Abstract
Background and aims Boron is essential for plant growth but hazardous when present in excess. As the antioxidant properties of hydrogen gas (H2) were recently described in plants, oxidative stress induced by excess boron was investigated along with other biological responses during rice (Oryza sativa) seed germination to study the beneficial role of H2.
Methods Rice seeds were pretreated with exogenous H2. Using physiological, pharmacological and molecular approaches, the production of endogenous H2, growth status, reactive oxygen species (ROS) balance and relative gene expression in rice were measured under boron stress to investigate mechanisms of H2-mediated boron toxicity tolerance.
Key Results In our test, boron-inhibited seed germination and seedling growth, and endogenous H2 production, were obviously blocked by exogenously applying H2. The re-establishment of ROS balance was confirmed by reduced lipid peroxidation and ROS accumulation. Meanwhile, activities of catalase (CAT) and peroxidase (POX) were increased. Suppression of pectin methylesterase (PME) activity and downregulation of PME transcripts by H2 were consistent with the alleviation of root growth inhibition caused by boron. Water status was improved as well. This result was confirmed by the upregulation of genes encoding specific aquaporins (AQPs), the maintenance of low osmotic potential and high content of soluble sugar. Increased transcription of representative AQP genes (PIP2;7 in particular) and BOR2 along with decreased BOR1 mRNA may contribute to lowering boron accumulation.
Conclusions Hydrogen provides boron toxicity tolerance mainly by improving root elongation, water status and ROS balance.
Keywords: Oryza sativa, boron toxicity, seed germination, root elongation, hydrogen gas, ROS balance, water status, aquaporins
INTRODUCTION
Although boron (B) is an essential micronutrient for plant growth, an excessive concentration of B due to arid and saline soils, as well as low rainfall and poor irrigation, usually produces toxicity in plants, including inhibition of seed germination and seedling growth and reduction of crop yield (e.g. Reid et al., 2004; Roessner et al., 2006; Miwa et al., 2007). The inhibition of root elongation has been found to be one of the most distinct symptoms among all the responses to B toxicity in plants (e.g. Chio et al., 2007; Tanaka and Fujiwara, 2008), and it has been reported that pectin methylesterase (PME) and osmotic potential are involved in this process (Chio et al., 2007; Tanaka and Fujiwara, 2008). Due to the excess B normally occurring in arid and semiarid areas, water stress is another serious problem (e.g. Ben-Gal and Shani, 2003; Reid et al., 2009; Pandey and Archana, 2013). Several genes encoding B transporters have been identified to play roles in B absorption or providing tolerance to B toxicity, including PIP2;4, PIP2;7 (Kumar et al., 2014), TIP5;1 (Pang et al., 2010) and BOR1 (Nakagawa et al., 2007), as well as Bot1 in barley (Sutton et al., 2007) and BOR4 in Arabidopsis (Miwa et al., 2007). Excess of B could also trigger the overproduction of reactive oxygen species (ROS) in plant cells, thus leading to oxidative damage in biomembrane lipids and other macromolecules (e.g. Cervilla et al., 2007, 2009). In response to ROS accumulation, activities of antioxidant enzymes, including superoxide dismutase (SOD), catalase (CAT), ascorbate peroxidase (APX) and guaiacol peroxidase (POX), were modulated. Thus, enhanced antioxidant enzyme activities have been shown to be closely associated with plant tolerance of excess B (e.g. Ardıc et al., 2009; Aftab et al., 2010).
The production of hydrogen gas (H2) in higher plants was discovered in 1964 (Renwick et al., 1964), and hydrogenase-like genes have been reported (Cavazza et al., 2008; Zeng et al., 2013). In animals, the antioxidant property of H2 was first described in 2007 owing to its ability to react directly with ROS (Ohsawa et al., 2007). Subsequently, a series of investigations showed that H2 exhibits multiple biological functions in clinical trials owing to its antioxidant ability (e.g. Buchholz et al., 2008; Taura et al., 2010). Ample evidence further confirmed that H2 exhibits potential as a new antioxidant and signalling molecule in preventive and therapeutic applications (e.g. Huang et al., 2010; Kawaguchi et al., 2014; Iuchi et al., 2016). Similar to the approach used in animals, hydrogen-rich water was regarded as a safe and easily available means of investigating the physiological function of endogenous H2 in plants. It has been shown that H2 might be a novel bioregulator involved in phytohormone signalling (Zeng et al., 2013), the delay of fruit senescence (Hu et al., 2014) and plant responses to various stresses, including paraquat (Jin et al., 2013), ultraviolet radiation (Su et al., 2014; Xie et al., 2015), drought (Xie et al., 2014), salinity (Xie et al., 2012; Xu et al., 2013), cadmium (Cui et al., 2013) and mercury exposure (Cui et al., 2014). However, whether H2 regulates plant adaptive responses to B toxicity is unknown. Most importantly, the above-mentioned beneficial responses in plants were mostly attributed to the antioxidant behaviour of H2.
