, Assadi-Porter FM, et al. 2013. Artificial sweeteners stimulate adipogenesis and suppress lipolysis independently of sweet taste receptors. J Biol Chem. 288:32475–32489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simon BR, Learman BS, Parlee SD, Scheller EL, Mori H, Cawthorn WP, Ning X, Krishnan V, Ma YL, Tyrberg B, et al. 2014. Sweet taste receptor deficient mice have decreased adiposity and increased bone mass. PLoS One. 9:e86454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith KR, Hussain T, Karimian Azari E, Steiner JL, Ayala JE, Pratley RE, Kyriazis GA. 2016. Disruption of the sugar sensing receptor T1R2 attenuates metabolic derangements associated with diet-induced obesity. Am J Physiol Endocrinol Metab. 310:E688–E698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Staels B, Dallongeville J, Auwerx J, Schoonjans K, Leitersdorf E, Fruchart JC. 1998. Mechanism of action of fibrates on lipid and lipoprotein metabolism. Circulation. 98(19):2088–2093. [DOI] [PubMed] [Google Scholar]
- Taniguchi K. 2004. Expression of the sweet receptor protein, T1R3, in the human liver and pancreas. J Vet Med Sci. 66(11):1311–1314. [DOI] [PubMed] [Google Scholar]
- Temizkan S, Deyneli O, Yasar M, Arpa M, Gunes M, Yazici D, Sirikci O, Haklar G, Imeryuz N, Yavuz DG. 2015. Sucralose enhances GLP-1 release and lowers blood glucose in the presence of carbohydrate in healthy subjects but not in patients with type 2 diabetes. Eur J Clin Nutr. 69(2):162–166. [DOI] [PubMed] [Google Scholar]
- Winnig M, Bufe B, Kratochwil NA, Slack JP, Meyerhof W. 2007. The binding site for neohesperidin dihydrochalcone at the human sweet taste receptor. BMC Struct Biol. 7:66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ye JM, Doyle PJ, Iglesias MA, Watson DG, Cooney GJ, Kraegen EW. 2001. Peroxisome proliferator-activated receptor (PPAR)-alpha activation lowers muscle lipids and improves insulin sensitivity in high fat-fed rats: comparison with PPAR-gamma activation. Diabetes. 50(2):411–417. [DOI] [PubMed] [Google Scholar]
- Yee KK, Sukumaran SK, Kotha R, Gilbertson TA, Margolskee RF. 2011. Glucose transporters and ATP-gated K+ (KATP) metabolic sensors are present in type 1 taste receptor 3 (T1r3)-expressing taste cells. Proc Natl Acad Sci USA. 108(13):5431–5436. [DOI] [PMC free article] [PubMed] [Google Scholar]