Abstract
BACKGROUND
More than 20 years ago, it was hypothesized that exposure to prenatal and early postnatal environmental xenobiotics with the potential to disrupt endogenous hormone signaling might be on the causal path to cryptorchidism, hypospadias, low sperm count and testicular cancer. Several consensus statements and narrative reviews in recent years have divided the scientific community and have elicited a call for systematic transparent reviews. We aimed to fill this gap in knowledge in the field of male reproductive disorders.
OBJECTIVE AND RATIONALE
The aim of this study was to systematically synthesize published data on the risk of cryptorchidism, hypospadias, low sperm counts and testicular cancer following in utero or infant exposure to chemicals that have been included on the European Commission's list of Category 1 endocrine disrupting chemicals defined as having documented adverse effects due to endocrine disruption in at least one intact organism.
SEARCH METHODS
A systematic literature search for original peer reviewed papers was performed in the databases PubMed and Embase to identify epidemiological studies reporting associations between the outcomes of interest and exposures documented by biochemical analyses of biospecimens including maternal blood or urine, placenta or fat tissue as well as amnion fluid, cord blood or breast milk; this was followed by meta-analysis of quantitative data.
OUTCOMES
The literature search resulted in 1314 references among which we identified 33 papers(28 study populations) fulfilling the eligibility criteria. These provided 85 risk estimates of links between persistent organic pollutants and rapidly metabolized compounds (phthalates and Bisphenol A) and male reproductive disorders. The overall odds ratio (OR) across all exposures and outcomes was 1.11 (95% CI 0.91–1.35). When assessing four specific chemical subgroups with sufficient data for meta-analysis for all outcomes, we found that exposure to one of the four compounds, p,p′-DDE, was related to an elevated risk: OR 1.35 (95% CI 1.04–1.74). The data did not indicate that this increased risk was driven by any specific disorder.
WIDER IMPLICATIONS
The current epidemiological evidence is compatible with a small increased risk of male reproductive disorders following prenatal and postnatal exposure to some persistent environmental chemicals classified as endocrine disruptors but the evidence is limited. Future epidemiological studies may change the weight of the evidence in either direction. No evidence of distortion due to publication bias was found, but exposure–response relationships are not evident. There are insufficient data on rapidly metabolized endocrine disruptors and on specific exposure–outcome relations. A particular data gap is evident with respect to delayed effects on semen quality and testicular cancer. Although high quality epidemiological studies are still sparse, future systematic and transparent reviews may provide pieces of evidence contributing to the narrative and weight of the evidence assessments in the field.
Keywords: cryptorchidism, endocrine disruption, hypospadias, infertility, prenatal exposure, sperm count, testicular cancer, xenobiotics
Introduction
As early as 1979, Henderson et al. hypothesized that a relative excess of estrogen, in particular at the time of testicle differentiation, is a major risk factor for testicular cancer (Henderson et al., 1979). Fourteen years later, Sharpe and Skakkebaek proposed that not only testicular cancer but also other male reproductive disorders such as cryptorchidism, hypospadias and low sperm counts may share a common fetal origin and that environmental exposure to chemicals with actions mimicking estrogens might play a pivotal role (Sharpe and Skakkebaek, 1993). The underlying mechanisms were thought to include increased negative feedback on the fetal pituitary resulting in reduced levels of gonadotrophins leading to disruption of normal development of the male fetal gonad during the late phase of the first trimester of pregnancy (Sharpe and Skakkebaek, 1993). This so-called estrogen hypothesis has been scrutinized by a large number of studies addressing the risk of male reproductive disorders in boys who, during pregnancy, were exposed to high or low levels of estrogens because of twin pregnancy, first parity, preeclampsia, hyperemesis or intended or incidental treatment with synthetic hormones during first trimester. Three literature reviews provide no support for the estrogen hypothesis except that testicular cancer seems related to high prenatal estrogen exposure (Sever et al., 1997; Storgaard et al., 2006; Martin et al., 2008). In particular, it is notable that prenatal exposure to the highly potent synthetic estrogen diethylstilbestrol is only associated with low sperm count at high doses (Gill et al., 1979; Leary et al., 1984). Subsequently a capacious amount of experimental research has indicated that adverse health effects, including male reproductive disorders, may be related to endocrine disruption of fetal development through xenobiotic interaction with steroid hormone receptors or through interference with the synthesis, secretion, transport, metabolism or degradation of natural hormones (European workshop on the impact of endocrine disrupters on human health and wildlife. Report of proceedings from a workshop held in Weybridge, UK, 2–4 December 1996; World Health Organisation (WHO) and United Nations Environment Program (UNEP), 2012). This broader endocrine disruption hypothesis with focus on environmental pollutants has gained tremendous public and scientific attention in the USA and the European Union over the past 15 years. Numerous narrative reviews and consensus statements have discussed supporting and contradicting evidence based on data of secular trends of male reproductive health outcomes, wildlife impact and exposure levels in various populations, in addition to the large body of in vitro and in vivo experimental data (European workshop on the impact of endocrine disrupters on human health and wildlife. Report of proceedings from a workshop held in Weybridge, UK, 2–4 December 1996; Bergman et al., 2013; Cantonwine et al., 2014; Diamanti-Kandarakis et al., 2009; Lamb et al., 2014; Nohynek et al., 2013; Sharpe, 2001; Sharpe and Irvine, 2004; Skakkebaek et al., 2001; Takahashi et al., 2004; Toppari et al., 2010; Vandenberg et al., 2012; World Health Organisation (WHO) and International Programme on Chemical Safety (IPCS), 2002; World Health Organisation (WHO) and United Nations Environment Program (UNEP), 2012). It is particularly noteworthy that biomonitoring data from all over the world provide unequivocal documentation of human exposure to a range of xenobiotic substances including persistent compounds (Govarts et al., 2012; Lenters et al., 2013; Gyalpo et al., 2016; Hung et al., 2016; Liu et al., 2016; Magulova and Priceputu, 2016) as well as rapidly metabolized and excreted compounds that reach steady state concentrations in human blood and tissues because of continuous exposure (Arbuckle et al., 2016; Zhang et al., 2016). These xenobiotic compounds have been released into the environment intentionally via the widespread application of pesticides or unintentionally by either degradation of industrial products and building materials or by leakage of packing materials into food items (Nam et al., 2010). New exposure pathways are described regularly. For instance, it has recently been reported that semi-volatile polychlorinated biphenyls (PCBs) represent an important inhalation exposure pathway for residents living in PCB contaminated homes containing PCB in calking material, paints and sealant used >40 years ago (Frederiksen et al., 2012; Meyer et al., 2013). Exposure by inhalation and possibly through the skin may more than double the PCB body burden in individuals exposed at the residence or at school (Meyer et al., 2013). Considering the mounting evidence that numerous of these environmental xenobiotics exhibit endocrine activity in in vitro and in vivo assays, there is basis for substantial concern regarding the potential health effects in humans (Gabrielsen and Tanrikut, 2016; McLachlan, 2016; Trasande et al., 2016), even if alarming reports on, for instance, declining sperm counts are circumstantial or not corroborated (Bonde et al., 2011) and the best evidence we have is reassuring (Jorgensen et al., 2012).
However, the epidemiological data on the adverse health effects are sparse, particularly regarding links with prenatal and postnatal exposure, and there is presently no consensus. In 2012, a Global Assessment of the State-of-the-Science of Endocrine Disrupters commissioned by the World Health Organization and United Nations Environment Program concluded that the direct evidence for endocrine disrupting effects with adverse health effects because of exposure to xenobiotic substances is limited in humans, but emphasized the need for continuous attention (European workshop on the impact of endocrine disrupters on human health and wildlife. Report of proceedings from a workshop held in Weybridge, UK, 2–4 December 1996; World Health Organisation (WHO) and United Nations Environment Program (UNEP), 2012). As several epidemiological studies addressing this issue have been published over the past 10 years, a systematic evaluation of the epidemiological evidence on effects of environmental endocrine disrupting xenobiotics including quantitative meta-analyses of data, where feasible, has now become timely (Beronius and Vandenberg, 2015).
Therefore, the objective of this review was to identify and evaluate the epidemiological evidence linking adverse reproductive health disorders with in utero or early postnatal exposure to xenobiotic endocrine disrupting compounds. The key eligibility criteria are documentation of exposure contrast by chemical measurements of the compounds in maternal blood or other biospecimens indicative of fetal or infant exposure. Specifically, we address the following four hypotheses on the risk of adverse male reproductive outcomes following prenatal and postnatal environmental exposure to industrial chemicals classified as endocrine disrupting substances (McCarthy, 2011): (i) that all compounds regardless of specific endocrine disrupting properties carry a risk of reproductive disorders considered as one outcome entity; (ii) that specific compounds are heterogeneous with respect to reproductive disorders considered as one entity; (iii) that all compounds regardless of specific endocrine disrupting properties carry a risk for specific outcomes; and (iv) that specific compounds carry a risk for specific outcomes.
Methods
The review was conducted and reported in accordance with the MOOSE guidelines for Meta-analyses and Systematic reviews of Observational Studies (Stroup et al., 2000).
Protocol and registration
A review protocol was registered at PROSPERO.org with registration number CRD4201603742 prior to initiation of the review process, on 12 April 2016 with amendments on 12 May 2016 (CRD420160374X). The amendments specified the four main hypotheses, provided details on sensitivity analyses and discarded the initial idea to analyze data according to estrogenic or anti-androgenic activity of measured compounds which proved non-feasible. The protocol was updated before the review process and data analysis were initiated.
Information sources
The databases PubMed and Embase were used as they cover the vast majority of relevant journals for the subject.
Eligibility criteria
We conducted a systematic search of original peer-reviewed original papers in English published between 1966 and 12 April 2016 to identify journal articles providing quantitative data on the association between xenobiotic endocrine disrupting chemicals and male reproductive disorders in humans. The complete search specification is provided in the online Supplementary Table 1. Eligibility criteria for inclusion in the systematic review were as follows.
Exposures: Chemicals that by the European Commission are classified as Category 1 endocrine disruptors with high concern in terms of human or wildlife exposure (McCarthy, 2011). Category 1 compounds are defined as chemicals where at least one study has shown endocrine effects in an intact organism. The EU report lists 60 Category 1 substances (29 chemical groups) that are highly persistent or have high current production volume (McCarthy, 2011). In addition we include the polyfluorinated chemicals, a group of ubiquitous biopersistent emerging endocrine disruptors (White et al., 2011) that are receiving increasing attention. Table I lists the specific chemical substances with abbreviations that are referenced in the text and in Tables II–VII.
Outcomes: Cryptorchidism (one or both testicles undescended) ascertained at birth or during childhood, hypospadias, testicular cancer regardless of histological subgroup, and sperm count (number of spermatozoa per volume or mass unit of seminal fluid).
Exposure to specific chemicals in utero or in the first year of life (postnatal exposure) documented by measurements in biological specimens (maternal blood or urine, placenta or fat tissue as well as amnion fluid, cord blood or breast milk). In addition serum concentrations in adult life as proxies for fetal or early life exposure were also included for testicular cancer.
Outcome ascertainment by medical standardized examination or antecedent medical records or reporting to health registries.
Risk estimates (rate risk [RR], OR, hazard ratio [HR]) for an outcome according to higher versus lower levels of prenatal and postnatal exposure defined by exposure contrasts within the given study. Studies reporting alternative measures of association such as difference in mean values of exposure levels in cases and controls were also included, and the authors were contacted to get risk estimates (Hosie et al., 2000; Damgaard et al., 2006; Main et al., 2007; Choi et al., 2012; Fenichel et al., 2012; Komarowska et al., 2015; Virtanen et al., 2012). Several authors responded positively but reanalysis of the original data could not be accomplished within the given time-frame and the studies were therefore not included in the present review.
Table III.
