Abstract
BACKGROUND
Initially identified for their capability to induce heterotopic bone formation, bone morphogenetic proteins (BMPs) are multifunctional growth factors that belong to the transforming growth factor β superfamily. Using cellular and molecular genetic approaches, recent studies have implicated intra-ovarian BMPs as potent regulators of ovarian follicular function. The bi-directional communication of oocytes and the surrounding somatic cells is mandatory for normal follicle development and oocyte maturation. This review summarizes the current knowledge on the physiological role and molecular determinants of these ovarian regulatory factors within the human germline-somatic regulatory loop.
OBJECTIVE AND RATIONALE
The regulation of ovarian function remains poorly characterized in humans because, while the fundamental process of follicular development and oocyte maturation is highly similar across species, most information on the regulation of ovarian function is obtained from studies using rodent models. Thus, this review focuses on the studies that used human biological materials to gain knowledge about human ovarian biology and disorders and to develop strategies for preventing, diagnosing and treating these abnormalities.
SEARCH METHODS
Relevant English-language publications describing the roles of BMPs or growth differentiation factors (GDFs) in human ovarian biology and phenotypes were comprehensively searched using PubMed and the Google Scholar database. The publications included those published since the initial identification of BMPs in the mammalian ovary in 1999 through July 2016.
OUTCOMES
Studies using human biological materials have revealed the expression of BMPs, GDFs and their putative receptors as well as their molecular signaling in the fundamental cells (oocyte, cumulus/granulosa cells (GCs) and theca/stroma cells) of the ovarian follicles throughout follicle development. With the availability of recombinant human BMPs/GDFs and the development of immortalized human cell lines, functional studies have demonstrated the physiological role of intra-ovarian BMPs/GDFs in all aspects of ovarian functions, from follicle development to steroidogenesis, cell–cell communication, oocyte maturation, ovulation and luteal function. Furthermore, there is crosstalk between these potent ovarian regulators and the endocrine signaling system. Dysregulation or naturally occurring mutations within the BMP system may lead to several female reproductive diseases. The latest development of recombinant BMPs, synthetic BMP inhibitors, gene therapy and tools for BMP-ligand sequestration has made the BMP pathway a potential therapeutic target in certain human fertility disorders; however, further clinical trials are needed. Recent studies have indicated that GDF8 is an intra-ovarian factor that may play a novel role in regulating ovarian functions in the human ovary.
WIDER IMPLICATIONS
Intra-ovarian BMPs/GDFs are critical regulators of folliculogenesis and human ovarian functions. Any dysregulation or variations in these ligands or their receptors may affect the related intracellular signaling and influence ovarian functions, which accounts for several reproductive pathologies and infertility. Understanding the normal and pathological roles of intra-ovarian BMPs/GDFs, especially as related to GC functions and follicular fluid levels, will inform innovative approaches to fertility regulation and improve the diagnosis and treatment of ovarian disorders.
Keywords: bone morphogenetic proteins, growth differentiation factors, activin receptor like-kinase, human ovary, human granulosa cells, human granulosa cell line, female infertility
Introduction
In humans, the ovarian follicle is the core functional unit of the female reproductive system, which may correspond to different developmental stages in the ovary. During the female reproductive cycle, these follicles progress through a highly coordinated regulatory process that involves several neural, neuroendocrine, endocrine and paracrine/autocrine control systems to achieve full ovulatory and steroidogenic capability (Gougeon, 1996). The primary roles of the pituitary gonadotrophin hormones, FSH and LH, GnRH and the gonadal hormones (estrogen, androgen and progesterone) in the female reproductive system are well established. However, normal ovarian function and follicular development also depend on a variety of locally produced growth factors and cytokines that exert their effects in a paracrine/autocrine fashion (Knight and Glister, 2006). In the last few decades, studies on a variety of species have demonstrated that members of the transforming growth factor-β (TGF-β) superfamily, which include bone morphogenetic proteins (BMPs), growth differentiation factors (GDFs), TGF-βs, activins and inhibins, and anti-Müllerian hormone (AMH), are expressed in the ovary and play essential roles in the regulation of folliculogenesis, oogenesis and ovarian functions (Gougeon, 1996; Juengel and McNatty, 2005; Knight and Glister, 2006). With over 20 members, BMPs/GDFs constitute the largest subfamily of the TGF-β superfamily and were initially identified as osteoinductive cytokines that may promote bone and cartilage formation (Wang et al., 1988; Wagner et al., 2010). Several publications have documented that locally produced BMPs are implicated in the formation and function of mammalian germ cells and that they participate in the regulation of ovarian functions (Yi et al., 2001; Shimasaki et al., 2004; Khan et al., 2016; Rossi et al., 2016). Moreover, naturally occurring gene mutations of BMP ligands and dysregulated BMP signaling are associated with prominent pathologies of human reproduction (Persani et al., 2014; Qin et al., 2015).
While the fundamental process of follicular development is highly similar across species, most information on the regulation of ovarian function is obtained from studies in rodents. Our understanding of the development and function of the human ovary has advanced only recently due to a combination of technologies including recombinant human BMPs/GDFs, immortalized human granulosa cell (GC) lines, tissue microarrays and pharmaceutical development. Thus a significant portion of this review is devoted to the human ovarian physiologies and human reproductive disorders associated with BMP/GDF signaling.
Methods
A comprehensive literature review was conducted using two databases, PubMed and Google Scholar to identify and retrieve information relevant to the field of BMP/GDF biology in the human ovary, since its initial identification in the mammalian ovary in 1999 (Shimasaki et al., 1999) through July 2016. A systematic review of English-language publications was carried out using the following keywords: BMP, GDF, human ovary, human GCs, human GC line, female infertility and therapeutic targets. The goal of this review is to provide an overview of the current knowledge of the physiological roles of BMPs and GDFs in human female reproduction and their potential therapeutic application for regulation of fertility.
BMPs and BMP receptors
BMP ligands
BMPs consist of an extensive subfamily of phylogenetically conserved growth factors in the TGF-β superfamily with critical physiological functions in organogenesis and morphogenesis in both invertebrates and vertebrates (Hogan, 1996; Bier and De Robertis, 2015). In the modern nomenclature, BMPs are regarded as encompassing growth factors that are either BMPs or GDFs due to the overlap between the two classification systems without significant separate lineages (Rider and Mulloy, 2010) (Fig. 1). Similar to other members of the TGF-β superfamily, BMPs are initially translated as a similar structure consisting of three components: a signal peptide, a prodomain and a mature peptide (mature domain). The signal peptide (20–30 amino acids long) is present at the N-terminal end of the newly synthesized large preproproteins, and it directs these ligands for proper secretion out of the cells (Veitia and Caburet, 2009). The BMPs share a conserved structure: the seven characteristically spaced cysteine residues indicative of the cysteine-knot motif of the TGF-β superfamily (Shi and Massague, 2003). Six of these cysteine residues can form intramolecular disulfide bonds known as cysteine knots, and the seventh cysteine is involved in the dimerization with another monomer via a disulfide bond to form homodimers or heterodimers (Sun and Davies, 1995). However, BMP15 and GDF9 are two of the few TGF-β members with only six cysteine residues in their cysteine-knot domain, each lacking the one cysteine residue responsible for covalent dimer formation (McIntosh et al., 2008). The inactive BMP precursors are proteolytically cleaved by a proprotein convertase family, proprotein convertase Subtilisin/Kexin, which is composed of nine members named PCSK1–PCSK9 (Seidah and Prat, 2012). At least one member of this family, PCSK3 (furin), recognizes the amino acid motif -Arg-X-Arg/Lys-Arg- at the linker site between the prodomain and the mature domain and activates the mature BMP homodimers or heterodimers (Harrison et al., 2011). Several BMP heterodimers, such as BMP2/BMP7, BMP2/BMP6, BMP4/BMP7 and BMP15/GDF9, have been suggested to exist and function both in vivo and in vitro (Israel et al., 1996; Little and Mullins, 2009; Valera et al., 2010; Peng et al., 2013). Most of these BMP heterodimers are more biologically potent than their respective homodimers in inducing cellular functions (Israel et al., 1996; Valera et al., 2010; Peng et al., 2013). The most likely explanation for these differential effects is that the heterodimer activity could be mediated by a different or an additional receptor subtype.
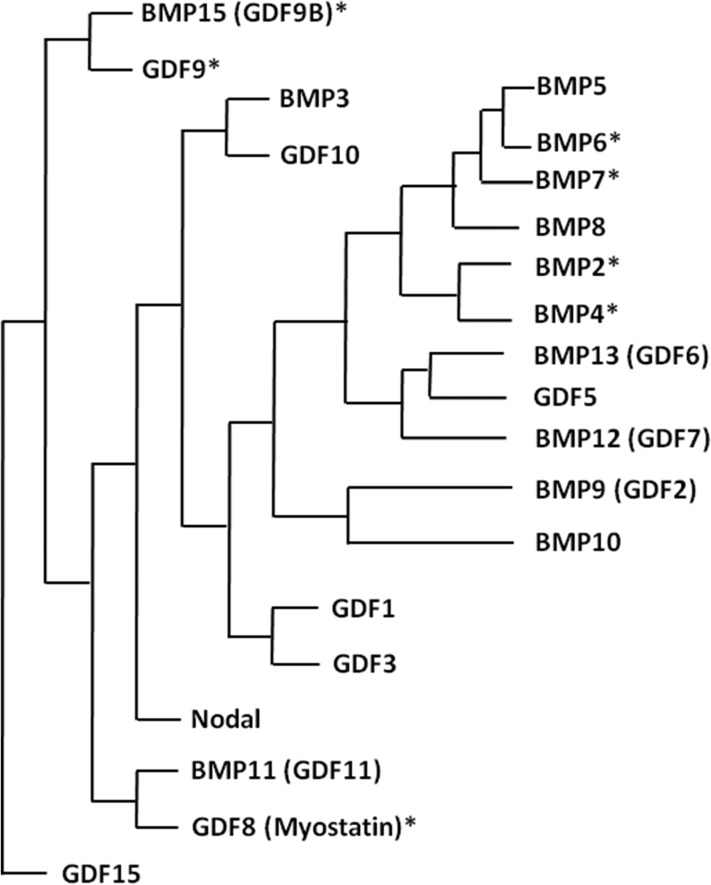
Figure 1.
Phylogenetic relationship of the BMP/GDF subfamily members. The alignment of 21 BMP/GDF-related ligands was based on published literature: (Ducy and Karsenty, 2000; Mazerbourg and Hsueh, 2006; Rider and Mulloy, 2010). Ligands with an asterisk are expressed in the human ovary. BMP/GDF, bone morphogenetic protein/growth differentiation factor.
It has been widely argued that the biological action of oocyte-derived BMP15 and GDF9 is redundant or synergistic. Evidence from a study of Bmp15 and Gdf9 double knockout mice demonstrated that there is a biological cooperation between these two growth factors (Yan et al., 2001). There is a great debate as to whether such cooperation results from the synergistic interaction of BMP15 and GDF9 homodimers or from the existence of a biologically active BMP15/GDF9 heterodimer (Mottershead et al., 2013; Peng et al., 2013; Persani et al., 2014). Interestingly, the BMP15/GDF9 heterodimer can be produced and secreted in 293 T cells (a specific cell line originally derived from human embryonic kidney cells) when two constructs are co-expressed (Liao et al., 2004). Indeed, the human BMP15/GDF9 heterodimer is approximately 1000- to 3000-fold more potent than the human BMP15 homodimer (Peng et al., 2013).
BMP receptors
TGF-β superfamily members signal through distinct sets of transmembrane serine/threonine kinase receptors (type I and type II receptors), which further regulate downstream gene expression by phosphorylating the Sma- and Mad-related protein (SMAD) transcription factors (Shi and Massague, 2003). To date, five distinct type II receptors (BMPR2, ACVR2A, ACVR2B, TβR2 and AMHR2) and seven type I receptors (also known as activin receptor like-kinase; ALK1–7) have been identified in mammals (Massague, 1998; Miyazono et al., 2001). All of these receptors share a very similar structure consisting of an extracellular domain (N-terminal), a transmembrane domain and an intracellular domain with Ser/Thr kinase activity (C-terminal). Each ligand of the TGF-β superfamily members can bind to the N-terminal extracellular domains of different combinations of type II and type I receptors and trigger downstream signaling complexes (Massague, 1998; Miyazono et al., 2001). Of the five type I receptors, three (BMPR2, ACVR2A and ACVR2B) are employed by one or more BMPs. Likewise, six (ALK2-7) of the seven type I receptors have been implicated in BMP-induced intracellular signaling (Macias-Silva et al., 1998; Kaivo-Oja et al., 2003; Moore et al., 2003; Mazerbourg and Hsueh, 2006; Miyagi et al., 2012). Receptors and signaling pathways utilized for various intra-ovarian BMPs/GDFs in human cells are listed in Table I.
Table I.
Putative receptors and signaling pathways of BMPs/GDFs in the human ovary.
| Ligand | Type II receptor | Type I receptor | SMAD | References |
|---|---|---|---|---|
| BMP2 | BMPR2, ACVR2A | ALK3/6 | SMAD1/5/8 | Mazerbourg and Hsueh (2006) |
| BMP4 | BMPR2, ACVR2A | ALK3/6 | SMAD1/5/8 | Miyagi et al. (2012) |
| Chang et al. (2015a, 2015c) | ||||
| ALK3/4/5 | SMAD2/3 | Zhang et al. (2016) | ||
| BMP6 | BMPR2, ACVR2A | ALK2/3/6 | SMAD1/5/8 | Mazerbourg and Hsueh (2006) |
| BMP7 | BMPR2, ACVR2A | ALK2/3/6 | SMAD1/5/8 | Macias-Silva et al. (1998) |
| Chang et al. (2015a, 2015b, 2015c) | ||||
| BMP15 | BMPR2 | ALK3/6 | SMAD1/5/8 | Moore et al. (2003) |
| Chang et al. (2013a) | ||||
| GDF8 | ACVR2A, ACVR2B | ALK5 | SMAD2/3 | Chang et al. (2015d) |
| Chang et al. (2016a, 2016b, 2016c) | ||||
| GDF9 | BMPR2 | ALK5 | SMAD2/3 | Mazerbourg and Hsueh (2006) |
| Kaivo-Oja et al. (2003) |
ACVR, activin receptor; ALK, activin receptor-like kinase; BMP, bone morphogenetic protein; BMPR, bone morphogenetic protein receptor; GDF, growth differentiation factor; SMAD, Sma- and Mad-related protein.
BMP signaling
Members of the TGF-β superfamily exert their functions by binding to type I and type II receptors, which further regulate downstream target gene expression by phosphorylating the receptor-regulatory SMAD (R-SMAD) transcription factors, SMAD1–8. Generally, two distinct models of the ligand–receptor interaction exist. BMPs, AMH and some GDFs activate SMAD1/5/8 via ALK2, ALK3 and/or ALK6; whereas, TGF-βs, activins and nodal activate SMAD2/3 via ALK4, ALK5 and/or ALK7 (Drummond, 2005; Mueller and Nickel, 2012). Upon phosphorylation of type I receptors, BMPs activate the canonical SMAD1/5/8 group, which then associate with the common SMAD (SMAD4), and this complex translocates to the nucleus to exert functions that regulate gene expression in most tissues.
