Abstract
Nicotine is the most abundant alkaloid in tobacco accounting for 95% of the alkaloid content. There are also several minor tobacco alkaloids, among these are nornicotine, anatabine and anabasine. We developed and applied a 96 well plate-based capillary LC-tandem mass spectrometry method for the analysis of nornicotine, anatabine and anabasine in urine. The method was validated with regard to accuracy and precision. Anabasine was quantifiable to low levels with a limit of quantitation (LOQ) of 0.2 ng/ml even when nicotine, which is isobaric, is present at concentrations >2500-fold higher than anabasine. This attribute of the method is important since anatabine and anabasine in urine have been proposed as biomarkers of tobacco use for individuals using nicotine replacement therapies. In the present study, we analyzed the three minor tobacco alkaloids in urine from 827 smokers with a wide range of tobacco exposures. Nornicotine (LOQ 0.6 ng/ml) was detected in all samples and anatabine (LOQ, 0.15 ng/ml) and anabasine were detected in 97.7% of the samples. The median urinary concentrations of nornicotine, anatabine and anabasine were 98.9, 4.02 and 5.53 ng/ml. Total nicotine equivalents (TNE) were well correlated with anatabine (r2 = 0.714) and anabasine (r2 = 0.760). TNE was most highly correlated with nornicotine, which is also a metabolite of nicotine. Urine samples from a subset of subjects (n = 110) were analyzed for the presence of glucuronide conjugates by quantifying any increase in anatabine and anabasine concentrations after β-glucuronidase treatment. The median ratio of the glucuronidated to free anatabine was 0.74 (range, 0.1 to 10.9) and the median ratio of glucuronidated to free anabasine was 0.3 (range, 0.1 to 2.9). To our knowledge this is the largest population of smokers for whom the urinary concentrations of these three tobacco alkaloids has been reported.
Introduction
Tobacco use is the number one preventable cause of death in the United States, and is responsible for one in five deaths.1 The complete cessation of tobacco use would be the ideal solution to this public health challenge. However due to the addictive properties of the nicotine present in tobacco maintaining abstinence from tobacco-use is challenging. Forty three percent of daily smokers make a quit attempt every year yet few are successful.2 In the United States 17.8 % of adults still smoke and 4% use smokeless tobacco products.2,3 It has been suggested that if the nicotine content of cigarettes were significantly reduced then this lethal product would become non-addictive.4
In 2009, the Food and Drug Administration (FDA) was granted the authority to reduce but not eliminate nicotine in tobacco. In collaboration with others we recently published the results of a 6-week randomized clinical trial in which nicotine exposure from reduced-nicotine cigarettes was compared to exposure from standard nicotine content cigarettes.5 Exposure, quantified by the urinary concentration of total nicotine equivalents (TNE, the sum of nicotine and six metabolites) was significantly reduced in users of cigarettes with nicotine content ≤ 2.4 mg/g compared to those using cigarettes with 15.8 mg nicotine per g, a level comparable to that in a Marlboro cigarette.6 Nicotine dependence was also reduced. These data suggest that if these effects are replicated in longer term studies, potentially with greater compliance to the use of low nicotine content cigarettes, then reduction of nicotine in cigarettes could lead to a reduction in smoking prevalence. The challenge is the assessment of compliance, particularly when compliant smokers may obtain nicotine from other sources, such as nicotine gum or lozenge, or by the use of electronic (e)-cigarettes.
Nicotine is the most abundant alkaloid in tobacco accounting for 95% of the alkaloid content. In addition, there are several minor tobacco alkaloids, among these are nornicotine, anatabine and anabasine.7 Nornicotine is also a metabolite of nicotine. Due to the relatively low level of minor tobacco alkaloids in pharmaceutical nicotine (99% purity and <0.5% of any minor alkaloid is the US Pharmacopeia standard), urinary levels of anatabine and anabasine have been proposed as biomarkers of tobacco use among purportedly abstinent individuals using nicotine replacement therapies.8,9 Due to common biosynthetic pathways, tobacco that is low in nicotine is also often low in the minor alkaloids, nornicotine, anatabine and anabasine.10 The anatabine and anabasine content in the lowest nicotine content tobacco (0.4 mg/g) used in our low nicotine cigarette trial were 6.5 and 31.5 μg/g, respectively. Whereas, in the 50 top selling cigarette brands in the United States the level of anabasine and anatabine ranged from 127 to 185 μg/g and 927 to 1390 μg/g.11 Therefore, these minor alkaloids may be useful biomarkers to assess the use of “normal” relative to low nicotine content tobacco products when nicotine is also obtained from non-tobacco sources.
Several different LC- tandem mass spectrometry (LC/MS/MS) methods have been applied to the analysis of anabasine and nornicotine in urine; yet only two of these analyzed anatabine.12-17 The limit of quantitation for one of the two methods was relatively high, 2.5 ng anatabine or anabasine/ml urine.14 The more recently developed method reports a limit of quantitation of 0.5 ng/ml anatabine and 0.6 ng/ml anabasine.12 All but one18 of the LC/MS/MS analyses reported to date have been carried out on analytical scale columns. We report here the characterization of a 96 well plate-based capillary LC/MS/MS method for the analysis of nornicotine, anatabine and anabasine in urine. The method was applied to quantify the concentration of these minor tobacco alkaloids in urine samples from 827 smokers with a wide range of nicotine exposure.
Experimental Design
Chemicals and reagents
Nornicotine, anatabine, anabasine, and their corresponding [pyridyl-d4]-analogs were obtained from Toronto Research Chemicals (New York, ON, Canada). All other solvents and reagents were of the highest analytical grade and purchased from Sigma-Aldrich (St. Louis, MO).