Characteristics of 10 case-referent studies (21 risk estimates) addressing the risk of hypospadias following prenatal and postnatal exposure to endocrine disrupting chemicals.
| Reference | Location | Study population | N Cases/ Referents | Exposure medium | Exposure contrast | Substance | OR | 95% CI | Meta- analysis | CRa | Biasb |
|---|---|---|---|---|---|---|---|---|---|---|---|
| Longnecker et al. (2002) | USA (12 centers) | Collaborative Perinatal Project | 199/552 | Third trimester serum | Highest versus lowest quintiles | p,p′-DDE | 1.2 | 0.60–2.40 | Yes | 8 | 0 |
| McGlynn et al. (2009a) | USA (12 centers) | Collaborative Perinatal Project | 201/593 | Third trimester serum | Highest versus lowest quartile | PCB (sum of 11 congeners) | 1.69 | 1.06–2.68 | Yes | 8 | 1 |
| Trabert et al. (2012) | USA (12 centers) | Collaborative Perinatal Project | 197/557 | Third trimester serum | Highest quartile versus lowest | trans-nonachlor | 1.08 | 0.62–1.89 | Yes | 9 | 0 |
| Oxychlordane | 1.24 | 0.69–2.22 | Yes | 9 | |||||||
| Bhatia et al. (2005) | USA, San Francisco | Child Health and Development Study | 66/283 | Maternal serum | Highest versus lowest quartile | p,p′-DDT | 0.79 | 0.33–1.89 | Yes | 9 | 0 |
| p,p′-DDE | 1.18 | 0.46–3.02 | Yes | 9 | 0 | ||||||
| Small et al. (2009) | USA, Michigan | Michigan Long-term PBB Study | 14/431 | Maternal serum | Highest versus lowest (<LOD). 3 categories | PBB-153 (includes cryptorchidism) | 0.71 | 0.1–3.8 | Yes | 4 | 1 |
| Carmichael et al. (2010) | USA, California | Pregnant women participating in a screening program | 20/28 | Maternal serum | OR for a 1–10 ng/g lipid change in the analyte | PBDE-100 | 1.02 | 0.74–1.35 | Yes | 5 | 1 |
| PCB-153 | 0.84 | 0.30–2.46 | Yes | 5 | 1 | ||||||
| HCB | 0.95 | 0.79–1.13 | Yes | 5 | 1 | ||||||
| p,p′-DDT | 1 | 0.99–1.01 | No | 5 | 1 | ||||||
| p,p′-DDE | 1 | 0.95–1.00 | No | 5 | 1 | ||||||
| Giordano et al. (2010) | Italy, Rome | Children enrolled at two Hospitals | 37/21 | Mid pregnancy serum | Above versus below median | p,p′-DDE | 1.18 | 0.47–6.91 | Yes | 5 | 1 |
| HCB | 5.5 | 1.25–24.31 | Yes | 5 | 1 | ||||||
| PCB (four congeners) | 1.89 | 0.51–6.93 | Yes | 5 | 1 | ||||||
| Rignell-Hydbom et al. (2012) | Sweden | Southen Sweden maternity cohort | 237/237 | Early pregnancy serum | Highest quartile versus lowest | PCB-153 | 0.60 | 0.30–1.19 | Yes | 10 | 0 |
| p,p′-DDE | 1.68 | 0.92–3.08 | Yes | 10 | 0 | ||||||
| HCB | 1.59 | 0.86–2.93 | Yes | 10 | 0 | ||||||
| Jensen et al. (2015) | Denmark | Pregnancy screening registry, Serum Institute | 75/300 | Second trimester amnion fluid | Highest tertile versus lowest | 5cx-MEPP | 0.89 | 0.44–1.80 | Yes | 9 | 1 |
| 7cx-MMeHP | 1.69 | 0.78–3.67 | Yes | 9 | 1 | ||||||
| Toft et al. (2016) | Denmark | Pregnancy screening registry, Serum Institute | 75/300 | Second trimester amnion fluid | Highest tertile versus lowest | PFOS | 0.69 | 0.35–1.38 | Yes | 9 | 1 |
aCR: completeness of reporting on a scale from 0 (low completeness) to 11 (high completeness).
bBias and confounding: 1 = higher risk of bias, 0 = lower risk of bias.
Criteria for exclusion of studies were as follows:
In vitro and in vivo experimental studies.
Studies addressing mechanisms and other outcomes related to endocrine disruption, for example, effects on sexual hormone levels in tissues and measures of semen quality other than sperm concentration.
Studies based upon chemical analysis of exposure after puberty except studies of testicular cancer.
Studies repeating risk estimates reported in previous publications for example, studies addressing risk according to gene polymorphisms for substances, where risk estimates for the entire population were provided earlier.
Ecological studies with exposure information at the population level rather than at the individual level.
Table I.
List of specific chemical substances with abbreviations.
| Bisphenol A | 4,4′-(Propan-2,2-diyl)diphenol |
| mBP | Mono-n-butyl-phthalate |
| Chlordane mix | cis-heptachlordane, cis-chlordane, ocychlordane, trans-nonachlordane, cis-nonachlordane |
| DBP | Di-n-butyl-phthalate |
| DDE | 1,1-Dichloro-2,2-bis(p-chlorophenyl)ethylene |
| o,p′-DDT | 1,1,1-Tricholoro-2-(p-chlororphenyl)-2-(o-chlorophenyl)ethane |
| p,p′-DDT | 1,1,1-Trichloro-2,2-bis(p-chlorophenyl)ethane |
| DEHP | Di-(2-ethylhexyl)phthalate |
| Dieldrin | Derivate of norbornadiene and hexachlorocyclopentadiene |
| HCB | Hexachlorobenzene |
| HCE | Heptachlor epoxide |
| HCCH | b-Hexachlorocyclohexane |
| MEHP | Mono-(2-ethylhexyl)phthalate |
| 5cx-MEPP | Di(2-ethylhexyl)phthalate [metabolite of DEHP] |
| 7cx-MMeHP | Mono(4-ethyl-7-carboxylheptyl)phthalate [metabolite of DiNP] |
| Mirex | Dimerization derivate of hexachlorocyclopentadiene |
| n-NP | n-Nonylphenol |
| t-OP | t-Octylphenol |
| PBB | Polybrominated biphenyl |
| PBDE | Polybrominated diphenyl ether |
| PCB | Polychlorinated biphenyl |
| PCDD/Fs | Polychlorinated dibenzo-p-dioxins/furans |
| PFOS | Perfluorooctane sulfonic acid |
| PFOA | Perfluorooctanoic acid |
| TCDD | 2,3,7,8-Tetrachlorodibenzo-p-dioxin |
Table II.
Characteristics and risk estimates for 10 case-referent studies (18 risk estimates) addressing the risk of cryptorchidism following prenatal and postnatal exposure to endocrine disrupting chemicals.
| Reference | Location | Study population | N Cases/ referents | Exposure medium | Exposure contrast | Substance | OR | 95% CI | CRa | Biasb |
|---|---|---|---|---|---|---|---|---|---|---|
| Longnecker et al. (2002) | USA (12 centers) | Collaborative Perinatal Project | 219/552 | Third trimester serum | Highest versus lowest quintiles | p,p′-DDE | 1.30 | 0.70–2.24 | 9 | 0 |
| Pierik et al. (2007) | USA (12 centers) | Collaborative Perinatal Project | 219/564 | Third trimester serum | 75–90 percentile versus 0–10 percentile | b-HCCH | 2.08 | 1.08–4.01 | 9 | 0 |
| HCE | 0.82 | 0.41–1.65 | ||||||||
| HCB | 0.85 | 0.52–1.40 | ||||||||
| McGlynn et al. (2009a) | USA (12 centers) | Collaborative Perinatal Project | 230/593 | Third trimester serum | Highest quartile versus lowest | PCB (sum of 11 congeners) | 1.41 | 0.90–2.20 | 8 | 0 |
| Trabert et al. (2012) | USA (12 centers) | Collaborative Perinatal Project | 217/557 | Third trimester serum | Highest quartile versus lowest | trans-nonachlor | 1.22 | 0.70–2.12 | 9 | 0 |
| Oxychlordane | 0.95 | 0.55–1.64 | ||||||||
| Bhatia et al. (2005) | USA, San Francisco | Child Health and Development Study | 75/283 | Maternal serum | Highest versus lowest quartile | p,p′- DDT | 1.01 | 0.44–2.28 | 9 | 1 |
| p,p′- DDE | 1.34 | 0.51–3.48 | ||||||||
| Brucker-Davis et al. (2008) | France, Nice | Newborns at maternity clinics in Nice and Grasse. | 56/69 | Colostrum | Highest versus lowest. Three categories | p,p′-DDE | 2.16 | 0.94–4.98 | 7 | 1 |
| PCB (seven congeners) | 2.74 | 1.15–6.53 | ||||||||
| mBP | 2.13 | 0.66–6.83 | ||||||||
| Small et al. (2009) | USA, Michigan | Michigan Long-term PBB Study | 9/464 | Maternal serum | Highest versus lowest (<LOD). Three categories | PBB-153 (Includes hypospadias) | 0.50 | 0.10–4.70 | 4 | 1 |
| Vesterholm et al. (2014) | Denmark and Finland | Danish–Finnish prospective Birth Cohort Study | 107/108 | Cord blood | Highest tertile versus lowest | PFOS | 0.83 | 0.39–1.79 | 9 | 0 |
| PFOA | 0.46 | 0.20–1.20 | 9 | 0 | ||||||
| Jensen et al. (2015) | Denmark | Pregnancy screening registry, Serum Institute | 270/300 | Second trimester amnion fluid | Highest tertile versus lowest | 5cx-MEPP | 0.90 | 0.57–1.41 | 9 | 1 |
| 7cx-MMeHP | 1.28 | 0.80–2.01 | ||||||||
| Toft et al. (2016) | Denmark | Pregnancy screening registry, Serum Institute | 270/300 | Second trimester amnion fluid | Highest tertile versus lowest | PFOS | 1.01 | 0.66–1.53 | 9 | 1 |
aCR: completeness of reporting on a scale from 0 (low completeness) to 11 (high completeness).
bBias and confounding: 1 = higher risk of bias, 0 = lower risk of bias.
Table VII.
ORs (95% CIs) of the summary estimate of analyses for associations between endocrine disrupting chemicals and cryptorchidism, hypospadias and testicular cancer. Strata with three or less risk estimates not eligible for meta-analysis. I2 only available for meta-analyses without bootstrapping (one risk estimate per study).
| Exposures | Outcomes | N studies | N risk estimates (min-max per study) | OR | 95% CI | I2 |
|---|---|---|---|---|---|---|
| All exposures | All outcomes | 19 | 70 (1–10) | 1.11 | 0.91–1.35 | |
| Cryptorchidism | 6 | 16 (1–7) | 1.03 | 0.72–1.47 | ||
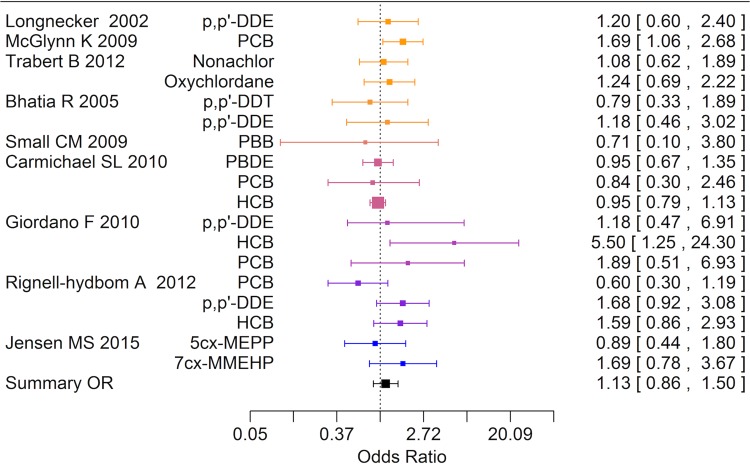
| Hypospadias | 7 | 18 (1–4) | 1.13 | 0.86–1.50 | ||
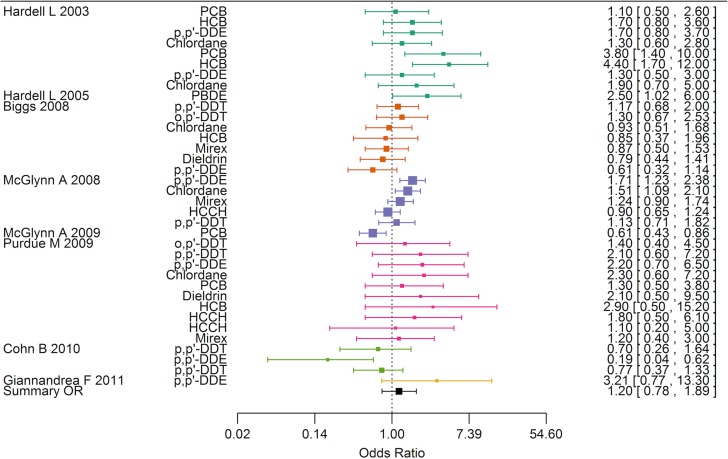
| Testicular cancer | 6 | 36 (2–10) | 1.20 | 0.78–1.89 | ||
| Bias less likely | All outcomes | 5 | 22 (2–7) | 1.12 | 0.81–1.57 | |
| Large studies | All outcomes | 9 | 34 (1–7) | 1.06 | 0.85–1.33 | |
| Excluding studies c. testis studies with adult exposure data | All outcomes | 13 | 34 (1–7) | 1.07 | 0.87–1.32 | |
| + Biopersistence | All outcomes | 17 | 66 (1–10) | 1.15 | 0.92–1.44 | |
| − Biopersistence | All outcomes | 3 | 4 (1–2) | 1.12 | 0.71–1.77 | |
| DDE | All outcomes | 13 | 13 (1) | 1.35 | 1.04–1.74 | 31.91 |
| Cryptorchidism | 3 | 3 (1) | ||||
| Hypospadias | 4 | 4 (1) | 1.38 | 0.93–2.04 | 0.00 | |
| Testicular cancer | 6 | 6 (1) | 1.19 | 0.60–2.36 | 78.05 | |
| DDT | All outcomes | 6 | 6 (1) | 1.07 | 0.81–1.42 | 0.00 |
| Cryptorchidism | 1 | 1 (1) | ||||
| Hypospadias | 1 | 1 (1) | ||||
| Testicular cancer | 4 | 4 (1) | 1.12 | 0.82–1.55 | 0.00 | |
| PCB | All outcomes | 9 | 9 (1) | 1.26 | 0.82–1.96 | 62.41 |
| Cryptorchidism | 2 | 2 (1) | ||||
| Hypospadias | 4 | 4 (1) | 1.11 | 0.61–2.01 | 54.45 | |
| Testicular cancer | 3 | 3 (1) | ||||
| HCB | All outcomes | 7 | 7 (1) | 1.19 | 0.87–1.62 | 40.94 |
| Cryptorchidism | 1 | 1 (1) | ||||
| Hypospadias | 3 | 3 (1) | ||||
| Testicular cancer | 3 | 3 (1) |
Search and study selection
We combined medical subject headings and generic terms for the exposures and outcomes (Supplementary Table 1) and obtained in total 1314 hits after removal of duplicates. Two authors (JPB and EVB) sifted titles and abstracts independently to assess eligibility and retrieved 110 papers for full text reading. Among these, several reports failed to provide quantitative data on exposure levels and others were excluded for other reasons detailed in Supplementary Table 2. Hand searches of the bibliographies of retrieved primary reports and reviews did not capture additional papers.
