SUMMARY
Ionizing radiation-mediated tumor regression depends on type I interferon (IFN) and the adaptive immune response, but the several pathways control I IFN induction. Here, we demonstrate that adaptor protein STING, but not MyD88, is required for type I IFN-dependent antitumor effects of radiation. In dendritic cells (DCs), STING was required for IFN-β induction in response to irradiated-tumor cells. The cytosolic DNA sensor cyclic GMP-AMP (cGAMP) synthase (cGAS) mediated sensing of irradiated-tumor cells in DCs. Moreover, STING was essential for radiation-induced adaptive immune responses, which relied on type I IFN signaling on DCs. Exogenous IFN-β treatment rescued the cross-priming by cGAS or STING-deficient DCs. Accordingly, activation of STING by a second messenger cGAMP administration enhanced antitumor immunity induced by radiation. Thus radiation-mediated antitumor immunity in immunogenic tumors requires a functional cytosolic DNA-sensing pathway and suggests cGAMP treatment may provide a new strategy to improve radiotherapy.
INTRODUCTION
Radiotherapy used alone or in combination with surgery or chemotherapy is employed to treat the primary and metastatic tumors in approximately 50-60% of all cancer patients (Begg et al., 2011; Liauw et al., 2013). The biological responses of tumors to radiation have been demonstrated to involve DNA damage, modulation of signal transduction, and alteration of the inflammatory tumor microenvironments (Begg et al., 2011; Liauw et al., 2013). Indeed, radiotherapy has been recently shown to induce antitumor adaptive immunity, leading to tumor control (Apetoh et al., 2007; Lee et al., 2009). Based on this concept, the blockade of immune checkpoints improves the efficacy of radiotherapy on local and distant tumors in experimental systems and more recently in clinical observations (Deng et al., 2014; Postow et al., 2012). Furthermore, radiotherapy sculpts innate immune response in a type I IFN-dependent manner to facilitate adaptive immune response (Burnette et al., 2011). However, the molecular mechanism for host type I IFN induction following local radiation has not yet been defined.
The innate immune system is the major contributor to host-defense in response to pathogens invasion or tissue damage (Takeuchi and Akira, 2010). The initial sensing of infection and injury is mediated by pattern recognition receptors (PRRs), which recognize pathogen-associated molecular patterns (PAMPs) and damage-associated molecular patterns (DAMPs) (Chen and Nunez, 2010; Desmet and Ishii, 2012; Kono and Rock, 2008). The first-identified and well-characterized of class of PRRs are the toll-like receptors (TLRs), which are responsible for detecting PAMPs and DAMPs outside the cell and in endosomes and lysosomes (O'Neill et al., 2013). Under the stress of chemotherapy and targeted therapies, the secretion of HMGB-1, which binds to TLR4, has been reported to contribute to antitumor effects (Apetoh et al., 2007; Park et al., 2010). However, whether the same mechanism dominates radiotherapy remains to be determined. Four endosomal TLRs (TLR3, TLR7, TLR8 and TLR9) that respond to microbial and host-mislocalized nucleic acids in cytoplasm have more recently been revealed (Desmet and Ishii, 2012). Through interaction of the adaptor proteins, myeloid differentiation primary-response protein 88 (MyD88) and TIR-domain-containing adaptor protein inducing IFN-β (TRIF), the activation of these four endosomal TLRs leads to significant induction of type I IFN production (Desmet and Ishii, 2012; O'Neill et al., 2013). Given that radiation induces production of type I IFNs, it is conceivable that radiation cause tumor cell nucleic acids and/or stress proteins to trigger the activation of TLRs and MyD88 and TRIF signaling.
A recently defined endoplasmic reticulum-associated protein STING (stimulator of interferon genes) has been demonstrated to be a mediator for type I IFN induction by intracellular exogenous DNA in a TLR-independent manner (Burdette and Vance, 2013). Cytosolic detection of DNA activates STING in the cytoplasm, which binds to TANK-binding kinase 1 (TBK1) and IκB kinase (IKK), that in turn activate the transcription factors interferon regulatory factor 3 (IRF3), signal transducer and activator of transcription (STAT6), and nuclear factor-κB (NF-κB), respectively (Paludan and Bowie, 2013). Subsequently, nuclear translocation of these transcription factors leads to the induction of type I IFNs and other cytokines that participate in host defense (Chen et al., 2011; Paludan and Bowie, 2013). In the past six years, STING has been demonstrated to be essential for the host protection against DNA pathogens through various mechanisms (Chen et al., 2011; Ishikawa and Barber, 2008; Ishikawa et al., 2009). STING is also a mediator for autoimmune diseases which are initiated by the aberrant cytoplasmic DNA (Ahn et al., 2012; Gall et al., 2012; Gehrke et al., 2013). Following the recognition of cytosolic DNA, cGAMP synthase (cGAS) catalyzes the generation of 2’ to 5’ cyclic GMP-AMP (cGAMP), which binds to and activates STING signaling (Li et al., 2013; Sun et al., 2013; Wu and Chen, 2014; Wu et al., 2013). More recently, cGAS has been considered as a universal cytosol DNA sensor for STING activation, such as in the setting of viral infection and lupus erythematosus (Gao et al., 2013a; Gehrke et al., 2013; Lahaye et al., 2013; Liang et al., 2013). Based on these considerations, it has become important to determine whether innate immune sensing following tumor radiation is mediated through TLR pathways or the alternative STING pathway.
Here, we demonstrate that innate immune sensing following radiotherapy is dominated by the cGAS-STING-dependent cytosolic DNA sensing pathway, which drives the adaptive immune response to radiation. Our study provides insight into better understanding mechanism of radiation-mediated tumor regression and forms the basis for new strategies to improve upon in radiotherapy efficacy in cancer patients.