Internal standard and stock solutions
Primary stock solutions of nornicotine, anatabine, anabasine and their d4-analogs were prepared in water at a concentration of 10 µg/ml. The concentrations of the primary stock solutions were confirmed by HPLC with UV detection and comparison to standard curves of known concentration. The HPLC analysis was carried out with a Phenomenex Luna C18 column (5μ, 250 × 4.6mm) and the minor alkaloids were eluted with 0.2% TFA in water and a flow of 1 ml/min (retention times, nornicotine, 4.2 min; anatabine 4.1 min; anabasine, 4.3 min). A working internal standard solution in PBS containing 0.02 ng/μl of each deuterated alkaloid was prepared in a Nalgene bottle and stored in 10 ml aliquots at −20°C.
Assay validations
To determine the limit of detection, two solutions were prepared. One which contained nornicotine, anatabine and anabasine and a second that contained the deuterated minor alkaloids at concentrations of 0.33 ng/μl each. Successive 1:10 serial dilutions of the solution of the non-deuterated compounds were prepared to final concentrations of 330 pg/μl, 33 pg/μl, 3.3 pg/μl, 0.33 pg/μl). Linear calibration curves (3 independent sets) were prepared keeping the concentration of the d4- compounds constant (25 pg/μl) and varying the concentration of nornicotine, anatabine and anabasine (25 pg/μl, 2.5 pg/μl, 0.25 pg/μl, 0.025 pg/μl and 0 pg/μl). The limit of detection and relative response of the instrument were determined from these calibration curves. The calibration curves were run prior to each series of LC/MS/MS analyses.
The limit of quantitation and linearity of the assay was determined by analyzing a series of samples with a 400 fold range of anatabine, anabasine and nornicotine concentrations. These samples were prepared either by adding standard minor alkaloids solutions to non-smokers urine or by serial dilution of a positive control smoker's urine sample with non-smoker's urine. The concentrations of anatabine, anabasine and nornicotine in the positive control sample were previously determined by a well-established GC-MS method.8,19
Urine Samples
The urine samples used in this study are the baseline samples from smokers who participated in a randomized trial of reduced nicotine content cigarettes. We previously analyzed the TNE concentration of these samples.5 At baseline the participants (n=839, 38% Black, 51% White and 5% Hispanic) were smoking their usual brand of cigarette and did not report using any other nicotine containing products in more than 9 of the last 30 days. Eligibility criteria for the trial included an age of 18 years, use of at least 5 cigarettes per day, either expired carbon monoxide levels >8ppm or urinary cotinine concentration greater than 100 ng/ml at the screening visit. Among the 839 baseline urine samples 11 with baseline cotinine levels less than 100 ng/ml, as they did not meet one of the screening criteria of the clinical trial (TNE of these samples was <2nmol/ml, reflecting very light smoking)were excluded from our minor alkaloid analysis. One additional sample that appeared to be contaminated with nicotine was also excluded, therefore a total of 827 urine samples were analyzed for nornicotine, anabasine and anatabine. A subset of samples (n=110) were reanalyzed for the minor tobacco alkaloids following overnight incubation at 37°C with 500 units of β-glucuronidase (bovine liver, Sigma-Aldrich). The concentrations of the glucuronide conjugates in the sample were calculated from the difference in the concentration of the minor alkaloid in the sample when analyzed with and without β-glucuronidase.
Sample Preparation
The urine samples (stored at −20°C prior to analysis) were thawed at room temperature, mixed well, and 50 μl added to a 96-well round bottom plate containing 150 μl 0.5% formic acid and internal standard (50 μl, 1 ng each d4-nornicotine, d4-anatabine, d4-anabasine). Fourteen wells in the plate were used for H2O blanks (n= 8) and positive control (n= 6). The positive controls were smokers’ urine samples. The plates were covered with Silicone Cap Mats and mixed; the entire content of each well was loaded by multichannel pipette onto an Oasis MCX 96 well plate (10 mg sorbent) previously conditioned sequentially with 0.3 ml methanol followed by 0.3 ml water and 0.3 ml 0.5% formic acid using a vacuum manifold. The MCX plate was washed with 0.3 ml 0.5% formic acid, 0.6 ml water, 0.3 ml methanol and 0.3 ml 60% acetonitrile: 40% methanol. The minor alkaloids were then eluted with 0.2 ml 2% ammonium hydroxide in 59% acetonitrile: 39% methanol, into a 2 ml True Taper 96-well plate (Analytical Sales and Service, Pompton Plains, NJ). The samples were concentrated (to 5 - 20 μl volume) under a gentle stream of N2 using a Quadra 4 SPE robot (TomTec, Hamden, CT). The plates were covered with a Silicone Cap Mat and stored at −20°C until LC/MS/MS analysis, at which time plates were brought to room temperature and 40 μl 25 mM ammonium bicarbonate, pH 6.5 was added to each well.