In this report, excess B-induced ROS imbalance and other biological responses during rice seed germination were used as excellent models in which to study the specific mechanism of action of H2. Our results showed that, besides the function of H2 in the re-establishment of ROS imbalance, tolerance to B toxicity is associated with reduced B accumulation and the improvement of water status. Alleviation of seed germination and root growth inhibition was also observed. Related mechanisms were primarily illustrated.
MATERIALS AND METHODS
Plant materials, growth conditions and experimental design
Rice (Oryza sativa, Nanjing 49) seeds were surface-sterilized with 5 % (v/v) hypochlorite (NaClO) for 15 min and rinsed extensively in distilled water for 30 min. Seeds were presoaked in hydrogen-rich water for 24 h and then transferred to Petri dishes containing 5 mL of distilled water or 10 mm boric acid (H3BO3) solution (B). All seeds were grown in a growth chamber in darkness and kept at 28 °C. After various treatments, the samples were harvested and used immediately. Alternatively, plant tissues were frozen in liquid nitrogen and stored at −80 °C until further analysis.
Seeds were supplied with H2 by adding hydrogen-rich water to the seed-bathing solution. Purified hydrogen gas (99·99 %, v/v) generated from a hydrogen gas generator (SHC-300; Saikesaisi Hydrogen Energy, Shandong, China) was bubbled into 1000 mL of distilled water at the rate of 150 mL min−1 for 30 min. Then, the hydrogen-saturated water was immediately diluted to the required concentrations [1, 10, 50 and 100 % saturation (v/v)]. The H2 concentration in freshly prepared solutions, analysed by gas chromatography (GC; Agilent 7890A, equipped with a thermal conductivity detector), was 0·008, 0·08, 0·39 and 0·78 mm, respectively, and maintained at a relatively constant level for at least 12 h.
Determination of endogenous H2 content
To analyse endogenous H2 content, headspace sampling of gas followed by GC (Agilent 7890A equipped with a thermal conductivity detector) was adopted with minor modifications according to a method described previously (Xie et al., 2014). Rice seedlings (0·2 g) were homogenized with 7 mL of distilled water and then placed in a vial, followed by the addition of 5 μL of octanol and 139 μL of concentrated sulphuric acid (H2SO4). Pure nitrogen (N2) was then bubbled into the vial to fully displace the air. After being capped and shaken vigorously for 1 min, the vial was heated at 70 °C for 1 h to liberate H2 before analysis.
Analysis of germination and growth
Germination tests were carried out using at least three replicates of 120 seeds each. After various treatments at the indicated time points, germination parameters (germination rate, germination energy and germination index) were recorded. Seed germination energy (%) was calculated as (number of germinating seeds/number of total seeds per treatment after germination for 2 d) × 100. The germination index (GI, %) was calculated as described by the Association of Official Seed Analysts (1983), using the following formula: GI = ∑(Gt/Dt), where Dt is the number of days to germination and Gt is the number of germinating seeds in correspondence to Dt. Seeds were considered to have germinated when the emerging root was approximately equal to the length of the seeds. We also determined root and shoot lengths and fresh and dry weights.
Additionally, soluble sugar content was determined as described by Dubois et al. (1956).
Analysis of osmotic potential and water status
Total water content was determined as fresh weight minus dry weight per plant. Water status of tissues, measured in terms of specific water content (SWC), relative water content (RWC), water uptake capacity (WUC) and water saturation deficit (WSD), was determined as described by Pandey and Archana (2013).
The osmotic potential in rice root tips (3 mm in length) was measured with a PSYPRO (C52; Wescor, South Logan, UT, USA), and calculated according to the van ’t Hoff equation.
Determination of boron content
Dried rice roots (∼100 mg) were digested with 2 mL of 68 % (v/v) nitric acid (HNO3) using a Microwave Digestion System (Milestone Ethos T, Italy) for 30 min. The B content was measured with an inductively coupled plasma optical emission spectrometer (ICP-OES; Perkin Elmer Optima 2100DV).
Analysis of thiobarbituric acid-reactive substances and ROS
Lipid peroxides were measured by measuring the concentration of thiobarbituric acid-reactive substances (TBARS) (Hodges et al., 1999). The absorbance of the supernatant was read at 532 nm and corrected by elimination of non-specific turbidity at 600 nm. The TBARS content was quantified by using an extinction coefficient of 155 mm−1 cm−1 and expressed as μmol g−1 dry weight.