RESULTS
STING signaling mediates antitumor effects of radiation
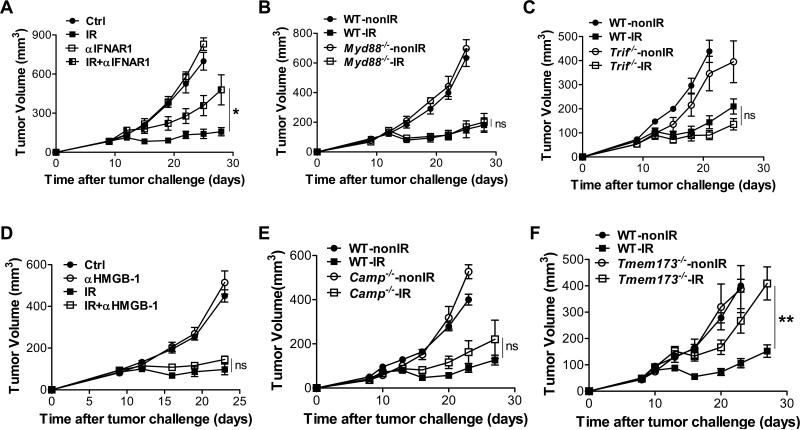
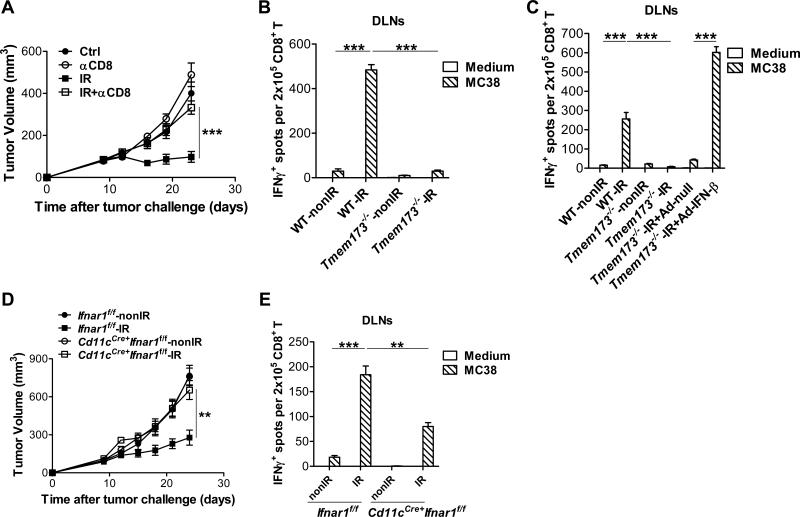
We previously demonstrated that antitumor effects of radiation were dependent on type I IFN signaling by utilizing IFNAR1-deficient mice (Burnette et al., 2011). To rule out the possibility that failure of tumors to respond to radiation was due to the intrinsic or developmental deficiency of IFNAR1-deficient mice, we administered blocking antibody against IFNAR1 in wild type (WT) mice following radiation. The results were similar to the effects observed in the knockout (KO) mice in that the antitumor effect of radiation was greatly attenuated by the neutralization of type I IFNs signaling with antibodies (Figure 1A). It has been demonstrated that MyD88 is essential for antitumor immunity of chemotherapy and targeted therapy with anti-HER2 (Apetoh et al., 2007; Park et al., 2010; Stagg et al., 2011). To investigate whether MyD88 is required to mediate response to radiation therapy, we implanted tumor cells on flanks of WT and MyD88-deficient mice. The inhibition of tumor growth post radiation was comparable between WT and MyD88-deficient mice (Figure 1B), demonstrating that host MyD88 was dispensable for the antitumor effect of radiation. To examine whether TRIF might be involved in the antitumor effects of radiation, we implanted tumor cells into WT and TRIF-deficient mice. Absence of host TRIF also failed to prevent the anti-tumor activity of radiation (Figure 1C), consistent with previous data (Burnette et al., 2011). Similar to chemotherapy and targeted antibody therapies, radiotherapy induces cell stress and result in the secretion of DAMPs. Next, we examined whether HMGB-1 secretion might be involved in the antitumor effect of radiation. We blocked HMGB-1 by administering specific antibodies along with radiation. However, tumor control by radiation was unaffected by anti-HMGB-1 treatment (Figure 1D), suggesting that HMGB-1 secretion is also not required for the antitumor effect of radiation. The cathelicidin-related antimicrobial peptide (CRAMP in mice and LL37 in human) has been identified as a mediator of type I IFN induction by binding self-DNA to trigger the TLR9-MyD88 pathway (Diana et al., 2013; Lande et al., 2007). To test the possibility that CRAMP might be responsible for the radiation response, we inoculated tumor cells into WT and CRAMP-deficient mice (CRAMP is encoded by Camp). Absence of host CRAMP also failed to prevent the antitumor effect of radiation (Figure 1E). Taken together, these data indicate that well-characterized TLR-dependent molecular mechanisms involved in chemotherapy and targeted antibody therapies are not responsible for the antitumor efficacy of radiation. Also, these results raise the possibility that a unique molecular mechanism which is TLRs-independent for type I IFN induction mediates the antitumor effect of radiation.
Figure 1. STING signaling is required for the antitumor effect of radiation.
MC38 tumors established in WT mice and KO mice were treated locally with one dose of 20Gy ionizing radiation (IR) or untreated. (A) 500μg anti-IFNAR1 was administered intratumorally in WT mice on day 0 and 2 after radiation. Tumor growth was monitored after radiation. (B) Tumor growth in WT and Myd88−/− mice after radiation. (C) Tumor growth in WT and Trif−/− mice after radiation. (D) 200μg anti-HMGB1 was administered i.p. in WT mice with tumors on day 0 and 3 after radiation. Tumor growth was monitored after radiation. (E) Tumor growth in WT and Camp−/− mice after radiation. (F) Tumor growth in WT and Tmem173−/− mice after radiation. STING-deficient mice are represented by Tmem173−/−, whereas CRAMP-deficient mice are represented by Camp−/−. Representative data are shown from three (A-F) experiments conducted with 5 (A-D) or 6 to 8 (E-F) mice per group. Data are represented as mean ± SEM. *P < 0.05, **P < 0.01 and ns No significant difference (Student's t test). See also Figure S1.
Recently, the STING-mediated cytosolic DNA sensing cascade has been demonstrated to be a major mechanism of TLR-independent type I IFN induction. To determine the role of STING in the radiation response, we implanted tumor cells on flanks of WT and STING-deficient mice (STING is encoded by Tmem 173) and monitored tumor growth. We found that, while tumor burden was significantly reduced by radiation in WT mice, absence of host STING significantly impaired the antitumor effect of radiation (Figure 1F), demonstrating that STING signaling is essential for the maximal antitumor effect of radiation. The antitumor effects of radiation were also impaired in STING-deficient mice when two doses of radiation treatment were utilized (Figure S1). Taken together, these results suggest that the STING-dependent cytosolic DNA sensing pathway is critical for the therapeutic effect of radiation in vivo.
STING signaling controls type I IFN induction and innate immune responses upon radiation
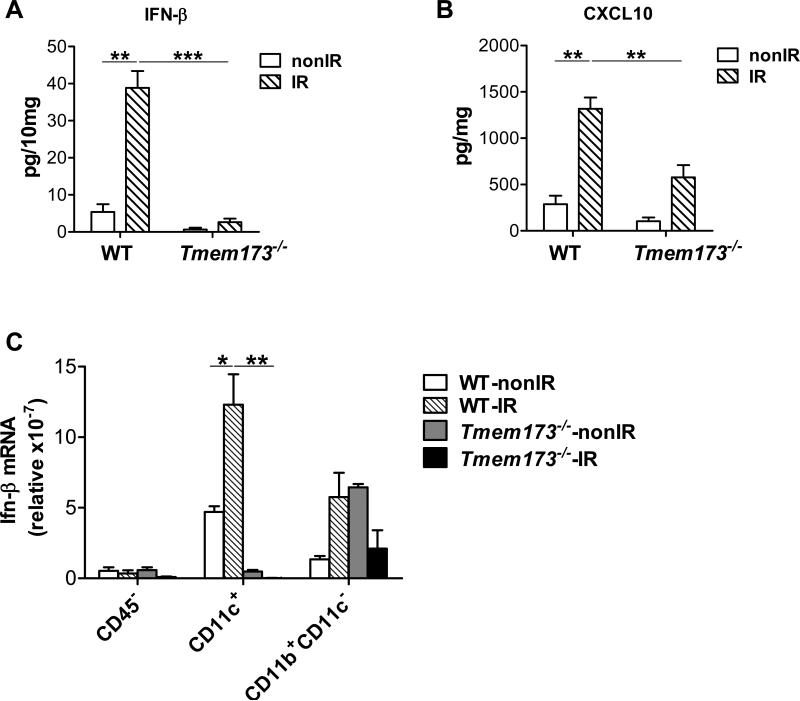
To test whether STING was responsible for type I IFN induction following radiation, we measured the amount of IFN-β protein in tumors. The induction of IFN-β in tumors was significantly abrogated in the absence of STING in the host after radiation (Figure 2A). As further confirmation, we found the amount of CXCL10, a type I IFN-stimulated gene (Ablasser et al., 2013; Holm et al., 2012), was also markedly diminished in tumors after radiation in STING-deficient hosts (Figure 2B). These results indicate that host STING is required for type I IFN induction by radiation. Next, to determine in which cell population STING mediates type I IFN induction, we performed quantitative real-time PCR assay of IFN-β in different sorted cell populations isolated from tumors after radiation. The phenotype of CD11c+ cells in tumors was characterized (Figure S2A). We observed that CD11c+ DCs were the major producer of IFN-β after radiation, compared to the CD45− population and the rest of myeloid cells (Figure 2C). In contrast, radiation-mediated induction of IFN-β mRNA by DCs was abolished in STING-deficient hosts (Figure 2C). Together, these data suggest that host STING controls radiation-mediated type I IFN induction in tumors and that the presence of STING in tumor-infiltrating DCs plays a major role in type I IFN induction after radiation.
Figure 2. STING signaling is essential for IFN-β induction by radiation.