LC–tandem mass spectrometry analysis
The resolution of nicotine and anabasine was determined on several capillary columns, including Phenomenex Luna C18 (100Å or 3μ, 150 × 0.5 mm); Millipore SeQuant Zic-HILIC (150 × 0.3 mm); Waters Atlantis HILIC, 5 μ (100 × 0.3 mm); Thermo Scientific Hypercarb, 3μ (100 × 0.5 mm), with various mobile phases (ammonium acetate, formic acid, ammonium formate, ammonium bicarbonate, acetonitrile, methanol), which varied in pH and concentration. Thermo Scientific Aquasil C18 (150 × 0.5 mm, 5 μ) was selected as optimal for the separation of nicotine and anabasine. LC/MS/MS analyses were carried out by positive electrospray ionization (ESI) on a Thermo Scientific TSQ Vantage triple quadrupole mass spectrometer (Thermo Fisher Scientific, Waltham, MA) interfaced to a Dionex Ultimate 3000 Capillary HPLC. The samples were injected (4 to 8 μl) onto the Aquasil capillary column (heated to 40°C). Nornicotine, anatabine and anabasine were eluted with 60% aqueous 20 mM ammonium formate and 40% acetonitrile. The flow rate was 20 μL/min. For the first 5 min the eluent was diverted to waste and for most injections diverted immediately after anatabine eluted. The electrospray source was operated at ambient temperature and the capillary temperature at 270°C. A spray voltage of 3200, a sheath gas flow of 35, and a collision gas pressure of 1.5 mTorr were used. The scan width was m/z 0.4 the scan time 0.10 seconds and the peak width for Q1 was set to m/z 0.4 . The mass transitions monitored were as follows: nornicotine, m/z 149.2→130.2 and m/z 149.2→117.2; d4-nornicotine, m/z 153.2→134.2 and m/z 153.2→121.2 (collision energy 17 V for m/z,130.2 and m/z 134.2 and 23V for m/z, 117.2 and m/z, 121.2); anatabine, m/z 161.2→144.2 and m/z 161.2→111.2; d4-anatabine, m/z 165.2→148.2 and m/z 165→115.2 (collision energy 16V for m/z, 144.2 and m/z, 148.2 and 25 V for m/z, 107.2 and m/z, 111.2); anabasine, m/z 163→144 and m/z 163→118; d4-anabasine, m/z 167→148 and m/z 167→122 (collision energy 21V for m/z, 144.2 and m/z, 148.2 and 22V for m/z, 118.2 and m/z, 122.2). Concentrations of the minor alkaloids were calculated from the ratio of the peak area obtained by selected reaction monitoring of the mass transitions for the analyte and its deuterated internal standard.
Results
We developed a high throughput capillary LC-ESI/MS/MS method for the analysis of the minor tobacco alkaloids; nornicotine, anatabine and anabasine in urine. The method was developed to allow the robust quantitation of relatively low levels of anatabine and anabasine (≤ 1ng/ml) in the presence of as much as 2000 fold higher concentrations of nicotine. This scenario may occur in smoking cessation studies or studies that switch smokers to low nicotine/anatabine/anabasine tobacco products, but allow NRT, or e-cigarettes use. Anabasine and nicotine are isobaric, and upon ESI many common fragment ions are formed (Supplemental Table S1). Anabasine generates a large number of daughter ions, whereas nicotine fragments into two major daughter ions m/z, 117.2 (90% abundance) and m/z, 130.2 (100% abundance). However, most of the major fragment ions of anabasine are present in daughter ion spectra of nicotine (m/z 92.2, 117.2, 118.2, 120.2, 130.2, 131.2, 144.2), albeit some at a low abundance. Therefore, chromatographic separation of these two alkaloids is critical to accurate quantitation of low levels of anabasine in the presence of nicotine.
The resolution of anabasine and nicotine on several capillary columns was determined with various mobile phases, which varied in pH and concentration. Reproducible baseline separation of nicotine and anabasine, and high quality peak shapes for nornicotine and anatabine at capillary flow rates was only obtained with the Thermo Scientific Aquasil C18 column. The separation between anabasine and nicotine was also dependent on the pH of the sample; therefore it was important that the samples were re-suspended in a solution with sufficient buffering capacity around pH 7. We found that 25 mM ammonium bicarbonate fit this criterion. A typical chromatogram obtained from LC/MS/MS analysis of a smoker's urine sample is presented in Figure 1. The mass transitions illustrated are the most abundant for each compound. Anatabine eluted first (9.32 min) followed by nornicotine (10.85 min) and anabasine (11.28 min). Nicotine eluted 2.5 min after anabasine. The chromatogram illustrated in Figure 2 presents the data collected for the two mass transitions that were monitored for anabasine, m/z, 163.2 → 118.2 and m/z, 163.2 → 144.2. Using the later transition the peak area for nicotine (12.54 min) is reduced more than 60 fold, whereas the anabasine peak area is reduced only by a factor of 3.4. Therefore, in the presence of high concentrations of nicotine using the m/z, 163.2 → 144.2 facilitates accurate integration of the anabasine peak. The concentration of anabasine in this urine sample was 0.38 ng/ml, calculated by either ion. The peak size increased the correct amount when anabasine was added to the urine prior to sample work –up (Figure 2A), and anabasine was well resolved even in the presence of nicotine at a concentration 2000 fold higher than anabasine (Figure 2B).
Figure 1.
LC-ESI-MS/MS chromatograms from analysis of a smoker's urine sample for anatabine (2.8 ng/ml), nornicotine (31.6 ng/ml) and anabasine (3.3 ng/ml), with select reaction monitoring for m/z 161 → 144, m/z 149→130 and m/z 163 → 118, respectively. The 100% abundance for m/z 161 → 144, is 5 × 104 compared to 7.8 × 103 for the other mass transitions. The total nicotine equivalents concentration of this urine sample was 16.4 nmol/ml. The nicotine concentration was 606 ng/ml.
Figure 2.
LC-ESI-MS/MS analysis of anabasine by selected reaction monitoring of m/z 163→118 (upper panels) and m/z 163→144 (lower panels). A) A smoker's sample containing 0.38 ng/ml anabasine and 38 ng/ml nicotine was analyzed twice, before and after the addition of 0.50 ng/ml anabasine to the urine, the chromatograms obtained for each analyses are overlaid. [The ratios of the peak area of the anabasine to the d4-anabasine internal standard were 0.042 and 0.098 in the two samples] B) The sample with the added anabasine was then co-injected with nicotine at a concentration 2000 times that of anabasine.