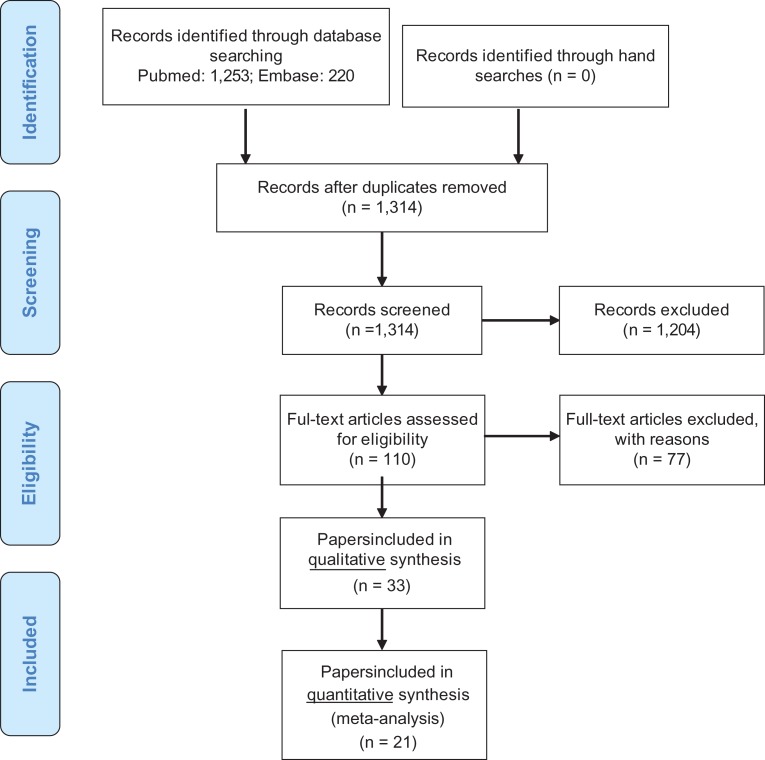
We selected 19 epidemiological studies (21 papers) that provided at least one risk estimate for a male reproductive disorder according to at least one measured xenobiotic classified as an endocrine disruptor. Moreover, nine studies (12 papers) that compared average exposure levels in cases and referents without providing risk estimates were included. Thus, the final database comprised 33 papers included for qualitative systematic analysis and 21 papers included for quantitative meta-analysis. No attempt was made to retrieve papers from the unpublished literature. The steps in the literature search are displayed in Fig. 1.
Figure 1.
Flow diagram showing results of a systematic literature search to identify peer review papers addressing risk of male reproductive disorders following prenatal and infancy exposure to environmental endocrine disrupting compounds.
Data extraction
Descriptive information (Tables II–V) was recorded from each publication using a standardized form constructed prior to the collection process. The standardized data extraction form is provided in Supplementary Table 3. Risk estimates with 95% CI were extracted for each measured compound and outcome. Sperm concentration (sperm count) was taken as the most relevant and robust measure of semen quality despite the substantial within- and between- individual variation (Schrader et al., 1988), while other indicators such as motility and morphology were ignored. When risk estimates were available for groups of chemicals, for example, PCB and PBDE congeners and phthalate metabolites, we used the summed risk estimate, if provided. Otherwise, we selected indicator congeners such as PCB-153 and PBDE-100 reflecting levels of all respective congeners. When risk according to several levels of exposure was reported, the highest level versus the reference category was chosen. In one study, the highest level was greater than the 90th percentile (Pierik et al., 2004); therefore, we used the next highest exposure category in that study to allow for theoretical inverse relationships. If the relevant relative risks were not reported but data were available, the authors of this paper computed risk estimates and CIs.
Table V.
Studies reporting associations between prenatal and postnatal exposure and cryptorchidism or hypospadias without risk estimates (not eligible for meta-analysis).
| Reference | Location | Study population | Study design | N population | Outcome | Bio- specimen | Measured xenobiotics | Results | CRa | Biasb |
|---|---|---|---|---|---|---|---|---|---|---|
| Hosie et al. (2000) | Germany | Chilldren undergoing surgery at one hospital | Cross-sectional | 18/30 | Cryptochidism | Fat samples | 26 persistent organochlorine pesticides and PCBs. | Median values of 21 among 26 chemicals were higher in cases and two compunds reached statistical significance (HCB and HCE) | 3 | 1 |
| Damgaard et al. (2006) | Denmark/Finland | Danish–Finnish prospective Birth Cohort Study | Case-referent | 62/68 | Cryptochidism | Breast milk | 27 biopersistent POPs | Median values of 14 among 27 chemicals were higher in cases. No single compound significantly elevated. Monte Carlo analysis of median values indicated higher levels in cases when the eight compounds with the highest was analysed (P = 0.03) | 9 | 0 |
| Main et al. (2007) | Denmark/Finland | Danish–Finnish prospective Birth Cohort Study | Case-referent | 95/185 placenta samples, 62/68 milk samples | Cryptochidism | Placenta tissue, breast milk | 14 PBDEs | Placenta: no difference between cases and controls for individual congeners or sum of 14 congeners | 9 | 0 |
| Breast milk: no difference between cases and controls for sum of 14 PBDE congeners, while seven individual congenres were significantly higher in cases | ||||||||||
| Virtanen et al. (2012) | Denmark/Finland | Danish–Finnish prospective Birth Cohort Study | Case-referent | 95/185 placenta samples, | Cryptochidism | Serum | 17 TCDDs and PCDD⁄ Fs) and 37 PCBs | No significant difference between cases and controls with respect to sum of 37 PCBs, PCDD or dioxin equivalents | 9 | 0 |
| Shekharyadav et al. (2011) | Northern India | Children with hypospadias and age-matched control children | Case-referent | 80/120 | Hypospadias | Serum | Three HCH isomers, aldrin, dieldrin, endo I, endo II, p,p′-DDT, p,p,-DDE | Higher serum concentrations of all nine pesticides in cases, b-hexachlorohexane (HCH), g-HCH, and p,p′-dichlorodiphenyl -dichloroethylene (p,p′-DDE) significantly higher | 5 | 1 |
| Choi et al. (2012) | Republic of Chorea | No data | Cross-sectional | 80/80 | Hypospadias | Urine Serum | DEHP, DBP, MEHP, MBP, PA, n-NP and t-OP | In urine five of eight substances higher in cases, in serum three substances higher, inconsistent with respect to DEHP (contamination?) | 2 | 1 |
| Fenichel et al. (2012) | Southern France | French studies on cryptorchidism in Nice and Grasse | Case-referent | 46/106 | Cryptochidism | Cord blood | Bisphenol A | Mean unconjugated Bisphenol A marginally higher in cases but not significant (P = 0.38) | 6 | 1 |
| Komarowska et al. (2015) | Poland | Children 0–4 years admitted to paediatric department | Case-referent | 98/57 | Cryptochidism | Serum | Bisphenol A | Free serum BPA level in cryptorchid boys and in the control group was not statistically significant but the conjugated and total serumBPA level was elevated in cryptorchid boys | 3 | 1 |
aCR: completeness of reporting on a scale from 0 (low completeness) to 11 (high completeness).
bBias and confounding: 1 = higher risk of bias, 0 = lower risk of bias.
Quality assessment
Reporting
Each publication was evaluated for completeness of reporting of the following 11 study characteristics modified after Bonzini et al., (2007): (i) study design, (ii) sampling frame and procedures, (iii) inclusion and exclusion criteria, (iv) population characteristics of exposed/unexposed or cases/referents, (v) response rates reported or given implicitly, (vi) methods for exposure measurements (reference to method for chemical analysis or detailed description), (vii) criteria for outcome ascertainment, (viii) external quality assurance program of biochemical analyses (certified laboratory and/or participating in analyses of spiked samples from other laboratories), (ix) detection level, recovery and precision (CV) provided on all issues, (x) statistical analysis, and (xi) reporting of exposure–response relationship (Supplementary Table 3).
We evaluated whether each of these study characteristics were described or not and assigned a value of one if the criterion was fulfilled and zero if not. Giving equal weight to each of the 11 items, we considered completeness of reporting as sufficient if the sum of the 0/1 scores for each paper was ≥8 (Bonzini et al., 2007). Completeness of reporting is not a direct measure of quality but high completeness is needed for adequate evaluation of bias and confounding.
Bias and confounding
The decision to limit the review to observational epidemiological studies applying biological monitoring for exposure assessment is considered of major importance to counteract biased findings because of differential recall of exposure often introduced by self-reports or interview information. For each study, other sources of bias were evaluated against a predefined list of seven potential sources of bias and confounding adapted from validated checklists in order to fit the type of studies and research questions addressed in this review (Shamliyan et al., 2010); these are as follows:
Reporting of tested hypotheses: Many studies perform multiple comparisons because numerous chemical compounds have been measured. A high risk of bias towards inflated risk estimates is likely if risk estimates are selectively reported compared to objectives or available data.
Sample size justification (power calculations and/or addressing sample size in discussion): Small numbers of cases and/or exposed may increase the risk of false negative reporting and thus cause bias towards the null.
Selection bias due to attrition in follow-up studies and non-response in case-referent studies: Attrition/non-response >20% or attrition/non-response differing by >10% in exposed/cases and unexposed/referents was considered as a potential cause of bias in an unpredictable direction.
Information bias (outcome ascertainment): Outcomes identified by patient recall in questionnaires, by interview or medical examination not blinded towards exposure status is a likely cause of inflated risk estimates because of differential exposure misclassification.
Confounding: Failure to account by study design or analysis for major potentially confounding factors including maternal age, parity, prematurity, social class and ethnicity was considered as high risk of bias in unpredictable directions. Other potentially confounding factors were considered on an outcome specific basis: Cryptorchidism is a strong risk factor for testis cancer and low sperm count, sexual abstinence and maternal smoking during pregnancy is related to sperm count and the sons age is related to risk of testis cancer. Weak and/or uncertain risk factors such as paternal age, maternal consumption of coffee and alcoholic beverages, infections, malformed uterus and poorly controlled diabetes were not considered.
Measuring of confounding factors: Potential bias in an unpredictable direction was considered if measures of confounding factors were invalid or inadequate.
Exposure contrast: Studies contrasting exposure levels by the median split rather than by at least exposure-tertiles were considered at risk of bias towards the null because of insufficient exposure contrast.
We considered selective reporting related to multiple testing (1), selection bias (3), information bias related to outcome ascertainment (4) and failure to account for major confounding factors (5) as the most important threats to validity. Accordingly, we categorized a study as at higher risk of bias if two or more of these sources of bias were present.
Risk of bias across studies was evaluated by means of funnel plots to assess risk of publication bias.
Meta-analysis
We applied a stepwise approach addressing four different scenarios for the association between chemical exposure and included outcomes with increasing level of specificity. Only cryptorchidism, hypospadias and testicular cancer were included in Steps (1)–(4) below, as none of the four papers addressing sperm count provided risk estimates. The following associations were analysed:
The association between all types of endocrine disrupting chemicals with all outcomes assuming that effects occur regardless of specific mechanisms and that the four outcomes are manifestations of the same underlying condition (testicular dysgenesis syndrome;Skakkebaek, 2002). This analysis assumes that all types of examined individual compounds are proxies for mixtures of substances that carry the same risk for the adverse reproductive outcomes. This assumption is very unlikely to be true, but the analysis is informative when considering global statements on the impact of endocrine disruptors that do not single out individual substances or mixtures of particular concern (Bergman et al., 2013).
The associations between specific chemicals and all outcomes. Hereby, we account for different endocrine disrupting actions and potencies exhibited by different compounds and address the testicular dysgenesis hypothesis stating that all male reproductive outcomes at least partly are manifestations of the same underlying causal agents operating in early life (Skakkebaek et al., 2001; Skakkebaek, 2003, 2004). One study reported multiple PCB and PBDE congener specific risk estimates but no summary estimate (Carmichael et al., 2010). For this study, we selected the PCB-153 and PBDE-100 as indicators of the total exposure to these compounds and did not include the other congener specific estimates (12 estimates). Another study reported the p,p′-DDE exposure estimate as well as an estimate for the sum of HCB and p,p′-DDE but no separate estimate for HCB (too few cases). In this case, we used the sum of the p,p′-DDE and HCB estimate (Giannandrea et al., 2011).
The associations between all exposures and specific outcomes, assuming that endocrine disrupting chemicals in general carry a risk for some but not other male reproductive disorders.
Each of the three outcomes individually in relation to specific compounds or group of compounds under the assumption of specific mechanisms and specific outcomes, but without an a priori ranking of more or less likely specific hypotheses. These exploratory analyses have a narrow scope because data are limited. We only included exposure–outcome estimates in the meta-analysis if four or more risk estimates were available.