The content of H2O2 was estimated according to the method described by Bellincampi et al. (2000). Rice seedlings were extracted with 200 mm perchloric acid (HClO4) and mixed with the substrate solution (500 μm ammonium ferrous sulphate, 50 mm H2SO4, 200 μm xylenol orange and 200 mm sorbitol) with incubation for 45 min. A calibration curve was obtained by adding various amounts of H2O2 to the substrate solution and measuring the respective absorbance values at 560 nm.
Superoxide anion (O2−•)-scavenging activity was measured according to the method of Nishikimi et al. (1972) with slight modifications. Extracts (0·1 g) were mixed with the reaction solution [1·3 μm riboflavin, 13 mm methionine, 63 μm nitroblue tetrazolium chloride (NBT), 100 μm ethylene diamine tetraacetic acid (EDTA) and 50 mm phosphate buffer (PBS), pH 7·8] and then incubated under 4000 lux illumination at 25 °C for 20 min. The absorbance values of the reaction mixtures were measured at 560 nm. The relative ()-scavenging activity (%) was calculated by using the formula: (1−A560 of sample/A560 of control) × 100.
The hydroxyl radical (•OH)-scavenging activity was also measured as described by Halliwell et al. (1987) with minor modifications. Homogenized samples (0.1 g) were added to the reaction solution [2·8 mm deoxyribose (DR), 50 μm FeCl3, 2·8 mm H2O2, 100 μm EDTA and 10 mm PBS], and incubated at 37 °C for 60 min after 100 μm ascorbic acid (ASA) had been added to start the reaction. The results are expressed as the percentage inhibition of DR attack, where 100 % attack is defined as absorbance of DR without addition of samples.
Analysis of enzyme activities
The activities of α-amylase and β-amylase were determined according to the starch–iodine method described by Collins et al. (1972). One unit of activity was taken as the quantity of enzyme giving 50 % of the original colour intensity. Protein concentration was determined by the method of Bradford (1976) using bovine serum albumin as the standard.
Pectin methylesterase was extracted using a high-salt buffer [0·1 m citrate, 0·2 m disodium hydrogen phosphate (Na2HPO4) and 1 m sodium chloride (NaCl), pH 5·0] (Ren and Kermode, 2000), and its activity was determined according to the method described by Richard et al. (1994). Extracts (8 μL) were added to 4 mL of substrate solution [0·5 % (w/v) citrus pectin (Sigma), 0·2 m NaCl and 0·15 % (w/v) methyl red, pH 6·8], followed by incubation at 37 °C for 2 h. A standard curve was obtained by adding 80–240 μL of 0·01 m hydrochloric acid (HCl) to 4 mL of substrate solution and measuring absorbance at 525 nm.
Frozen rice plants (0·2 g) were homogenized in 2 mL of 50 mm PBS (pH 7·0) containing 1 mm EDTA and 1 % (w/v) polyvinylpyrrolidone for SOD, POX and CAT assays, or the combination with the addition of 1 mm ASA for the APX assay. Activity of SOD was analysed by measuring its capacity to inhibit the photochemical reduction of NBT (Beauchamp et al., 1971). One unit of SOD activity was defined as the amount of crude enzyme extract required to inhibit the reduction rate of NBT by 50 %. Activity of APX was determined by monitoring the decrease at 290 nm (extinction coefficient 2·8 mm−1 cm−1) (Nakano and Asada, 1981). Activity of CAT was measured by monitoring the consumption of H2O2 (extinction coefficient 39·4 mm−1 cm−1) at 240 nm for at least 3 min (Durner et al., 1996). Activity of POX was determined by measuring the oxidation of guaiacol (extinction coefficient 26·6 mm−1 cm−1) at 470 nm (Hammerschmidt et al., 1982).
Gel electrophoresis
The isozymes of SOD, APX, CAT and POX were separated on discontinuous polyacrylamide gels (stacking gel 5 % and separating gel 10 %) under non-denaturing conditions. Isozyme activities on the gel were visualized (Woodbury et al., 1971; Pinhero et al., 1997; Janda et al., 1999). Gels were scanned in transmission black-and-white mode, and band intensity was calculated by using Quantity One v4.4.0 software (Bio-Rad, Hercules, CA, USA).
Real-time quantitative reverse transcription–polymerase chain reaction analysis
Total RNAs were extracted by using Trizol reagent (Invitrogen, Gaithersburg, MD, USA). Further real-time quantitative reverse transcription–polymerase chain reaction (qRT–PCR) reactions were performed using a Mastercycler® ep realplex real-time PCR system (Eppendorf, Hamburg, Germany) with SYBR® Premix Ex TaqTM (TaKaRa Bio, Dalian, China). A list of the oligonucleotide primers used is shown in Supplementary Data Table S1. All genes were amplified by initial heating at 95 °C for 10 min followed by 40 cycles at 95 °C for 10 s, x °C (different for individual genes) for 20 s and 72 °C for 20 s. Melting curves were analysed at the dissociation step to examine the specificity of amplification. Relative expression level was expressed as the value relative to that of the corresponding control samples at the indicated times, after normalization to actin1 transcript levels. Data were obtained in three independent experiments with three replicates for each.