Nornicotine and anabasine standards in ammonium bicarbonate had a linear response over a 2000-fold range of concentrations (1 pg – 2000 pg for on column), and the linear response for anatabine was from 0.2 to 2000 pg. The limit of quantitation and the correlation coefficient of the expected nornicotine, anatabine and anabasine concentrations vs the measured values are presented in Table 1. The LOQ were 0.6 ng/ml, 0.15 ng/ml and 0.2 ng/ml for nornicotine, anatabine, and anabasine, respectively. Precision was determined from positive control samples included in each 96-well plate and is presented in Table 2.
Table 1.
Determination of assay accuracya
| Nornicotine | Anatabine | Anabasine | |
|---|---|---|---|
| Limit of quantitation (ng/ml urine)b | 0.6 | 0.15 | 0.2 |
| Linearity | y = 0.93x - 0.334 | y = 0.93x - 0.013 | y = 1.04x - 0.023 |
| Correlation | 0.9996 | 0.9997 | 0.9995 |
Determined from 4 independent graphs of expected vs measured values. Each sample was analyzed in duplicate. The concentrations used were 0.33, 1.32, 1.99, 6.62, 13.2, 66.2 ng/ml for nornicotine, 0.055, 0.11, 0.21, 0.32, 1.05, 2.10, 10.5 ng/ml for anatabine, and 0.09, 0.18, 0.26, 0.88, 1.76, 8.80 ng/ml for anabasine.
LOQ determined from accuracy of measured values compared to expected values. The LOQ accuracy is >95%.
Table 2.
Inter- and intraday precisiona
| Nornicotine | Anatabine | Anabasine | ||
|---|---|---|---|---|
|
Coefficient of Variance (%) |
||||
| Interday | Sample 1 (n = 20) | 5.9 | 6.5 | 4.2 |
| Intradayb | Sample 1 | 4.1 ± 1.5 | 3.7 ± 1.7 | 6.4 ± 2.2 |
| Sample 2 | 1.3 ± 1.3 | 2.3 ± 2.7 | 2.0 ± 1.8 | |
On the basis of positive control sample #1 (65.6 ng/ml nornicotine, 10.6 ng/ml anatabine, 8.9 ng/ml anabasine) and #2 (104 ng/ml nornicotine, 3.2 ng/ml anatabine, 4.2 ng/ml anabasine).
Average of intraday (inter-plate) CVs from 10 plates containing 4-6 positive control samples.
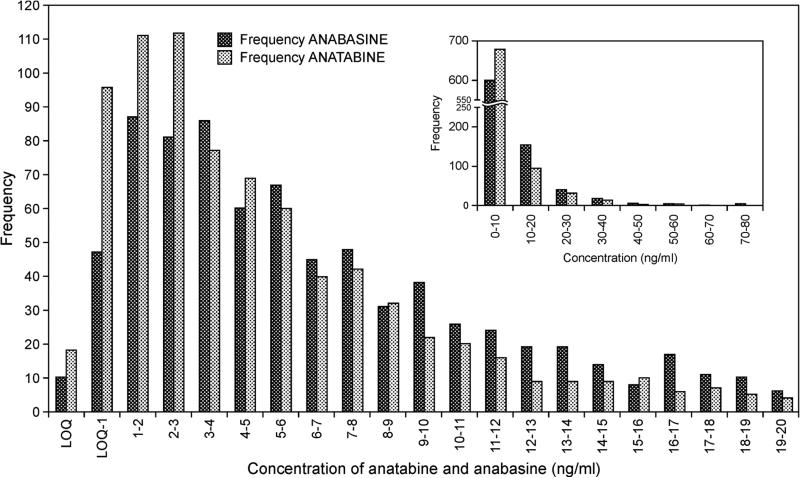
Urine samples (previously analyzed for TNE) from smokers (n=827), who reported smoking between 5 and 59 cigarettes per day (mean ± SD, 15 ± 7) were analyzed for nornicotine, anabasine and anatabine. The results are presented in Table 3. Mean nornicotine, anatabine and anabasine concentrations were 78.3, 6.38 and 8.56 ng/ml respectively. Anatabine and anabasine concentrations were highly correlated (r2=0.919). TNE was also well correlated with anatabine (r2 = 0.714) and with anabasine (r2 = 0.760). TNE was most highly correlated with nornicotine, which is also a metabolite of nicotine.20 The median urinary concentrations of anatabine and anabasine were 4.02 and 5.53 ng/ml. The distribution of anatabine and anabasine urinary concentrations for all smokers is presented in Figure 3. Anatabine was detected in all but 18 smokers (TNE concentration for these smokers ranged from 2.5 to 52.5 nmol/ml). Six of these smokers also had anabasine levels below our limit of quantitation; an additional four smokers had non-detectable levels of anabasine. Four smokers had anatabine levels greater than 50 ng/ml and two of these plus two others had anabasine levels greater than 70 ng/ml.
Table 3.
Urinary concentrations of total nicotine equivalents, anatabine, anabasine and nornicotine (n=827)
| TNE (nmol/ml)a | Nornicotine | Anatabine | Anabasine | |
|---|---|---|---|---|
|
ng/ml (pmol/ml) |
||||
| Mean±SD | 56.4 ± 40.1 | 78.3 ± 72.8 (529 ± 422) | 6.38 ± 7.67 (39.9 ± 27.9) | 8.56 ± 9.73 (52.8 ± 60.0) |
| Median | 45.4 | 58.9 (398) | 4.02 (25.1) | 5.53 (34.1) |
| Range | 2.47 - 256 | 1.0 - 638 (6.77 - 4310) | ND - 57.7 (ND - 361) | ND - 81.5 (ND - 503) |
| Correlations | ||||
| Nornicotine | 0.827 | |||
| Anatabine | 0.714 | 0.796 | ||
| Anabasine | 0.769 | 0.868 | 0.919 | |
TNE, total nicotine equivalents were reported previously5
Figure 3.