In sensitivity analyses, we: (i) regrouped compounds into persistent or rapidly metabolised substances with half-lives in the range of hours; (ii) excluded studies with blood sampling after the diagnosis of testicular cancer; (iii) examined the effect of each study on the overall estimate by excluding the studies one at a time; (iv) performed separate analyses of studies with high completeness of reporting and lower risk of bias and confounding as defined above; and (v) performed separate analyses of large case-referent studies with at least 75 cases.
We computed a common risk estimate across studies by weighing the risk ratio (RR) or equivalent (OR or HR) by the inverse variance computed from the mean of the provided confidence limits. This is justified because the outcomes are rare (prevalence <5%). Random effects estimates are presented regardless of tests for heterogeneity since all studies are conceptually heterogeneous. Nevertheless we also provide I2 statistics as a formal measure of heterogeneity. Bootstrapping techniques were used in analyses where the same study yielded more than one risk estimate, to account for multiple risk estimates per person in studies where risk estimates were reported for several compounds or outcomes. Bootstrapping with 500 repeats was applied by sampling among all included risk estimates, ensuring that each study only contributed one risk estimate to each bootstrapping step. Mean values across bootstrapped steps were used as overall measures of common risk estimates and confidence limits. These analyses were repeated for each outcome to account for heterogeneity in the exposure–outcome relation.
All statistical analyses were performed using R and a significance level of 0.05. We also used this software to create funnel plots of the standard error by the logarithm of the RR separately for each of the exposures and for the higher quality studies, and inspected these plots for evidence of publication bias.
Results
The study base
We identified 33 papers that were based upon 28 independent studies reported 89 associations between measured levels of endocrine disrupting chemicals and one or more of the outcomes: cryptorchidism (Hosie et al., 2000; Longnecker et al., 2002; Pierik et al., 2004, 2007; Bhatia et al., 2005; Damgaard et al., 2006; Main et al., 2007; Brucker-Davis et al., 2008; McGlynn et al., 2009a; Fenichel et al., 2012; Chevalier et al., 2015; Jensen et al., 2015; Komarowska et al., 2015; Small et al., 2009; Trabert et al., 2012; Vesterholm et al., 2014; Toft et al., 2016; Virtanen et al., 2012); hypospadias (Longnecker et al., 2002; Bhatia et al., 2005; Small et al., 2009; McGlynn et al., 2009a; Carmichael et al., 2010; Giordano et al., 2010; Choi et al., 2012; Jensen et al., 2015; Rignell-Hydbom et al., 2012; Trabert et al., 2012; Toft et al., 2016); testicular cancer (Hardell et al., 2003, 2004; Biggs et al., 2008; Cohn et al., 2010; Giannandrea et al., 2011; McGlynn et al., 2008; 2009b; Purdue et al., 2009); and sperm count (Mocarelli et al., 2011; Axelsson et al., 2015; Vested et al., 2013, 2014).
There were 12 papers, based upon nine different study populations, which compared exposure levels in cases and referents but did not provide risk estimates. An overview of eligible studies and risk estimates are given in Tables II–IV while the results of three studies (four papers) addressing sperm count and six studies (eight papers) addressing cryptorchidism and hypospadias without risk estimates are displayed in Tables V and VI. Most studies addressed persistent organochlorines such as DDE, the metabolite of DDT (18 associations), PCBs (12 associations), DDT and HCB (each 12 associations), while studies on rapidly metabolized compounds such as phthalate ethers and Bisphenol were sparse. The systematic literature search seems to have been efficient since no additional papers fulfilling the eligibility criteria were identified from hand searches of reference lists or reviews. The majority of the studies were case-referent studies, many nested within large well-defined cohorts, while two studies of sperm count were cohort studies (Vested et al., 2013, 2014) and two were cross-sectional studies (Mocarelli et al., 2011; Axelsson et al., 2015). The case-referent studies included a total of 2446 cases of which 671 cases were cryptorchidism, 570 cases were hypospadias and 1205 cases were testicular cancer. In addition 324 men took part in studies of semen quality. Of all of the studies, 19 were published within the past 10 years, of these 13 were carried out in the United States of America or Scandinavia. The completeness of reporting assessed by 11 items were considered high (sum score ≥8 of 11 items) for over 65% of the papers. But less than half of the studies were considered at low risk of bias and confounding. Only five case-referent studies included >75 cases. Using the form in Supplementary Table 3, we extracted detailed information on each study and including our evaluation of completeness of reporting and potential biases (data available on request). In the following sections, we first present the results of the quantitative meta-analysis and next we review papers that did not report risk estimates.
Table IV.
Characteristics of six case-referent studies (36 risk estimates) addressing the risk of testicular cancer following exposure to endocrine disrupting chemicals.
| Reference | Location | Study population | N Cases/referents | Exposure medium | Exposure contrast | Substance | RR | 95% CI | CRa | Biasb |
|---|---|---|---|---|---|---|---|---|---|---|
| Hardell et al. (2003) | Sweden | Patients referred to hospital departments in 5 Swedish cities | 58/61 | Blood, diagnosed men | Above versus below median in referents | PCB (sum of 38 congeners) | 1.10 | 0.50–2.60 | 9 | 1 |
| HCB | 1.70 | 0.80–3.60 | 9 | 1 | ||||||
| p,p′-DDE | 1.70 | 0.80–3.70 | 9 | 1 | ||||||
| Chlordane | 1.30 | 0.60–2.80 | 9 | 1 | ||||||
| 44/45 | Blood, mothers at time of sons diagnosis | Above versus below median in referents | PCB (sum of 38 congeners) | 3.80 | 1.40–10.0 | 9 | 1 | |||
| HCB | 4.40 | 1.70–12.0 | 9 | 1 | ||||||
| p,p′-DDE | 1.30 | 0.50–3.00 | 9 | 1 | ||||||
| Chlordane | 1.90 | 0.70–5.00 | 9 | 1 | ||||||
| Hardell et al. (2006) | Sweden | Patients referred to hospital departments in 5 Swedish cities | 44/45 | Blood, mothers at time of sons diagnosis | Above versus below median in referents | PBDE 47, 99, 153 | 2.50 | 1.02–6.00 | 9 | 1 |
| Biggs et al. (2008) | USA, Washing-ton State | The general population | 246/630 | Blood, men when diagnosed | Above 85th percentile versus below 50th percentile in referents | p,p′-DDT | 1.17 | 0.68–2.00 | 9 | 1 |
| o,p′-DDT | 1.30 | 0.67–2.53 | 9 | 1 | ||||||
| Chlordane (six substances) | 0.93 | 0.51–1.68 | 9 | 1 | ||||||
| HCB | 0.85 | 0.37–1.96 | 9 | 1 | ||||||
| Mirex | 0.87 | 0.50–1.53 | 9 | 1 | ||||||
| Dieldrin | 0.79 | 0.44–1.41 | 9 | 1 | ||||||
| DDE | 0.61 | 0.32–1.14 | 9 | 1 | ||||||
| McGlynn et al. (2008) | USA | US servicemen who had donated serum between 1987 and 2002 | 739/915 | Serum, adult males (bio-banked, (mean storage time 14.2 years)) | Highest quartile versus lowest | DDT | 1.13 | 0.71–1.82 | 11 | 0 |
| DDE | 1.71 | 1.23–2.38 | 11 | 0 | ||||||
| Chlordane | 1.51 | 1.09–2.10 | 11 | 0 | ||||||
| Mirex | 1.24 | 0.90–1.74 | 11 | 0 | ||||||
| HCCH | 0.90 | 0.65–1.24 | 11 | 0 | ||||||
| McGlynn et al. (2009b) | USA | US servicemen who had donated serum between 1987 and 2002 | 736/913 | Serum, adult males (bio-banked, (mean storage time 14.2 years)) | Highest quartile versus lowest | PCB (sum of 11 congeners) | 0.61 | 0.43–0.86 | 11 | 0 |
| Purdue et al. (2009) | Norway | Citizens with serum stored in the Norwegian cancer Registry | 49/51 | Serum, men (pre-diagnostic serum samples from between 1972 and 1978 in the Norwegian Janus Serum Bank) | Highest tertile versus lowest | o,p′-DDT | 1.40 | 0.40–4.50 | 7 | 1 |
| p,p′-DDT | 2.10 | 0.60–7.20 | 7 | 1 | ||||||
| p,p′-DDE | 2.20 | 0.70–6.50 | 7 | 1 | ||||||
| Chlordane | 2.30 | 0.60–7.20 | 7 | 1 | ||||||
| Total PCB | 1.30 | 0.50–3.80 | 7 | 1 | ||||||
| Dieldrin | 2.10 | 0.50–9.50 | 7 | 1 | ||||||
| HCB | 2.90 | 0.50–15.20 | 7 | 1 | ||||||
| β-HCCH | 1.80 | 0.50–6.10 | 7 | 1 | ||||||
| λ-HCCH | 1.10 | 0.20–5.00 | 7 | 1 | ||||||
| Mirex | 1.20 | 0.40–3.00 | 7 | 1 | ||||||
| Cohn et al. (2010) | USA, North California | The Child Health and Development Studies | 15/45 | Early postpartum maternal serum | Continuous contrasting 25th–75th interquartile range in controls | p,p′-DDT | 0.70 | 0.26–1.64 | 8 | 1 |
| p,p′-DDE | 0.19 | 0.04–0.62 | 8 | |||||||
| o,p′-DDT | 0.77 | 0.37–1.33 | ||||||||
| Giannandrea et al. (2011) | Italy, Rome | Hospital cases and controls undergoing evaluation for fertility status | 50/48 | Serum, extrapolated backwards from male adult values | Above versus below LOD (0.2 ng/mL) | Sum of p,p′-DDE and HCB | 3.34 | 1.09–10.2 | 7 | 1 |
aCR: completeness of reporting on a scale from 0 (low completeness) to 11 (high completeness).
bBias and confounding: 1 = higher risk of bias, 0 = lower risk of bias.
Table VI.
Studies reporting associations between prenatal exposure and sperm count without risk estimates (not eligible for meta-analysis).
| Reference | Location | Study population | Study design | N population | Biospecimen | Measured xenobiotics | Results | CRa | Biasb |
|---|---|---|---|---|---|---|---|---|---|
| Mocarelli et al. (2011) | Italy, Seveso | Exposed men are sons of women living near the Seveso chemical plant at the time of the accident in 1976. Controls were blood donors whose mothers were not exposed | Mixed design | 39 exposed /58 referents | Maternal serum in exposed men. Referents had an assumed exposure of 10 ppt | Serum samples TCDD | Average sperm counts were almost halved in the exposed group and the effect was most pronounced among breast fed men | 8 | 1 |
| Vested et al. (2013) | Denmark | Pregnancy cohort, male offspring | Follow-up | 169 | Maternal serum | PFOS | PFOA but not PFOS was associated with lower adjusted sperm concentration (p-trend = 0.01) | 11 | 0 |
| PFOA | |||||||||
| Vested et al. (2014) | Denmark | Pregnancy cohort, male offspring | Follow-up | 173 | Maternal serum | PCB (six congeners) | Sum PCB and p,p′-DDE was not associated with sperm count | 11 | 0 |
| DDE | |||||||||
| Axelsson et al. (2015) | Sweden | Mainly young men presenting for military health board but also a few from the general population (through announcements in schools) | Follow-up | 112 | Maternal serum | Three DEHP metabolites Three DiNP metabolites | None of the six phthalate metabolites were associated with Sperm count | 7 | 1 |
aCR: completeness of reporting on a scale from 0 (low completeness) to 11 (high completeness).
bBias and confounding: 1 = higher risk of bias, 0 = lower risk of bias.
Global assessment: generic exposures and outcomes as one entity (Hypothesis 1)
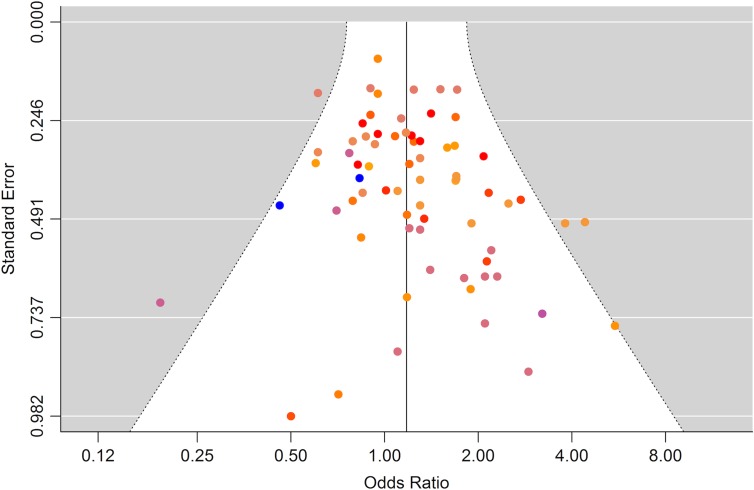
The meta-analysis, including all 70 eligible estimates addressing the risk for cryptorchidism, hypospadias or testicular cancer and giving equal weight to each of the xenobiotic exposure indicators, showed an overall risk estimate marginally above unity with rather narrow 95% confidence limits including unity, OR 1.11 (95% CI 0.91–1.35) (Table VII), and the estimate was similar in studies less likely to be biased, OR 1.12 (95% CI 0.81–1.57). A funnel plot revealed some indication of publication bias as studies with few cases and large standard errors seemed overrepresented among studies with elevated risk estimates (Fig. 2). In a subsequent analysis excluding studies with <75 cases, we found an attenuated summary estimate (OR 1.06, 95% CI 0.85–1.33) (Table VII, ‘Large studies’) but with no indication of publication bias in the corresponding funnel plot (data not shown). Leaving out five estimates originating from studies addressing xenobiotics with rapid metabolism and excretion (phthalates) produced similar results (Table VII‘+biopersistent/-biopersistent’). No single study or exposure had a major impact on the summary risk estimate. Studies addressing p,p-DDT, p,p′-DDE and HCB contributed the most.