Quite recently, novel non-canonical SMAD2/3 signaling (commonly induced by TGF-βs and activins) activated by BMPs to regulate cancer progression and hormone production has been given considerable attention (Holtzhausen et al., 2014; Wang et al., 2014). In human granulosa tumor cells and mouse GCs, recombinant human BMP15, but not mouse BMP15, mildly phosphorylated SMAD2/3 and stimulated mouse cumulus–oocyte complex (COC) expansion (Peng et al., 2013). In line with these reports challenging the standard guideline that BMPs exert cellular functions solitarily through canonical SMAD1/5/8 signaling, our most recent studies showed that BMPs-induced hyaluronan synthase type 2 (HAS2) expression is mediated by noncanonical SMAD2/3 signaling through binding to ALK4/5/7 in immortalized human GCs (Zhang et al., 2016). Differences in receptor expression levels and receptor binding properties between different BMPs (homodimers or heterodimers) most likely contribute to the differential activation of noncanonical SMAD2/3 signaling in certain cell types.
In addition to the SMAD signaling, several SMAD-independent (non-SMAD) signaling pathways for BMPs have been identified in specific tissues. It is widely believed that the mitogen-activated protein kinase (MAPK) signal pathway is the major SMAD-independent pathway induced by BMPs. BMP4 has been found to phosphorylate TGF-β activated kinase 1, a serine/threonine kinase of the MAPKKK family (Yamaguchi et al., 1995; Derynck and Zhang, 2003). Previous studies indicate that BMPs are also able to activate several signaling pathways, including phosphoinositide 3-kinase, extracellular signal-regulated kinase (ERK), protein kinase (PK) A, PKC and PKD (PKCμ) (Yamaguchi et al., 1995; Nakamura et al., 1999; von Bubnoff and Cho, 2001). Interestingly, our recent study showed that the BMP subfamily member GDF8 decreases steroidogenic acute regulatory protein (StAR) expression by activating both the SMAD3 and ERK signaling pathways (Fang et al., 2015). Upon ligand–receptor binding and interaction, the subsequent pathway activated by BMPs is most likely dependent on other cellular activity, the extracellular environment and crosstalk with other signaling pathways.
Intra-ovarian BMPs and oocyte–somatic cell interactions
Human GC lines
To clearly understand the underlying mechanisms by which hormones or growth factors regulate folliculogenesis and ovarian steroidogenesis, available cell models that are suitable for in vitro studies are warranted. GC-based in vitro cell model systems have provided valuable tools for studying ovarian biology. The major source of human GCs for in vitro studies is usually from infertile patients undergoing IVF. However, these cells are obtainable only in small numbers, which make it difficult to conduct extensive experiments related to detailed molecular analysis. In addition, the clinically obtained GCs are generally luteinized because of their extensive stimulation with FSH/LH and hCG prior to cell isolation. Therefore, these GCs have a limited life span with a slow proliferation rate, and they do not survive in vitro for many passages (Breckwoldt et al., 1996). Furthermore, primary GCs derived from different patients may exhibit wide variability, which adds to the difficulty of obtaining reproducible results. Because of these obstacles, a substitute human GC line has become an attractive option. In the last few years, there has been a growing interest in developing several cell lines from human GCs (Table II). These cell lines were generated through various techniques, including oncogenic transformation (Rainey et al., 1994; Lie et al., 1996; Hosokawa et al., 1998; Nitta et al., 2001; Tajima et al., 2002; Okamura et al., 2003), transfection with site-directed mutagenesis (Bayasula et al., 2012) and explants of human tumors (Ishiwata et al., 1984; van den Berg-Bakker et al., 1993; Nishi et al., 2001). Notably, each cell line may have different cell properties with regard to steroidogenic functions, gonadotrophin (FSH and LH) responsiveness, cAMP responsiveness, BMP responsiveness, and mitogenic and differential capabilities (Havelock et al., 2004) (Table II). Therefore, it is essential to choose the appropriate cell line for individual studies. For instance, the SVOG cell line was developed by transfection of human granulosa-lutein (hGL) cells using SV40 large T antigen (Lie et al., 1996) (Table II). Primary hGL cells were used to generate the immortalized cells and the two display similar biological responses to many different treatments, such as LH, hCG, cAMP and various growth factors (Lie et al., 1996). As demonstrated in numerous previous publications from our laboratory as well as those of other investigators, these cells are a widely accepted model to study the ovarian response during the periovulatory and early luteal phases (Havelock et al., 2004; Chang et al., 2015a, 2015c, 2016c, 2016a; Fang et al., 2016a). COV434 and KGN are two widely used GC lines originating from ovarian GC tumors (van den Berg-Bakker et al., 1993; Zhang et al., 2000; Nishi et al., 2001). Unfortunately, the development of an androgen-producing thecal cell line has faced a tough challenge with limited success (Magoffin and Erickson, 1988; McAllister et al., 1989).
Table II.
The cellular properties of human ovarian GC lines.
| Cell line | Origin | Transfection | Progesterone production | Aromatase activity | Steroidogenic enzyme | FSH response | LH/hCG response | cAMP response | BMP response | References |
|---|---|---|---|---|---|---|---|---|---|---|
| SVOG | Luteinized GC | SV40 T | (+) | (−) | (+) | (−) | (+) | (+) | (+) | Lie et al. (1996) |
| HGL5 | Luteinized GC | HPV16E6/E7 | (+) | (+) | (+) | (−) | (−) | (+) | (+) | Rainey et al. (1994) |
| HO-23 | Luteinized GC | SV40 T, Ha-ras p53 | (+) | ND | (+) | ND | (−) | (+) | ND | Hosokawa et al. (1998) |
| HGP53 | Luteinized GC | Ha-ras & p53val135 | (+) | ND | (+) | (+) | ND | (+) | ND | Takebayashi et al. (2000) |
| GC1a | Non-luteinized GC | Mouse SF-1 | (−) | (−) | (−) | (−) | (−) | ND | ND | Nitta et al. (2001) |
| Okamura et al. (2003) | ||||||||||
| HGrC1 | Non-luteinized GC | HPV16E6/E7, CDK4R24C, cyclin D1 | (+) | (+) | (+) | (+) | (+) | ND | (+) | Bayasula et al. (2012) |
| COV434 | Metastatic GC tumor | (+) | (+) | (−) | (+) | (−) | (+) | (+) | van den Berg-Bakker et al. (1993) | |
| KGN | GC carcinoma | (+) | (+) | (−) | (+) | (−) | (+) | (+) | Nishi et al. (2001) | |
| HTOG | Granulosa-theca cell tumor | (+) | (+) | ND | ND | ND | ND | ND | Ishiwata et al. (1984) |
GC, granulosa cell; ND, Not documented.
Expression of BMPs and BMP receptors in the human ovary
A number of studies performed over the past decade have provided important information about the expression of the BMP system in the mammalian ovary, with the most comprehensive study using adult cycling rats (Erickson and Shimasaki, 2003; Shimasaki et al., 2004). However, the expression pattern of BMPs in the rat ovary may not necessarily apply to other species. For instance, BMP4 and BMP7 are primarily expressed by theca and stroma cells in rats (Erickson and Shimasaki, 2003); however, both BMP4 and BMP7 have been detected in human oocytes (Abir et al., 2008). GDF9 has been identified in hGL cells and oocytes (Aaltonen et al., 1999; Teixeira Filho et al., 2002; Huang et al., 2009; Shi et al., 2009), but GDF9 is exclusively expressed in the oocytes of ovine and bovine ovaries (Bodensteiner et al., 1999; Hayashi et al., 1999). Likewise, BMP15, which was previously thought to be oocyte-derived (Aaltonen et al., 1999), has been detected in human cumulus cells (Li et al., 2014), GCs and stroma cells in girls and adults (Margulis et al., 2009). The development and physiological functions of the human reproductive system are mostly influenced by the tissue-specific and time-dependent expression of BMP subfamily members (Shimasaki et al., 2004), even though the spatiotemporal expression pattern of BMPs in the human ovary remains largely unknown. The expression of BMP/GDF ligands and their receptors in human ovarian tissues is listed in Table III. In the human ovary, BMP2, BMP4, BMP5, BMP6, BMP7 and BMP8A are expressed in the GCs from normally cycling and polycystic ovary syndrome (PCOS) women (Khalaf et al., 2013). In concert with this study, a study using isolated human pre-antral follicles that compared the expression levels of BMPs in five size-matched populations demonstrated that BMP6 and BMP15 are the most abundantly expressed ligands (Kristensen et al., 2014). In the human corpus luteum, BMP2, BMP4 and BMP6 are highly expressed in the granulosa-lutein and theca-lutein cells and are involved in the process of luteolysis (Nio-Kobayashi et al., 2015).
Table III.
Localization of BMPs/GDFs and receptors in the human ovary.
| Ligands | Localization | Expression | Detection method | References |
|---|---|---|---|---|
| BMP2 | Luteinized GC | mRNA | RT-qPCR | Khalaf et al. (2013) |
| CL | mRNA | RT-qPCR | Nio-Kobayashi et al. (2015) | |
| FF (1–115 ng/ml) | Protein | ELISA | Sugiyama et al. (2010) | |
| BMP4 | Luteinized GC | mRNA & Protein | RT-qPCR, IHC | Khalaf et al. (2013) |
| Oocytea | Protein | IHC | Abir et al. (2008) | |
| Theca/stroma cellsa | Protein | IHC | Abir et al. (2008) | |
| GCa | Protein | IHC | Abir et al. (2008) | |
| CL | mRNA & Protein | RT-qPCR, IHC | Nio-Kobayashi et al. (2015) | |
| FF (1–20 pg/ml) | Protein | ELISA | Chang et al. (2015a) | |
| BMP5 | Luteinized GC | mRNA | RT-qPCR | Khalaf et al. (2013) |
| BMP6 | Luteinized GC | mRNA & Protein | RT-qPCR, IHC | Khalaf et al. (2013) |
| CL | mRNA & Protein | RT-qPCR, IHC | Nio-Kobayashi et al. (2015) | |
| BMP7 | Luteinized GC | mRNA & Protein | RT-qPCR, IHC | Khalaf et al. (2013) |
| Oocyte | Protein | IHC | Abir et al. (2008) | |
| Theca/stroma cells | Protein | IHC | Abir et al. (2008) | |
| GC | Protein | IHC | Abir et al. (2008) | |
| FF (50–130 ng/ml) | Protein | ELISA | Sugiyama et al. (2010) | |
| BMP8A | Luteinized GC | mRNA | RT-qPCR | Khalaf et al. (2013) |
| BMP15 | Pre-antral follicles (>75 μm) | mRNA | Tissue microarray | Kristensen et al. (2014) |
| Cumulus cells | mRNA | RT-qPCR | Li et al. (2014) | |
| Oocyte | mRNA & Protein | In situ hybridization, RT-qPCR, IHC | Margulis et al. (2009) | |
| Aaltonen et al. (1999) | ||||
| Teixeira Filho et al. (2002) | ||||
| Theca/stroma cells | mRNA & Protein | In situ hybridization, IHC | Margulis et al. (2009) | |
| GC | mRNA & Protein | In situ hybridization, IHC | Margulis et al. (2009) | |
| FF | Protein | Western blot | Wu et al. (2012) | |
| GDF8 | Luteinized GC | mRNA | RT-qPCR | Chang et al. (2015d) |
| FF (2.01–4.17 ng/ml) | Protein | ELISA | Chang et al. (2015d) | |
| GDF9 | Pre-antral follicles | mRNA | Tissue microarray | Kristensen et al. (2014) |
| Cumulus cells | mRNA | RT-qPCR | Li et al. (2014) | |
| Oocyte | mRNA & Protein | In situ hybridization, IHC | Aaltonen et al. (1999) | |
| Teixeira Filho et al. (2002) | ||||
| Luteinized GC | mRNA & Protein | RT-PCR, Western blot | Huang et al. (2009) | |
| FF (0.83–53.9 ng/ml) | Protein | ELISA | ||
| ALK2 | Luteinized GC | mRNA | RT-qPCR | Khalaf et al. (2013) |
| ALK3 | Luteinized GC | mRNA & Protein | RT-qPCR, IHC | Khalaf et al. (2013) |
| GC | Protein | IHC | Abir et al. (2008) | |
| Oocyte | Protein | IHC | Abir et al. (2008) | |
| CL | mRNA & Protein | RT-qPCR, IHC | Nio-Kobayashi et al. (2015) | |
| ALK5 | Luteinized GC | mRNA | RT-qPCR | Khalaf et al. (2013) |
| ALK6 | Luteinized GC | mRNA & Protein | RT-qPCR, IHC | Khalaf et al. (2013) |
| Oocyte | Protein | IHC | Abir et al. (2008) | |
| Stroma cells | Protein | IHC | Abir et al. (2008) | |
| GC | Protein | IHC | Abir et al. (2008) | |
| CL | mRNA & Protein | RT-qPCR, IHC | Nio-Kobayashi et al. (2015) | |
| BMPR2 | Luteinized GC | mRNA & Protein | RT-qPCR, IHC | Khalaf et al. (2013) |
| Oocyte | Protein | IHC | Abir et al. (2008) | |
| Stroma cells | Protein | IHC | Abir et al. (2008) | |
| GC | Protein | IHC | Abir et al. (2008) | |
| CL | mRNA & Protein | RT-qPCR, IHC | Nio-Kobayashi et al. (2015) |
BMR, bone morphogenetic proteins; GDF, growth differentiation factors; CL, corpora lutea; ELISA, enzyme-linked immunosorbent assay; FF, follicular fluid; IHC, immunohistochemistry; RT-qPCR, quantitative real-time PCR.
aExpressed only in fetal ovary.
During the antral follicular stage, the growth and maturation of the oocyte are dependent on several intra-ovarian factors present in the follicular fluid (Hsieh et al., 2009). The concentrations of some of the follicular fluid growth factors are correlated with the serum levels of these factors (Qiao and Feng, 2011). The imbalance of any of these intra-ovarian factors may lead to abnormal follicular development and dysfunction of oocyte maturation in humans (Franks et al., 2008; Qiao and Feng, 2011). Recent studies have shown that BMP2, BMP4, BMP7, BMP15, GDF8 and GDF9 are detectable in the follicular fluid (Table III) (Sugiyama et al., 2010; Wu et al., 2012; Chang et al., 2015a; Chang et al., 2015d). Among these follicular fluid BMPs, the results from clinical data suggested that BMP2 and BMP15 may potentially be used as indicators of oocyte fertilization and/or oocyte maturation (Wu et al., 2007; Sugiyama et al., 2010).