Distribution of urinary anatabine and anabasine concentrations in smokers. Samples with anatabine and anabasine concentrations from the limit of quantitation (LOQ) to 20 ng/ml are included in the main figure. The insert shows the distribution for all 827 subjects. LOQ: Anabasine, 0.2ng/ml, Anatabine, 0.15 ng/ml.
It has been previously reported that urinary anatabine and anabasine levels below 2 ng/ml were in concordance with self-reported cessation of tobacco use.9 Among the more than 800 smokers in this study 27% had urinary anatabine concentrations that were less than 2 ng/ml (n=225) and 17.4% (n=144) had anabasine levels less than 2 ng/ml. No smoker with TNE levels < 10 nmol/ml (n=33 or 4%) had either anatabine or anabasine levels > 2 ng/ml. However, sixteen percent of the smokers in our study had both anatabine and anabasine levels below 2 ng/ml. The TNE levels for these 132 smokers are presented in Figure 4, they ranged from 2.5 to 69.1 nmol/ml and the median was 19.1. This compares to a median TNE level of 45 nmol/ml for all 827 smokers (Table 3). Anatabine levels were less than 1 ng/ml in 98 smokers (11.8%) and anabasine levels less than 1 ng/ml in 57 smokers (6.9%). The highest TNE levels for the smokers in these two groups were 59.2 and 52.5 nmol/ml, and the median TNE levels were 17.1 and 12.7 nmol/ml, respectively (Figure 4).
Figure 4.
Total nicotine equivalents of smokers with urinary anatabine and anabasine concentrations <2 ng/ml, or < 1 ng/ml anatabine, or anabasine < 1 ng/ml. The dashed lines indicate the median total nicotine concentrations in the 3 groups (19.1, 17.1 and 12.7nmol/ml).
Urine samples from a subset of subjects (n = 110) were analyzed for the presence of glucuronide conjugates by quantifying any increase in anatabine and anabasine concentrations after β-glucuronidase treatment. A summary of these data are presented in Table 4. The concentration of anatabine increased more than 10% in all but four samples. The calculated urinary concentration of the presumed anatabine glucuronide ranged from 0.4 to 17.5 ng/ml (median, 2.9 ng/ml) and the median ratio of the glucuronidated to free anatabine was 0.74 (range, 0.1 to 10.9). The median ratios of glucuronidated to free anatabine for samples with anatabine concentrations above and below the median anatabine concentration (4.02ng/ml) were determined. The median ratio of the anatabine glucuronide to anatabine for the samples with anatabine concentrations <4.02 ng/ml was 3 times higher than the ratio for samples with anatabine concentrations >4.02 ng/ml (p<0.001).
Table 4.
Urinary concentrations of anatabine and anabasine before and after β-glucuronidase treatment (n=110)
| Anatabine (ng/ml) | Anabasine (ng/ml) | TNE (nmol/ml)a | |
|---|---|---|---|
|
Median (range) |
|||
| Totalb | 7.5 (0.3 - 54.4) | 7.5 (0.3 - 49.7) | 48.9 (3.4 - 230) |
| Free c | 3.9 (ND - 40.6) | 6.5 (0.2 - 43.7) | na |
| Glucuronide | 2.9 (0.4 - 17.5)d | 1.8 (0.3 - 11.8)e | na |
| Ratio of glucuronide to free | 0.74 (0.1 - 10.9)d | 0.30 (0.1 - 2.9)e | na |
TNE, total nicotine equivalents were reported previously,5 na not applicable.
Total refers to the sum of the alkaloid and its glucuronide conjugate
Free refers to the concentration of the alkaloid prior to β-glucuronidase treatment
Values were calculated for samples with different concentrations of free and total anatabine, n = 107
Values were calculated for samples with different concentrations of free and total anabasine, n = 93
The urinary anabasine concentration after β-glucuronidase treatment increased much less than did anatabine. The median increase was 20%, and no increase was observed for 16 samples. Among the other 94 samples, the median concentration of the presumed anabasine glucuronide was 1.8 ng/ml (range, 0.3 to 11.8 ng/ml) and the median ratio of glucuronidated to free anabasine was 0.3 (range, 0.1 to 2.9). In contrast to anatabine glucuronidation, the median ratio of glucuronidated to free anabasine was not significantly different at high and low concentrations of anabasine.
Discussion
We report here the quantification of anatabine, anabasine and nornicotine in smokers’ urine by a newly developed capillary LC-ESI MS/MS method. Our method is a 96 well plate based assay and allows the efficient and accurate analysis of low concentrations of these three minor tobacco alkaloids. Anabasine is quantifiable to low levels even when nicotine, which is isobaric with anabasine, is present at high concentrations (>2500 fold higher than anabasine). This attribute of the method is important since anatabine and anabasine in urine have been proposed as biomarkers of tobacco use for the confirmation of abstinence in individuals using nicotine replacement therapies.9 More recently, we have proposed that these minor alkaloids also may be used to confirm compliance with the use of very low nicotine cigarettes, particularly when there are other sources of nicotine exposure. Low nicotine content cigarettes are also low in anatabine and anabasine 5,21. In an on-going study we will analyze urinary anatabine and anabasine levels in a subset of the subjects in the current study after they have switched to smoking low nicotine cigarettes. In the present study, we analyzed urine samples from 827 smokers for anatabine, anabasine and nornicotine. To our knowledge this is the largest population of smokers for whom the urinary concentrations of these tobacco alkaloids has been reported. These data will be useful to compare to alkaloid levels in users of nicotine replacement therapies and e-cigarettes and to establish the anatabine and anatabine levels that will result in the greatest specificity and sensitivity to distinguish smokers, from non-smokers using other nicotine containing products.