(A-B) Tumors were excised on day 3 after radiation and homogenized in PBS with protease inhibitor. After homogenization, Triton X-100 was added to obtain lysates. ELISA assay was performed to measure IFN-β (A) and CXCL10 (B). (C) 72 hours after radiation, single cell suspensions from tumors in WT and Tmem173−/−mice were stained with 7-AAD and conjugated antibodies against CD45, CD11c and CD11b, and then sorted into different cell populations by flow cytometry. Ifn-β mRNA in different cell subsets was quantified by real-time PCR assay. STING-deficient mice are represented by Tmem173−/−. Representative data are shown from three experiments conducted with 4 mice per group. Data are represented as mean ± SEM. *P<0.05, **P < 0.01 and ***P < 0.001 (Student's t test). See also Figure S2.
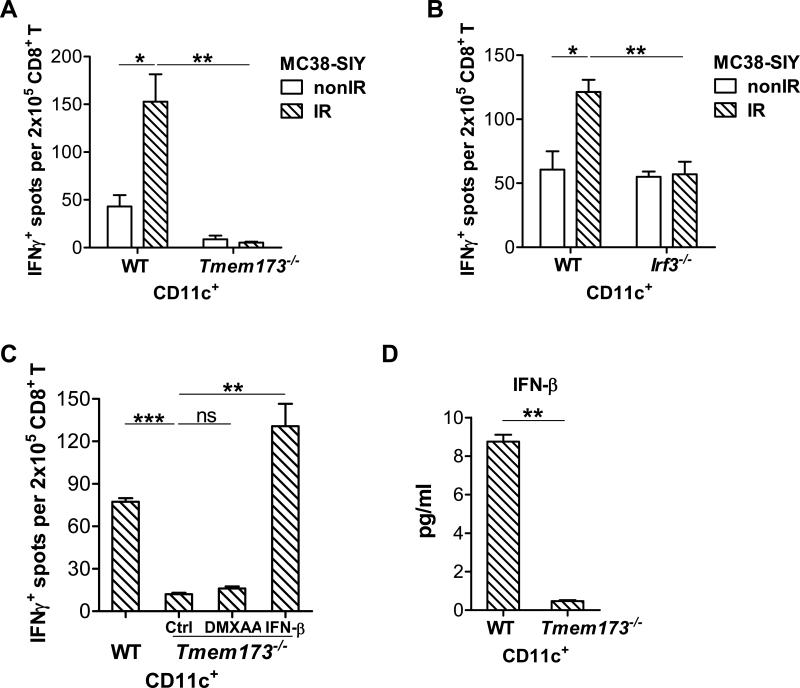
To determine whether STING signaling is activated by irradiated-tumor cells and whether it is essential for DC-mediated cross-priming of CD8+ T cells, a cross-priming assay was conducted with BMDCs from WT and STING-deficient mice. The phenotype of CD11c+ cells from GM-CSF stimulated bone marrow cells was characterized (Figure S2B). The functional capability of DCs to cross-present antigen was augmented by the stimulation of irradiated-tumor cells compared to non-irradiated-tumor cells, whereas the deficiency of STING in DCs resulted in failed responses of DCs to cross-prime T cells (Figure 3A). In contrast, CD19+ B cells isolated from the spleen of naïve mice were unable to cross prime T cells (Figure S3A). To determine whether DCs differentiated in vivo were also functionally affected by STING, we isolated CD11c+ cells from the spleen of WT and STING-deficient mice to perform cross-priming assays. Similar to in vitro generated DC, splenic DCs were impaired in the absence of STING (Figure S3B). To confirm whether IRF3 is essential to the function of DCs by the stimulation of irradiated-tumor cells, we performed cross-priming assay with WT BMDCs and IRF3-deficient BMDCs. Similar to STING-deficient BMDC, IRF3-deficient BMDCs failed to cross-prime CD8+ T cells in response to stimulation with irradiated-tumor cells (Figure 3B). These results indicate that the STING-IRF3 axis in DCs is activated by irradiated-tumor cells and is the predominant innate signaling pathway needed for cross-priming by DCs.
Figure 3. STING-IRF3 axis in DCs is activated by irradiated-tumor cells.
(A-C) BMDCs were cultured with 40Gy-pretreated MC38-SIY cells or non-irradiated-MC38-SIY cells. Subsequently purified CD11c+ cells were co-cultured with isolated CD8+ T cells from naive 2C mice for three days and analyzed by ELISPOT assays. (A) BMDCs from WT or Tmem173−/− mice were used for co-culture with irradiated or non-irradiated MC38-SIY cells. DC cross-priming activity was analyzed by ELISPOT assays. (B) BMDCs from WT or Irf3−/− mice were used for co-culture with irradiated or nonirradiated MC38-SIY cells. DC cross-priming activity was analyzed by ELISPOT assays. (C) WT and Tmem173−/− BMDCs were cultured with 40Gy-pretreated MC38-SIYhi cells. 10ng/ml IFN-β was added into the co-culture of Tmem173−/− BMDC and irradiated-MC38-SIY cells. 100μg/ml DMXAA was added to isolated Tmem173−/− CD11c+ cells for additional three hours incubation. DC cross-priming activity was analyzed by ELISPOT assays. (D) WT and Tmem173−/− BMDCs were co-cultured with 40Gy-pretreated MC38-SIY cells. The purified CD11c+ cells were incubated for additional two days and the supernatants were collected to measure IFN-β by ELISA assay. STING-deficient mice are represented by Tmem173−/−. Representative data are shown from three (A-D) experiments. Data are represented as mean ± SEM. *P<0.05, **P < 0.01, ***P < 0.001 and ns No significant difference (Student's t test). See also Figure S2 and S3.
To determine whether exogenous IFN-β treatment rescues the functions of STING-deficient BMDCs, we added IFN-β to co-cultured BMDCs and irradiated-tumor cells. The ability of STING-deficient BMDCs to cross-prime specific T cells was restored in the presence of exogenous IFN-β treatment (Figure 3C). Recently, it has been demonstrated that DMXAA, as a small molecular inducing cytokines production and disrupting tumor vascularization, binds to murine STING and activates STING signaling to induce type I IFN production (Gao et al., 2013b). DMXAA failed to rescue the function of STING-deficient BMDCs, confirming activation of STING is required to increase cross-priming through IFN pathway (Figure 3C). Next, to rule out the possibility that the impaired capacity of STING-deficient DCs and IRF3-deficient DCs for priming is due to intrinsic defects of these cells, a direct priming assay was performed with peptide stimulation. No difference was observed between WT BMDCs and STING-deficient BMDCs function in priming 2C cells with the stimulation of SIY peptide (Figure S3C). This result suggests that STING-deficient DCs do not have an intrinsic defect in direct priming of T cells. IRF3-deficient DCs also retained the ability to directly prime 2C T cells with SIY peptide stimulation (Figure S3C). To determine whether STING signaling might be activated by irradiated-tumor cells, we assessed the production of IFN-β by WT and STING-deficient BMDCs stimulated by irradiated-tumor cells. While the amount of IFN-β induction in response to irradiated-tumor cells was less than that induced by STING pathway agonists such as DMXAA (data not shown), we were nonetheless able to characterize the molecular requirements for this induction. The amount of IFN-β induced by irradiated-tumor cells (MC38) in vitro was reduced in STING-deficient BMDCs compared to WT BMDCs (Figure 3D). A similar difference was also observed when WT and STING-deficient BMDCs were stimulated by another tumor cell line (B16) in vitro (Figure S3D). These results suggest that activation of STING by irradiated-tumor cells controls type I IFN induction in DCs and that this process is crucial for the ability of DCs to cross-prime CD8+ T cells. These results also raise the possibility that STING molecules in DCs are stimulated by a component provided by irradiated-tumor cells, presumably DNA.