Previous research has documented the expression of the mRNA and protein of BMP receptors in various follicular compartments of pre-antral and antral follicles of the human ovary (Khalaf et al., 2013; Kristensen et al., 2014). Among these receptors, BMPR2 is the most abundantly expressed type II receptor (Kristensen et al., 2014). In comparison with the type I receptors ALK1, ALK2 and ALK7, four type I receptors ALK3, ALK4, ALK5 and ALK6 are expressed at modest levels in the human pre-antral follicles (Kristensen et al., 2014). A recent study showed that the dysregulation of ALK6 in human GCs is associated with reduced ovarian reserve and the age-related decline in fertility (Regan et al., 2016).
BMPs and primordial germ cell development
Primordial germ cells (PGCs), the precursors of the sperm and egg in the adult, are a group of germline stem cells that develop only during the early embryonic stage (McLaren, 2003). These stem cells migrate to the primitive gonadal fold and mitotically proliferate to increase cell numbers, which subsequently differentiate into primordial follicles (oocytes associate with the surrounding somatic cells) (Pepling and Spradling, 2001). Members of the BMP subfamily play critical roles in regulating ovarian development and function (Shimasaki et al., 2004; Knight and Glister, 2006). However, the developmental processes are differentially regulated in rodents and humans with respect to the spatiotemporal organization (Lawson et al., 1999; Childs et al., 2010). In mouse embryos, the extraembryonic ectoderm-derived BMP4 and BMP8B collaboratively induce PGC formation (Ying et al., 2002). In human fetal ovaries, BMP4 and BMP signaling modulates post-migratory PGC numbers by promoting apoptosis (Childs et al., 2010). During oogenesis in humans, GDF9 is transiently secreted by oocytes before follicle formation, which is accompanied by activin βA signaling from somatic cells to determine selective germ cell survival (Bayne et al., 2015).
BMPs and intra-ovarian cell–cell communication
The ovarian follicle is the principal functional unit of the female reproductive system. A coordinated interplay within this biological compartment between the oocyte and the follicular cells (cumulus cells/GCs and theca/stroma cells) depends heavily on functional gap junctions (Granot and Dekel, 2002). These connexin-coupled cell junctions directly mediate cell–cell communication by allowing the passage of small molecules (ions, metabolites, nutrition and small signaling molecules) between two adjacent cells (Caspar et al., 1977; Makowski et al., 1977). In the growing follicles in humans, connexin 43 (Cx43) primarily contributes to the formation of gap junctions between cumulus cells/GCs, whereas gap junctions that connect the oocyte to the surrounding cumulus cells are mainly composed of connexin 37 (Cx37) (Furger et al., 1996; Tsai et al., 2003; Gershon et al., 2008). Studies in knockout mice demonstrated that mice lacking Cx43 exhibit a phenotype of reduced germ cell numbers in the fetal gonads, retarded growth of oocytes and fertilization failure (Ackert et al., 2001). In addition, the ablation of Cx37 leads to an abolition of intercellular coupling between oocytes and cumulus cells, disruption of follicle development at the antral stage, incompetent oocytes and ovulatory dysfunction (Simon et al., 1997).
Cx43 is abundantly expressed in the GCs throughout all follicular stages and its expression is required for GC proliferation (Ackert et al., 2001; Gittens et al., 2005). The cyclic expression of Cx43 in the GCs is developmentally and hormonally regulated by gonadotrophins (FSH and LH) and steroid hormones (estrogen, progesterone and androgen) in many species, including humans (Petrocelli and Lye, 1993; Granot and Dekel, 2002; Wu et al., 2010). Our recent studies have shown that three intra-ovarian BMPs (BMP4, BMP7 and BMP15) and TGF-β1 may act in a paracrine/autocrine manner to modulate Cx43 expression and intercellular communication in human GCs (Fig. 2) (Chang et al., 2013b, 2014b, 2015). Specifically, BMP-mediated signaling suppresses Cx43 expression, whereas TGF-β1 increases Cx43 expression, indicating competing regulatory roles for these paracrine/autocrine factors. This functional discrepancy between the growth factors belonging to the TGF-β superfamily of differential signaling molecules (TGF-βs and BMPs) may influence a broad range of cellular actions.
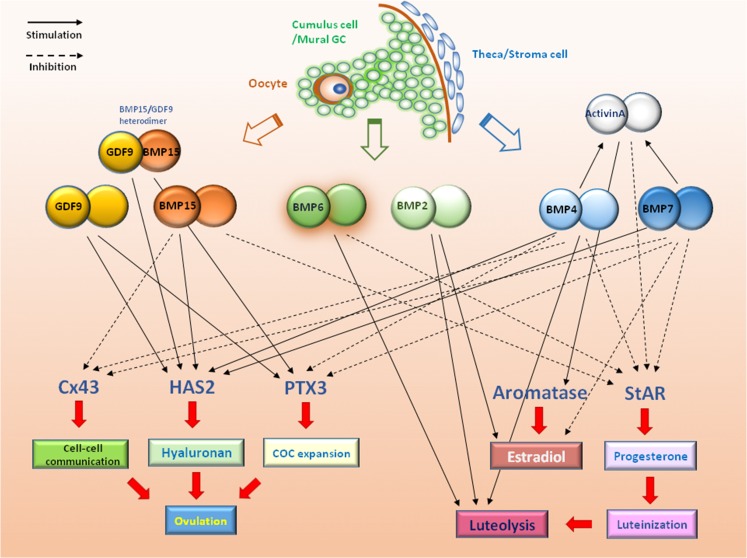
Figure 2.
Schematic diagram summarizing functional roles of BMPs and GDF9 in the human ovary. The potential physiological roles of intra-ovarian BMPs in regulating human ovarian functions, including steroidogenesis, activin production, cumulus–oophorus complex formation and expansion, cell–cell communication, ovulation and luteolysis are shown. BMP, bone morphogenetic protein; COC, cumulus–oophorus complex; Cx43, connexin 43; GC, granulosa cell; HAS2, hyaluronan synthase type 2; PTX3, pentraxin 3; StAR, steroidogenic acute regulatory protein.
Oocyte-somatic cell interactions
In the mammalian ovary, oocytes do not merely reside inside the follicles and passively receive the developmental signals from the surrounding cumulus cells/GCs, but they actively govern and modulate follicular development and ovulation. Within the follicular microenvironment, oocytes and the supporting somatic cells coordinately control the development and maturation of the follicle as well as the acquisition of a meiotically competent oocyte (Eppig, 2001). The results obtained from animal studies indicated that oocytes might promote follicular development and cell differentiation by releasing some growth factors that act in a paracrine manner to affect the neighboring cumulus cells/GCs. These paracrine effects, in turn, modulate oocyte development and maturation (Eppig, 2001). Studies in sheep and mice have shown that the experimental ablation (removal, absence or destruction) of the oocytes results in impaired folliculogenesis, indicating a critical role of the oocyte factors or oocyte-induced paracrine signaling in follicular development (Nekola and Nalbandov, 1971; Gilchrist et al., 2004). An in vivo study has revealed that ovectomy can lead to the spontaneous transformation of the Graafian follicle into the corresponding corpus luteum (el-Fouly et al., 1970). In vitro experiments have demonstrated that oocyte-secreted factors may potently promote DNA synthesis and cell proliferation in the surrounding cumulus/mural GCs (Vanderhyden et al., 1992; Gilchrist et al., 2001). In addition, these oocyte-derived potent mitogens can also augment the effects of several GC regulators (FSH, insulin-like growth factor-I and androgen) on cell growth activities (Armstrong et al., 1996; Fraidenraich et al., 1998; Li et al., 2000; Hickey et al., 2004).
Aside from the mitogenic effects, oocytes are potent modulators of cumulus cell/GC differentiation in various species, including humans (Eppig, 2001). In particular, oocytes modulate the FSH-stimulated estradiol and progesterone production by GCs (Vanderhyden, 1993; Coskun et al., 1995; Vanderhyden and Tonary, 1995), whereas they suppress the expression of LH receptor (LHR) induced by FSH in GCs (Eppig et al., 1997). Apart from the gonadotrophins secreted by the pituitary gland, oocyte-derived BMP15 and GDF9 also participate in the modulation of certain target genes related to ovulation and luteinization (Pangas and Matzuk, 2005; Diaz et al., 2007). In human GCs, oocyte-derived BMP15 decreases progesterone production by down-regulating the expression of StAR (Chang et al., 2014b). Collectively, these results support a previously proposed hypothesis that the oocyte is capable of inhibiting follicular luteinization (Fig. 2) (el-Fouly et al., 1970).
Data collected from the cumulus cell transcriptome revealed that bi-directional communication between the human oocyte and cumulus cells is essential for the production of a competent oocyte (Huang and Wells, 2010). In this regard, the oocyte acts as a central regulator of neighboring follicular cell function by secreting various growth factors and cytokines to modulate cell proliferation, cell differentiation, apoptosis and luteinization (Gilchris et al., 2008). At ovulation, cumulus expansion is a complicated process that is required for the optimal extrusion of the cumulus–oocyte cell mass from the follicle (Russell and Robker, 2007). The cumulus expansion process and oocyte maturation are highly dependent on the interactions of two signals induced by epidermal growth factor (EGF)-like peptides (triggered by LH or hCG) and an oocyte-derived paracrine factor (Hsieh et al., 2009; Fang et al., 2013). Specifically, the oocyte-derived factor capacitates the response of cumulus cells to three EGF-like peptides, which induces the expression of several target genes [HAS2, tumor necrosis factor alpha-induced protein 6 (TNFAIP6), pentraxin 3 (PTX3) and prostaglandin-endoperoxide synthase 2 (PTGS2)] related to extracellular matrix formation and stability (Diaz et al., 2007). Indeed, the in vitro studies have shown that oocyte factors, GDF9 and, to a lesser extent, BMP4 and BMP7 induce HAS2 expression and hyaluronan synthesis as well as prostaglandin E2 production, which is essential for normal ovulation (Elvin et al., 1999; Zhang et al., 2016). The knowledge obtained from cumulus–oocyte interactions during the periovulatory stage will enable clinicians to design optimal procedures for ART, especially the IVM protocol.
BMPs prevent premature luteinization
In humans, the LH surge stimulates multiple intra-follicular activities and triggers ovulation (Russell and Robker, 2007). At the time of ovulation, a series of morphological transitions and tissue remodeling cause the ruptured ovarian follicle to develop into the corpus luteum, a temporary endocrine structure that secretes progesterone (Oon and Johnson, 2000). The release of progesterone targets and prepares the reproductive tract for initiation of fertilization and maintenance of early pregnancy (Niswender et al., 2000). Premature luteinization refers to an elevation of serum progesterone levels on (or before) the day of hCG administration in patients undergoing controlled ovarian stimulation (Al-Azemi et al., 2012). The premature rise of progesterone can shift the implantation window (synchronization between embryonic development and endometrial receptivity), which may hamper embryo implantation and decrease the pregnancy rate (Achache and Revel, 2006). During the antral follicle stage, one of the most important physiological functions is the prevention of premature luteinization, which maintains follicular growth and somatic cell proliferation (Baerwald et al., 2012).
Evidence from animal studies has shown that the regulation of steroid hormones by BMPs is species- and stage-specific in the pre-antral and antral follicular stages. In humans, some inconsistent results have been published regarding the regulatory effects of BMPs on ovarian steroid hormone synthesis. In a human granulosa tumor cell line (KGN), BMP4 and BMP7 suppressed forskolin- or cAMP-induced progesterone production without affecting forskolin- or cAMP-induced estradiol production (Miyoshi et al., 2006). In contrast, a cohort study using GCs from normal healthy women and PCOS women showed that BMP7, but not BMP4, suppressed basal estradiol production, and both BMP4 and BMP7 had no effect on FSH-induced estradiol production in the normal women (Khalaf et al., 2013). Studies using primary GCs obtained from IVF patients showed that BMP2, BMP4 and BMP6 suppressed StAR expression, whereas BMP2 induced aromatase expression (Shi et al., 2011; Nio-Kobayashi et al., 2015). Consistent with some of the previous studies, our recent results showed that BMP4, BMP7 and BMP15 all decreased progesterone production by down-regulating ALK3-mediated StAR expression in immortalized GCs (SVOG cells), but not in KGN cells (Chang et al., 2013; Zhang et al., 2015). Moreover, BMP4 and BMP7 increased the synthesis of bioactive activin A by up-regulating the production and proteolytical processing of the inhibin βA subunit in human immortalized GCs (Chang et al., 2015c). Activin A is another potent luteinization inhibitor because of its ability to decrease the basal and FSH- or LH-induced progesterone production in hGL cells (Chang et al., 2014a). GDF9, a close relative of BMP15, has divergent roles in the regulation of human steroid production. In human GCs and theca cells, GDF9 suppresses 8-bromo-cAMP-induced StAR expression and progesterone production without affecting basal StAR protein levels or progesterone production (Yamamoto et al., 2002; Shi et al., 2010).
Taken together, all of these studies in the human follicle cells suggest that the secretion of BMPs from oocytes, GCs or theca/stroma cells inhibits StAR expression and, in turn, decreases progesterone production. On the other hand, oocyte-derived GDF9 interacts with pituitary gonadotrophins to further reduce progesterone production (Fig. 2). After ovulation, the dramatic decrease in GDF9 and BMP15 levels in the corpus luteum leads to elevated StAR expression and a subsequent increase in progesterone production.
BMPs modulate COC formation and expansion
During the antral follicle stage (characterized by the formation of a fluid-filled antrum), the original GCs that surround the oocyte differentiate into two functionally and anatomically distinct sublineages, the cumulus cells and mural GCs. The cumulus cells intimately interact with the oocyte to form an elaborate structure called the COC, while mural GCs are layered against the follicle wall and close to the basement membrane and theca/stroma cells (Albertini et al., 2001). During the periovulatory stage, these two types of GC exhibit highly divergent characteristics. In general, cumulus cells are enriched for transcripts involved in cell proliferation and metabolism, whereas mural GCs are enriched for transcripts associated with cell differentiation and signaling (Wigglesworth et al., 2015). In contrast to mural GCs, cumulus cells display a higher cell proliferation rate, a higher AMH expression level, a lower steroidogenic capacity, a lower LHR expression level and have the ability to secrete hyaluronic acid for COC expansion (cumulus expansion) (Armstrong et al., 1996; Eppig et al., 1997; Li et al., 2000; Grondahl et al., 2011). The cumulus cells associate with the oocyte and the extended viscoelastic extracellular matrix to form a unique structure, the hyaluronan-rich COC matrix (Russell and Robker, 2007). Hyaluronan is the structural backbone of this matrix, which is further stabilized by a complex network of binding proteins, including versican, tumor necrosis factor-stimulated gene 6 protein (TSG-6), inter-α trypsin inhibitor and PTX3 (Russell and Salustri, 2006; Baranova et al., 2014).