There are a number of LC/MS/MS methods for the analysis of anabasine and nornicotine in urine, two of these also analyzed anatabine.12,14-18,22 Several of the methods have relatively high limits of quantitation (1-2 ng/ml) and all but one use analytical grade LC columns. The capillary column used previously was a HILIC column,18 which we have found to be an excellent column for the analysis of nicotine and nicotine N-oxide.23,24 However, we were unable to find a mobile phase that allowed both reproducible resolution of nicotine and anabasine, and low limits of quantitation for all three minor tobacco alkaloids.
There are several studies in the literature that report the urinary anabasine concentrations in smokers, a few also quantify anatabine.8,9,12,16,17,19,25 Most of the studies are small and focus on the analytical method, the two largest report data for 99 and 94 smokers.9,12 The first of these reported a mean urinary concentrations of 22 ng/ml for both anabasine and anatabine.9 More recently, McGuffy et al12 reported mean levels of total anabasine of 6.12 ng/ml and total anatabine of 15.2 ng/ml, where “total” refers to the urinary concentration of the alkaloid, after β-glucuronidase treatment. The mean concentration of anabasine we report here for more than 800 smokers was similar, 8.56 ng/ml; the mean concentration of anatabine (6.38 ng/ml), was less than half the total anatabine concentration reported by McGuffy et al. However, we found an almost two-fold increase in the median level of anatabine in the samples we analyzed after β-glucuronidase treatment, suggesting the mean total anatabine in the 827 smokers would likely have been about 13 ng/ml.
As previously reported, the relative amounts of nornicotine, anabasine and anatabine in the urine of smokers were quite different from their relative levels in tobacco. In the first analysis of these alkaloids in smokers’ urine (n=22) the ratio of the mean concentrations of nornicotine to anabasine to anatabine was 6.5 to 1 to 1.5.25 However, the most abundant of these minor tobacco alkaloids in the 50 top selling cigarettes in the United States is anatabine and the mean ratio of nornicotine to anabasine to anatabine is 5.8 to 1.0 to 7.5.11 In the smokers of our study the ratio of the median levels of the three alkaloids was 10.6 to 1 to 0.8. The difference in the distribution of these minor alkaloids in tobacco and urine is in part due to differences in the transfer of the alkaloid from tobacco to the smoke.26 There is little data on the smoke level of these tobacco alkaloids in commercial cigarettes. The amounts of nornicotine, anabasine and anatabine in smoke from reference cigarettes are 73, 7 and 18 μg/g tobacco.27 The relative levels of nornicotine and anabasine in tobacco smoke are similar to their relative concentrations in urine. The lower concentration of anatabine than anabasine in most smokers’ urine may be due to greater metabolism of anatabine. We confirm here that one significant pathway of anatabine metabolism is glucuronidation, but other pathways are also likely to contribute.
Both anatabine and anabasine were detected in 97.7% of the smokers’ urine samples in our study, and only 6 samples had non-detectable levels of either of these tobacco alkaloids. Anatabine and anabasine were both well correlated with TNE. But, for a specific concentration of the minor alkaloid a range of TNE were observed. For example, whereas no smoker with TNE below 10 had either anatabine or anabasine levels > 2 ng/ml, TNE concentrations ranged from 2.5 to 69.1 nmol/ml in smokers with both anatabine and anabasine concentrations below 2 ng/ml. The association of a wide range of TNE levels among smokers with similar urinary anabasine and anatabine concentrations may be due to differences in the amount of the minor alkaloids relative to nicotine present in the cigarettes smoked. Alternatively, the relatively high TNE in some smokers with low levels of anatabine or anabasine may be due to the variability in the time of the last cigarette and individual differences between the half-life of nicotine and its metabolites compared to the minor alkaloids. Nicotine metabolism and clearance is well studied.20,24,28 However, there is little to no data on the metabolism of anatabine and anabasine in smokers. A study a number of years ago suggests there is little metabolism of anabasine; when anabasine was administered orally about 70% of the dose was excreted unchanged in the urine.29
If anabasine and anatabine are to be used as biomarker of tobacco use in abstinent smokers, then ideally these tobacco alkaloids should not be present in non-smokers. There is one report of the analysis of anabasine in a large number of urine samples. The 19,000 samples were de-identified but classified as non-smoker urine based on cotinine and 3-hydroxycotinine levels below 5 and 50 ng/ml, respectively,30 anabasine (limit of quantitation >3 ng/ml) was detected in only 86 samples (0.4%). The authors suggest these individuals may have been exposed to anabasine in insecticides. However, it seems more likely this relatively high level of anabasine is due to exposure to or use of Nicotiana glauca, a tobacco plant with little nicotine and 1000-fold higher levels of anabasine compared to Nicotiana tabacum, which is used in commercial tobacco products.11,31 Nicotiana glauca referred to as the tobacco tree grows wild throughout California and the southwest of the United States and has been used in medicinal and “alternate tobacco” products. There is much less data on anatabine levels in non-smokers, but it is not used as a pesticide and its levels are similar in Nicotiana tabacum and Nicotiana glauca.11,31
The relatively low limit of quantitation for anabasine and anatabine by the LC/MS/MS method reported here will allow the determination of the concentration of anatabine and/or anabasine that will provide the best selectivity and sensitivity to confirm smoking abstinence. It will be useful to establish new cut points. If the previously suggested concentration of <2 ng/ml anatabine and anabasine was applied to the smokers in our study then 16% of the self-reported smokers would be considered non-smokers. The use of total anatabine would also improve the selectivity. The high throughput nature of our method will facilitate the analysis of large numbers of urine samples. In addition to applying this method to the analysis of urine from non-smokers and smokers of “normal” nicotine level cigarettes, we will determine the level of these minor alkaloids in the urine of individuals who smoke very low nicotine content cigarettes.