cGAS mediates DC sensing of irradiated-tumor cells
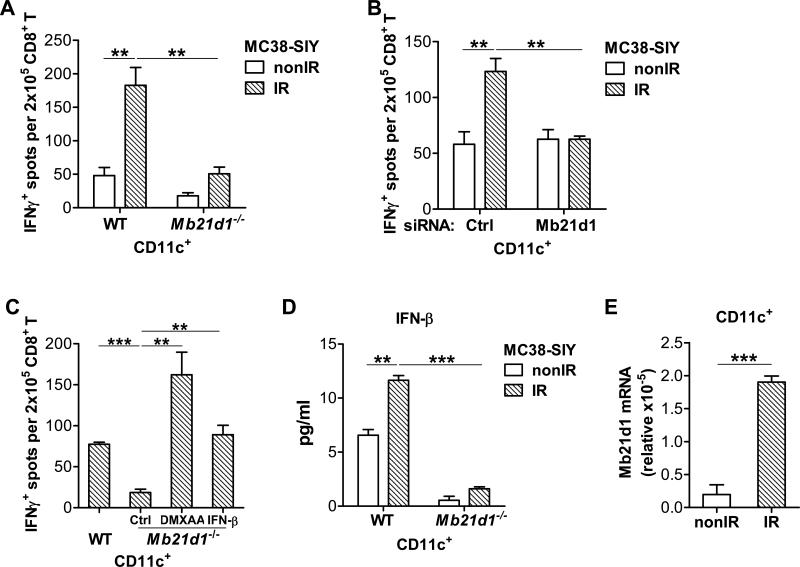
To interrogate whether cGAS (encoded by Mb21d1) is required for DCs sensing of irradiated-tumor cells to stimulate adaptive immunity, we compared the function of BMDCs from WT and cGAS-deficient mice. In contrast to WT BMDCs, cGAS-deficient BMDCs failed to cross-prime 2C cells in response to stimulation by irradiated-tumor cells (Figure 4A). To validate that the phenotype of cGAS-deficient BMDCs is not due to intrinsic or developmental defects, we silenced cGAS in WT BMDCs using siRNA. The silencing of cGAS in BMDCs diminished cross-primng by DCs compared to the silencing of non-target controls, when stimulated with irradiated-tumor cells (Figure 4B). The results confirmed that cGAS is essential for sensing of irradiated-tumor cells by DC. To map whether the cGAS-STING-type I IFN axis is needed for cross-priming by BMDCs, we performed bypass experiments in which either DCs were co-cultured with irradiated-tumor cells in the presence of exogenous IFN-β or isolated DCs from the co-culture were additionally stimulated with DMAXX. The cross-priming by cGAS-deficient BMDCs were restored with IFN-β and DMXAA treatment, respectively (Figure 4C). To further assess whether cGAS is required for sensing of irradiated-tumor cells by BMDC, we determined the production of IFN-β by WT BMDCs and by cGAS-deficient BMDCs after stimulation with irradiated-tumor cells. Indeed, the amount of IFN-β induced by irradiated-tumor cells was decreased in cGAS-deficient BMDCs compared to WT BMDCs (Figure 4D). Mb21d1 mRNA was detected in CD11c+ cells from tumors and increased after radiation in vivo (Figure 4E). We also performed the cross-priming assay using irradiated-human tumor cells expressing SIY and again found the cross-priming by DCs was impaired in the absence of STING or cGAS (Figure S4A). Thus cGAS responds to irradiated-murine and -human tumor cells and initiates type I IFN production to enhance DC cross-priming activity.
Figure 4. cGAS is essential for DC sensing of irradiated-tumor cells.
(A-C) BMDCs were cultured with 40Gy-pretreated MC38-SIY or non-irradiated-MC38-SIY cells. Subsequently purified CD11c+ cells were co-cultured with isolated CD8+ T cells from naive 2C mice for three days. (A) BMDCs from WT and Mb21d1−/− mice were used for co-culture with irradiated and nonirradiated MC38-SIY cells. DC cross-priming activity was analyzed by ELISPOT assays. (B) BMDCs were transfected with a siRNA-non-targeting control or siRNA-Mb21d1. Two days later after the transfection, the BMDCs were harvested for the co-culture with irradiated and non-irradiated MC38-SIY cells. DC cross-priming activity was analyzed by ELISPOT assays. (C) WT and Mb21d1−/− BMDCs were cultured with 40Gy-pretreated MC38-SIY cells. 10ng/ml IFN-β was added into the co-culture of Mb21d1−/− BMDC and irradiated-MC38-SIY cells. 100μg/ml DMXAA was added to isolated Mb21d1−/− CD11c+ cells for additional three hours incubation. DC cross-priming activity was analyzed by ELISPOT assays. (D) CD11c+ cells from WT or Mb21d1−/− BMDCs after co-culture with irradiated or non-irradiated MC38-SIY cells were incubated for 2 days, and then subjected to ELISA assays for IFNβ level. (E) CD11c+ cells were sorted from tumors in WT mice at 72 hour after radiation. Real-time PCR assay was performed to quantify the Mb21d1 mRNA. cGAS-deficient mice are represented by Mb21d1−/−. Representative data are shown from three (A-E) experiments. Data are represented as mean ± SEM. **P < 0.01 and ***P < 0.001 (Student's t test). See also Figure S4.
The results suggest that DNA from irradiated-tumor cells may gain access to the cytosolic DNA sensing pathway to trigger STING-dependent type I IFN induction. DNA from irradiated-tumor cells could be delivered into the cytosol of DCs as free DNA or as membrane-associated DNA transferred by membrane fusion. The priming ability of DCs in response to irradiated-tumor cells was not impaired by the presence of DNase I (Figure S4B), suggesting that DCs do not engulf free DNA fragments. To test whether DNA delivery is contact-dependent, BMDCs were separated from irradiated-tumor cells via a trans-well screen that only allows particles under 0.4μm in diameter to travel freely between compartments. Under these setting DC cross-priming activity was abolished (Figure S4C), indicating DNA delivery is mediated by direct cell-cell contact. Furthermore, the addition of Latrunculin B, an actin polymerization inhibitor, in the co-culture led to a dramatic reduction in the ability of DCs to induce cross-priming (Figure S4D). Production of IFN-β by DCs in response to irradiated tumor cells was also greatly decreased by application of a physical barrier or an actin polymerization inhibitor (Figure S4E). Taken together, these results suggest that DNA from irradiated-tumor cells is sensed by host cGAS during a cell-cell contact-mediated process.
STING signaling promotes adaptive immune responses upon radiation
Our previous studies have shown that adaptive immune responses play a role in the anti-tumor effect of radiation alone or combined with immunotherapy (Deng et al., 2014; Lee et al., 2009; Liang et al., 2013). To validate the role of CD8+ T cells after radiation in the MC38 tumor model, depleting antibodies against CD8+ T cells were administrated after radiation, and in agreement with our previous reports, the anti-tumor effect of radiation was reduced (Figure 5A) similar to the tumor growth curve in STING-deficient mice post radiation. To examine whether the failure of STING-deficient mice to respond to radiation is due to impaired CD8+ T cell function, we performed an ELISPOT assay with purified CD8+ T cells from tumor inguinal draining lymph nodes (DLNs). Radiation induced robust tumor antigen-specific CD8+ T cell responses in WT mice, whereas the antigen-specific CD8+ T cell responses in STING-deficient mice after radiation was diminished (Figure 5B). CD8+ T cells purified from mice that received radiation were reactivated with MC38 cells, but not B16F10 cells, confirming the assay detects tumor-specific T cell responses (Figure S5). To determine whether impaired CD8+ T cell responses in STING-deficient mice post-radiation were due to the insufficient induction of type I IFNs, STING-deficient mice received intratumoral treatment with Adenovirus (Ad)-IFN-β after radiation. Exogenous IFN-β treatment was able to restore the CD8+ T cell function in STING-deficient mice after radiation (Figure 5C). The CD8+ T cell response in STING-deficient and WT mice was demonstrated previously to be equivalent (Ishikawa et al., 2009). These data show reduced production of type I IFNs rather than intrinsic defects in CD8+ T cells accounts for impaired adaptive immunity in STING-deficient mice after radiation.
Figure 5. STING signaling is required for effective adaptive immune responses mediated by type I IFN signaling on DCs after radiation.