At mid-cycle, the LH surge initiates the ovulatory process by decreasing cell–cell communication, resuming oocyte meiotic maturation, generating tissue remodeling and inducing the expansion of the COC (Russell and Robker, 2007). The cumulus expansion, characterized by a process of morphological change involved in the proliferation and dispersion of cumulus cells, is initiated under the control of several endocrine, paracrine and oocyte-derived factors (Eppig, 1980; Varani et al., 2002; Russell and Robker, 2007; Fang et al., 2013). In vitro studies have demonstrated the requirement of either the oocyte or its conditioned medium for the response of cumulus cells to EGF, FSH or cAMP in the synthesis of hyaluronan (Buccione et al., 1990; Salustri et al., 1990). In cumulus cells, the key enzyme responsible for the polymerization and elongation of the hyaluronan chains to localize into the intercellular space is a transmembrane protein, HAS2 (Weigel et al., 1997). Indeed, the human BMP15 homodimer and mouse GDF9 are able to up-regulate the cumulus expansion-related genes (Has2, Ptx3 and Ptgs2) in mouse GCs and promote cumulus expansion in vitro (Peng et al., 2013).
In the structure of the hyaluronan-based extracellular matrix, PTX3 plays a critical role in the assembly process. In this stable and sustainable hyaluronan network, PTX3 acts as an aggregating reagent to link to a molecule, TSG-6, which is further bound to the distinct hyaluronan strand (Baranova et al., 2014). The knockout mouse model has been used to highlight the essential roles of PTX3 and cumulus expansion in the processes of oocyte maturation and ovulation, efficient transportation of the oocyte through the oviducts and in vivo fertilization (Vanderhyden and Armstrong, 1989). Mice lacking Ptx3 display subfertility, structural defects of the COC and failed in vivo fertilization (Varani et al., 2002). Our recent study showed that BMP4 and BMP7 down-regulated the expression and protein production of PTX3 in human GCs, indicating that BMPs may participate in extracellular matrix formation and tissue remodeling. Collectively, we can speculate that oocyte-secreted factors (mainly BMP15 and GDF9) maintain the cumulus cell phenotype by promoting cumulus expansion, whereas theca/stroma-derived BMPs (mainly BMP4 and BMP7) decompose the structure of the extracellular matrix in the neighboring mural GCs to facilitate the separation of COC and mural GCs in the human ovary (Fig. 2).
BMPs and luteal function
In the absence of pregnancy, the corpus luteum begins to regress at the end of the luteal phase, a degradation process called luteolysis (Hussein, 2005). Luteolysis is a complicated process involving a loss of the structural and functional integrity of the corpus luteum accompanied by a decrease in progesterone production (Hussein, 2005). Studies using human ovarian tissues demonstrated that the expression levels of BMP2, BMP4 and BMP6 are increased during luteal regression and they are differentially regulated by hCG, suggesting that these BMPs may be involved in the process of luteolysis (Nio-Kobayashi et al., 2015) (Fig. 2).
Role of BMPs in female reproductive pathology
Any abnormality in the intra-ovarian BMPs or BMP signaling may negatively affect oocyte–somatic cell interactions, steroidogenesis, GC proliferation, oocyte maturation, cumulus expansion, ovulation, embryonic quality and luteal function, leading to female infertility and reproductive pathology. Knocking out of Bmp4 or the depletion of Alk2 in mouse embryos results in a lack of PGC formation (Lawson et al., 1999). Genetic depletion of the Bmp6 gene in female mice causes a decrease in number of ovulated eggs and reduced litter size (Sugiura et al., 2010). Oocyte-derived BMP15 and GDF9 are the prominent BMP/GDF associated with various ovarian functions and ovulation rate (Persani et al., 2014). Abnormal expression of these two factors may be related to female infertility (Gilchrist et al., 2008). The results from clinical data have suggested that BMP15 levels in the follicular fluid may be used as an indicator of oocyte quality and subsequent fertilization ability (Wu et al., 2007). In addition, a couple of BMP receptors have been shown to be involved in implantation and maternal–fetal interactions during human pregnancy. Disruption of BMPR2-mediated signaling in the uterine decidua leads to placental abruption, fetal demise and female sterility (Nagashima et al., 2013). During mouse pregnancy, mice carrying a conditional ablation of Alk2 in the uterus (Alk2 cKO mice) exhibit delayed embryo invasion into the uterine stroma, failed uterine decidualization and sterility. Likewise, ablation of ALK2 using siRNA targeted to ALK2 in human uterine stromal cells may alter uterine decidualization and embryo implantation, leading to female sterility (Clementi et al., 2013).
Polycystic ovary syndrome
Using immunohistochemical staining, the expression levels of BMP15 and GDF9 proteins in the oocyte and GCs of follicles in PCOS ovaries are reduced and delayed during the early follicular stage (Wei et al., 2014). Compared with the control group, the expression levels of BMP15 and GDF9 proteins in the oocytes tended to be higher in women with PCOS, which could be the result of PCOS follicular dysplasia (Zhao et al., 2010). However, the expression levels of GDF9 protein in cumulus cells are lower in PCOS women, which may result in premature luteinization, poor oocyte competence and luteal dysfunction, leading to higher miscarriage rates in PCOS patients (Zhao et al., 2010). These results provide evidence of two valuable intra-ovarian growth factors in the regulation of oocyte maturation in the follicular microenvironment and will help in the design of a potential technique to improve IVM protocols for oocytes from women with PCOS (Qiao and Feng, 2011). Most serum BMP protein levels are either undetectable or at the lower limit of detection in PCOS women, and therefore provide limited information about this disease (van Houten et al., 2013). The data generated from genome-wide association studies indicate that the involvement of BMP15 or GDF9 genetic variations in the etiology of PCOS remains controversial (Takebayashi et al., 2000; Gonzalez et al., 2008; Liu et al., 2011; Persani et al., 2014).
Primary ovarian insufficiency
Several BMP15 gene mutations have been reported in primary ovarian insufficiency (POI) patients with primary or secondary amenorrhea (Takebayashi et al., 2000; Di Pasquale et al., 2004, 2006; Chand et al., 2006; Dixit et al., 2006; Persani et al., 2010; Tiotiu et al., 2010; Auclair et al., 2013; Ferrarini et al., 2013). The first missense mutation in the BMP15 gene was identified in two Italian sisters with familial ovarian dysgenesis and primary amenorrhea (Di Pasquale et al., 2004). Compared to the normal human BMP15 protein, a recombinant BMP15 developed from this mutant gene fails to stimulate GC growth activity and is unable to inhibit progesterone production in primary human GCs (Auclair et al., 2013). Like BMP15, several cohort studies involving different populations with POI have been conducted to screen for GDF9 gene mutations (Dixit et al., 2005; Laissue et al., 2006; Kovanci et al., 2007; Wang et al., 2013; Simpson et al., 2014). However, various missense mutations encoding different GDF9 mutant proteins may display contrasting results in functional studies. Some variants significantly decrease mature GDF9 protein production and diminish the ability of GDF9 to stimulate GC proliferation (Wang et al., 2013). In contrast, other variants may encode a mutant GDF9 that significantly increases GC proliferation (Simpson et al., 2014).
Endometriosis
Endometriosis is defined as the presence of endometrial tissue outside the uterine cavity, which affects 10% of reproductive-aged women (Vigano et al., 2004). Although many theories have been proposed, the precise pathogenesis and molecular mechanism of this common disease remain unknown (Bulun et al., 2015). Immunohistochemical staining of endometriotic lesions obtained from 85 patients revealed that BMP6 is strongly expressed in both the epithelial and stromal cells (Athanasios et al., 2012). A genome-wide profiling analysis comparing the gene expression of ectopic and eutopic endometrial tissues showed that BMP4 and its antagonist, GREM1, are dysregulated in endometriosis (Crispi et al., 2013). Future studies aimed at addressing the functional roles of BMPs in the development of endometriosis will be of great interest.
Novel role of GDF8 in the human ovary
Originally identified in the musculoskeletal system, GDF8 is one of the members of the TGF-β superfamily (McPherron et al., 1997). GDF8 and BMP11 (GDF11) are two closely related growth factors that share a similar structure and function (Gamer et al., 1999). Also known as myostatin, GDF8 is a potent inhibitor of skeletal muscle growth and development (McPherron et al., 1997). Mice carrying a targeted mutation of the Gdf8 gene have an ~25–30% increase in muscle mass because of muscle fiber hypertrophy and hyperplasia (McPherron et al., 1997). Naturally occurring GDF8 gene mutations leading to increased muscle mass have been reported in several species, including cattle, sheep, dogs and humans (McPherron and Lee, 1997; Schuelke et al., 2004; Clop et al., 2006; Mosher et al., 2007). In the last few years, there has been a growing interest in the investigation of GDF8’s functional roles outside of the musculoskeletal systems. Notably, the expression and potential functions of GDF8 have recently been investigated in several reproductive organs, including the uterus and placenta (Islam et al., 2014; Peiris et al., 2014). Furthermore, data from clinical samples have highlighted the possible involvement of GDF8 in the pathogenesis of certain reproductive disorders, such as uterine myoma, pre-eclampsia and PCOS (Chen et al., 2012; Guo et al., 2012; Islam et al., 2014).
GDF8 and ovarian steroidogenesis
In chicken embryos, GDF8 is extensively expressed in various tissues, including the gonads (testis and ovary) (Kubota et al., 2007). In bovine ovaries, microarray analysis revealed the expression of GDF8 in the GCs of different sizes of antral follicles (Skinner et al., 2008). Our most recent immunohistochemistry analysis revealed that GDF8 and its putative functional receptors (ACVR2A, ACVR2B and ALK5) are expressed in the GCs of growing follicles in the human ovary (unpublished data). Moreover, the mature GDF8 protein is detectable in the follicular fluid obtained from IVF patients (Chang et al., 2015d). In addition, recent studies have identified a novel role for GDF8 in the regulation of human GC steroidogenesis. Specifically, GDF8 regulates steroid production by increasing aromatase/estradiol production while decreasing StAR/progesterone production in primary hGL cells (Chang et al., 2016b). Analysis of the data from clinical samples supports these findings, as there is a negative correlation between GDF8 concentrations and progesterone concentrations in the serum and follicular fluid (Fang et al., 2015, 2016b). Moreover, GDF8 may enhance the FSH-induced aromatase/estradiol production, whereas GDF8 suppresses the LH-induced StAR/progesterone production in hGL cells (Chang et al., 2016b). In this regard, GDF8 exerts a regulatory function by modulating GC responsiveness to gonadotrophins. Notably, GDF8 enhances GC responsiveness to FSH by up-regulating FSH receptor (FSHR) expression while suppressing GC responsiveness to LH by down-regulating LHR expression (Chang et al., 2016b). In line with these results, our previous studies showed that activins (activin A, B and AB) may act in a similar manner to modulate steroidogenesis in the same cell model (Chang et al., 2015b). The downstream signaling pathway may account for the similar cellular effects, as both GDF8 and activins act through SMAD2/3-SMAD4-mediated target gene regulation in human GCs (Fang et al., 2015; Chang et al., 2015b).
GDF8 and GC proliferation
During follicular development, GC proliferation and GC terminal differentiation are the key processes essential for oocyte maturation, ovulation and luteinization (Baerwald et al., 2012). At the periovulatory stage, various regulatory endocrine and paracrine factors meticulously modulate the functional switch of GCs from a highly proliferative status to a non-proliferative, terminally differentiated status (McGee and Hsueh, 2000). Beyond its role in regulating steroid production, an in vitro study showed that GDF8 suppresses human GC proliferation (Chang et al., 2016). This negative regulatory effect is mediated by another growth factor, connective tissue growth factor (CTGF), indicating that GDF8 and CTGF may be involved in the control of highly proliferative and non-proliferative events during human follicular development (Chang et al., 2016).
GDF8 and extracellular matrix formation
Lysyl oxidase (LOX) is the key enzyme for the final assembly and stabilization of the extracellular matrix that is essential for follicle and oocyte maturation (Kagan and Trackman, 1991; Woodruff and Shea, 2007). In the rat ovary, the LOX expression level is positively correlated with oocyte quality (Jiang et al., 2010). In many organs, CTGF is a principle mediator of extracellular matrix-related tissue remodeling (Lipson et al., 2012). Both CTGF and LOX are expressed in human GCs, and CTGF mediates the GDF8-induced increase in LOX activity and LOX protein expression (Chang et al., 2016c). Interestingly, the GDF8-induced up-regulation of CTGF expression in human GCs occurs through the ALK5-mediated SMAD2/3-SMAD4 signaling pathway (Chang et al., 2016c). This finding is inconsistent with a previous study demonstrating that GDF8 signaling is mainly dependent on ALK4, but not ALK5, in mouse C2C12 myoblasts, indicating that the type I receptor-mediated GDF8 downstream signaling is cell-type specific (Kemaladewi et al., 2012). Specifically, GDF8 acts through ALK4 in myoblast cells, while ALK5 mediates the GDF8-induced cellular action in non-myogenic cells.
GDF8 and COC expansion
The occurrence of COC and the extent of cumulus expansion have been linked to oocyte competence and are potentially useful as indicators for oocyte selection in the IVF program (Ball et al., 1983; Foote, 1987). The combined results of the in vitro studies and the clinical data also revealed a positive correlation between PTX3 expression levels in cumulus cells and their corresponding oocyte quality and subsequent fertilization rates (Zhang et al., 2005; Huang et al., 2013). Consistent with the effects of BMP4 and BMP7, GDF8 might modulate cumulus expansion by down-regulating the key linking protein, PTX3, in human GCs (Chang et al., 2015d).
Taken together, GDF8 is expressed in human GCs, and its mature proteins are detectable in follicular fluid. A series of functional studies have demonstrated the roles of GDF8 in regulating steroidogenesis, gonadotrophin responsiveness, cell proliferation, LOX expression, LOX activity and PTX3 expression in human GCs (Fig. 3). These findings suggest that this unique TGF-β superfamily member might play a critical role in modulation of the final differentiation processes in the growing follicles, most likely by acting as both a maturation stimulator and a luteinization inhibitor.
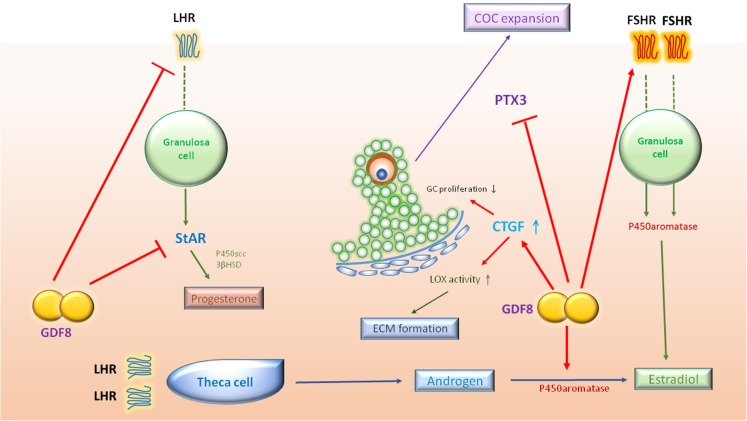
Figure 3.