Figure 2.
Funnel plot of all risk estimates from all studies included in the meta-analysis (n = 70) of the association between prenatal and postnatal exposure to endocrine disrupting environmental chemicals and male reproductive disorders. Risk estimates with identical colors are derived from same study.
Specific exposures and outcomes as one entity (Hypothesis 2)
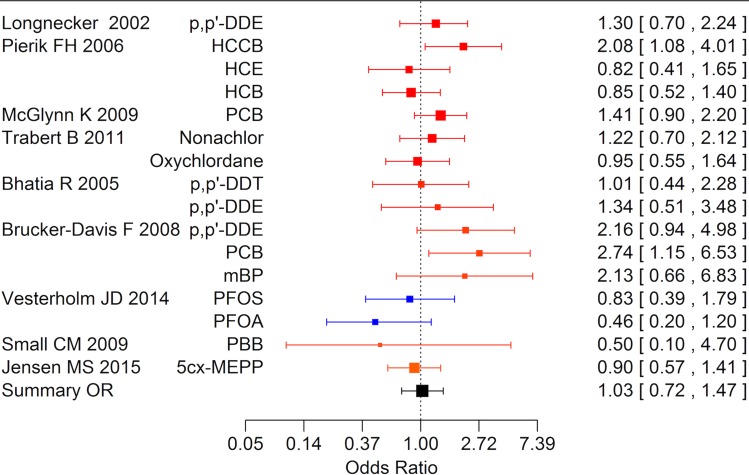
Data were available for substance specific analyses of risk of any of the reproductive disorders for the most frequently examined compounds (p,p′-DDT, p,p′-DDE, PCB and HCB). Results are summarized in Table VII. The summary estimate was above unity of all exposures but only statistically significantly elevated for p,p′-DDE (Fig. 3).
Figure 3.
Forest plot of risk estimates (95% CI) from studies included in the boot strap meta-analysis of the association between prenatal and postnatal exposure to DDE and cryptorchidism, hypospadias or testicular cancer (N = 13).
Generic exposures and specific outcomes (Hypothesis 3)
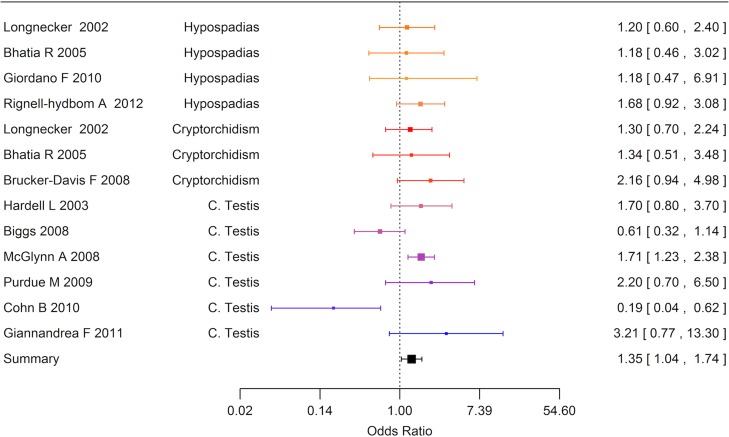
Cryptorchidism
Ten case-referent studies based upon six study populations provided 16 risk estimates for cryptorchidism (Fig. 4). The most frequently studied exposures were persistent organochlorine pesticides and PCBs which were measured in maternal pregnancy or postpartum blood samples, or cord blood or breast milk, and one study used amnion fluid to assess exposure to phthalates and perfluorooctane sulfonic acid (PFOS). Among the 16 risk estimates, nine were above unity and four exceeded a relative risk of 2. The meta-analytic summary estimate was close to unity, OR 1.03 (95% CI 0.72–1.47), Fig. 4. Funnel plots did not indicate publication bias (data not shown).
Figure 4.
Forest plot of the risk estimates from studies included in the meta-analysis of the association between prenatal and postnatal exposure to endocrine disrupting environmental chemicals and cryptorchidism (n = 16).
Hypospadias
Nine case-referent studies based upon seven study populations addressed links between xenobiotics and hypospadias and all studies except one measured xenobiotics in maternal serum (Fig. 5). Five of the studies also reported on cryptorchidism. Among 18 risk estimates, 11 were above unity and seven were below and one relative risk exceeded 2. The summary estimate was close to unity, OR 1.13 (95% CI 0.86–1.50), Table VII. Funnel plots did not indicate publication bias (data not shown).
Figure 5.
Forest plot of risk estimates (95% CI) from studies included in the boot strap meta-analysis of the association between prenatal and postnatal exposure to endocrine disrupting environmental chemicals and hypospadias (n = 18).
Testicular cancer
Eight case-referent studies based upon six study populations examined risk of testicular cancer according to environmental exposure to potential endocrine disruptive chemicals (Fig. 6). Two early Swedish studies measured xenobiotics at the time of diagnosis in the men and in a subset of their mothers at the same time (Hardell et al., 2003, 2006), while a few later studies targeted the hypothesis of prenatal origins of testicular cancer assuming that persistent organic pollutants in prediagnostic serum samples of young men was a useful proxy of in utero exposure and early infancy exposure (Biggs et al., 2008; Giannandrea et al., 2011; McGlynn et al., 2008). Only a single study was based on maternal serum samples (Cohn et al., 2010). Altogether, the eight case-referent studies provided 36 risk estimates with DDT being the most frequently examined compound (seven estimates). Of these, 26 estimates were above unity and nine exceeded a relative risk of 2. The summary estimate was slightly elevated but not statistically significantly different from one, OR 1.20 (95% CI 0.78–1.89). The funnel plot was compatible with publication bias since small studies contrary to large studies reported a non-proportional large number of elevated risk estimates (data not shown).
Figure 6.
Forest plot of risk estimates (95% CI) from studies included in the boot strap meta-analysis of the association between exposure to endocrine disrupting environmental chemicals and testicular cancer (n = 36).
Sperm count
No risk estimates available.
Specific exposures and specific outcomes (Hypothesis 4)
Available data for specific exposure and specific outcomes are limited. Four or more risk estimates were only available for four exposure–outcome combinations. Results of the conventional meta-analysis without bootstrapping (since risk estimates are independent) are shown in Table VII. The summary relative risk estimates were slightly above unity but none were statistically significant.
Studies not eligible for meta-analysis
Cryptorchidism
Six case-referent studies based upon four independent study populations did not provide risk estimates but compared concentrations of xenobiotics in fat samples, breast milk, placenta tissue, maternal serum or cord blood in cases and controls (Hosie et al., 2000; Damgaard et al., 2006; Main et al., 2007; Fenichel et al., 2012; Komarowska et al., 2015; Virtanen et al., 2012), Table V. Altogether these papers report 104 exposure–outcome comparisons, ignoring that some studies examined compounds in more than one type of biological medium. The findings with respect to biopersistent organochlorines results are largely consistent with the findings in the quantitative meta-analysis, showing limited evidence of associations. This also applies to studies with high completeness of reporting and less likely risk of bias, that addressed PBDE, TCDD, PCDD and organochlorine pesticides (Table V). Bootstrapping analyses of the subset of the seven substances with highest breast milk concentrations indicated higher median values in cases but this may be a chance finding since the overall analysis of all compounds was non-significant (Damgaard et al., 2006). The study on brominated flame retardants found increased levels in breast milk samples among cases, but this result was not confirmed in analyses based upon corresponding placenta tissue samples, which a priori were thought to provide the most accurate measure of fetal exposure (Main et al., 2007). Findings in other studies mainly addressing rapidly metabolized contaminants were also reassuring, but these studies were considered at high risk of bias (Table V).
Hypospadias
Two studies, not providing best practice reporting and considered at higher risk of bias, did not provide risk estimates but compared concentrations of xenobiotics in blood and urine samples in cases and controls (Shekharyadav et al., 2011; Choi et al., 2012; Table V). In the first study, compounds with short biological half-lives were examined and three out of eight substances were elevated in serum samples compared to controls but these results were not entirely consistent with results based upon urine samples (Choi et al., 2012). The other study examined nine biopersistent pesticides and observed higher concentrations in serum from cases for them all (Shekharyadav et al., 2011).
Sperm count
To date only three studies (four papers) explicitly studying xenobiotic prenatal exposure to potential endocrine disrupting compounds relative to semen quality in adult men have been published (Table VI). These studies are highly heterogeneous with respect to design and xenobiotics and a meta-analysis is not appropriate. Rather than providing risk estimates for reduced sperm count these studies report measures of average sperm counts according to the level of xenobiotic. Sons of women living near a chemical plant exposed to dioxin due to an accident in 1976 that contaminated the surroundings were identified in a seminal study and serum dioxin concentration at the time of conception during the period 1977–1984 was estimated based upon bio-banked maternal serum samples. Sperm counts in the exposed group of sons were compared with counts from men with assumed background exposure. Average sperm counts were almost halved in the exposed group and the effect was most pronounced among men who had been breastfed during childhood (Mocarelli et al., 2011). The sample included only 39 exposed men and was too small to allow for exposure-response analysis. In a Danish study, sons of mothers with biobanked serum samples provided semen samples when they were 19–21 years of age and sperm count was examined according to tertiles of maternal serum concentration of a range of compounds. There was no indication that PCBs, the DDT metabolite DDE or PFOS were associated with reduced sperm count, but high level PFOA exposure was significantly associated with reduced sperm concentration (Vested et al., 2013, 2014). Finally, a Swedish cross-sectional study using biobanked maternal serum samples did not show associations between sperm count and a range of phthalate metabolites (Axelsson et al., 2015).
Discussion
This is the first systematic review with meta-analysis that has rigorously evaluated the epidemiological evidence on prenatal and postnatal exposure to endocrine disrupting compounds and male reproductive disorders. A total of 33 papers provided 89 risk estimates on which we found no strong support for a global effect as a whole or on any specific outcome. However, one of four specific compounds with sufficient data to allow for meta-analysis were related to a moderate increased risk of all outcomes taken together (p,p′-DDE). Although only limited data were available for specific exposure–outcome associations, these findings do not seem to be due to increased risk of any single included disorder (cryptorchidism, hypospadias or testicular cancer). Thus findings provide some support to the hypothesis of shared prenatal etiology of these outcomes, but they also point to heterogeneity of compounds classified as endocrine disruptors with respect to potential effects on male reproduction.
The strength of this review in comparison with narrative reviews and global assessments is primarily that it includes, to our knowledge, all published epidemiological evidence fulfilling predefined criteria using a systematic and transparent search of the literature. Although the findings of this review provide some evidence for environmental endocrine disruption of male reproductive function, the limitations of observational epidemiology and the outcomes reported in the few available high quality studies precludes strong concluding statements.
Exposure assessment
Reliable exposure assessment is essential in environmental epidemiology and measurements of the compounds of interest are considered as the gold standard. We therefore only included studies with actual measurements of the chemicals in tissues as exposure assessment based on external determinants (such as job title) is crude when it comes to endocrine disrupting chemicals. Analyses of blood or tissue concentrations of chemicals were performed by gas chromatography/mass spectrometry and most studies provided data on recovery and between batch coefficients of variation. Although differences in sample preparation, analytical technique and units may invalidate comparisons of absolute exposure levels across studies, this is not expected to affect risk estimates that are derived from within-study contrasts of exposure levels.
Misclassification of exposure at the susceptible periods of development most likely results in bias towards the null. Most studies measured the contaminants late in pregnancy, at birth or during lactation and not precisely at the time of differentiation of the male gonads, that based upon animal studies in the rat, is assumed to take place in gestational week 8–14 (Welsh et al., 2008; Macleod et al., 2010). Considering the long half-lives of the persistent chemicals with little expected fluctuation of tissue levels across a few months, measurements around the time of birth seems a reasonable proxy for exposure levels during early pregnancy although distribution kinetics during pregnancy may be an issue (Verner et al., 2013). This is entirely different for compounds with a rapid metabolism and excretion such as phthalates and bisphenol A, which fluctuate markedly within individuals as shown in studies with repeated sampling. Thus, in a study where three blood samples were taken during pregnancy the intra-class coefficient of variation were for most phthalate metabolites between 20% and 40% (Cantonwine et al., 2014). However, at the group level serum levels of metabolites were remarkably stable. The direction of bias may be in either direction in the individual study but tends, on average, to cause bias towards null. One of the included studies measuring phthalate metabolites in amnion fluid in the second trimester found no association with cryptorchidism and hypospadias even though one DEHP metabolite was related to higher fetal testosterone and lower insulin-like factor III (Jensen et al., 2015).
Assessment of fetal exposure using maternal blood or milk samples assumes that the placenta is not an efficient barrier for chemical transfer from the maternal to the fetal circulation and that fetal exposure is proportional to maternal exposure. It is therefore noteworthy that knowledge regarding the transport of persistent xenobiotics over the placenta to the fetus has been known for decades (Needham et al., 1999; Mazdai et al., 2003). More recent findings indicate that rapidly metabolized compounds also pass through the placenta and these have been detected in cord blood and amnion fluid (Jensen et al., 2015).