Statistical analysis
Results were expressed as the means ± s.e. of three independent experiments with at least three replicates for each. Statistical analysis was performed using SPSS 10·0 software according to Duncan’s multiple comparison.
RESULTS
Boron inhibited rice seed germination in a concentration- and time-dependent manner
Rice seed germination rate was examined to evaluate the toxic effect of excess B. Results showed that the addition of different concentrations of H3BO3 (B) for 5 d inhibited rice seed germination rate in a concentration- and time-dependent manner (Supplementary Data Fig. S1). For instance, in respect to the B-free control samples, 5 and 10 mm H3BO3 treatments for 5 d brought about ∼13·0 and ∼74 % reduction in germination rate, respectively. Since 20 mm H3BO3 severely inhibited seed germination up to ∼90 % (regarded as a lethal dose), 10 mm H3BO3 (an excess B condition) was applied in the following experiments.
Excess boron decreased endogenous H2 production in germinating rice seeds
We tested whether the toxic effect of excess B was related to the production of endogenous H2 in rice plants. By using GC we observed that, in comparison with the control samples, B treatment for 24 h significantly inhibited endogenous H2 production in germinating seeds (Fig. 1A). This result suggested a possible role of endogenous H2 in the regulation of B toxicity, which was assessed in the following experiments.
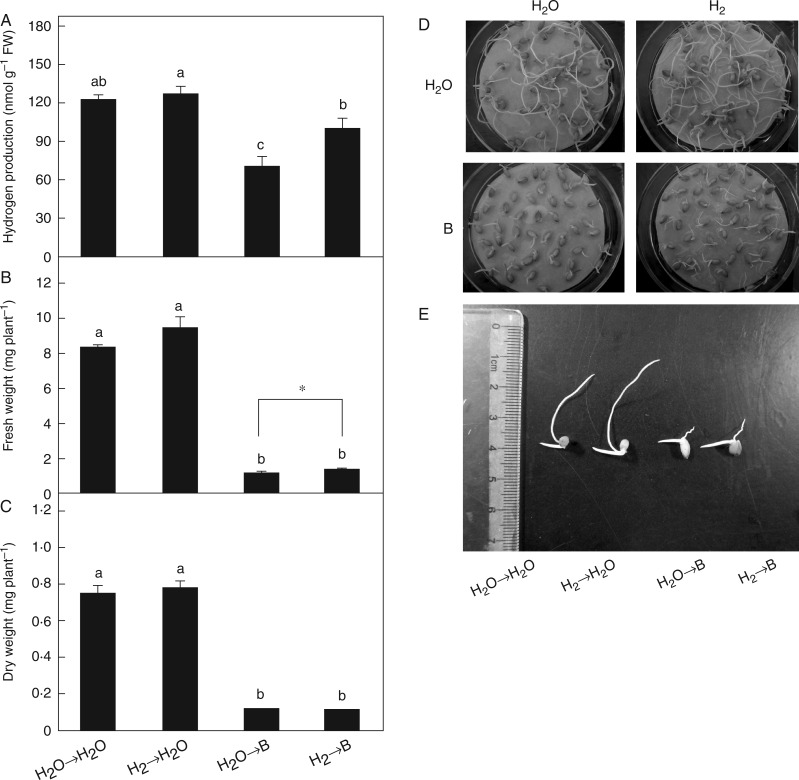
Fig. 1.
Changes in endogenous H2 production and the alleviation of growth inhibition induced by excess boron mediated by H2. Rice seeds were presoaked in water in the presence or absence of 0·39 mm H2 for 24 h and then transferred to water (→H2O) or 10 mm H3BO3 solution (→B). Hydrogen production in rice seedlings (A), fresh weight (B) and dry weight (C) were measured after 24 or 72 h of the different treatments. Whole (D) and selected phenotypes (E) were photographed after 72 h of the treatments. Scale bar = 1 cm. Values are means ± s.e. of three independent experiments with at least three replicates for each. Different letters and * denote differences significant at P < 0·05 according to Duncan’s multiple comparison test.