Schematic diagram summarizing potential roles of GDF8 in a human growing follicle. In this follicular microenvironment, the locally produced GDF8 may promote aromatase/estradiol and FSHR expression, suppress StAR/progesterone and LHR expression and down-regulate PTX3 expression. In addition, GDF8 induces the expression of CTGF, which contributes to the suppression of GC proliferation and the increase in LOX activity. 3βHSD, 3β-hydroxysteroid dehydrogenase; CTGF, connective tissue growth factor; ECM, extracellular matrix; LOX, lysyl oxidase; P450scc, P450 side-chain cleavage enzyme; PTX3, pentraxin 3; FSHR, FSH receptor; LHR, LH receptor.
Perspectives and therapeutic potential
A more comprehensive understanding of the physiological roles of BMPs/GDFs in the human ovary will provide significant insights into ovarian pathology and lead to new methods of approaching fertility regulation, whether the goal is to develop alternative forms of contraception, to diagnose and treat human infertility, or to develop safer and reliable protocols of inducing ovulation in ART. The involvement of BMP/GDF signaling pathways in a wide range of developmental and pathophysiological processes of reproductive biology makes targeting these pathways a potential therapeutic approach to overcome female infertility. At present, several recombinant human BMPs/GDFs and their inhibitors or antagonists have undergone clinical trials or have received US Food and Drug Administration (FDA) approval in human skeletal and muscular systems (Gautschi et al., 2007; Kinouchi et al., 2008; Lissenberg-Thunnissen et al., 2011; Smith and Lin, 2013; Lee and Wikesjo, 2014; Schmidt-Bleek et al., 2016). Furthermore, the latest development of gene therapy and BMP-ligand trapping approaches are currently attracting more attention (Kinouchi et al., 2008; Hawinkels et al., 2013; Sherman et al., 2013; Spiekerkoetter et al., 2013). All of the above information clearly reflects the availability of potential new medications acting on the BMP/GDF pathways for the treatment of a variety of human diseases, including female reproductive pathologies. However, further human clinical trials are warranted to investigate the efficacy, safety and administration routes of these pharmaceutical applications.
Conclusion
In the last decade, research regarding locally produced growth factors and intra-follicular signaling between the oocyte and the follicle cells has been of considerable interest. The accumulated data indicate that the intra-ovarian BMP/GDF system is of great importance in controlling female reproduction, including PGC development, cell–cell communication, steroidogenesis, COC formation and expansion, oocyte maturation, ovulation and luteolysis. Thus, the relationship between the oocyte and its supporting cells (GCs and theca/stroma cells) should be regarded as a synchronous partnership. During follicular development, all of these cell types play essential, yet sometimes distinct, roles in regulating ovarian functions to generate a competent oocyte for embryo development. A comprehensive understanding of the expression, actions and underlying molecular mechanisms of the BMP/GDF system in the human ovary is critical to the development of clinical approaches (diagnosis and/or treatment) for women suffering from infertility and ovulation dysfunction.
Authors’ roles
H.-M.C. collected the information, designed the pictures and wrote the manuscript. J.Q. collected the information and critically revised the manuscript. P.C.K.L. collected the information, designed the pictures and critically revised the manuscript. All the authors have seen and approved the final version.
Funding
An operating grant from the Canadian Institutes of Health Research (#143317) to P.C.K.L. Beijing Advanced Innovation Center for Genomics and the National Natural Science Foundation of Key Program (#31230047) to J.Q.
Conflict of interest
We declare that Hsun-Ming Chang, Jie Qiao and Peter C.K. Leung have no conflict of interest with the contents of this manuscript.
References
- Aaltonen J, Laitinen MP, Vuojolainen K, Jaatinen R, Horelli-Kuitunen N, Seppa L, Louhio H, Tuuri T, Sjoberg J, Butzow R et al. Human growth differentiation factor 9 (GDF-9) and its novel homolog GDF-9B are expressed in oocytes during early folliculogenesis. J Clin Endocrinol Metab 1999;84:2744–2750. [DOI] [PubMed] [Google Scholar]
- Abir R, Ben-Haroush A, Melamed N, Felz C, Krissi H, Fisch B. Expression of bone morphogenetic proteins 4 and 7 and their receptors IA, IB, and II in human ovaries from fetuses and adults. Fertil Steril 2008;89:1430–1440. [DOI] [PubMed] [Google Scholar]
- Achache H, Revel A. Endometrial receptivity markers, the journey to successful embryo implantation. Hum Reprod Update 2006;12:731–746. [DOI] [PubMed] [Google Scholar]
- Ackert CL, Gittens JE, O'Brien MJ, Eppig JJ, Kidder GM. Intercellular communication via connexin43 gap junctions is required for ovarian folliculogenesis in the mouse. Dev Biol 2001;233:258–270. [DOI] [PubMed] [Google Scholar]
- Al-Azemi M, Kyrou D, Kolibianakis EM, Humaidan P, Van Vaerenbergh I, Devroey P, Fatemi HM. Elevated progesterone during ovarian stimulation for IVF. Reprod Biomed Online 2012;24:381–388. [DOI] [PubMed] [Google Scholar]
- Albertini DF, Combelles CM, Benecchi E, Carabatsos MJ. Cellular basis for paracrine regulation of ovarian follicle development. Reproduction 2001;121:647–653. [DOI] [PubMed] [Google Scholar]
- Armstrong DT, Xia P, de Gannes G, Tekpetey FR, Khamsi F. Differential effects of insulin-like growth factor-I and follicle-stimulating hormone on proliferation and differentiation of bovine cumulus cells and granulosa cells. Biol Reprod 1996;54:331–338. [DOI] [PubMed] [Google Scholar]
- Athanasios F, Afrodite N, Effstratios P, Demetrios K. Co-expression of bone morphogenetic protein 6 with estrogen receptor a in endometriosis. Arch Gynecol Obstet 2012;285:1001–1007. [DOI] [PubMed] [Google Scholar]
- Auclair S, Rossetti R, Meslin C, Monestier O, Di Pasquale E, Pascal G, Persani L, Fabre S. Positive selection in bone morphogenetic protein 15 targets a natural mutation associated with primary ovarian insufficiency in human. PLoS One 2013;8:e78199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baerwald AR, Adams GP, Pierson RA. Ovarian antral folliculogenesis during the human menstrual cycle: a review. Hum Reprod Update 2012;18:73–91. [DOI] [PubMed] [Google Scholar]
- Ball GD, Leibfried ML, Lenz RW, Ax RL, Bavister BD, First NL. Factors affecting successful in vitro fertilization of bovine follicular oocytes. Biol Reprod 1983;28:717–725. [DOI] [PubMed] [Google Scholar]
- Baranova NS, Inforzato A, Briggs DC, Tilakaratna V, Enghild JJ, Thakar D, Milner CM, Day AJ, Richter RP. Incorporation of pentraxin 3 into hyaluronan matrices is tightly regulated and promotes matrix cross-linking. J Biol Chem 2014;289:30481–30498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bayasula, Iwase A, Kiyono T, Takikawa S, Goto M, Nakamura T, Nagatomo Y, Nakahara T, Kotani T, Kobayashi H et al. Establishment of a human nonluteinized granulosa cell line that transitions from the gonadotropin-independent to the gonadotropin-dependent status. Endocrinology 2012;153:2851–2860. [DOI] [PubMed] [Google Scholar]
- Bayne RA, Kinnell HL, Coutts SM, He J, Childs AJ, Anderson RA. GDF9 is transiently expressed in oocytes before follicle formation in the human fetal ovary and is regulated by a novel NOBOX transcript. PLoS One 2015;10:e0119819. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bier E, De Robertis EM. EMBRYO DEVELOPMENT. BMP gradients: a paradigm for morphogen-mediated developmental patterning. Science 2015;348:aaa5838. [DOI] [PubMed] [Google Scholar]
- Bodensteiner KJ, Clay CM, Moeller CL, Sawyer HR. Molecular cloning of the ovine growth/differentiation factor-9 gene and expression of growth/differentiation factor-9 in ovine and bovine ovaries. Biol Reprod 1999;60:381–386. [DOI] [PubMed] [Google Scholar]
- Breckwoldt M, Selvaraj N, Aharoni D, Barash A, Segal I, Insler V, Amsterdam A. Expression of Ad4-BP/cytochrome P450 side chain cleavage enzyme and induction of cell death in long-term cultures of human granulosa cells. Mol Hum Reprod 1996;2:391–400. [DOI] [PubMed] [Google Scholar]
- Buccione R, Vanderhyden BC, Caron PJ, Eppig JJ. FSH-induced expansion of the mouse cumulus oophorus in vitro is dependent upon a specific factor(s) secreted by the oocyte. Dev Biol 1990;138:16–25. [DOI] [PubMed] [Google Scholar]
- Bulun SE, Monsivais D, Kakinuma T, Furukawa Y, Bernardi L, Pavone ME, Dyson M. Molecular biology of endometriosis: from aromatase to genomic abnormalities. Semin Reprod Med 2015;33:220–224. [DOI] [PubMed] [Google Scholar]
- Caspar DL, Goodenough DA, Makowski L, Phillips WC. Gap junction structures. I. Correlated electron microscopy and x-ray diffraction. J Cell Biol 1977;74:605–628. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chand AL, Ponnampalam AP, Harris SE, Winship IM, Shelling AN. Mutational analysis of BMP15 and GDF9 as candidate genes for premature ovarian failure. Fertil Steril 2006;86:1009–1012. [DOI] [PubMed] [Google Scholar]
- Chang HM, Cheng JC, Fang L, Qiu X, Klausen C, Taylor EL, Leung PC. Recombinant BMP4 and BMP7 downregulate pentraxin 3 in human granulosa cells. J Clin Endocrinol Metab 2015. a;100:E365–374. [DOI] [PubMed] [Google Scholar]
- Chang HM, Cheng JC, Huang HF, Shi FT, Leung PC. Activin A, B and AB decrease progesterone production by down-regulating StAR in human granulosa cells. Mol Cell Endocrinol 2015. b;412:290–301. [DOI] [PubMed] [Google Scholar]
- Chang HM, Cheng JC, Klausen C, Leung PC. BMP15 suppresses progesterone production by down-regulating StAR via ALK3 in human granulosa cells. Mol Endocrinol 2013. a;27:2093–2104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chang HM, Cheng JC, Klausen C, Leung PC. Recombinant BMP4 and BMP7 increase activin A production by up-regulating inhibin betaA subunit and furin expression in human granulosa-lutein cells. J Clin Endocrinol Metab 2015. c;100:E375–386. [DOI] [PubMed] [Google Scholar]
- Chang HM, Cheng JC, Klausen C, Taylor EL, Leung PC. Effects of recombinant activins on steroidogenesis in human granulosa-lutein cells. J Clin Endocrinol Metab 2014;99:E1922–1932. [DOI] [PubMed] [Google Scholar]
- Chang HM, Cheng JC, Leung PC. Theca-derived BMP4 and BMP7 down-regulate connexin43 expression and decrease gap junction intercellular communication activity in immortalized human granulosa cells. J Clin Endocrinol Metab 2013. b;98:E437–445. [DOI] [PubMed] [Google Scholar]
- Chang HM, Cheng JC, Liu Y, Klausen C, Xu C, Leung PC. Activin A-induced increase in LOX activity in human granulosa-lutein cells is mediated by CTGF. Reproduction 2016. a;152:293–301. [DOI] [PubMed] [Google Scholar]
- Chang HM, Cheng JC, Taylor E, Leung PC. Oocyte-derived BMP15 but not GDF9 down-regulates connexin43 expression and decreases gap junction intercellular communication activity in immortalized human granulosa cells. Mol Hum Reprod 2014;20:373–383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chang HM, Fang L, Cheng JC, Klausen C, Sun YP, Leung PC. Growth differentiation factor 8 down-regulates pentraxin 3 in human granulosa cells. Mol Cell Endocrinol 2015. d;404:82–90. [DOI] [PubMed] [Google Scholar]
- Chang HM, Fang L, Cheng JC, Taylor EL, Sun YP, Leung PC. Effects of growth differentiation factor 8 on steroidogenesis in human granulosa-lutein cells. Fertil Steril 2016. b;105:520–528. [DOI] [PubMed] [Google Scholar]
- Chang HM, Fang Y, Liu PP, Cheng JC, Yang X, Leung PC. Connective tissue growth factor mediates growth differentiation factor 8-induced increase of lysyl oxidase activity in human granulosa-lutein cells. Mol Cell Endocrinol 2016. c;434:186–198. [DOI] [PubMed] [Google Scholar]
- Chang HM, Pan HH, Cheng JC, Zhu YM, Leung PC. Growth differentiation factor 8 suppresses cell proliferation by up-regulating CTGF expression in human granulosa cells. Mol Cell Endocrinol 2016. c;422:9–17. [DOI] [PubMed] [Google Scholar]
- Chen MJ, Han DS, Yang JH, Yang YS, Ho HN, Yang WS. Myostatin and its association with abdominal obesity, androgen and follistatin levels in women with polycystic ovary syndrome. Hum Reprod 2012;27:2476–2483. [DOI] [PubMed] [Google Scholar]
- Chen YC, Chang HM, Cheng JC, Tsai HD, Wu CH, Leung PC. Transforming growth factor-beta1 up-regulates connexin43 expression in human granulosa cells. Hum Reprod 2015;30:2190–2201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Childs AJ, Kinnell HL, Collins CS, Hogg K, Bayne RA, Green SJ, McNeilly AS, Anderson RA. BMP signaling in the human fetal ovary is developmentally regulated and promotes primordial germ cell apoptosis. Stem Cells 2010;28:1368–1378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clementi C, Tripurani SK, Large MJ, Edson MA, Creighton CJ, Hawkins SM, Kovanci E, Kaartinen V, Lydon JP, Pangas SA et al. Activin-like kinase 2 functions in peri-implantation uterine signaling in mice and humans. PLoS Genet 2013;9:e1003863. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clop A, Marcq F, Takeda H, Pirottin D, Tordoir X, Bibe B, Bouix J, Caiment F, Elsen JM, Eychenne F et al. A mutation creating a potential illegitimate microRNA target site in the myostatin gene affects muscularity in sheep. Nat Genet 2006;38:813–818. [DOI] [PubMed] [Google Scholar]