Exposure contrast within and across studies
The most prevalent persistent organochlorine contaminants were banned in high income countries during the 1970s and subsequently their tissue levels have declined substantially. However, the studies included in this review encompass studies based upon samples biobanked >50 years ago such as the Collaborative Perinatal Study with relatively high serum concentrations (Longnecker et al., 2002; Pierik et al., 2007; McGlynn et al., 2008, 2009b; Trabert et al., 2012) as well as studies based upon sampling much later (Damgaard et al., 2006; Main et al., 2007; Virtanen et al., 2012). Unfortunately, data are too sparse to allow for a formal meta-analysis based upon the variation in exposure levels in reference populations throughout time; however, no individual studies with large exposure contrast found evidence for a relationship between exposure and response.
Since this review is focused upon prenatal exposures, we excluded a wealth of cross-sectional studies linking occupational and environmental exposure in adulthood with semen quality (for a recent review and a large collaborate study summary, see Bonde et al., 2008; Bonde and Giwercman, 2014). In contrast to testicular cancer, it is well established that spermatogenesis is susceptible to short-term effects of reproductive toxicants not least in the workplace.
Outcome ascertainment
The male reproductive disorders addressed in this review are distinct diseases with different pathologies, clinical characteristics and age of appearance. Cryptorchidism and hypospadias were either defined by specified criteria applied in systematic examinations of newborns in prospective studies or from medical records. Although criteria for both disorders may differ substantially and contribute to apparent secular trends and spatial shifts in prevalence rates, it is unlikely that differences in outcome ascertainment across studies disrupt the internal validity and the relative risk estimates. No studies used participant questionnaires or interviews to define outcomes and therefore recall bias is not an issue in studies included in this review.
The testicular dysgenesis syndrome hypothesis
We analyzed the male reproductive disorders, with the exception of low sperm count where no risk estimates were available, as one entity with reference to the testicular dysgenesis syndrome hypothesis on shared etiology of the four male reproductive disorders (Skakkebaek, 2002) and with reference to experimental evidence suggesting multiple mechanistic pathways of endocrine disruption (Toppari, 2002). The strength of the testicular dysgenesis syndrome hypothesis and the applicability of this concept as explanation of the pathogenesis of the majority of its components is an important pre-requisite for obtaining statistically significant associations when testing hypotheses I and II, where cryptorchidism, hypospadias and testicular cancer were merged together as end points in statistical analyses. However, the heterogeneity of the pathogenesis underlying those conditions and their relative weak or moderate mutual clinical associations might indicate that only a minor proportion of these male reproductive abnormalities fit into the testicular dysgenesis syndrome concept (Thorup et al., 2010), which may explain the generally non-significant or only weakly statistically significant findings of our analyses.
Studies addressing testicular cancer
Although focus was on developmental disorders induced by chemical exposure during critical time windows in early life (Lee and Jacobs, 2015), there only exists one small study using prenatal or postnatal bio-specimens to assess testicular cancer (Cohn et al., 2010). We therefore included six studies on testicular cancer assessing exposure by measurement of chemicals in serum samples obtained after puberty. Hereby, we assume that serum levels of persistent chemicals in young men or their mothers are reasonable proxies for prenatal and postnatal exposure. There is however some evidence to support this strong assumption. First of all, the biological half-lives of many of these compounds are counted in decades (Hagmar et al., 2006). Moreover, studies in a Faroese birth cohort demonstrated strong intra-individual correlations between persistent chemicals in cord blood and serum concentrations at 7 and 14 years of age, respectively (Barr et al., 2006). Another study found high correlations of persistent chemical levels in serum samples in adult men obtained up to 10 years apart (Hagmar et al., 2006). Finally, a history of breastfeeding was related to elevated serum levels in men up to 40 years later in one study (Biggs et al., 2008). Although adult serum concentrations of persistent chemicals may to some extent reflect prenatal and early postnatal exposure, it is obvious that varying rates of metabolism as well as additional lifetime exposures during infancy, childhood and adulthood also contribute to exposure levels. The resulting misclassification most is most likely is towards the null. Accordingly it is not surprising that the overall risk estimate was attenuated in the analysis excluding studies on testis cancer with adult serum data only.
Studies addressing sperm count
Although the effects of endocrine disrupting chemicals on male fertility has been the focus of heated debate (Bonde et al., 2011), only three human studies have to date linked prenatal and postnatal exposure to xenobiotics with sperm count in adults (Mocarelli et al., 2011; Axelsson et al., 2015; Vested et al., 2013). This is probably explained by the difficult and costly logistics of population-based semen studies and the need for maternal biospecimens or cord blood stored decades before follow-up takes place. In the study by Mocarelli et al., the findings of halved sperm counts in men whose mothers were exposed to higher levels of dioxin when pregnant are intriguing but call for cautious interpretation. Exposure levels in women living in the contaminated area are overlapping the background exposure, participants are few, referents are from a convenience sample and significant results were limited to the subgroup of men breast-fed as infants (Mocarelli et al., 2011). The pertinent question is whether sperm counts in the exposed group are low (in the range of 45 mill/mL) or whether sperm counts in the reference group are high (in the range of 80 mill/mL). It is well established that small cross-sectional semen studies are highly vulnerable to selection bias (Larsen et al., 1998). Unfortunately it is hardly possible to replicate the findings. Results from the sole study that was explicitly designed to test the hypothesis on delayed effects on sperm count are reassuring with respect to organochlorines (Vested et al., 2014), but less so with respect to the much less studied but ubiquitous perfluorinated hydrocarbons (Vested et al., 2013). The Swedish study addressing phthalate metabolites did not reveal adverse effects but exposure assessment of the rapidly metabolized compounds with high within-day and between-days variation within individuals is an issue. Nevertheless single spot urine samples may distinguish different exposure levels at the population level if study populations are sufficiently large (Hagmar et al., 2006; Preau et al., 2010; Ye et al., 2011). Obviously there is a need to design future studies with sufficient power to examine exposure–response associations. With the aging large European birth cohorts with stored maternal serum samples, this will become feasible in a few years.
Methodological issues
Many of the included studies examined several compounds for which risk estimates are not independent. This was accounted for by the use of bootstrapping statistical techniques by which analyses are iterated several hundred times randomly including only one risk estimate per population in each analysis and providing an average risk estimate giving equal weight to studies with few and many risk estimates. This method does not provide statistics for heterogeneity which is considered less of a problem since study populations and risk estimates obviously are heterogeneous. Therefore, we used random effects models rather than fixed effects models in all analyses. In spite of strong heterogeneity across studies with respect to study populations, exposures and outcomes, it is remarkable that all summary risk estimates from substance and outcome specific supplementary analyses are of similar magnitude spanning a relative risk from 1.03 to 1.26 when ignoring one outlying observation.
The number of studies addressing specific chemicals and outcomes were few and did not permit robust meta-analysis. Therefore, this review is primarily informative with respect to the overall association and the hypothesis on shared etiology. A number of studies did not report risk estimates but used other measures of associations. However, those findings did not seem to deviate from the overall picture.
Since we only included studies that documented exposure by objective analysis of compounds in biological media, the often encountered risk of bias due to differential recall of exposure or outcome was bypassed. Selection bias related to prior knowledge about levels of contaminants in biological tissues among patients or researchers therefore also seems unlikely. Few, if any, know their own tissue concentrations of the investigated contaminants to which all persons are exposed. Moreover, the nested case-referent design adopted by the majority of the included studies promotes comparability of cases and referents with respect to social and behavioral factors that may be related to exposure levels and thus indirectly cause bias. The two main reasons for rating some studies at a higher risk of confounding was either due to lack of or insufficient adjustment for potential confounding factors such as maternal age and prematurity in case of congenital malformations with unpredictable direction of bias or small study size that was not justified by power calculations which may increase the risk of publication bias. In fact funnel plots indicated overrepresentation of higher risk estimates in small studies with larger standard errors. Selective reporting did not appear to be a problem. All studies seemed to provide data on all measured substances regardless of association. Finally, only a few studies corrected for weight gain during pregnancy which may cause false positive associations due to dilution of the distribution volume for biopersistent chemicals (Verner et al., 2013), an issue if the outcome is related to lower birthweight or congenital malformations.
Nevertheless, the findings must be interpreted in the light of the complexity of this research field. Thus, the meta-analyses strongly violates basic assumptions in that populations, chemical substances and outcomes differ across studies (Rothman and Greenland, 1998), which necessarily must result in substantial heterogeneity disregarding results of any statistical test. The meta-analytic summary estimates and their confidence limits are not quantitatively reliable measures of specific exposure–outcome relations but merely serve to provide an overview of the available limited evidence.
Cocktail effects
Humans are simultaneously exposed to a plethora of hundreds of xenobiotics. Some in vitro and in vivo animal studies have demonstrated combined effects of endocrine disrupting chemicals at levels at which the individual chemicals do not induce observable effects (Kortenkamp, 2007; Kortenkamp, 2014). Because of this ‘cocktail effect’ of mixtures, scientist have criticized epidemiological studies for most often addressing effects of individual chemicals one by one. However, this criticism ignores to some extent that persistent organic pollutants are highly correlated and epidemiological studies addressing one indicator chemical, for example PCB-153, are in fact reflecting exposure to a mixture of numerous other chemicals. For example, in a study of persistent organic pollutants and fertility, it was not possible to distinguish between the potential effects of PCBs and the DDT metabolite DDE (Axmon et al., 2006).
Non-monotonic dose–response relationships
Another concern is the possibility of non-monotonic dose–response relationships which may explain apparently inconsistent findings across studies because of different levels and ranges of exposures (Vandenberg et al., 2012). This is particularly a potential problem when extrapolating from high occupational exposure levels to low environmental levels and in risk assessment with a need to extrapolate from high doses in in vitro and in vivo animal studies to many-fold lower environmental levels that humans encounter. However, the possibility of non-monotonic dose–response relationship is less of a problem in large population-based studies capturing a broad low-level exposure range (Lee and Jacobs, 2015). None of the large studies included in this review with substantial exposure contrast and comprehensive evaluation of risk according to exposure strata indicate the existence of such non-monotonic exposure–response relationships (Longnecker et al., 1997, 2002).
Wider implications and conclusion
The widely stated view that ubiquitous endocrine disrupting chemicals in our environment play a substantial role in the development of male reproductive disorders through prenatal and perinatal mechanisms is to some extent challenged by this review. Although the current epidemiological evidence is compatible with a small increased risk of male reproductive disorders following prenatal and postnatal exposure to some persistent environmental chemicals classified as endocrine disruptors, the evidence is limited. In this light, estimates of the burden of disease and costs of exposure to endocrine chemicals (Trasande et al., 2016) seem highly speculative, at least with respect to male reproductive disorders. Future epidemiological studies may change the weight of the evidence in either direction and the need for appropriate risk assessment of chemicals based upon experimental evidence should not be ignored. There are insufficient data on rapidly metabolized endocrine disruptors and specific exposure–outcome relations. A particular data gap is evident with respect to delayed effects on semen quality and testicular cancer.
Supplementary data
Supplementary data are available at http://humupd.oxfordjournals.org/.
Acknowledgements
Research secretary Hanne Tulinius is thanked for typing an early version of the manuscript.
Authors’ roles
Jens Peter Bonde and Elvira Braüner conceived and organized the study, sifted titles and abstracts and independently included and excluded papers. Esben Meulengracht Flachs carried out the statistical analysis. Clara Helene Glazer, Karin Sørig Hougaard, Birgit Bjerre Høyer, Katia Keglberg Hærvig, Sesilje Bondo Petersen and Ina Olmer Specht reviewed and rated the individual papers with respect to data extraction, completeness of reporting, bias and confounding. All of the above-mentioned authors as well as Aleksander Giwercman, Cecilia Høst Ramlau-Hansen, Lars Rylander and Gunnar Toft contributed to the design and provided critical comments. Susie Rimborg did the systematic literature search. Jens Peter Bonde drafted the manuscript to which all authors contributed and approved the final version format.
Funding
The review was funded by InterReq ReproUnion (NYPS 20200407).
Conflict of interest
None declared.