MC38 tumors established in WT, Tmem173−/−, Cd11cCre+ Ifnarf/f and Ifnarf/f mice were treated locally with one dose of 20Gy. (A) 300μg anti-CD8 mAb was administered i.p. in WT mice every three days for a total of four times starting from the day of radiation. Tumor growth was monitored. (B) Eight days after radiation, tumor draining inguinal lymph nodes (DLNs) were removed from WT and Tmem173−/− mice. Tumor antigen-specific CD8+ T cells function was measured by ELISPOT assays by co-culturing purified CD8+ cells with IFN-γ-treated MC38 tumor cells. (C) 1×1010 viral particles of Ad-null or Ad-IFN-β was administered intratumorally on day 2 after radiation. Tumor DLNs were removed on day 8 after radiation. Isolated CD8+ cells were subjected to ELISPOT assay with the presence of IFN-γ-treated MC38 tumor cells. (D) Tumor growth curves were analyzed in Cd11cCre+ Ifnarf/f and Ifnarf/f mice after radiation. (E) Tumor DLNs from Cd11cCre+ Ifnarf/f and Ifnarf/f mice were removed on day 8 after radiation. ELISPOT assay were performed with purified CD8+ cells and IFN-γ-treated MC38 tumor cells. STING-deficient mice are represented by Tmem173−/−. Representative data are shown from three (A-E) experiments conducted with 5-6 (A and D) or 3-4 (B-C and E) mice per group. Data are represented as mean ± SEM. **P<0.01 and ***P <0.001 (Student's t test). See also Figure S5.
To further determine whether DCs are directly responsible for type I IFN signaling after radiation, we implanted tumor cells into Cd11cCre+-Ifnar1f/f mice and Ifnar1f/f mice. Conditional deletion of Ifnar1 in DCs hampered the antitumor effect of radiation (Figure 5D), demonstrating that type I IFN signaling on DCs are responsible for antitumor effects of radiation. CD8+ T cell function in the DLNs after irradiation was compromised in Cd11cCre+-Ifnar1f/f mice versus Ifnar1f/f mice after radiation (Figure 5E). These results indicate that type I IFN signaling on DCs is required for antitumor efficacy of radiation by boosting adaptive immune responses.
cGAMP treatment and radiation synergistically amplify the antitumor immune response
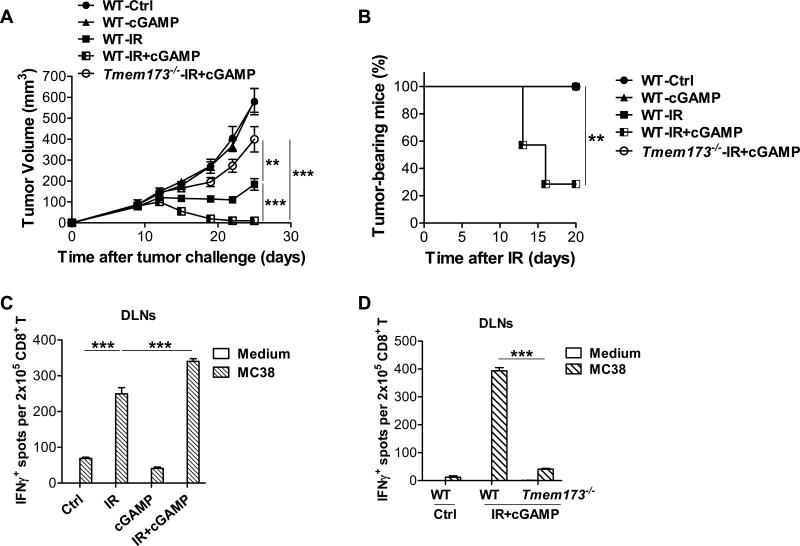
It has been demonstrated that 2’3’-cGAMP (cyclic [G(2’,5’)pA(3’,5’)p]) is generated in mammalian cells by cGAS in response to double-stranded DNA in the cytoplasm (Gao et al., 2013a; Wu et al., 2013; Zhang et al., 2013). We hypothesized that exogenous 2’3’-cGAMP treatment might improve the antitumor effect of radiation by enhancing STING activation. To test this hypothesis, 2’3’-cGAMP was intratumorally administrated after radiation. Treatment with a combination of 2’3’-cGAMP and radiation more effectively reduced tumor burden compared to 2’3’-cGAMP or radiation alone in WT mice, suggesting cGAMP treatment can potentiate the effect of radiation (Figure 6A). In addition, about 70% of mice completely rejected the tumors at the completion of combination treatment (Figure 6B). In contrast, the synergy of 2’3’-cGAMP and radiation was abrogated in STING-deficient mice (Figure 6A and 6B). Together, these data indicate boosting activation of STING signaling is able to inhibit tumor growth after radiation. To address whether the combination of 2’3’-cGAMP and radiation enhanced tumor-specific T cell responses, ELISPOT assays were performed with isolated CD8+ T cells from DLNs, co-cultured with IFN-γ-treated MC38. The number of tumor-specific IFN-γ-producing CD8+ T cells was increased in DLNs of mice that received combination treatment compared with those received radiation or 2’3’-cGAMP alone (Figure 6C). However, the robust antitumor CD8+ T cell response induced by the combination of 2’3’-cGAMP and radiation was dampened in STING-deficient hosts (Figure 6D). Together, these results indicate that 2’3’-cGAMP treatment potentiates the therapeutic effect of radiation by further enhancing tumor-specific CD8+ T cell functions and that the synergy is dependent on the presence of STING in the host.
Figure 6. cGAMP treatment promotes the antitumor effect of radiation in a STING-dependent manner.
MC38 tumors were established in WT and Tmem173−/− mice and treated locally with one dose of 20Gy. (A-B) 10μg 2’3’-cGAMP was administered intratumorally on day 2 and 6 after radiation. Tumor volume (A) and tumor-bearing mice frequency after IR (B) in WT and Tmem173−/− mice were monitored. (C-D) 10μg 2’3’-cGAMP was administered intratumorally in WT and Tmem173−/− mice on day 2 after radiation. Tumor DLNs were removed on day 8 after radiation, and purified CD8+ T cells were subjected to ELISPOT assay with the presence of IFNγ-treated MC38 cells. (C) IFN-γ-producing CD8+ T cells were evaluated in WT mice that were treated with IR alone, cGAMP alone or the combination of IR and cGAMP. (D) IFN-γ-producing CD8+ T cells were evaluated in WT and Tmem173−/− mice that were treated with the combinaiton of IR and cGAMP. STING-deficient mice are represented by Tmem173−/−. Representative data are shown from three experiments conducted with 5-7 (A-B) or 3-4 (C-D) mice per group. Data are represented as mean ± SEM. **P<0.01 and ***P <0.001 (Student's t test in A, C and D, and log rank (Mantel-Cox) test in B).
DISCUSSION
Radiation has been demonstrated to induce adaptive immune responses to support tumor regression (Apetoh et al., 2007; Lee et al., 2009). The induction of type I IFNs by radiation is essential for the function of CD8+ T cells (Burnette et al., 2011). Although the importance of type I IFNs has been elucidated using mice lacking IFNAR1 in all tissues, the identity of the immune cells that are responsible for type I IFN responses after radiation has been unclear. In addition, because of the diverse range of stimuli able to generate type I IFN production, it is necessary to discern the mechanism responsible for type I IFN induction by radiation in order to develop potential therapeutics that targets this pathway. Various nucleic acid-sensing pathways from different subcellular compartments have been reported to play a critical role in inducing type I IFNs in response to pathogen infection and tissue injury (Desmet and Ishii, 2012; Wu and Chen, 2014). Indeed, radiation induces cell stress and causes excess DNA breaks, indicating that nucleic acid-sensing pathways could feasibly account for the induction of type I IFNs upon radiation. We identified that the cGAS- and STING-dependent cytosolic DNA sensing pathway in DCs is required for type I IFN induction after radiation, and that type I IFN signaling on DCs determines the radiation-mediated adaptive immune responses. In addition, enhancing STING signaling by exogenous cGAMP treatment facilitated the antitumor effect of radiation. Therefore, the STING pathway is a key mediator of tumor immune responses to therapeutic radiation (Figure S6).