Hydrogen alleviated inhibition of rice seed germination and seedling growth caused by excess boron
To test whether endogenous H2 has any role in the alleviation of B toxicity, rice seeds pretreated with different concentrations of H2 (using hydrogen-rich water) followed by 10 mm H3BO3 stress were used to compare growth status. Table 1 shows that rice seed germination (assessed using germination rate, germination energy and germination index) and seedling growth were markedly inhibited after being exposed to excess B, with more distinct inhibition of root length than of shoot length. However, pretreatments with H2 ranging from 0·008 to 0·78 mm differentially alleviated the reduction of root and shoot lengths compared with samples subjected to B stress alone. Among the pretreatments, 0·39 mm H2 exhibited the most significant rescuing effect (except changes in germination energy and shoot length). Time-course analysis of seed germination rate exhibited similar tendencies (Supplementary Data Fig. S2), and 0·39 mm H2 was therefore selected for further experiments. We also noticed that the application of 0·08 and 0·39 mM H2 alone clearly boosted germination energy, germination index (but not germination rate), and seedling growth with respect to the control samples (except germination rate; Table 1 and Fig. S2).
Table 1.
Alleviation of excess of boron-induced inhibition of rice seed germination and root and shoot length by H2
| Treatment | Germination rate (%, 5 d) | Germination energy (%, 2 d) | Germination index(%) | Root length (%, 3 d) | Shoot length (%, 3 d) |
|---|---|---|---|---|---|
| 0 mm H2→H2O | 93·33 ± 1·76A | 66·00 ± 3·46B | 53·06 ± 0·91B | 3·62 ± 0·11B | 1·28 ± 0·05A |
| 0·008 mm H2→H2O | 94·67 ± 2·3A | 79·33 ± 6·40A | 56·91 ± 1·59A | 3·78 ± 0·1AB | 1·29 ± 0·05A |
| 0·08 mm H2→H2O | 98·00 ± 1·14A | 88·00 ± 1·13A | 60·38 ± 0·74A | 3·91 ± 0·11A | 1·38 ± 0·05A |
| 0·39 mm H2→H2O | 96·67 ± 1·75A | 85·33 ± 1·33A | 59·19 ± 1·01A | 3·96 ± 0·08A | 1·38 ± 0·08A |
| 0·78 mm H2→H2O | 96·00 ± 1·14A | 81·33 ± 5·70A | 57·93 ± 1·095A | 3·63 ± 0·07B | 1·28 ± 0·04A |
| 0 mm H2→B | 24·00 ± 2·31c | 0·67 ± 0·67c | 8·12 ± 1·15c | 0·49 ± 0·03b | 0·91 ± 0·01b |
| 0·008 mm H2→B | 45·33 ± 2·67b | 0·67 ± 0·67c | 14·73 ± 0·09b | 0·61 ± 0·03b | 0·96 ± 0·06ab |
| 0·08 mm H2→B | 46·00 ± 3·05b | 3·33 ± 0·67a | 17·88 ± 1·57b | 0·67 ± 0·03b | 1·02 ± 0·03a |
| 0·39 mm H2→B | 64·67 ± 5·21a | 1·33 ± 0·67bc | 24·02 ± 1·48a | 0·84 ± 0·03a | 0·91 ± 0·07b |
| 0·78 mm H2→B | 46·00 ± 3·46b | 1·33 ± 0·67bc | 16·46 ± 0·05b | 0·65 ± 0·03b | 0·92 ± 0·03b |
Seeds were presoaked in water in the presence or absence of 0·008, 0·08, 0·39 and 0·78 mm H2 for 24 h and then transferred to H2O or 10 mm H3BO3 solution (B) for another 5 d.
Values are means ± s.d. of three independent experiments with at least three replicates for each.
Within each set of experiments, uppercase letters denote significant differences among different H2 pretreatments followed by H2O treatments, and lower-case letters denote significant differences among different H2 pretreatments followed by B treatments, at P < 0·05 according to Duncan’s multiple comparison test.
Subsequent results showed that the addition of 0·39 mm H2 could block B-inhibited H2 production in rice plants (Fig. 1A). Similar to our previous results (Table 1 and Fig. S2), B-triggered inhibition of seed germination and root growth was lessened by H2 (Fig. 1D, E). In particular, the inhibition of fresh weight rather than dry weight per plant was alleviated to some extent (Fig. 1B, C). Consistent with the improvement in seed germination inhibition (Table 1), we discovered that 0·39 mm H2 pretreatment was able to increase the activities of α/β-amylase in B-stressed rice seeds, which was further confirmed by the accumulation of soluble sugar (Fig. 2A, B).