- Coskun S, Uzumcu M, Lin YC, Friedman CI, Alak BM. Regulation of cumulus cell steroidogenesis by the porcine oocyte and preliminary characterization of oocyte-produced factor(s). Biol Reprod 1995;53:670–675. [DOI] [PubMed] [Google Scholar]
- Crispi S, Piccolo MT, D'Avino A, Donizetti A, Viceconte R, Spyrou M, Calogero RA, Baldi A, Signorile PG. Transcriptional profiling of endometriosis tissues identifies genes related to organogenesis defects. J Cell Physiol 2013;228:1927–1934. [DOI] [PubMed] [Google Scholar]
- Derynck R, Zhang YE. Smad-dependent and Smad-independent pathways in TGF-beta family signalling. Nature 2003;425:577–584. [DOI] [PubMed] [Google Scholar]
- Di Pasquale E, Beck-Peccoz P, Persani L. Hypergonadotropic ovarian failure associated with an inherited mutation of human bone morphogenetic protein-15 (BMP15) gene. Am J Hum Genet 2004;75:106–111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Di Pasquale E, Rossetti R, Marozzi A, Bodega B, Borgato S, Cavallo L, Einaudi S, Radetti G, Russo G, Sacco M et al. Identification of new variants of human BMP15 gene in a large cohort of women with premature ovarian failure. J Clin Endocrinol Metab 2006;91:1976–1979. [DOI] [PubMed] [Google Scholar]
- Diaz FJ, Wigglesworth K, Eppig JJ. Oocytes determine cumulus cell lineage in mouse ovarian follicles. J Cell Sci 2007;120:1330–1340. [DOI] [PubMed] [Google Scholar]
- Dixit H, Rao LK, Padmalatha V, Kanakavalli M, Deenadayal M, Gupta N, Chakravarty B, Singh L. Mutational screening of the coding region of growth differentiation factor 9 gene in Indian women with ovarian failure. Menopause 2005;12:749–754. [DOI] [PubMed] [Google Scholar]
- Dixit H, Rao LK, Padmalatha VV, Kanakavalli M, Deenadayal M, Gupta N, Chakrabarty B, Singh L. Missense mutations in the BMP15 gene are associated with ovarian failure. Hum Genet 2006;119:408–415. [DOI] [PubMed] [Google Scholar]
- Drummond AE. TGFbeta signalling in the development of ovarian function. Cell Tissue Res 2005;322:107–115. [DOI] [PubMed] [Google Scholar]
- Ducy P, Karsenty G. The family of bone morphogenetic proteins. Kidney Int 2000;57:2207–2214. [DOI] [PubMed] [Google Scholar]
- el-Fouly MA, Cook B, Nekola M, Nalbandov AV. Role of the ovum in follicular luteinization. Endocrinology 1970;87:286–293. [PubMed] [Google Scholar]
- Elvin JA, Clark AT, Wang P, Wolfman NM, Matzuk MM. Paracrine actions of growth differentiation factor-9 in the mammalian ovary. Mol Endocrinol 1999;13:1035–1048. [DOI] [PubMed] [Google Scholar]
- Eppig JJ. Regulation of cumulus oophorus expansion by gonadotropins in vivo and in vitro. Biol Reprod 1980;23:545–552. [DOI] [PubMed] [Google Scholar]
- Eppig JJ. Oocyte control of ovarian follicular development and function in mammals. Reproduction 2001;122:829–838. [DOI] [PubMed] [Google Scholar]
- Eppig JJ, Wigglesworth K, Pendola F, Hirao Y. Murine oocytes suppress expression of luteinizing hormone receptor messenger ribonucleic acid by granulosa cells. Biol Reprod 1997;56:976–984. [DOI] [PubMed] [Google Scholar]
- Erickson GF, Shimasaki S. The spatiotemporal expression pattern of the bone morphogenetic protein family in rat ovary cell types during the estrous cycle. Reprod Biol Endocrinol 2003;1:9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fang L, Chang HM, Cheng JC, Yu Y, Leung PC, Sun YP. Growth differentiation factor-8 decreases StAR expression through ALK5-mediated Smad3 and ERK1/2 signaling pathways in luteinized human granulosa cells. Endocrinology 2015;156:4684–4694. [DOI] [PubMed] [Google Scholar]
- Fang L, Cheng JC, Chang HM, Sun YP, Leung PC. EGF-like growth factors induce COX-2-derived PGE2 production through ERK1/2 in human granulosa cells. J Clin Endocrinol Metab 2013;98:4932–4941. [DOI] [PubMed] [Google Scholar]
- Fang L, Yu Y, Zhang R, He J, Sun YP. Serum GDF-8 levels change dynamically during controlled ovarian hyperstimulation in patients undergoing IVF/ICSI-ET. Sci Rep 2016. b;6:28036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fang Y, Chang HM, Cheng JC, Klausen C, Leung PC, Yang X. Transforming growth factor-beta1 increases lysyl oxidase expression by downregulating MIR29A in human granulosa lutein cells. Reproduction 2016. a;152:205–213. [DOI] [PubMed] [Google Scholar]
- Ferrarini E, Russo L, Fruzzetti F, Agretti P, De Marco G, Dimida A, Gianetti E, Simoncini T, Simi P, Baldinotti F et al. Clinical characteristics and genetic analysis in women with premature ovarian insufficiency. Maturitas 2013;74:61–67. [DOI] [PubMed] [Google Scholar]
- Foote RH. In vitro fertilization and embryo transfer in domestic animals: applications in animals and implications for humans. J In Vitro Fert Embryo Transf 1987;4:73–88. [DOI] [PubMed] [Google Scholar]
- Fraidenraich D, Lang R, Basilico C. Distinct regulatory elements govern Fgf4 gene expression in the mouse blastocyst, myotomes, and developing limb. Dev Biol 1998;204:197–209. [DOI] [PubMed] [Google Scholar]
- Franks S, Stark J, Hardy K. Follicle dynamics and anovulation in polycystic ovary syndrome. Hum Reprod Update 2008;14:367–378. [DOI] [PubMed] [Google Scholar]
- Furger C, Cronier L, Poirot C, Pouchelet M. Human granulosa cells in culture exhibit functional cyclic AMP-regulated gap junctions. Mol Hum Reprod 1996;2:541–548. [DOI] [PubMed] [Google Scholar]
- Gamer LW, Wolfman NM, Celeste AJ, Hattersley G, Hewick R, Rosen V. A novel BMP expressed in developing mouse limb, spinal cord, and tail bud is a potent mesoderm inducer in Xenopus embryos. Dev Biol 1999;208:222–232. [DOI] [PubMed] [Google Scholar]
- Gautschi OP, Frey SP, Zellweger R. Bone morphogenetic proteins in clinical applications. ANZ J Surg 2007;77:626–631. [DOI] [PubMed] [Google Scholar]
- Gershon E, Plaks V, Dekel N. Gap junctions in the ovary: expression, localization and function. Mol Cell Endocrinol 2008;282:18–25. [DOI] [PubMed] [Google Scholar]
- Gilchrist RB, Lane M, Thompson JG. Oocyte-secreted factors: regulators of cumulus cell function and oocyte quality. Hum Reprod Update 2008;14:159–177. [DOI] [PubMed] [Google Scholar]
- Gilchrist RB, Ritter LJ, Armstrong DT. Mouse oocyte mitogenic activity is developmentally coordinated throughout folliculogenesis and meiotic maturation. Dev Biol 2001;240:289–298. [DOI] [PubMed] [Google Scholar]
- Gilchrist RB, Ritter LJ, Armstrong DT. Oocyte-somatic cell interactions during follicle development in mammals. Anim Reprod Sci 2004;82-83:431–446. [DOI] [PubMed] [Google Scholar]
- Gittens JE, Barr KJ, Vanderhyden BC, Kidder GM. Interplay between paracrine signaling and gap junctional communication in ovarian follicles. J Cell Sci 2005;118:113–122. [DOI] [PubMed] [Google Scholar]
- Gonzalez A, Ramirez-Lorca R, Calatayud C, Mendoza N, Ruiz A, Saez ME, Moron FJ. Association of genetic markers within the BMP15 gene with anovulation and infertility in women with polycystic ovary syndrome. Fertil Steril 2008;90:447–449. [DOI] [PubMed] [Google Scholar]
- Gougeon A. Regulation of ovarian follicular development in primates: facts and hypotheses. Endocr Rev 1996;17:121–155. [DOI] [PubMed] [Google Scholar]
- Granot I, Dekel N. The ovarian gap junction protein connexin43: regulation by gonadotropins. Trends Endocrinol Metab 2002;13:310–313. [DOI] [PubMed] [Google Scholar]
- Grondahl ML, Nielsen ME, Dal Canto MB, Fadini R, Rasmussen IA, Westergaard LG, Kristensen SG, Yding Andersen C. Anti-Mullerian hormone remains highly expressed in human cumulus cells during the final stages of folliculogenesis. Reprod Biomed Online 2011;22:389–398. [DOI] [PubMed] [Google Scholar]
- Guo J, Tian T, Lu D, Xia G, Wang H, Dong M. Alterations of maternal serum and placental follistatin-like 3 and myostatin in pre-eclampsia. J Obstet Gynaecol Res 2012;38:988–996. [DOI] [PubMed] [Google Scholar]
- Harrison CA, Al-Musawi SL, Walton KL. Prodomains regulate the synthesis, extracellular localisation and activity of TGF-beta superfamily ligands. Growth Factors 2011;29:174–186. [DOI] [PubMed] [Google Scholar]
- Havelock JC, Rainey WE, Carr BR. Ovarian granulosa cell lines. Mol Cell Endocrinol 2004;228:67–78. [DOI] [PubMed] [Google Scholar]
- Hawinkels LJ, Garcia de Vinuesa A, Ten Dijke P. Activin receptor-like kinase 1 as a target for anti-angiogenesis therapy. Expert Opin Investig Drugs 2013;22:1371–1383. [DOI] [PubMed] [Google Scholar]
- Hayashi M, McGee EA, Min G, Klein C, Rose UM, van Duin M, Hsueh AJ. Recombinant growth differentiation factor-9 (GDF-9) enhances growth and differentiation of cultured early ovarian follicles. Endocrinology 1999;140:1236–1244. [DOI] [PubMed] [Google Scholar]
- Hickey TE, Marrocco DL, Gilchrist RB, Norman RJ, Armstrong DT. Interactions between androgen and growth factors in granulosa cell subtypes of porcine antral follicles. Biol Reprod 2004;71:45–52. [DOI] [PubMed] [Google Scholar]
- Hogan BL. Bone morphogenetic proteins: multifunctional regulators of vertebrate development. Genes Dev 1996;10:1580–1594. [DOI] [PubMed] [Google Scholar]
- Holtzhausen A, Golzio C, How T, Lee YH, Schiemann WP, Katsanis N, Blobe GC. Novel bone morphogenetic protein signaling through Smad2 and Smad3 to regulate cancer progression and development. FASEB J 2014;28:1248–1267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hosokawa K, Dantes A, Schere-Levy C, Barash A, Yoshida Y, Kotsuji F, Vlodavsky I, Amsterdam A. Induction of Ad4BP/SF-1, steroidogenic acute regulatory protein, and cytochrome P450scc enzyme system expression in newly established human granulosa cell lines. Endocrinology 1998;139:4679–4687. [DOI] [PubMed] [Google Scholar]
- Hsieh M, Zamah AM, Conti M. Epidermal growth factor-like growth factors in the follicular fluid: role in oocyte development and maturation. Semin Reprod Med 2009;27:52–61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang HY, Wang HS, Chan SH, Lee CL, Wang CW, Soong YK. Granulosa-lutein cell growth differentiation factor-9 (GDF-9) messenger RNA and protein expression in in vitro fertilization (IVF) cycles: relation to characteristics of ovulation induction and IVF. Fertil Steril 2009;91:1583–1585. [DOI] [PubMed] [Google Scholar]
- Huang X, Hao C, Shen X, Zhang Y, Liu X. RUNX2, GPX3 and PTX3 gene expression profiling in cumulus cells are reflective oocyte/embryo competence and potentially reliable predictors of embryo developmental competence in PCOS patients. Reprod Biol Endocrinol 2013;11:109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang Z, Wells D. The human oocyte and cumulus cells relationship: new insights from the cumulus cell transcriptome. Mol Hum Reprod 2010;16:715–725. [DOI] [PubMed] [Google Scholar]
- Hussein MR. Apoptosis in the ovary: molecular mechanisms. Hum Reprod Update 2005;11:162–177. [DOI] [PubMed] [Google Scholar]
- Ishiwata I, Ishiwata C, Soma M, Kobayashi N, Ishikawa H. Establishment and characterization of an estrogen-producing human ovarian granulosa tumor cell line. J Natl Cancer Inst 1984;72:789–800. [PubMed] [Google Scholar]
- Islam MS, Catherino WH, Protic O, Janjusevic M, Gray PC, Giannubilo SR, Ciavattini A, Lamanna P, Tranquilli AL, Petraglia F et al. Role of activin-A and myostatin and their signaling pathway in human myometrial and leiomyoma cell function. J Clin Endocrinol Metab 2014;99:E775–785. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Israel DI, Nove J, Kerns KM, Kaufman RJ, Rosen V, Cox KA, Wozney JM. Heterodimeric bone morphogenetic proteins show enhanced activity in vitro and in vivo. Growth Factors 1996;13:291–300. [DOI] [PubMed] [Google Scholar]
- Jiang JY, Xiong H, Cao M, Xia X, Sirard MA, Tsang BK. Mural granulosa cell gene expression associated with oocyte developmental competence. J Ovarian Res 2010;3:6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Juengel JL, McNatty KP. The role of proteins of the transforming growth factor-beta superfamily in the intraovarian regulation of follicular development. Hum Reprod Update 2005;11:143–160. [DOI] [PubMed] [Google Scholar]
- Kagan HM, Trackman PC. Properties and function of lysyl oxidase. Am J Respir Cell Mol Biol 1991;5:206–210. [DOI] [PubMed] [Google Scholar]
- Kaivo-Oja N, Bondestam J, Kamarainen M, Koskimies J, Vitt U, Cranfield M, Vuojolainen K, Kallio JP, Olkkonen VM, Hayashi M et al. Growth differentiation factor-9 induces Smad2 activation and inhibin B production in cultured human granulosa-luteal cells. J Clin Endocrinol Metab 2003;88:755–762. [DOI] [PubMed] [Google Scholar]
- Kemaladewi DU, de Gorter DJ, Aartsma-Rus A, van Ommen GJ, ten Dijke P, tHoen PA, Hoogaars WM. Cell-type specific regulation of myostatin signaling. FASEB J 2012;26:1462–1472. [DOI] [PubMed] [Google Scholar]
- Khalaf M, Morera J, Bourret A, Reznik Y, Denoual C, Herlicoviez M, Mittre H, Benhaim A. BMP system expression in GCs from polycystic ovary syndrome women and the in vitro effects of BMP4, BMP6, and BMP7 on GC steroidogenesis. Eur J Endocrinol 2013;168:437–444. [DOI] [PubMed] [Google Scholar]
- Khan DR, Fournier E, Dufort I, Richard FJ, Singh J, Sirard MA. Meta-analysis of gene expression profiles in granulosa cells during folliculogenesis. Reproduction 2016;151:R103–110. [DOI] [PubMed] [Google Scholar]