We found that, while type I IFN responses in DCs dictated the efficacy of antitumor radiation, no evidence for involvement of HMGB-1 release or MyD88 signaling was detected. In contrast, chemotherapeutic agents and anti-HER2 antibody treatments have previously been demonstrated to depend on a distinct immune mechanism to trigger adaptive immune responses (Apetoh et al., 2007; Park et al., 2010). Anti-HER2 treatment and chemotherapy require HMGB-1 release from dying tumor cells, as well as TLR4 and its adaptor MyD88 on DCs. The interaction of HMGB-1 and TLR4 potentiates the processing of dying tumor cells by DCs, leading to efficient cross-priming of CD8+ T cells. However, the antitumor effects of some chemotherapy agents have been shown to depend on MyD88 signaling but not TLR4 (Iida et al., 2013). Although MyD88 signaling has been shown to be necessary for responses to vaccination with irradiated-tumor cells, it was unanticipated that this signaling pathway is dispensable for radiation treatment of established tumors. Nevertheless, our study demonstrates that the induction of type I IFNs by radiation depends on STING pathway signaling, validating this particular molecular mechanism mediates antitumor immune responses to radiation.
The cGAS-STING pathway is a key component for activation of innate immune response to DNA from various pathogens, including viruses, bacteria, and parasites (Gao et al., 2013a; Lahaye et al., 2013; Liang et al., 2013; Lippmann et al., 2011; Sharma et al., 2011). In addition to pathogens, the cGAS-STING signaling pathway might play a dominant role in response to transfected DNA. Two groups have linked this signaling with DNA vaccines performed by intramuscular electroporation. One report found that TBK1 mediates antigen-specific B cell and T cell immune responses after DNA vaccination through type I IFN induction (Ishii et al., 2008). Another report pointed out that STING is essential for DNA vaccine-induced adaptive immune responses (Ishikawa et al., 2009). The release of DNA from dying host cells has been shown to stimulate adaptive immune responses in the TBK1-IRF3-type I IFN-dependent manner, leading to alum adjuvant activity (Marichal et al., 2011). In addition, oxidized self-DNA released from dying cells has been demonstrated to activate the cGAS-STING pathway as a mechanism to sense UV-exposed skin lesions (Bernard et al., 2012). Our results have revealed that the cGAS-STING-dependent cytosolic DNA sensing pathway mediates the efficacy of therapeutic radiation. It is likely that DNA derived from irradiated-tumor cells is a mediator of cGAS-STING signaling in DCs in vivo.
How DNA from irradiated-tumor cells is delivered into the cytosol of DCs remains unknown. DNA binding proteins such as LL37 are prevalent in neutrophil extracellular traps (NETs) and believed to enhance cytoplasmic delivery of DNA (Diana et al., 2013; Lande et al., 2007). Indeed, several reports have shown that STING signaling is activated by a DNA-LL37 complex (Chamilos et al., 2012; Gehrke et al., 2013). However, we have not been able to find that DNA is delivered either by free floating form or by complex forms. It is therefore possible that DNA from irradiated-tumor cells is delivered into the cytosol of DCs during cell-cell contact process. Moreover, radiation is able to induce tumor cells and phagocytes to generate reactive oxygen species (ROS), and then oxidated DNA modified by ROS is resistant to cytosolic exonuclease TREX-1-mediated degradation (Gehrke et al., 2013; Moeller et al., 2004). It is conceivable that radiation-induced ROS maintains the stability of tumor cell DNA during delivery into the cytosol of DCs. Elucidating the mechanism by which tumor-derived DNA finds access to the cytosol of host DCs in vivo will be of interest to carry out in future studies.
Our study not only reveals a previously unknown mechanism by which cytosolic DNA-cGAS-STING pathway controls radiation-mediated antitumor immunity, but also indicates that the combination of radiation and the STING agonist cGAMP reduces radioresistence and synergistically increases the antitumor host response. Although the free radical generation involved in DNA damage upon irradiation is short, the multiple integral events (especially in the microenvironment) generated by radiation can persist over longer time periods (3-10 days). The components of immune responses to radiation include release of danger signals, recruitment of myeloid cells, modulation of signal transduction, and alteration of innate and adaptive immune responses. It is likely that the activation of STING signaling by radiation occurs in newly replenished myeloid cells with high cross-priming activity, whereas the activation of STING by cGAMP alone occurs in the tolerized immune cells with low cross-priming activity. In addition, delivery of cGAMP into the cytosol from injection might not be very effective, and retention of cGAMP by injection could be much shorter than sustained release of DNA over periods of days induced by radiation. It is therefore conceivable that the antitumor effects of radiation are unable to be reproduced by treatment of cGAMP alone. Whether radiation also promotes the delivery of cGAMP remains to be determined.
In summary, we demonstrate that the adaptor protein STING, and not MyD88 or TRIF, is required for the antitumor effect of radiation and the induction of type I IFNs. This mechanism appears to involve cGAS for sensing of DNA by DCs in response to irradiated-tumor cells. The cGAS-STING-IRF3-Type I IFN cascade mediates a robust adaptive immune responses to radiation. In addition, exogenous cGAMP treatment synergizes with radiation to control tumors. Therefore, our findings reveal a novel molecular mechanism of radiation-mediated antitumor immunity and highlight the potential to improve radiotherapy by cGAMP administration.
EXPERIMENTAL PROCEDURES
Mice
Six- to eight-week old C57BL/6J mice were purchased from Harlan. Myd88−/−, Trif−/−, Camp−/−, 2C CD8+ T cell receptor (TCR)-Tg, Cd11cCre+-Tg mice were purchased from The Jackson Laboratory. Ifnar1flox/flox mice were kindly provided by Dr. Ulrich Kalinke of the Institute for Experimental Infection Research, Hanover, Germany. Tmem173−/− mice were kindly provided by Dr. Glen N. Barber of University of Miami School of Medicine, Miami. Irf3−/− mice were kindly provided by T. Taniguchi of University of Tokyo, Tokyo, Japan. All the mice were maintained under specific pathogen free conditions and used in accordance to the animal experimental guidelines set by the Institute of Animal Care and Use Committee. This study has been approved by the Institutional Animal Care and Use Committee of the University of Chicago.
Tumor Growth and Treatments
1×106 MC38 tumor cells were subcutaneously injected into the flank of mice. Tumors were measured and irradiated at 20 Gy or 2×15Gy as described in (Deng et al., 2014). For type I IFN blockade experiments, 200μg anti-IFAR1 mAb was intratumorally injected on day 0 and 2 after radiation. For HMGB-1 blockade experiments, 200μg anti-HMGB-1 mAb was administered i.p. on day 0 and 3 after radiation. For CD8+ T cell depletion experiments, 300μg anti-CD8 mAb was delivered 5 times by i.p. injection every three days starting one day before radiation. For exogenous IFN-β treatment experiments, 1×1010 viral particles of Adenovirus (Ad) -IFN-β were intratumorally administered on day 2 after radiation. Ad-null was used as negative control. For cGAMP treatment experiments, 10μg 2’3’-cGAMP in PBS was intratumorally administered on day 2 and 6 after radiation.
In vitro culture and function assay of BMDCs
Single-cell suspensions of bone marrow cells were obtained from C57BL/6J, Tmem173−/− and Irf3−/− mice. Bone marrow from Mb21d1−/− mice was kindly provided by Dr. Zhijian J. Chen of University of Texas Southwestern Medical Center, Dallas (Li et al., 2013). The cells were placed in 10cm petri dish and cultured in RPMI-1640 medium containing 10% FBS (DENVILLE), supplemented with 20ng/ml GM-CSF. Fresh media with GM-CSF was added into culture on day 3. BMDCs were harvest for stimulation assay on day 7. 8×106 MC38-SIYhi cells were plated into 10cm cell culture dishes overnight, and then pretreated with 40Gy and incubated for 5 hours. BMDCs were added and co-cultured with MC38-SIYhi cells at the ratio of 1:1 in the presence of fresh GM-CSF for additional 6-8 hours. Subsequently purified CD11c+ cells with EasySep™ Mouse CD11c Positive Selection Kit II (STEMCELL) were incubated with isolated CD8+ T cells from naive 2C mice for three days. For the bypassing assay, 10ng/ml murine IFN-β was added in the co-culture of BMDCs and tumor cells, or 100μg/ml DMXAA was added into isolated CD11c+ cells with additional 3h incubation prior to co-culture with CD8+ T cells. For IFN-β detection, 1x106 cells/ml purified CD11c+ cells from co-culture were seeded into 96-well plates and the supernatants were harvest after two-day incubation.