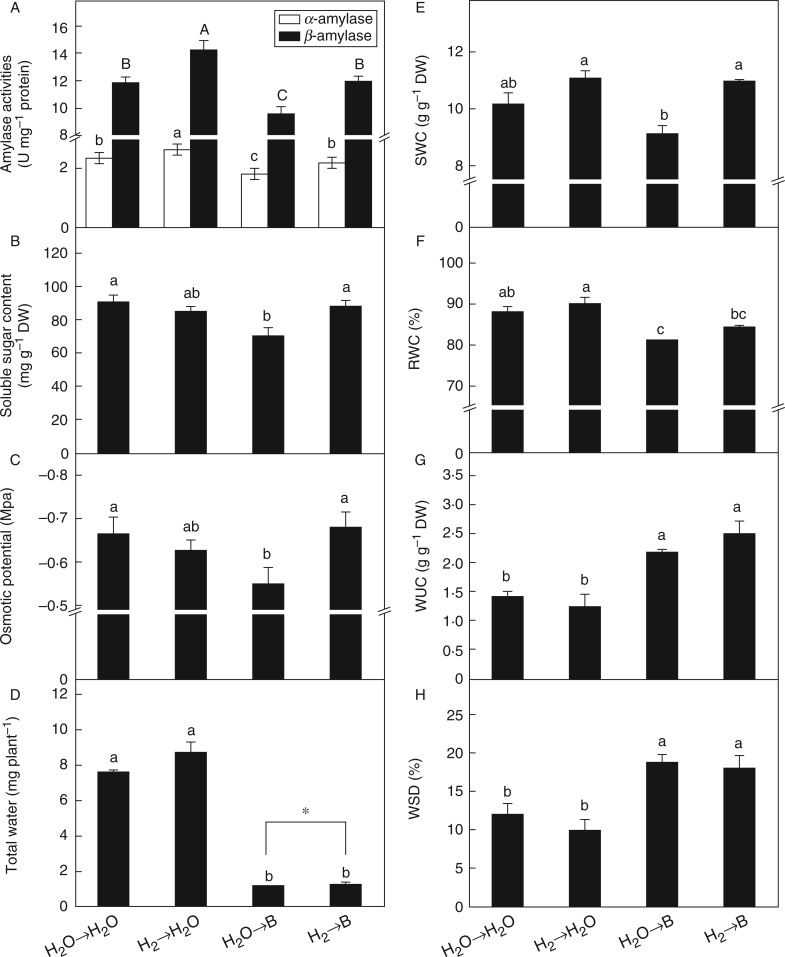
Fig. 2.
Hydrogen modulates amylase activities, soluble sugar content, osmotic potential and water status in rice seedling roots under boron toxicity. Seeds were presoaked in water in the presence or absence of 0·39 mm H2 for 24 h and then transferred to water (→H2O) or 10 mm H3BO3 solution (→B). Activities of α-amylase and β-amylase (A) were measured after 48 h of different treatments. Soluble sugar content (B), osmotic potential (C) and the water status parameters of total water (D), specific water content (SWC; E), relative water content (RWC; F), water uptake capacity (WUC; G) and water saturation deficit (WSD; H) were measured after 72 h of the treatments. Within each set of experiments, values are the means ± s.e. of three independent experiments with at least three replicates for each. Different letters and * denote significant differences at P < 0·05 according to Duncan’s multiple comparison test.
Hydrogen improved water status
Normally, excess B can lead to water deficiency in plants, but low osmotic potential in plant cells can enhance water uptake and maintain root elongation under low water potential condition. As expected, higher osmotic potential was observed in rice roots when supplied with excess B, and this was arrested by H2 pretreatment (Fig. 2C). Reductions in total water content, SWC and RWC in rice roots were also observed under excess B, while WUC and WSD were increased (Fig. 2D–H). By contrast, H2 pretreatment differentially increased total water content and SWC under B toxicity, indicating that water status in rice roots was partly improved.