References
- Arbuckle TE, Fisher M, Macpherson S, Lang C, Provencher G, LeBlanc A, Hauser R, Feeley M, Ayotte P, Neisa A et al. Maternal and early life exposure to phthalates: the Plastics and Personal-care Products use in Pregnancy (P4) study. Sci Total Environ 2016;13:344–356. [DOI] [PubMed] [Google Scholar]
- Axelsson J, Rylander L, Rignell-Hydbom A, Lindh CH, Jonsson BA, Giwercman A. Prenatal phthalate exposure and reproductive function in young men. Environ Res 2015;138:264–270. [DOI] [PubMed] [Google Scholar]
- Axmon A, Thulstrup AM, Rignell-Hydbom A, Pedersen HS, Zvyezday V, Ludwicki JK, Jonsson BA, Toft G, Bonde JP, Hagmar L. Time to pregnancy as a function of male and female serum concentrations of 2,2′4,4′5,5′-hexachlorobiphenyl (CB-153) and 1,1-dichloro-2,2-bis (p-chlorophenyl)-ethylene (p,p’-DDE). Hum Reprod 2006;21:657–665. [DOI] [PubMed] [Google Scholar]
- Barr DB, Weihe P, Davis MD, Needham LL, Grandjean P. Serum polychlorinated biphenyl and organochlorine insecticide concentrations in a Faroese birth cohort. Chemosphere 2006;62:1167–1182. [DOI] [PubMed] [Google Scholar]
- Bergman A, Heindel JJ, Kasten T, Kidd KA, Jobling S, Neira M, Zoeller RT, Becher G, Bjerregaard P, Bornman R et al. The impact of endocrine disruption: a consensus statement on the state of the science. Environ Health Perspect 2013;121:A104–A106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beronius A, Vandenberg LN. Using systematic reviews for hazard and risk assessment of endocrine disrupting chemicals. Rev Endocr Metab Disord 2015;16:273–287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bhatia R, Shiau R, Petreas M, Weintraub JM, Farhang L, Eskenazi B. Organochlorine pesticides and male genital anomalies in the child health and development studies. Environ Health Perspect 2005;113:220–224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Biggs ML, Davis MD, Eaton DL, Weiss NS, Barr DB, Doody DR, Fish S, Needham LL, Chen C, Schwartz SM. Serum organochlorine pesticide residues and risk of testicular germ cell carcinoma: a population-based case-control study. Cancer Epidemiol Biomarkers Prev 2008;17:2012–2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bonde JP, Giwercman A. Environmental xenobiotics and male reproductive health. Asian J Androl 2014;16:3–4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bonde JP, Ramlau-Hansen CH, Olsen J. Trends in sperm counts: the saga continues. Epidemiology 2011;22:617–619. [DOI] [PubMed] [Google Scholar]
- Bonde JP, Toft G, Giwercman A, Rylander L, Rignell-Hydbom A, Giwercman A, Spano M, Manicardi GC, Bizarro D, Ludwicki JK et al. Fertility and markers of male reproductive function in inuit and European populations spanning large contrasts in blood levels of persistent organochlorines. Environ Health Perspect 2008;116:269–277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bonzini M, Coggon D, Palmer KT. Risk of prematurity, low birthweight and pre-eclampsia in relation to working hours and physical activities: a systematic review. Occup Environ Med 2007;64:228–243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brucker-Davis F, Wagner-Mahler K, Delattre I, Ducot B, Ferrari P, Bongain A, Kurzenne JY, Mas JC, Fenichel P. Cryptorchidism at birth in Nice area (France) is associated with higher prenatal exposure to PCBs and DDE, as assessed by colostrum concentrations. Hum Reprod 2008;23:1708–1718. [DOI] [PubMed] [Google Scholar]
- Cantonwine DE, Cordero JF, Rivera-Gonzalez LO, Anzalota Del Toro LV, Ferguson KK, Mukherjee B, Calafat AM, Crespo N, Jimenez-Velez B, Padilla IY et al. Urinary phthalate metabolite concentrations among pregnant women in Northern Puerto Rico: distribution, temporal variability, and predictors. Environ Int 2014;62:1–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carmichael SL, Herring AH, Sjodin A, Jones R, Needham L, Ma C, Ding K, Shaw GM. Hypospadias and halogenated organic pollutant levels in maternal mid-pregnancy serum samples. Chemosphere 2010;80:641–646. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chevalier N, Brucker-Davis F, Lahlou N, Coquillard P, Pugeat M, Pacini P, Panaia-Ferrari P, Wagner-Mahler K, Fenichel P. A negative correlation between insulin-like peptide 3 and bisphenol A in human cord blood suggests an effect of endocrine disruptors on testicular descent during fetal development. Hum Reprod 2015;30:447–453. [DOI] [PubMed] [Google Scholar]
- Choi H, Kim J, Im Y, Lee S, Kim Y. The association between some endocrine disruptors and hypospadias in biological samples. J Environ Sci Health A Tox Hazard Subst Environ Eng 2012;47:2173–2179. [DOI] [PubMed] [Google Scholar]
- Cohn BA, Cirillo PM, Christianson RE. Prenatal DDT exposure and testicular cancer: a nested case-control study. Arch Environ Occup Health 2010;65:127–134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Damgaard IN, Skakkebaek NE, Toppari J, Virtanen HE, Shen H, Schramm KW, Petersen JH, Jensen TK, Main KM. Persistent pesticides in human breast milk and cryptorchidism. Environ Health Perspect 2006;114:1133–1138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Diamanti-Kandarakis E, Bourguignon JP, Giudice LC, Hauser R, Prins GS, Soto AM, Zoeller RT, Gore AC. Endocrine-disrupting chemicals: an Endocrine Society scientific statement. Endocr Rev 2009;30:293–342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- European workshop on the impact of endocrine disrupters on human health and wildlife Report of Proceedings from a Workshop Held in Weybridge, UK, 2–4 December 1996. In European Commission, DGXII, Brussels, Belgium. [Google Scholar]
- Fenichel P, Dechaux H, Harthe C, Gal J, Ferrari P, Pacini P, Wagner-Mahler K, Pugeat M, Brucker-Davis F. Unconjugated bisphenol A cord blood levels in boys with descended or undescended testes. Hum Reprod 2012;27:983–990. [DOI] [PubMed] [Google Scholar]
- Frederiksen M, Meyer HW, Ebbehoj NE, Gunnarsen L. Polychlorinated biphenyls (PCBs) in indoor air originating from sealants in contaminated and uncontaminated apartments within the same housing estate. Chemosphere 2012;89:473–479. [DOI] [PubMed] [Google Scholar]
- Gabrielsen JS, Tanrikut C. Chronic exposures and male fertility: the impacts of environment, diet, and drug use on spermatogenesis. Andrology 2016;4:648–661. [DOI] [PubMed] [Google Scholar]
- Giannandrea F, Gandini L, Paoli D, Turci R, Figa-Talamanca I. Pesticide exposure and serum organochlorine residuals among testicular cancer patients and healthy controls. J Environ Sci Health B 2011;46:780–787. [DOI] [PubMed] [Google Scholar]
- Gill WB, Schumacher GF, Bibbo M, Straus FH, Schoenberg HW. Association of diethylstilbestrol exposure in utero with cryptorchidism, testicular hypoplasia and semen abnormalities. J Urol 1979;122:36–39. [DOI] [PubMed] [Google Scholar]
- Giordano F, Abballe A, De FE, di DA, Ferro F, Grammatico P, Ingelido AM, Marra V, Marrocco G, Vallasciani S et al. Maternal exposures to endocrine disrupting chemicals and hypospadias in offspring. Birth Defects Res A Clin Mol Teratol 2010;88:241–250. [DOI] [PubMed] [Google Scholar]
- Govarts E, Nieuwenhuijsen M, Schoeters G, Ballester F, Bloemen K, de Boer M, Chevrier C, Eggesbo M, Guxens M, Kramer U et al. Birth weight and prenatal exposure to polychlorinated biphenyls (PCBs) and dichlorodiphenyldichloroethylene (DDE): a meta-analysis within 12 European Birth Cohorts. Environ Health Perspect 2012;120:162–170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gyalpo T, Scheringer M, Hungerbuhler K. Recommendations for evaluating temporal trends of persistent organic pollutants in breast milk. Environ Health Perspect 2016;124:881–885. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hagmar L, Wallin E, Vessby B, Jonsson BA, Bergman A, Rylander L. Intra-individual variations and time trends 1991–2001 in human serum levels of PCB, DDE and hexachlorobenzene. Chemosphere 2006;64:1507–1513. [DOI] [PubMed] [Google Scholar]
- Hardell L, Bavel B, Lindstrom G, Eriksson M, Carlberg M. In utero exposure to persistent organic pollutants in relation to testicular cancer risk. Int J Androl 2006;29:228–234. [DOI] [PubMed] [Google Scholar]
- Hardell L, Malmqvist N, Ohlson CG, Westberg H, Eriksson M. Testicular cancer and occupational exposure to polyvinyl chloride plastics: a case-control study. Int J Cancer 2004;109:425–429. [DOI] [PubMed] [Google Scholar]
- Hardell L, Van BB, Lindstrom G, Carlberg M, Dreifaldt AC, Wijkstrom H, Starkhammar H, Eriksson M, Hallquist A, Kolmert T. Increased concentrations of polychlorinated biphenyls, hexachlorobenzene, and chlordanes in mothers of men with testicular cancer. Environ Health Perspect 2003;111:930–934. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Henderson BE, Benton B, Jing J, Yu MC, Pike MC. Risk factors for cancer of the testis in young men. Int J Cancer 1979;23:598–602. [DOI] [PubMed] [Google Scholar]
- Hosie S, Loff S, Witt K, Niessen K, Waag KL. Is there a correlation between organochlorine compounds and undescended testes. Eur J Pediatr Surg 2000;10:304–309. [DOI] [PubMed] [Google Scholar]
- Hung H, Katsoyiannis AA, Brorstrom-Lunden E, Olafsdottir K, Aas W, Breivik K, Bohlin-Nizzetto P, Sigurdsson A, Hakola H, Bossi R et al. Temporal trends of Persistent Organic Pollutants (POPs) in arctic air: 20 years of monitoring under the Arctic Monitoring and Assessment Programme (AMAP). Environ Pollut 2016;10 doi:10.1016/j.envpol.2016.01.079. [Epub ahead of print]. [DOI] [PubMed] [Google Scholar]
- Jensen MS, Anand-Ivell R, Norgaard-Pedersen B, Jonsson BA, Bonde JP, Hougaard DM, Cohen A, Lindh CH, Ivell R, Toft G. Amniotic fluid phthalate levels and male fetal gonad function. Epidemiology 2015;26:91–99. [DOI] [PubMed] [Google Scholar]
- Jorgensen N, Joensen UN, Jensen TK, Jensen MB, Almstrup K, Olesen IA, Juul A, Andersson AM, Carlsen E, Petersen JH et al. Human semen quality in the new millennium: a prospective cross-sectional population-based study of 4867 men. BMJ Open 2012. DOI:10.1136/bmjopen-2012-000990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Komarowska MD, Hermanowicz A, Czyzewska U, Milewski R, Matuszczak E, Miltyk W, Debek W. Serum Bisphenol A Level in Boys with Cryptorchidism: A Step to Male Infertility? 410 Park Avenue, 15th Floor, 287 pmb) New York NY 10022, United States: Hindawi Publishing Corporation, 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kortenkamp A. Ten years of mixing cocktails: a review of combination effects of endocrine-disrupting chemicals. Environ Health Perspect 2007;115:98–105. doi:10.1289/ehp.9357.:98-105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kortenkamp A. Low dose mixture effects of endocrine disrupters and their implications for regulatory thresholds in chemical risk assessment. Curr Opin Pharmacol 2014;19:105–111. [DOI] [PubMed] [Google Scholar]
- Lamb JC, Boffetta P, Foster WG, Goodman JE, Hentz KL, Rhomberg LR, Staveley J, Swaen G, Van Der Kraak G, Williams AL. Critical comments on the WHO-UNEP State of the Science of Endocrine Disrupting Chemicals - 2012. Regul Toxicol Pharmacol 2014;69:22–40. [DOI] [PubMed] [Google Scholar]
- Larsen SB, Abell A, Bonde JP. Selection bias in occupational sperm studies. Am J Epidemiol 1998;147:681–685. [DOI] [PubMed] [Google Scholar]
- Leary FJ, Resseguie LJ, Kurland LT, O'Brien PC, Emslander RF, Noller KL. Males exposed in utero to diethylstilbestrol. JAMA 1984;252:2984–2989. [PubMed] [Google Scholar]
- Lee DH, Jacobs DR Jr. Methodological issues in human studies of endocrine disrupting chemicals. Rev Endocr Metab Disord 2015;16:289–297. [DOI] [PubMed] [Google Scholar]
- Lenters V, Thomsen C, Smit LA, Jonsson BA, Pedersen HS, Ludwicki JK, Zviezdai V, Piersma AH, Toft G, Bonde JP et al. Serum concentrations of polybrominated diphenyl ethers (PBDEs) and a polybrominated biphenyl (PBB) in men from Greenland, Poland and Ukraine. Environ Int 2013;61:8–16. [DOI] [PubMed] [Google Scholar]
- Liu LY, Ma WL, Jia HL, Zhang ZF, Song WW, Li YF. Research on persistent organic pollutants in China on a national scale: 10 years after the enforcement of the Stockholm Convention. Environ Pollut 2016;10 DOI:10.1016/j.envpol.2015.12.056. [DOI] [PubMed] [Google Scholar]
- Longnecker MP, Klebanoff MA, Brock JW, Zhou H, Gray KA, Needham LL, Wilcox AJ. Maternal serum level of 1,1-dichloro-2,2-bis(p-chlorophenyl)ethylene and risk of cryptorchidism, hypospadias, and polythelia among male offspring. Am J Epidemiol 2002;155:313–322. [DOI] [PubMed] [Google Scholar]
- Longnecker MP, Rogan WJ, Lucier G. The human health effects of DDT (dichlorodiphenyltrichloroethane) and PCBS (polychlorinated biphenyls) and an overview of organochlorines in public health. Annu Rev Public Health 1997;18:211–244. [DOI] [PubMed] [Google Scholar]
- Macleod DJ, Sharpe RM, Welsh M, Fisken M, Scott HM, Hutchison GR, Drake AJ, van den Driesche S. Androgen action in the masculinization programming window and development of male reproductive organs. Int J Androl 2010;33:279–287. [DOI] [PubMed] [Google Scholar]