- Kinouchi N, Ohsawa Y, Ishimaru N, Ohuchi H, Sunada Y, Hayashi Y, Tanimoto Y, Moriyama K, Noji S. Atelocollagen-mediated local and systemic applications of myostatin-targeting siRNA increase skeletal muscle mass. Gene Ther 2008;15:1126–1130. [DOI] [PubMed] [Google Scholar]
- Knight PG, Glister C. TGF-beta superfamily members and ovarian follicle development. Reproduction 2006;132:191–206. [DOI] [PubMed] [Google Scholar]
- Kovanci E, Rohozinski J, Simpson JL, Heard MJ, Bishop CE, Carson SA. Growth differentiating factor-9 mutations may be associated with premature ovarian failure. Fertil Steril 2007;87:143–146. [DOI] [PubMed] [Google Scholar]
- Kristensen SG, Andersen K, Clement CA, Franks S, Hardy K, Andersen CY. Expression of TGF-beta superfamily growth factors, their receptors, the associated SMADs and antagonists in five isolated size-matched populations of pre-antral follicles from normal human ovaries. Mol Hum Reprod 2014;20:293–308. [DOI] [PubMed] [Google Scholar]
- Kubota K, Sato F, Aramaki S, Soh T, Yamauchi N, Hattori MA. Ubiquitous expression of myostatin in chicken embryonic tissues: its high expression in testis and ovary. Comp Biochem Physiol A Mol Integr Physiol 2007;148:550–555. [DOI] [PubMed] [Google Scholar]
- Laissue P, Christin-Maitre S, Touraine P, Kuttenn F, Ritvos O, Aittomaki K, Bourcigaux N, Jacquesson L, Bouchard P, Frydman R et al. Mutations and sequence variants in GDF9 and BMP15 in patients with premature ovarian failure. Eur J Endocrinol 2006;154:739–744. [DOI] [PubMed] [Google Scholar]
- Lawson KA, Dunn NR, Roelen BA, Zeinstra LM, Davis AM, Wright CV, Korving JP, Hogan BL. Bmp4 is required for the generation of primordial germ cells in the mouse embryo. Genes Dev 1999;13:424–436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee J, Wikesjo UM. Growth/differentiation factor-5: pre-clinical and clinical evaluations of periodontal regeneration and alveolar augmentation--review. J Clin Periodontol 2014;41:797–805. [DOI] [PubMed] [Google Scholar]
- Li R, Norman RJ, Armstrong DT, Gilchrist RB. Oocyte-secreted factor(s) determine functional differences between bovine mural granulosa cells and cumulus cells. Biol Reprod 2000;63:839–845. [DOI] [PubMed] [Google Scholar]
- Li Y, Li RQ, Ou SB, Zhang NF, Ren L, Wei LN, Zhang QX, Yang DZ. Increased GDF9 and BMP15 mRNA levels in cumulus granulosa cells correlate with oocyte maturation, fertilization, and embryo quality in humans. Reprod Biol Endocrinol 2014;12:81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liao WX, Moore RK, Shimasaki S. Functional and molecular characterization of naturally occurring mutations in the oocyte-secreted factors bone morphogenetic protein-15 and growth and differentiation factor-9. J Biol Chem 2004;279:17391–17396. [DOI] [PubMed] [Google Scholar]
- Lie BL, Leung E, Leung PC, Auersperg N. Long-term growth and steroidogenic potential of human granulosa-lutein cells immortalized with SV40 large T antigen. Mol Cell Endocrinol 1996;120:169–176. [DOI] [PubMed] [Google Scholar]
- Lipson KE, Wong C, Teng Y, Spong S. CTGF is a central mediator of tissue remodeling and fibrosis and its inhibition can reverse the process of fibrosis. Fibrogenesis Tissue Repair 2012;5:S24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lissenberg-Thunnissen SN, de Gorter DJ, Sier CF, Schipper IB. Use and efficacy of bone morphogenetic proteins in fracture healing. Int Orthop 2011;35:1271–1280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Little SC, Mullins MC. Bone morphogenetic protein heterodimers assemble heteromeric type I receptor complexes to pattern the dorsoventral axis. Nat Cell Biol 2009;11:637–643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu J, Wang B, Wei Z, Zhou P, Zu Y, Zhou S, Wen Q, Wang J, Cao Y, Ma X. Mutational analysis of human bone morphogenetic protein 15 in Chinese women with polycystic ovary syndrome. Metabolism 2011;60:1511–1514. [DOI] [PubMed] [Google Scholar]
- Macias-Silva M, Hoodless PA, Tang SJ, Buchwald M, Wrana JL. Specific activation of Smad1 signaling pathways by the BMP7 type I receptor, ALK2. J Biol Chem 1998;273:25628–25636. [DOI] [PubMed] [Google Scholar]
- Magoffin DA, Erickson GF. Purification of ovarian theca-interstitial cells by density gradient centrifugation. Endocrinology 1988;122:2345–2347. [DOI] [PubMed] [Google Scholar]
- Makowski L, Caspar DL, Phillips WC, Goodenough DA. Gap junction structures. II. Analysis of the x-ray diffraction data. J Cell Biol 1977;74:629–645. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Margulis S, Abir R, Felz C, Nitke S, Krissi H, Fisch B. Bone morphogenetic protein 15 expression in human ovaries from fetuses, girls, and women. Fertil Steril 2009;92:1666–1673. [DOI] [PubMed] [Google Scholar]
- Massague J. TGF-beta signal transduction. Annu Rev Biochem 1998;67:753–791. [DOI] [PubMed] [Google Scholar]
- Mazerbourg S, Hsueh AJ. Genomic analyses facilitate identification of receptors and signalling pathways for growth differentiation factor 9 and related orphan bone morphogenetic protein/growth differentiation factor ligands. Hum Reprod Update 2006;12:373–383. [DOI] [PubMed] [Google Scholar]
- McAllister JM, Kerin JF, Trant JM, Estabrook RW, Mason JI, Waterman MR, Simpson ER. Regulation of cholesterol side-chain cleavage and 17 alpha-hydroxylase/lyase activities in proliferating human theca internal cells in long term monolayer culture. Endocrinology 1989;125:1959–1966. [DOI] [PubMed] [Google Scholar]
- McGee EA, Hsueh AJ. Initial and cyclic recruitment of ovarian follicles. Endocr Rev 2000;21:200–214. [DOI] [PubMed] [Google Scholar]
- McIntosh CJ, Lun S, Lawrence S, Western AH, McNatty KP, Juengel JL. The proregion of mouse BMP15 regulates the cooperative interactions of BMP15 and GDF9. Biol Reprod 2008;79:889–896. [DOI] [PubMed] [Google Scholar]
- McLaren A. Primordial germ cells in the mouse. Dev Biol 2003;262:1–15. [DOI] [PubMed] [Google Scholar]
- McPherron AC, Lawler AM, Lee SJ. Regulation of skeletal muscle mass in mice by a new TGF-beta superfamily member. Nature 1997;387:83–90. [DOI] [PubMed] [Google Scholar]
- McPherron AC, Lee SJ. Double muscling in cattle due to mutations in the myostatin gene. Proc Natl Acad Sci USA 1997;94:12457–12461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miyagi M, Mikawa S, Sato T, Hasegawa T, Kobayashi S, Matsuyama Y, Sato K. BMP2, BMP4, noggin, BMPRIA, BMPRIB, and BMPRII are differentially expressed in the adult rat spinal cord. Neuroscience 2012;203:12–26. [DOI] [PubMed] [Google Scholar]
- Miyazono K, Kusanagi K, Inoue H. Divergence and convergence of TGF-beta/BMP signaling. J Cell Physiol 2001;187:265–276. [DOI] [PubMed] [Google Scholar]
- Miyoshi T, Otsuka F, Suzuki J, Takeda M, Inagaki K, Kano Y, Otani H, Mimura Y, Ogura T, Makino H. Mutual regulation of follicle-stimulating hormone signaling and bone morphogenetic protein system in human granulosa cells. Biol Reprod 2006;74:1073–1082. [DOI] [PubMed] [Google Scholar]
- Moore RK, Otsuka F, Shimasaki S. Molecular basis of bone morphogenetic protein-15 signaling in granulosa cells. J Biol Chem 2003;278:304–310. [DOI] [PubMed] [Google Scholar]
- Mosher DS, Quignon P, Bustamante CD, Sutter NB, Mellersh CS, Parker HG, Ostrander EA. A mutation in the myostatin gene increases muscle mass and enhances racing performance in heterozygote dogs. PLoS Genet 2007;3:e79. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mottershead DG, Harrison CA, Mueller TD, Stanton PG, Gilchrist RB, McNatty KP. Growth differentiation factor 9:bone morphogenetic protein 15 (GDF9:BMP15) synergism and protein heterodimerization. Proc Natl Acad Sci USA 2013;110:E2257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mueller TD, Nickel J. Promiscuity and specificity in BMP receptor activation. FEBS Lett 2012;586:1846–1859. [DOI] [PubMed] [Google Scholar]
- Nagashima T, Li Q, Clementi C, Lydon JP, DeMayo FJ, Matzuk MM. BMPR2 is required for postimplantation uterine function and pregnancy maintenance. J Clin Invest 2013;123:2539–2550. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakamura K, Shirai T, Morishita S, Uchida S, Saeki-Miura K, Makishima F. p38 mitogen-activated protein kinase functionally contributes to chondrogenesis induced by growth/differentiation factor-5 in ATDC5 cells. Exp Cell Res 1999;250:351–363. [DOI] [PubMed] [Google Scholar]
- Nekola MV, Nalbandov AV. Morphological changes of rat follicular cells as influenced by oocytes. Biol Reprod 1971;4:154–160. [DOI] [PubMed] [Google Scholar]
- Nio-Kobayashi J, Trendell J, Giakoumelou S, Boswell L, Nicol L, Kudo M, Sakuragi N, Iwanaga T, Duncan WC. Bone morphogenetic proteins are mediators of luteolysis in the human corpus luteum. Endocrinology 2015;156:1494–1503. [DOI] [PubMed] [Google Scholar]
- Nishi Y, Yanase T, Mu Y, Oba K, Ichino I, Saito M, Nomura M, Mukasa C, Okabe T, Goto K et al. Establishment and characterization of a steroidogenic human granulosa-like tumor cell line, KGN, that expresses functional follicle-stimulating hormone receptor. Endocrinology 2001;142:437–445. [DOI] [PubMed] [Google Scholar]
- Niswender GD, Juengel JL, Silva PJ, Rollyson MK, McIntush EW. Mechanisms controlling the function and life span of the corpus luteum. Physiol Rev 2000;80:1–29. [DOI] [PubMed] [Google Scholar]
- Nitta M, Katabuchi H, Ohtake H, Tashiro H, Yamaizumi M, Okamura H. Characterization and tumorigenicity of human ovarian surface epithelial cells immortalized by SV40 large T antigen. Gynecol Oncol 2001;81:10–17. [DOI] [PubMed] [Google Scholar]
- Okamura H, Katabuchi H, Ohba T. What we have learned from isolated cells from human ovary. Mol Cell Endocrinol 2003;202:37–45. [DOI] [PubMed] [Google Scholar]
- Oon VJ, Johnson MR. The regulation of the human corpus luteum steroidogenesis: a hypothesis. Hum Reprod Update 2000;6:519–529. [DOI] [PubMed] [Google Scholar]
- Pangas SA, Matzuk MM. The art and artifact of GDF9 activity: cumulus expansion and the cumulus expansion-enabling factor. Biol Reprod 2005;73:582–585. [DOI] [PubMed] [Google Scholar]
- Peiris HN, Salomon C, Payton D, Ashman K, Vaswani K, Chan A, Rice GE, Mitchell MD. Myostatin is localized in extravillous trophoblast and up-regulates migration. J Clin Endocrinol Metab 2014;99:E2288–2297. [DOI] [PubMed] [Google Scholar]
- Peng J, Li Q, Wigglesworth K, Rangarajan A, Kattamuri C, Peterson RT, Eppig JJ, Thompson TB, Matzuk MM. Growth differentiation factor 9:bone morphogenetic protein 15 heterodimers are potent regulators of ovarian functions. Proc Natl Acad Sci USA 2013;110:E776–785. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pepling ME, Spradling AC. Mouse ovarian germ cell cysts undergo programmed breakdown to form primordial follicles. Dev Biol 2001;234:339–351. [DOI] [PubMed] [Google Scholar]
- Persani L, Rossetti R, Cacciatore C. Genes involved in human premature ovarian failure. J Mol Endocrinol 2010;45:257–279. [DOI] [PubMed] [Google Scholar]
- Persani L, Rossetti R, Di Pasquale E, Cacciatore C, Fabre S. The fundamental role of bone morphogenetic protein 15 in ovarian function and its involvement in female fertility disorders. Hum Reprod Update 2014;20:869–883. [DOI] [PubMed] [Google Scholar]
- Petrocelli T, Lye SJ. Regulation of transcripts encoding the myometrial gap junction protein, connexin-43, by estrogen and progesterone. Endocrinology 1993;133:284–290. [DOI] [PubMed] [Google Scholar]
- Qiao J, Feng HL. Extra- and intra-ovarian factors in polycystic ovary syndrome: impact on oocyte maturation and embryo developmental competence. Hum Reprod Update 2011;17:17–33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Qin Y, Jiao X, Simpson JL, Chen ZJ. Genetics of primary ovarian insufficiency: new developments and opportunities. Hum Reprod Update 2015;21:787–808. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rainey WH, Sawetawan C, Shay JW, Michael MD, Mathis JM, Kutteh W, Byrd W, Carr BR. Transformation of human granulosa cells with the E6 and E7 regions of human papillomavirus. J Clin Endocrinol Metab 1994;78:705–710. [DOI] [PubMed] [Google Scholar]
- Regan SL, Knight PG, Yovich JL, Stanger JD, Leung Y, Arfuso F, Dharmarajan A, Almahbobi G. Dysregulation of granulosal bone morphogenetic protein receptor 1B density is associated with reduced ovarian reserve and the age-related decline in human fertility. Mol Cell Endocrinol 2016;425:84–93. [DOI] [PubMed] [Google Scholar]
- Rider CC, Mulloy B. Bone morphogenetic protein and growth differentiation factor cytokine families and their protein antagonists. Biochem J 2010;429:1–12. [DOI] [PubMed] [Google Scholar]
- Rossi RO, Costa JJ, Silva AW, Saraiva MV, Van den Hurk R, Silva JR. The bone morphogenetic protein system and the regulation of ovarian follicle development in mammals. Zygote 2016;24:1–17. [DOI] [PubMed] [Google Scholar]
- Russell DL, Robker RL. Molecular mechanisms of ovulation: co-ordination through the cumulus complex. Hum Reprod Update 2007;13:289–312. [DOI] [PubMed] [Google Scholar]
- Russell DL, Salustri A. Extracellular matrix of the cumulus-oocyte complex. Semin Reprod Med 2006;24:217–227. [DOI] [PubMed] [Google Scholar]