Hydrogen suppressed B accumulation by regulating expression of BOR1 and aquaporin (AQP) genes
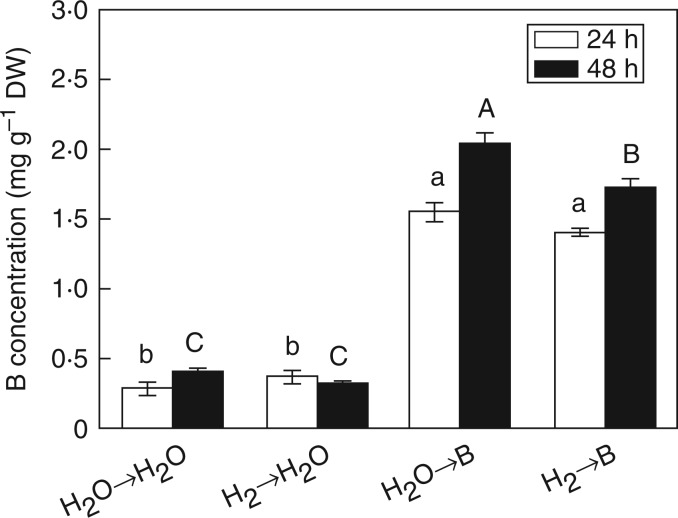
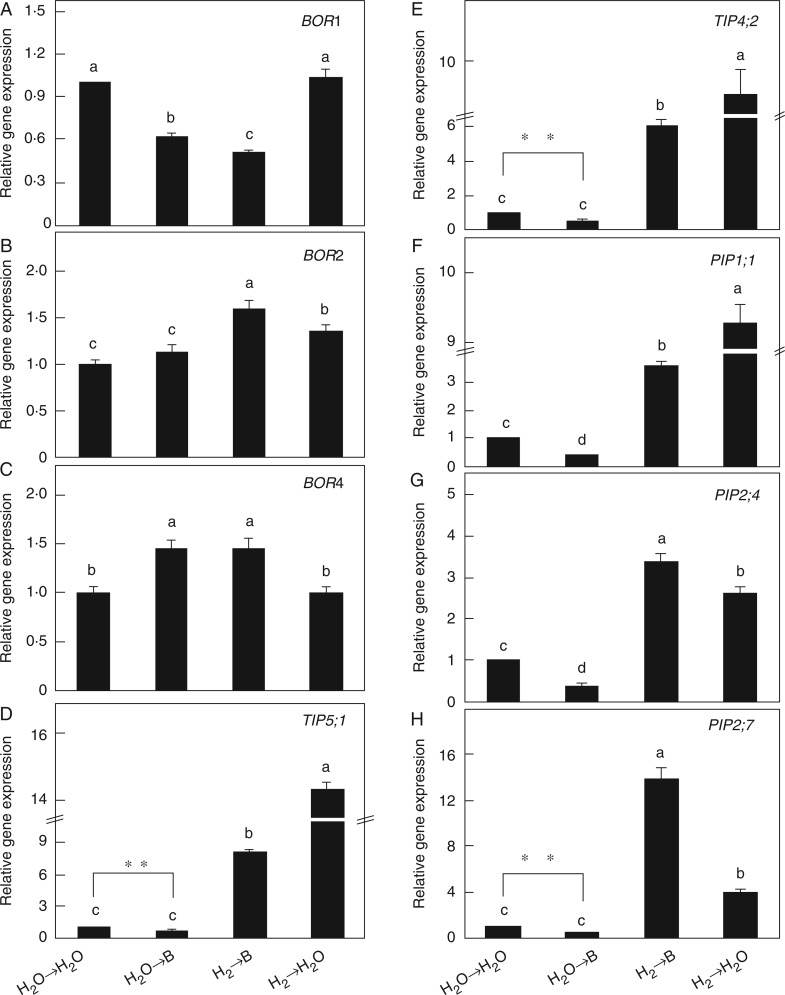
In our experimental conditions, excess B treatment for 48 h led to rapid uptake of B in root tissues, while pretreatment with H2 significantly suppressed the accumulation of B (Fig. 3). We also noticed that in the initial 24 h no significant difference in B content was observed between the presence and absence of H2. Transcription of the BOR1 gene, encoding an efflux B transporter in rice roots (Nakagawa et al., 2007), was further analysed. As expected, downregulation of BOR1 associated with excess B was markedly increased by H2 (Fig. 4A). Transcription of BOR2 (a barley homologue of Bot1) and BOR4 (an Arabidopsis homologue of BOR4) was also analysed. Pretreatment with H2 clearly upregulated the expression of BOR2 under B toxicity (Fig. 4B). However, there was no significant difference in the transcription of BOR4 between H2 pretreatment and control samples (Fig. 4C). These results suggest the possible role of H2 in the suppression of B accumulation by regulation of BOR1 and BOR2.
Fig. 3.
Changes in boron accumulation in rice seedling roots. Seeds were presoaked in water in the presence or absence of 0·39 mm H2 for 24 h and then transferred to water (→H2O) or 10 mm H3BO3 solution (→B). Boron concentration was measured after 24 and 48 h of the different treatments. Within each set of experiments, values are means ± s.e. of three independent experiments with at least three replicates for each. Different letters denote significant differences at P < 0·05 according to Duncan’s multiple comparison test.
Fig. 4.
Hydrogen modulates gene expression of BOR1 (A), BOR2 (B), BOR4 (C), TIP5;1 (D), TIP4;2 (E), PIP1;1 (F), PIP2;4 (G) and PIP2;7 (H) in rice seedling roots under boron toxicity. Seeds were presoaked in water in the presence or absence of 0·39 mm H2 for 24 h and then transferred to water (→H2O) or 10 mm H3BO3 solution (→B) for another 24 h. Values are means ± s.e. of three independent experiments with three replicates for each. Different letters denote significant differences at P < 0·05, and ** denotes significant differences at P < 0·01 according to Duncan’s multiple comparison test.