Supplementary Material
Acknowledgements
The following individuals contributed to the recruiting and collection of the urine samples analyzed: Mustafa al'Absi, Neal Benowitz, Paul Cinciripini, Rachel Denlinger, David Drobes, Joni Jensen, Joseph Koopmeiners, Tonya Lane, Joseph McClernon, Jason Robinson, Maxine Stitzer, Andrew Strasser, Jennifer Tidey, Hilary Tindle, Ryan Vandrey, and all the students, fellows, and staff involved in the Center for the Evaluation of Nicotine in Cigarettes. The content is solely the responsibility of the authors and does not necessarily represent the official views of the NIH or the Food and Drug Administration.
Funding Sources.
This study was supported by a grant from the National Institute on Drug Abuse and the Food and Drug Administration Center for Tobacco Products, U54 DA031659. The LC/MS/MS analysis was carried out in the Analytical Biochemistry Core of the University of Minnesota Cancer Center supported in part by NIH CA77598.
Abbreviations
- TNE
Total nicotine equivalents
Footnotes
Supporting Information Available
Table of electrospray ionization MS/MS fragment ions of nornicotine, anatabine, and anabasine and their deuterated analogs. This material is available free of charge via the Internet at http://pubs.acs.org.
Reference List
- 1.United States Department of Health and Human Services . The Health Consequences of Smoking: 50 Years of Progress. A Report of the Surgeon General. U.S. Department of Health and Human Services, Centers for Disease Control and Prevention, National Center for Chronic Disease Prevention and Health Promotion, Office on Smoking and Health; Atlanta, GA.: 2014. Smoking attributable morbidity, mortality and economic cost; pp. 647–680. [Google Scholar]
- 2.U.S.Department of Health and Human Services . The Health Consequences of Smoking: 50 Years of Progress. A Report of the Surgeon General. U.S. Department of Health and Human Services, Centers for Disease Control and Prevention, National Center for Chronic Disease Prevention and Health Promotion, Office on Smoking and Health; Atlanta, GA.: 2014. Patterns of Tobacco Use Among U.S. Youth, Young Adults and Adults. pp. 701–770. [Google Scholar]
- 3.Current Cigarette smoking among adults -- United States, 2005-2013. Morbidity and Mortality Weekly Report. 2014;63:1108–1114. [PMC free article] [PubMed] [Google Scholar]
- 4.Benowitz NL, Henningfield JE. Reducing the nicotine content to make cigarettes less addictive. Tob. Control. 2013;1(22 Suppl):i14–i17. doi: 10.1136/tobaccocontrol-2012-050860. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Donny EC, Denlinger RL, Tidey JW, Koopmeiners JS, Benowitz NL, Vandrey RG, al'Absi M, Carmella SG, Cinciripini PM, Dermody SS, Drobes DJ, Hecht SS, Jensen J, Lane T, Le CT, McClernon FJ, Montoya ID, Murphy SE, Robinson JD, Stitzer ML, Strasser AA, Tindle H, Hatsukami DK. Randomized trial of reduced-nicotine standards for cigarettes. N. Engl. J. Med. 2015;373:1340–1349. doi: 10.1056/NEJMsa1502403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Wu W, Ashley DL, Watson CH. Determination of nicotine and other minor alkaloids in international cigarettes by solid-phase microextraction and gas chromatography/mass spectrometry. Anal. Chem. 2002;74:4878–4884. doi: 10.1021/ac020291p. [DOI] [PubMed] [Google Scholar]
- 7.Gorrod JW, Wahren J, editors. Nicotine and related alkaloids: absorption, diistribution, metabolism and excretion. Chapman and Hall; London: 1993. Biosynthesis and metabolism of nicotine and related alkaloids. pp. 1–30. [Google Scholar]
- 8.Jacob P, III, Yu L, Shulgin AT, Benowitz NL. Minor tobacco alkaloids as biomarkers for tobacco use: comparison of users of cigarettes, smokeless tobacco, cigars, and pipes. Am. J Public Health. 1999;89:731–736. doi: 10.2105/ajph.89.5.731. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Jacob P, III, Hatsukami D, Severson H, Hall S, Yu L, Benowitz NL. Anabasine and anatabine as biomarkers for tobacco use during nicotine replacement therapy. Cancer Epidemiol. Biomarkers Prev. 2002;11:1668–1673. [PubMed] [Google Scholar]
- 10.Dewey RE, Xie J. Molecular genetics of alkaloid biosynthesis in Nicotiana tabacum. Phytochemistry. 2013;94:10–27. doi: 10.1016/j.phytochem.2013.06.002. [DOI] [PubMed] [Google Scholar]
- 11.Lisko JG, Stanfill SB, Duncan BW, Watson CH. Application of GC-MS/MS for the analysis of tobacco alkaloids in cigarette filler and various tobacco species. Anal. Chem. 2013;85:3380–3384. doi: 10.1021/ac400077e. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.McGuffey JE, Wei B, Bernert JT, Morrow JC, Xia B, Wang L, Blount BC. Validation of a LC-MS/MS method for quantifying urinary nicotine, six nicotine metabolites and the minor tobacco alkaloids--anatabine and anabasine--in smokers' urine. PLoS One. 2014;9:e101816. doi: 10.1371/journal.pone.0101816. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Wei B, Feng J, Rehmani IJ, Miller S, McGuffey JE, Blount BC, Wang L. A high-throughput robotic sample preparation system and HPLC-MS/MS for measuring urinary anatabine, anabasine, nicotine and major nicotine metabolites. Clin. Chim. Acta. 2014;436:290–297. doi: 10.1016/j.cca.2014.06.