- Salustri A, Ulisse S, Yanagishita M, Hascall VC. Hyaluronic acid synthesis by mural granulosa cells and cumulus cells in vitro is selectively stimulated by a factor produced by oocytes and by transforming growth factor-beta. J Biol Chem 1990;265:19517–19523. [PubMed] [Google Scholar]
- Schmidt-Bleek K, Willie BM, Schwabe P, Seemann P, Duda GN. BMPs in bone regeneration: less is more effective, a paradigm-shift. Cytokine Growth Factor Rev 2016;27:141–148. [DOI] [PubMed] [Google Scholar]
- Schuelke M, Wagner KR, Stolz LE, Hubner C, Riebel T, Komen W, Braun T, Tobin JF, Lee SJ. Myostatin mutation associated with gross muscle hypertrophy in a child. N Engl J Med 2004;350:2682–2688. [DOI] [PubMed] [Google Scholar]
- Seidah NG, Prat A. The biology and therapeutic targeting of the proprotein convertases. Nat Rev Drug Discov 2012;11:367–383. [DOI] [PubMed] [Google Scholar]
- Sherman ML, Borgstein NG, Mook L, Wilson D, Yang Y, Chen N, Kumar R, Kim K, Laadem A. Multiple-dose, safety, pharmacokinetic, and pharmacodynamic study of sotatercept (ActRIIA-IgG1), a novel erythropoietic agent, in healthy postmenopausal women. J Clin Pharmacol 2013;53:1121–1130. [DOI] [PubMed] [Google Scholar]
- Shi FT, Cheung AP, Huang HF, Leung PC. Effects of endogenous growth differentiation factor 9 on activin A-induced inhibin B production in human granulosa-lutein cells. J Clin Endocrinol Metab 2009;94:5108–5116. [DOI] [PubMed] [Google Scholar]
- Shi FT, Cheung AP, Klausen C, Huang HF, Leung PC. Growth differentiation factor 9 reverses activin A suppression of steroidogenic acute regulatory protein expression and progesterone production in human granulosa-lutein cells. J Clin Endocrinol Metab 2010;95:E172–180. [DOI] [PubMed] [Google Scholar]
- Shi J, Yoshino O, Osuga Y, Koga K, Hirota Y, Nose E, Nishii O, Yano T, Taketani Y. Bone morphogenetic protein-2 (BMP-2) increases gene expression of FSH receptor and aromatase and decreases gene expression of LH receptor and StAR in human granulosa cells. Am J Reprod Immunol 2011;65:421–427. [DOI] [PubMed] [Google Scholar]
- Shi Y, Massague J. Mechanisms of TGF-beta signaling from cell membrane to the nucleus. Cell 2003;113:685–700. [DOI] [PubMed] [Google Scholar]
- Shimasaki S, Moore RK, Otsuka F, Erickson GF. The bone morphogenetic protein system in mammalian reproduction. Endocr Rev 2004;25:72–101. [DOI] [PubMed] [Google Scholar]
- Shimasaki S, Zachow RJ, Li D, Kim H, Iemura S, Ueno N, Sampath K, Chang RJ, Erickson GF. A functional bone morphogenetic protein system in the ovary. Proc Natl Acad Sci USA 1999;96:7282–7287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simon AM, Goodenough DA, Li E, Paul DL. Female infertility in mice lacking connexin 37. Nature 1997;385:525–529. [DOI] [PubMed] [Google Scholar]
- Simpson CM, Robertson DM, Al-Musawi SL, Heath DA, McNatty KP, Ritter LJ, Mottershead DG, Gilchrist RB, Harrison CA, Stanton PG. Aberrant GDF9 expression and activation are associated with common human ovarian disorders. J Clin Endocrinol Metab 2014;99:E615–624. [DOI] [PubMed] [Google Scholar]
- Skinner MK, Schmidt M, Savenkova MI, Sadler-Riggleman I, Nilsson EE. Regulation of granulosa and theca cell transcriptomes during ovarian antral follicle development. Mol Reprod Dev 2008;75:1457–1472. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith RC, Lin BK. Myostatin inhibitors as therapies for muscle wasting associated with cancer and other disorders. Curr Opin Support Palliat Care 2013;7:352–360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spiekerkoetter E, Tian X, Cai J, Hopper RK, Sudheendra D, Li CG, El-Bizri N, Sawada H, Haghighat R, Chan R et al. FK506 activates BMPR2, rescues endothelial dysfunction, and reverses pulmonary hypertension. J Clin Invest 2013;123:3600–3613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sugiura K, Su YQ, Eppig JJ. Does bone morphogenetic protein 6 (BMP6) affect female fertility in the mouse. Biol Reprod 2010;83:997–1004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sugiyama R, Fuzitou A, Takahashi C, Akutagawa O, Ito H, Nakagawa K, Sugiyama R, Isaka K. Bone morphogenetic protein 2 may be a good predictor of success in oocyte fertilization during assisted reproductive technology. Hum Cell 2010;23:83–88. [DOI] [PubMed] [Google Scholar]
- Sun PD, Davies DR. The cystine-knot growth-factor superfamily. Annu Rev Biophys Biomol Struct 1995;24:269–291. [DOI] [PubMed] [Google Scholar]
- Tajima K, Hosokawa K, Yoshida Y, Dantes A, Sasson R, Kotsuji F, Amsterdam A. Establishment of FSH-responsive cell lines by transfection of pre-ovulatory human granulosa cells with mutated p53 (p53val135) and Ha-ras genes. Mol Hum Reprod 2002;8:48–57. [DOI] [PubMed] [Google Scholar]
- Takebayashi K, Takakura K, Wang H, Kimura F, Kasahara K, Noda Y. Mutation analysis of the growth differentiation factor-9 and −9B genes in patients with premature ovarian failure and polycystic ovary syndrome. Fertil Steril 2000;74:976–979. [DOI] [PubMed] [Google Scholar]
- Teixeira Filho FL, Baracat EC, Lee TH, Suh CS, Matsui M, Chang RJ, Shimasaki S, Erickson GF. Aberrant expression of growth differentiation factor-9 in oocytes of women with polycystic ovary syndrome. J Clin Endocrinol Metab 2002;87:1337–1344. [DOI] [PubMed] [Google Scholar]
- Tiotiu D, Alvaro Mercadal B, Imbert R, Verbist J, Demeestere I, De Leener A, Englert Y, Vassart G, Costagliola S, Delbaere A. Variants of the BMP15 gene in a cohort of patients with premature ovarian failure. Hum Reprod 2010;25:1581–1587. [DOI] [PubMed] [Google Scholar]
- Tsai MY, Lan KC, Huang KE, Huang FJ, Kung FT, Chang SY. Significance of mRNA levels of connexin37, connexin43, and connexin45 in luteinized granulosa cells of controlled hyperstimulated follicles. Fertil Steril 2003;80:1437–1443. [DOI] [PubMed] [Google Scholar]
- Valera E, Isaacs MJ, Kawakami Y, Izpisua Belmonte JC, Choe S. BMP-2/6 heterodimer is more effective than BMP-2 or BMP-6 homodimers as inductor of differentiation of human embryonic stem cells. PLoS One 2010;5:e11167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van den Berg-Bakker CA, Hagemeijer A, Franken-Postma EM, Smit VT, Kuppen PJ, van Ravenswaay Claasen HH, Cornelisse CJ, Schrier PI. Establishment and characterization of 7 ovarian carcinoma cell lines and one granulosa tumor cell line: growth features and cytogenetics. Int J Cancer 1993;53:613–620. [DOI] [PubMed] [Google Scholar]
- van Houten EL, Laven JS, Louwers YV, McLuskey A, Themmen AP, Visser JA. Bone morphogenetic proteins and the polycystic ovary syndrome. J Ovarian Res 2013;6:32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vanderhyden BC. Species differences in the regulation of cumulus expansion by an oocyte-secreted factor(s). J Reprod Fertil 1993;98:219–227. [DOI] [PubMed] [Google Scholar]
- Vanderhyden BC, Armstrong DT. Role of cumulus cells and serum on the in vitro maturation, fertilization, and subsequent development of rat oocytes. Biol Reprod 1989;40:720–728. [DOI] [PubMed] [Google Scholar]
- Vanderhyden BC, Telfer EE, Eppig JJ. Mouse oocytes promote proliferation of granulosa cells from preantral and antral follicles in vitro. Biol Reprod 1992;46:1196–1204. [DOI] [PubMed] [Google Scholar]
- Vanderhyden BC, Tonary AM. Differential regulation of progesterone and estradiol production by mouse cumulus and mural granulosa cells by A factor(s) secreted by the oocyte. Biol Reprod 1995;53:1243–1250. [DOI] [PubMed] [Google Scholar]
- Varani S, Elvin JA, Yan C, DeMayo J, DeMayo FJ, Horton HF, Byrne MC, Matzuk MM. Knockout of pentraxin 3, a downstream target of growth differentiation factor-9, causes female subfertility. Mol Endocrinol 2002;16:1154–1167. [DOI] [PubMed] [Google Scholar]
- Veitia RA, Caburet S. Extensive sequence turnover of the signal peptides of members of the GDF/BMP family: exploring their evolutionary landscape. Biol Direct 2009;4:22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vigano P, Parazzini F, Somigliana E, Vercellini P. Endometriosis: epidemiology and aetiological factors. Best Pract Res Clin Obstet Gynaecol 2004;18:177–200. [DOI] [PubMed] [Google Scholar]
- von Bubnoff A, Cho KW. Intracellular BMP signaling regulation in vertebrates: pathway or network. Dev Biol 2001;239:1–14. [DOI] [PubMed] [Google Scholar]
- Wagner DO, Sieber C, Bhushan R, Borgermann JH, Graf D, Knaus P. BMPs: from bone to body morphogenetic proteins. Sci Signal 2010;3:mr1. [DOI] [PubMed] [Google Scholar]
- Wang EA, Rosen V, Cordes P, Hewick RM, Kriz MJ, Luxenberg DP, Sibley BS, Wozney JM. Purification and characterization of other distinct bone-inducing factors. Proc Natl Acad Sci USA 1988;85:9484–9488. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang TT, Ke ZH, Song Y, Chen LT, Chen XJ, Feng C, Zhang D, Zhang RJ, Wu YT, Zhang Y et al. Identification of a mutation in GDF9 as a novel cause of diminished ovarian reserve in young women. Hum Reprod 2013;28:2473–2481. [DOI] [PubMed] [Google Scholar]
- Wang Y, Ho CC, Bang E, Rejon CA, Libasci V, Pertchenko P, Hebert TE, Bernard DJ. Bone morphogenetic protein 2 stimulates noncanonical SMAD2/3 signaling via the BMP type 1A receptor in gonadotrope-like cells: implications for FSH synthesis. Endocrinology 2014;155:1970–1981. [DOI] [PubMed] [Google Scholar]
- Wei LN, Huang R, Li LL, Fang C, Li Y, Liang XY. Reduced and delayed expression of GDF9 and BMP15 in ovarian tissues from women with polycystic ovary syndrome. J Assist Reprod Genet 2014;31:1483–1490. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weigel PH, Hascall VC, Tammi M. Hyaluronan synthases. J Biol Chem 1997;272:13997–14000. [DOI] [PubMed] [Google Scholar]
- Wigglesworth K, Lee KB, Emori C, Sugiura K, Eppig JJ. Transcriptomic diversification of developing cumulus and mural granulosa cells in mouse ovarian follicles. Biol Reprod 2015;92:23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Woodruff TK, Shea LD. The role of the extracellular matrix in ovarian follicle development. Reprod Sci 2007;14:6–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu CH, Yang JG, Yang JJ, Lin YM, Tsai HD, Lin CY, Kuo PL. Androgen excess down-regulates connexin43 in a human granulosa cell line. Fertil Steril 2010;94:2938–2941. [DOI] [PubMed] [Google Scholar]
- Wu YT, Tang L, Cai J, Lu XE, Xu J, Zhu XM, Luo Q, Huang HF. High bone morphogenetic protein-15 level in follicular fluid is associated with high quality oocyte and subsequent embryonic development. Hum Reprod 2007;22:1526–1531. [DOI] [PubMed] [Google Scholar]
- Wu YT, Wang TT, Chen XJ, Zhu XM, Dong MY, Sheng JZ, Xu CM, Huang HF. Bone morphogenetic protein-15 in follicle fluid combined with age may differentiate between successful and unsuccessful poor ovarian responders. Reprod Biol Endocrinol 2012;10:116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamaguchi K, Shirakabe K, Shibuya H, Irie K, Oishi I, Ueno N, Taniguchi T, Nishida E, Matsumoto K. Identification of a member of the MAPKKK family as a potential mediator of TGF-beta signal transduction. Science 1995;270:2008–2011. [DOI] [PubMed] [Google Scholar]
- Yamamoto N, Christenson LK, McAllister JM, Strauss JF III. Growth differentiation factor-9 inhibits 3′5′-adenosine monophosphate-stimulated steroidogenesis in human granulosa and theca cells. J Clin Endocrinol Metab 2002;87:2849–2856. [DOI] [PubMed] [Google Scholar]
- Yan C, Wang P, DeMayo J, DeMayo FJ, Elvin JA, Carino C, Prasad SV, Skinner SS, Dunbar BS, Dube JL et al. Synergistic roles of bone morphogenetic protein 15 and growth differentiation factor 9 in ovarian function. Mol Endocrinol 2001;15:854–866. [DOI] [PubMed] [Google Scholar]
- Yi SE, LaPolt PS, Yoon BS, Chen JY, Lu JK, Lyons KM. The type I BMP receptor BmprIB is essential for female reproductive function. Proc Natl Acad Sci USA 2001;98:7994–7999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ying Y, Qi X, Zhao GQ. Induction of primordial germ cells from pluripotent epiblast. Sci World J 2002;2:801–810. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang H, Klausen C, Zhu H, Chang HM, Leung PC. BMP4 and BMP7 suppress StAR and progesterone production via ALK3 and SMAD1/5/8-SMAD4 in human granulosa-lutein cells. Endocrinology 2015;156:4269–4280. [DOI] [PubMed] [Google Scholar]
- Zhang H, Tian S, Klausen C, Zhu H, Liu R, Leung PC. Differential activation of noncanonical SMAD2/SMAD3 signaling by bone morphogenetic proteins causes disproportionate induction of hyaluronan production in immortalized human granulosa cells. Mol Cell Endocrinol 2016;428:17–27. [DOI] [PubMed] [Google Scholar]
- Zhang H, Vollmer M, De Geyter M, Litzistorf Y, Ladewig A, Durrenberger M, Guggenheim R, Miny P, Holzgreve W, De Geyter C. Characterization of an immortalized human granulosa cell line (COV434). Mol Hum Reprod 2000;6:146–153. [DOI] [PubMed] [Google Scholar]
- Zhang X, Jafari N, Barnes RB, Confino E, Milad M, Kazer RR. Studies of gene expression in human cumulus cells indicate pentraxin 3 as a possible marker for oocyte quality. Fertil Steril 2005;83:1169–1179. [DOI] [PubMed] [Google Scholar]
- Zhao SY, Qiao J, Chen YJ, Liu P, Li J, Yan J. Expression of growth differentiation factor-9 and bone morphogenetic protein-15 in oocytes and cumulus granulosa cells of patients with polycystic ovary syndrome. Fertil Steril 2010;94:261–267. [DOI] [PubMed] [Google Scholar]