ELISA
Tumor tissues were excised on day 3 after radiation and homogenized in PBS with protease inhibitor. After homogenization, Triton X-100 was added to obtain lysates. Cell culture supernatants were obtained from isolated CD11c+ cells after 48h-incubation with fresh GM-CSF. The concentration of IFN-β and CXCL10 was measured with VeriKine-HS™ Mouse Interferon Beta Serum ELISA Kit (PBL Assay Science) and mouse CXCL10 Quantikine ELISA kit (R&D) in accordance with the manufacturer's instructions, respectively.
Measurement of IFNγ-Secreting CD8+T Cells by ELISPOT Assay
For bone-marrow CD11c+ cells functional assay, 2×104 purified CD11c+ cells with were incubated with isolated CD8+ T cells from naive 2C mice with EasySep™ Mouse CD8α Positive Selection Kit (STEMCELL) for three days at the ratio of 1:10. For tumor-specific CD8+ T cells functional assay, eight days after radiation, tumor DLNs were removed and CD8+ T cells were purified. MC38 tumor cells were exposed to 20ng/ml murine IFN-γ for 24 hr prior to plating with purified CD8+ T. 2×105 CD8+ T cells were incubated with MC38 at the ratio of 10:1 for 48 hours. ELISPOT assays were performed to detect the cytokine spots of IFN-γ according to product protocol (Millipore).
RNA interference
siRNAs (Mission siRNA) against murine cGAS and control siRNA were purchased from Sigma as described. BMDCs were transfected with siRNA by Lipofectamine RNAiMAX Reagent (Invitrogen) at a final concentration of 50nM: mmcGAS 5’-GAGGAAAUCCGCUGAGUCAdTdT-3’ (Ablasser et al., 2013); MissionsiRNA Universal Negative control 1. Forty-eight hours after transfection, cells were used for further experiments.
RNA extraction and quantitative real-time RT-PCR
Total RNA from sorted cells was extracted with the RNeasy Micro Kit (QIAGEN) and reversed-transcribed with Seniscript Reverse Transcription Kit (QIAGEN). Real-time RT-PCR was performed with SSoFast EvaGreen supermix (Bio-Rad) according to the manufacturer's instructions and different primer sets on StepOne Plus (Applied Biosystems). Data were normalized by the level of 18S expression in each individual sample. 2-ΔΔCt method was used to calculate relative expression changes.
Supplementary Material
HIGHLIGHTS.
STING but not MyD88 or TRIF is essential for therapeutic radiation
cGAS-STING axis mediates dendritic cell sensing of irradiated-tumor cells
STING is required for effective adaptive immune responses to radiation
Exogenous cGAMP treatment promotes antitumor efficacy of radiation
ACKOWLEDGMENTS
We acknowledge Joanna A. Wroblewska, Xiaohuan Guo and Yuan Zhang for helpful scientific discussion. We thank Rolando Torres for expert technical assistance. This work was in part supported by US National Institutes of Health grants CA141975 and CA CA134563 to Y.X.F, CA111423 to R.R.W., R01 CA181160 to T.F.G., a grant from the Ludwig Foundation to R.R.W., and a generous gift from The Foglia Foundation (Y-X.F., and R.R.W.).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
REFERENCES
- Ablasser A, Schmid-Burgk JL, Hemmerling I, Horvath GL, Schmidt T, Latz E, Hornung V. Cell intrinsic immunity spreads to bystander cells via the intercellular transfer of cGAMP. Nature. 2013;503:530–534. doi: 10.1038/nature12640. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ahn J, Gutman D, Saijo S, Barber GN. STING manifests self DNA-dependent inflammatory disease. Proc Natl Acad Sci U S A. 2012;109:19386–19391. doi: 10.1073/pnas.1215006109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Apetoh L, Ghiringhelli F, Tesniere A, Obeid M, Ortiz C, Criollo A, Mignot G, Maiuri MC, Ullrich E, Saulnier P, et al. Toll-like receptor 4-dependent contribution of the immune system to anticancer chemotherapy and radiotherapy. Nat Med. 2007;13:1050–1059. doi: 10.1038/nm1622. [DOI] [PubMed] [Google Scholar]
- Begg AC, Stewart FA, Vens C. Strategies to improve radiotherapy with targeted drugs. Nat Rev Cancer. 2011;11:239–253. doi: 10.1038/nrc3007. [DOI] [PubMed] [Google Scholar]
- Bernard JJ, Cowing-Zitron C, Nakatsuji T, Muehleisen B, Muto J, Borkowski AW, Martinez L, Greidinger EL, Yu BD, Gallo RL. Ultraviolet radiation damages self noncoding RNA and is detected by TLR3. Nat Med. 2012;18:1286–1290. doi: 10.1038/nm.2861. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burdette DL, Vance RE. STING and the innate immune response to nucleic acids in the cytosol. Nat Immunol. 2013;14:19–26. doi: 10.1038/ni.2491. [DOI] [PubMed] [Google Scholar]
- Burnette BC, Liang H, Lee Y, Chlewicki L, Khodarev NN, Weichselbaum RR, Fu YX, Auh SL. The efficacy of radiotherapy relies upon induction of type i interferon-dependent innate and adaptive immunity. Cancer Res. 2011;71:2488–2496. doi: 10.1158/0008-5472.CAN-10-2820. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chamilos G, Gregorio J, Meller S, Lande R, Kontoyiannis DP, Modlin RL, Gilliet M. Cytosolic sensing of extracellular self-DNA transported into monocytes by the antimicrobial peptide LL37. Blood. 2012;120:3699–3707. doi: 10.1182/blood-2012-01-401364. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen GY, Nunez G. Sterile inflammation: sensing and reacting to damage. Nat Rev Immunol. 2010;10:826–837. doi: 10.1038/nri2873. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen H, Sun H, You F, Sun W, Zhou X, Chen L, Yang J, Wang Y, Tang H, Guan Y, et al. Activation of STAT6 by STING is critical for antiviral innate immunity. Cell. 2011;147:436–446. doi: 10.1016/j.cell.2011.09.022. [DOI] [PubMed] [Google Scholar]
- Deng L, Liang H, Burnette B, Beckett M, Darga T, Weichselbaum RR, Fu YX. Irradiation and anti-PD-L1 treatment synergistically promote antitumor immunity in mice. J Clin Invest. 2014;124:687–695. doi: 10.1172/JCI67313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Desmet CJ, Ishii KJ. Nucleic acid sensing at the interface between innate and adaptive immunity in vaccination. Nat Rev Immunol. 2012;12:479–491. doi: 10.1038/nri3247. [DOI] [PubMed] [Google Scholar]
- Diana J, Simoni Y, Furio L, Beaudoin L, Agerberth B, Barrat F, Lehuen A. Crosstalk between neutrophils, B-1a cells and plasmacytoid dendritic cells initiates autoimmune diabetes. Nat Med. 2013;19:65–73. doi: 10.1038/nm.3042. [DOI] [PubMed] [Google Scholar]
- Gall A, Treuting P, Elkon KB, Loo YM, Gale M, Jr., Barber GN, Stetson DB. Autoimmunity initiates in nonhematopoietic cells and progresses via lymphocytes in an interferon-dependent autoimmune disease. Immunity. 2012;36:120–131. doi: 10.1016/j.immuni.2011.11.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gao P, Ascano M, Wu Y, Barchet W, Gaffney BL, Zillinger T, Serganov AA, Liu Y, Jones RA, Hartmann G, et al. Cyclic [G(2′,5′)pA(3′,5′)p] is the metazoan second messenger produced by DNA-activated cyclic GMP-AMP synthase. Cell. 2013a;153:1094–1107. doi: 10.1016/j.cell.2013.04.046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gao P, Ascano M, Zillinger T, Wang W, Dai P, Serganov AA, Gaffney BL, Shuman S, Jones RA, Deng L, et al. Structure-function analysis of STING activation by c[G(2′,5′)pA(3′,5′)p] and targeting by antiviral DMXAA. Cell. 2013b;154:748–762. doi: 10.1016/j.cell.2013.07.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gehrke N, Mertens C, Zillinger T, Wenzel J, Bald T, Zahn S, Tuting T, Hartmann G, Barchet W. Oxidative damage of DNA confers resistance to cytosolic nuclease TREX1 degradation and potentiates STING-dependent immune sensing. Immunity. 2013;39:482–495. doi: 10.1016/j.immuni.2013.08.004. [DOI] [PubMed] [Google Scholar]