- Magulova K, Priceputu A. Global monitoring plan for persistent organic pollutants (POPs) under the Stockholm Convention: triggering, streamlining and catalyzing global POPs monitoring. Environ Pollut 2016;217:82–84. [DOI] [PubMed] [Google Scholar]
- Main KM, Kiviranta H, Virtanen HE, Sundqvist E, Tuomisto JT, Tuomisto J, Vartiainen T, Skakkebaek NE, Toppari J. Flame retardants in placenta and breast milk and cryptorchidism in newborn boys. Environ Health Perspect 2007;115:1519–1526. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martin OV, Shialis T, Lester JN, Scrimshaw MD, Boobis AR, Voulvoulis N. Testicular dysgenesis syndrome and the estrogen hypothesis: a quantitative meta-analysis. Environ Health Perspect 2008;116:149–157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mazdai A, Dodder NG, Abernathy MP, Hites RA, Bigsby RM. Polybrominated diphenyl ethers in maternal and fetal blood samples. Environ Health Perspect 2003;111:1249–1252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McCarthy M. European Chemical Agency (ECHA) List of Pre-Registered Substances. In Brussels: European Commission, 2011. [Google Scholar]
- McGlynn KA, Guo X, Graubard BI, Brock JW, Klebanoff MA, Longnecker MP. Maternal pregnancy levels of polychlorinated biphenyls and risk of hypospadias and cryptorchidism in male offspring. Environ Health Perspect 2009. a;117:1472–1476. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McGlynn KA, Quraishi SM, Graubard BI, Weber JP, Rubertone MV, Erickson RL. Persistent organochlorine pesticides and risk of testicular germ cell tumors. J Natl Cancer Inst 2008;100:663–671. [DOI] [PubMed] [Google Scholar]
- McGlynn KA, Quraishi SM, Graubard BI, Weber JP, Rubertone MV, Erickson RL. Polychlorinated biphenyls and risk of testicular germ cell tumors. Cancer Res 2009. b;69:1901–1909. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McLachlan JA. Environmental signaling: from environmental estrogens to endocrine-disrupting chemicals and beyond. Andrology 2016;4:684–694. [DOI] [PubMed] [Google Scholar]
- Meyer HW, Frederiksen M, Goen T, Ebbehoj NE, Gunnarsen L, Brauer C, Kolarik B, Muller J, Jacobsen P. Plasma polychlorinated biphenyls in residents of 91 PCB-contaminated and 108 non-contaminated dwellings-an exposure study. Int J Hyg Environ Health 2013;216:755–762. [DOI] [PubMed] [Google Scholar]
- Mocarelli P, Gerthoux PM, Needham LL, Patterson DG Jr, Limonta G, Falbo R, Signorini S, Bertona M, Crespi C, Sarto C et al. Perinatal exposure to low doses of dioxin can permanently impair human semen quality. Environ Health Perspect 2011;119:713–718. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nam SH, Seo YM, Kim MG. Bisphenol A migration from polycarbonate baby bottle with repeated use. Chemosphere 2010;79:949–952. [DOI] [PubMed] [Google Scholar]
- Needham LL, Gerthoux PM, Patterson DG Jr, Brambilla P, Smith SJ, Sampson EJ, Mocarelli P. Exposure assessment: serum levels of TCDD in Seveso, Italy. Environ Res 1999;80:S200–S206. [DOI] [PubMed] [Google Scholar]
- Nohynek GJ, Borgert CJ, Dietrich D, Rozman KK. Endocrine disruption: fact or urban legend. Toxicol Lett 2013;223:295–305. [DOI] [PubMed] [Google Scholar]
- Pierik FH, Burdorf A, Deddens JA, Juttmann RE, Weber RF. Maternal and paternal risk factors for cryptorchidism and hypospadias: a case-control study in newborn boys. Environ Health Perspect 2004;112:1570–1576. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pierik FH, Klebanoff MA, Brock JW, Longnecker MP. Maternal pregnancy serum level of heptachlor epoxide, hexachlorobenzene, and beta-hexachlorocyclohexane and risk of cryptorchidism in offspring. Environ Res 2007;105:364–369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Preau JL Jr, Wong LY, Silva MJ, Needham LL, Calafat AM. Variability over 1 week in the urinary concentrations of metabolites of diethyl phthalate and di(2-ethylhexyl) phthalate among eight adults: an observational study. Environ Health Perspect 2010;118:1748–1754. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Purdue MP, Engel LS, Langseth H, Needham LL, Andersen A, Barr DB, Blair A, Rothman N, McGlynn KA. Prediagnostic serum concentrations of organochlorine compounds and risk of testicular germ cell tumors. Environ Health Perspect 2009;117:1514–1519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rignell-Hydbom A, Lindh CH, Dillner J, Jonsson BA, Rylander L. A nested case-control study of intrauterine exposure to persistent organochlorine pollutants and the risk of hypospadias. PLoS One 2012;7:e44767. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rothman KJ, Greenland S. Modern Epidemiology. Philadelphia: Lippincott-Raven, 1998. [Google Scholar]
- Schrader SM, Turner TW, Breitenstein MJ, Simon SD. Longitudinal study of semen quality of unexposed workers. I. Study overview. Reprod Toxicol 1988;2:183–190. [DOI] [PubMed] [Google Scholar]
- Sever LE, Arbuckle TE, Sweeney A. Reproductive and developmental effects of occupational pesticide exposure: the epidemiologic evidence. Occup Med 1997;12:305–325. [PubMed] [Google Scholar]
- Shamliyan TA, Kane RL, Ansari MT, Raman G, Berkman ND, Grant M, Janes G, Maglione M, Moher D, Nasser M et al. A systematic review of tools used to assess the quality of observational studies that examine incidence or prevalence and risk factors for diseases. J Clin Epidemiol 2010;63:1061–1070. [DOI] [PubMed] [Google Scholar]
- Sharpe RM. Hormones and testis development and the possible adverse effects of environmental chemicals. Toxicol Lett 2001;120:221–232. [DOI] [PubMed] [Google Scholar]
- Sharpe RM, Irvine DS. How strong is the evidence of a link between environmental chemicals and adverse effects on human reproductive health. BMJ 2004;328:447–451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sharpe RM, Skakkebaek NE. Are oestrogens involved in falling sperm counts and disorders of the male reproductive tract. Lancet 1993;341:1392–1395. [DOI] [PubMed] [Google Scholar]
- Shekharyadav C, Bajpai M, Kumar V, Ahmed RS, Gupta P, Banerjee BD. Polymorphism in CYP1A1, GSTMI, GSTT1 genes and organochlorine pesticides in the etiology of hypospadias. Hum Exp Toxicol 2011;30:1464–1474. [DOI] [PubMed] [Google Scholar]
- Skakkebaek NE. Endocrine disrupters and testicular dysgenesis syndrome. Horm Res 2002;57:43. [DOI] [PubMed] [Google Scholar]
- Skakkebaek NE. Testicular dysgenesis syndrome. Horm Res 2003;60:49. [DOI] [PubMed] [Google Scholar]
- Skakkebaek NE. Testicular dysgenesis syndrome: new epidemiological evidence. Int J Androl 2004;27:189–191. [DOI] [PubMed] [Google Scholar]
- Skakkebaek NE, Rajpert-De ME, Main KM. Testicular dysgenesis syndrome: an increasingly common developmental disorder with environmental aspects. Hum Reprod 2001;16:972–978. [DOI] [PubMed] [Google Scholar]
- Small CM, DeCaro JJ, Terrell ML, Dominguez C, Cameron LL, Wirth J, Marcus M. Maternal exposure to a brominated flame retardant and genitourinary conditions in male offspring. Environ Health Perspect 2009;117:1175–1179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Storgaard L, Bonde JP, Olsen J. Male reproductive disorders in humans and prenatal indicators of estrogen exposure. A review of published epidemiological studies. Reprod Toxicol 2006;21:4–15. [DOI] [PubMed] [Google Scholar]
- Stroup DF, Berlin JA, Morton SC, Olkin I, Williamson GD, Rennie D, Moher D, Becker BJ, Sipe TA, Thacker SB. Meta-analysis of observational studies in epidemiology: a proposal for reporting. Meta-analysis Of Observational Studies in Epidemiology (MOOSE) group. JAMA 2000;283:2008–2012. [DOI] [PubMed] [Google Scholar]
- Takahashi K, Hanaoka T, Pan G. Male reproductive health in relation to occupational exposure to endocrine disrupting and other potent chemicals, a review of the epidemiologic literature. J UOEH 2004;26:23–40. [DOI] [PubMed] [Google Scholar]
- Thorup J, McLachlan R, Cortes D, Nation TR, Balic A, Southwell BR, Hutson JM. What is new in cryptorchidism and hypospadias--a critical review on the testicular dysgenesis hypothesis. J Pediatr Surg 2010;45:2074–2086. [DOI] [PubMed] [Google Scholar]
- Toft G, Jonsson BA, Bonde JP, Norgaard-Pedersen B, Hougaard DM, Cohen A, Lindh CH, Ivell R, Anand-Ivell R, Lindhard MS. Perfluorooctane sulfonate concentrations in amniotic fluid, biomarkers of fetal leydig cell function, and cryptorchidism and hypospadias in Danish boys (1980–1996). Environ Health Perspect 2016;124:151–156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Toppari J. Environmental endocrine disrupters and disorders of sexual differentiation. Semin Reprod Med 2002;20:305–312. [DOI] [PubMed] [Google Scholar]
- Toppari J, Virtanen HE, Main KM, Skakkebaek NE. Cryptorchidism and hypospadias as a sign of testicular dysgenesis syndrome (TDS): environmental connection. Birth Defects Res A Clin Mol Teratol 2010;88:910–919. [DOI] [PubMed] [Google Scholar]
- Trabert B, Longnecker MP, Brock JW, Klebanoff MA, McGlynn KA. Maternal pregnancy levels of trans-nonachlor and oxychlordane and prevalence of cryptorchidism and hypospadias in boys. Environ Health Perspect 2012;120:478–482. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Trasande L, Zoeller RT, Hass U, Kortenkamp A, Grandjean P, Myers JP, DiGangi J, Hunt PM, Rudel R, Sathyanarayana S et al. Burden of disease and costs of exposure to endocrine disrupting chemicals in the European Union: an updated analysis. Andrology 2016;4:565–572. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vandenberg LN, Colborn T, Hayes TB, Heindel JJ, Jacobs DR Jr, Lee DH, Shioda T, Soto AM, vom Saal FS, Welshons WV et al. Hormones and endocrine-disrupting chemicals: low-dose effects and nonmonotonic dose responses. Endocr Rev 2012;33:378–455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Verner MA, McDougall R, Glynn A, Andersen ME, Clewell HJ III, Longnecker MP. Is the relationship between prenatal exposure to PCB-153 and decreased birth weight attributable to pharmacokinetics. Environ Health Perspect 2013;121:1219–1224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vested A, Ramlau-Hansen CH, Olsen SF, Bonde JP, Kristensen SL, Halldorsson TI, Becher G, Haug LS, Ernst EH, Toft G. Associations of in utero exposure to perfluorinated alkyl acids with human semen quality and reproductive hormones in adult men. Environ Health Perspect 2013;121:453–458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vested A, Ramlau-Hansen CH, Olsen SF, Bonde JP, Stovring H, Kristensen SL, Halldorsson TI, Rantakokko P, Kiviranta H, Ernst EH et al. In utero exposure to persistent organochlorine pollutants and reproductive health in the human male. Reproduction 2014;148:635–646. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vesterholm JD, Christensen J, Virtanen HE, Skakkebaek NE, Main KM, Toppari J, Veje CW, Andersson AM, Nielsen F, Grandjean P et al. No association between exposure to perfluorinated compounds and congenital cryptorchidism: a nested case-control study among 215 boys from Denmark and Finland 1. Reproduction 2014;147:411–417. [DOI] [PubMed] [Google Scholar]
- Virtanen HE, Koskenniemi JJ, Sundqvist E, Main KM, Kiviranta H, Tuomisto JT, Tuomisto J, Viluksela M, Vartiainen T, Skakkebaek NE et al. Associations between congenital cryptorchidism in newborn boys and levels of dioxins and PCBs in placenta. Int J Androl 2012;35:283–293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Welsh M, Saunders PT, Fisken M, Scott HM, Hutchison GR, Smith LB, Sharpe RM. Identification in rats of a programming window for reproductive tract masculinization, disruption of which leads to hypospadias and cryptorchidism. J Clin Invest 2008;118:1479–1490. [DOI] [PMC free article] [PubMed] [Google Scholar]
- White SS, Fenton SE, Hines EP. Endocrine disrupting properties of perfluorooctanoic acid . J Steroid Biochem Mol Biol 2011;127:16–26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- World Health Organisation (WHO) and International Programme on Chemical Safety (IPCS) (In: Damstra T, Barlow S, Bergman A, Kavlock R, Van Der Kraak G (eds). Global Assessment of the State-of-the-Science of Endocrine Disrupters. Geneva: World Health Organisation, 2002:1–133. [Google Scholar]
- World Health Organisation (WHO) and United Nations Environment Program (UNEP) Bergman Å, Heindel JJ, Jobling S, Kidd KA, Zoeller RT (eds). State of the Science of Endocrine Disrupting Chemicals. Geneva: WHO-UNEP, 2012:1–296. [Google Scholar]
- Ye X, Wong LY, Bishop AM, Calafat AM. Variability of urinary concentrations of bisphenol A in spot samples, first morning voids, and 24-hour collections. Environ Health Perspect 2011;119:983–988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang J, Liu L, Wang X, Huang Q, Tian M, Shen H. Low-level environmental phthalate exposure associates with urine metabolome alteration in a Chinese Male Cohort. Environ Sci Technol 2016;50:5953–5960. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.