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Miller EI, Norris HR, Rollins DE, Tiffany ST, Wilkins DG. A novel validated procedure for the determination of nicotine, eight nicotine metabolites and two minor tobacco alkaloids in human plasma or urine by solid-phase extraction coupled with liquid chromatography-electrospray ionization-tandem mass spectrometry. J. Chromatogr. B Analyt. Technol. Biomed. Life Sci. 2010;878:725–737. doi: 10.1016/j.jchromb.2009.12.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Hoofnagle AN, Laha TJ, Rainey PM, Sadrzadeh SM. Specific detection of anabasine, nicotine, and nicotine metabolites in urine by liquid chromatography-tandem mass spectrometry. Am. J. Clin. Pathol. 2006;126:880–887. doi: 10.1309/LQ8U3UL956ET324X. [DOI] [PubMed] [Google Scholar]
- 16.Xu X, Iba MM, Weisel CP. Simultaneous and sensitive measurement of anabasine, nicotine, and nicotine metabolites in human urine by liquid chromatography-tandem mass spectrometry. Clin. Chem. 2004;50:2323–2330. doi: 10.1373/clinchem.2004.038489. [DOI] [PubMed] [Google Scholar]
- 17.Moyer TP, Charlson JR, Enger RJ, Dale LC, Ebbert JO, Schroeder DR, Hurt RD. Simultaneous analysis of nicotine, nicotine metabolites, and tobacco alkaloids in serum or urine by tandem mass spectrometry, with clinically relevant metabolic profiles. Clin. Chem. 2002;48:1460–1471. [PubMed] [Google Scholar]
- 18.Yue B, Kushnir MM, Urry FM, Rockwood AL. Quantitation of nicotine, its metabolites, and other related alkaloids in urine, serum, and plasma using LC-MS-MS. Methods Mol. Biol. 2010;603:389–398. doi: 10.1007/978-1-60761-459-3_38. [DOI] [PubMed] [Google Scholar]
- 19.Murphy SE, Link CA, Jensen J, Le C, Puumala S, Hecht SS, Carmella SG, Losey L, Hatsukami DK. A comparison of urinary biomarkers of tobacco and carcinogen exposure in smokers. Cancer Epidemiol. Biomarkers Prev. 2004;13:1617–1623. [PubMed] [Google Scholar]
- 20.Hukkanen J, Jacob P, III, Benowitz NL. Metabolism and disposition kinetics of nicotine. Pharmacol. Rev. 2005;57:79–115. doi: 10.1124/pr.57.1.3. [DOI] [PubMed] [Google Scholar]
- 21.Denlinger RL, Smith T, Murphy SE, Koopmeiners JS, Benowitz NL, Hatsukami DK, Pacek LR, Collino C, Cwalina SN, Donny EC. Characterizing biomarkers of nicotine exposure when smoking very low nicotine content cigarettes in a controlled access environment. Tob. Control. 2015 submitted. [Google Scholar]
- 22.Wei B, Feng J, Rehmani IJ, Miller S, McGuffey JE, Blount BC, Wang L. A high-throughput robotic sample preparation system and HPLC-MS/MS for measuring urinary anatabine, anabasine, nicotine and major nicotine metabolites. Clin. Chim. Acta. 2014;436:290–297. doi: 10.1016/j.cca.2014.06.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Murphy SE, Villalta P, Ho SW, von Weymarn LB. Analysis of [3′,3′-d(2)]-nicotine and [3′,3′-d(2)]-cotinine by capillary liquid chromatography-electrospray tandem mass spectrometry. J. Chromatogr. B Analyt. Technol. Biomed. Life Sci. 2007;857:1–8. doi: 10.1016/j.jchromb.2007.06.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Murphy SE, Park SS, Thompson EF, Wilkens LR, Patel Y, Stram DO, Le Marchand L. Nicotine N-glucuronidation relative to N-oxidation and C-oxidation and UGT2B10 genotype in five ethnic/racial groups. Carcinogenesis. 2014;35:2526–2533. doi: 10.1093/carcin/bgu191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Jacob P, III, Yu L, Liang G, Shulgin AT, Benowitz NL. Gas chromatographic mass spectrometric method for determination of anabasine, anatabine and other tobacco alkaloids in urine of smokers and smokeless tobacco users. J. Chromatog. Biomed. Appl. 1993;619:49–61. doi: 10.1016/0378-4347(93)80445-a. [DOI] [PubMed] [Google Scholar]
- 26.Davis DL, Bush LP, Grunwald C, Cornelius PL. Transfer of exogenously applied and endogenous alkaloids and sterols from tobacco to its smoke condensate. J. Agric. Food Chem. 1977;25:752–756. doi: 10.1021/jf60212a017. [DOI] [PubMed] [Google Scholar]
- 27.Perfetti TA, Coleman WM, Smith WS. Determination of mainstream and sidestream cigarette smoke components for cigarettes of different tobacco types and a set of reference cigarettes. Beitr Tabakforsch. 1998;18:95–113. [Google Scholar]
- 28.Benowitz NL, Hukkanen J, Jacob P., III Nicotine chemistry, metabolism, kinetics and biomarkers. Handb. Exp. Pharmacol. 2009;192:29–60. doi: 10.1007/978-3-540-69248-5_2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Beckett AH, Gorrod JW, Jenner P. A possible relation between pKa 1 and lipid solubility and the amounts excreted in urine of some tobacco alkaloids given to man. J. Pharm. Pharmacol. 1972;24:115–120. doi: 10.1111/j.2042-7158.1972.tb08943.x. [DOI] [PubMed] [Google Scholar]
- 30.Suh-Lailam BB, Haglock-Adler CJ, Carlisle HJ, Ohman T, McMillin GA. Reference interval determination for anabasine: a biomarker of active tobacco use. J. Anal. Toxicol. 2014;38:416–420. doi: 10.1093/jat/bku059. [DOI] [PubMed] [Google Scholar]
- 31.Plumlee KH, Holstege DM, Blanchard PC, Fiser KM, Galey FD. Nicotiana glauca toxicosis of cattle. J. Vet. Diagn. Invest. 1993;5:498–499. doi: 10.1177/104063879300500340. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.