- Holm CK, Jensen SB, Jakobsen MR, Cheshenko N, Horan KA, Moeller HB, Gonzalez-Dosal R, Rasmussen SB, Christensen MH, Yarovinsky TO, et al. Virus-cell fusion as a trigger of innate immunity dependent on the adaptor STING. Nat Immunol. 2012;13:737–743. doi: 10.1038/ni.2350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Iida N, Dzutsev A, Stewart CA, Smith L, Bouladoux N, Weingarten RA, Molina DA, Salcedo R, Back T, Cramer S, et al. Commensal bacteria control cancer response to therapy by modulating the tumor microenvironment. Science. 2013;342:967–970. doi: 10.1126/science.1240527. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ishii KJ, Kawagoe T, Koyama S, Matsui K, Kumar H, Kawai T, Uematsu S, Takeuchi O, Takeshita F, Coban C, Akira S. TANK-binding kinase-1 delineates innate and adaptive immune responses to DNA vaccines. Nature. 2008;451:725–729. doi: 10.1038/nature06537. [DOI] [PubMed] [Google Scholar]
- Ishikawa H, Barber GN. STING is an endoplasmic reticulum adaptor that facilitates innate immune signalling. Nature. 2008;455:674–678. doi: 10.1038/nature07317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ishikawa H, Ma Z, Barber GN. STING regulates intracellular DNA-mediated, type I interferon-dependent innate immunity. Nature. 2009;461:788–792. doi: 10.1038/nature08476. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kono H, Rock KL. How dying cells alert the immune system to danger. Nat Rev Immunol. 2008;8:279–289. doi: 10.1038/nri2215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lahaye X, Satoh T, Gentili M, Cerboni S, Conrad C, Hurbain I, El Marjou A, Lacabaratz C, Lelievre JD, Manel N. The capsids of HIV-1 and HIV-2 determine immune detection of the viral cDNA by the innate sensor cGAS in dendritic cells. Immunity. 2013;39:1132–1142. doi: 10.1016/j.immuni.2013.11.002. [DOI] [PubMed] [Google Scholar]
- Lande R, Gregorio J, Facchinetti V, Chatterjee B, Wang YH, Homey B, Cao W, Su B, Nestle FO, Zal T, et al. Plasmacytoid dendritic cells sense self-DNA coupled with antimicrobial peptide. Nature. 2007;449:564–569. doi: 10.1038/nature06116. [DOI] [PubMed] [Google Scholar]
- Lee Y, Auh SL, Wang Y, Burnette B, Meng Y, Beckett M, Sharma R, Chin R, Tu T, Weichselbaum RR, Fu YX. Therapeutic effects of ablative radiation on local tumor require CD8+ T cells: changing strategies for cancer treatment. Blood. 2009;114:589–595. doi: 10.1182/blood-2009-02-206870. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li XD, Wu J, Gao D, Wang H, Sun L, Chen ZJ. Pivotal roles of cGAS-cGAMP signaling in antiviral defense and immune adjuvant effects. Science. 2013;341:1390–1394. doi: 10.1126/science.1244040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liang H, Deng L, Chmura S, Burnette B, Liadis N, Darga T, Beckett MA, Lingen MW, Witt M, Weichselbaum RR, Fu YX. Radiation-induced equilibrium is a balance between tumor cell proliferation and T cell-mediated killing. J Immunol. 2013;190:5874–5881. doi: 10.4049/jimmunol.1202612. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liauw SL, Connell PP, Weichselbaum RR. New paradigms and future challenges in radiation oncology: an update of biological targets and technology. Sci Transl Med. 2013;5:173sr172. doi: 10.1126/scitranslmed.3005148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lippmann J, Muller HC, Naujoks J, Tabeling C, Shin S, Witzenrath M, Hellwig K, Kirschning CJ, Taylor GA, Barchet W, et al. Dissection of a type I interferon pathway in controlling bacterial intracellular infection in mice. Cell Microbiol. 2011;13:1668–1682. doi: 10.1111/j.1462-5822.2011.01646.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marichal T, Ohata K, Bedoret D, Mesnil C, Sabatel C, Kobiyama K, Lekeux P, Coban C, Akira S, Ishii KJ, et al. DNA released from dying host cells mediates aluminum adjuvant activity. Nat Med. 2011;17:996–1002. doi: 10.1038/nm.2403. [DOI] [PubMed] [Google Scholar]
- Moeller BJ, Cao Y, Li CY, Dewhirst MW. Radiation activates HIF-1 to regulate vascular radiosensitivity in tumors: role of reoxygenation, free radicals, and stress granules. Cancer Cell. 2004;5:429–441. doi: 10.1016/s1535-6108(04)00115-1. [DOI] [PubMed] [Google Scholar]
- O'Neill LA, Golenbock D, Bowie AG. The history of Toll-like receptors - redefining innate immunity. Nat Rev Immunol. 2013;13:453–460. doi: 10.1038/nri3446. [DOI] [PubMed] [Google Scholar]
- Paludan SR, Bowie AG. Immune sensing of DNA. Immunity. 2013;38:870–880. doi: 10.1016/j.immuni.2013.05.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Park S, Jiang Z, Mortenson ED, Deng L, Radkevich-Brown O, Yang X, Sattar H, Wang Y, Brown NK, Greene M, et al. The therapeutic effect of anti-HER2/neu antibody depends on both innate and adaptive immunity. Cancer Cell. 2010;18:160–170. doi: 10.1016/j.ccr.2010.06.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Postow MA, Callahan MK, Barker CA, Yamada Y, Yuan J, Kitano S, Mu Z, Rasalan T, Adamow M, Ritter E, et al. Immunologic correlates of the abscopal effect in a patient with melanoma. N Engl J Med. 2012;366:925–931. doi: 10.1056/NEJMoa1112824. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sharma S, DeOliveira RB, Kalantari P, Parroche P, Goutagny N, Jiang Z, Chan J, Bartholomeu DC, Lauw F, Hall JP, et al. Innate immune recognition of an AT-rich stem-loop DNA motif in the Plasmodium falciparum genome. Immunity. 2011;35:194–207. doi: 10.1016/j.immuni.2011.05.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stagg J, Loi S, Divisekera U, Ngiow SF, Duret H, Yagita H, Teng MW, Smyth MJ. Anti-ErbB-2 mAb therapy requires type I and II interferons and synergizes with anti-PD-1 or anti-CD137 mAb therapy. Proc Natl Acad Sci U S A. 2011;108:7142–7147. doi: 10.1073/pnas.1016569108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sun L, Wu J, Du F, Chen X, Chen ZJ. Cyclic GMP-AMP synthase is a cytosolic DNA sensor that activates the type I interferon pathway. Science. 2013;339:786–791. doi: 10.1126/science.1232458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takeuchi O, Akira S. Pattern recognition receptors and inflammation. Cell. 2010;140:805–820. doi: 10.1016/j.cell.2010.01.022. [DOI] [PubMed] [Google Scholar]
- Wu J, Chen ZJ. Innate immune sensing and signaling of cytosolic nucleic acids. Annu Rev Immunol. 2014;32:461–488. doi: 10.1146/annurev-immunol-032713-120156. [DOI] [PubMed] [Google Scholar]
- Wu J, Sun L, Chen X, Du F, Shi H, Chen C, Chen ZJ. Cyclic GMP-AMP is an endogenous second messenger in innate immune signaling by cytosolic DNA. Science. 2013;339:826–830. doi: 10.1126/science.1229963. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang X, Shi H, Wu J, Sun L, Chen C, Chen ZJ. Cyclic GMP-AMP containing mixed phosphodiester linkages is an endogenous high-affinity ligand for STING. Mol Cell. 2013;51:226–235. doi: 10.1016/j.molcel.2013.05.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.