Since AQPs, which are membrane-intrinsic proteins, can mediate the transport of water and some low molecular weight solutes, including B (Javot and Maurel, 2002; Pang et al., 2010; Kumar et al., 2014), transcription of five AQP genes was analysed. In our experimental conditions, the expression of TIP4;2, TIP5;1, PIP1;1, PIP2;4 and PIP2;7 was decreased by excess B (Fig. 4D–H), which was consistent with the water depletion under B stress (Fig. 2D–H). However, H2 pretreatment significantly increased the expression of the AQP genes, especially PIP2;7, in B-stressed plants.
Hydrogen modulated PME activity and expression of PME genes
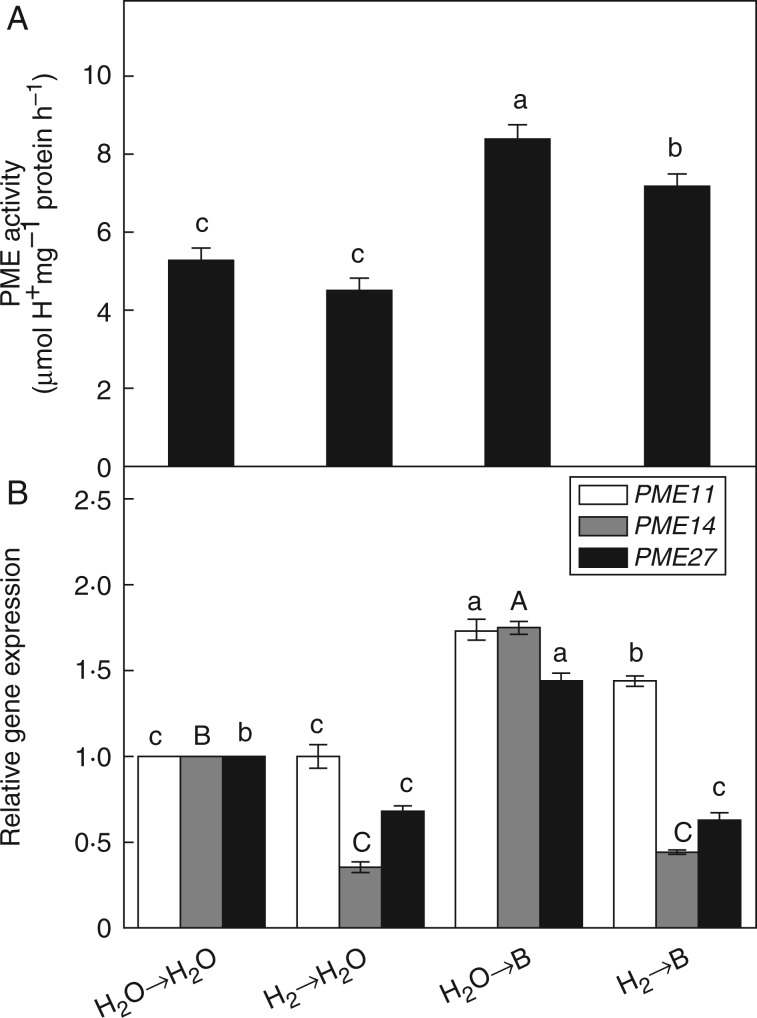
Increases in PME activity and PME gene expression may stiffen the cell wall and lead to the inhibition of root elongation under B toxicity (Wang et al., 2010). To examine whether the alleviating effect of H2 on root growth inhibition was related to PME, further research was conducted. As expected, a significant increase in PME activity observed after 48 h of exposure of rice seeds to excess B was counteracted by H2 pretreatment (Fig. 5A). The results of qRT-PCR further showed that B toxicity stimulated the gene expression of PME11, PME14 and PME27 (Fig. 5B). However, H2 pretreatment partly abolished the induction by B of PME genes, especially PME14.
Fig. 5.
Hydrogen modulates PME activity (A) and relative expression of PME genes (B) in rice seedling roots under boron toxicity. Seeds were presoaked in water in the presence or absence of 0·39 mm H2 for 24 h and then transferred to water (→H2O) or 10 mm H3BO3 solution (→B). PME activity and expression of PME genes were measured after 48 and 24 h of the treatments. Within each set of experiments, values are the means ± s.e. of three independent experiments with three replicates for each. Different letters denote significant differences at P < 0·05 according to Duncan’s multiple comparison test.
Hydrogen modulated ROS homeostasis
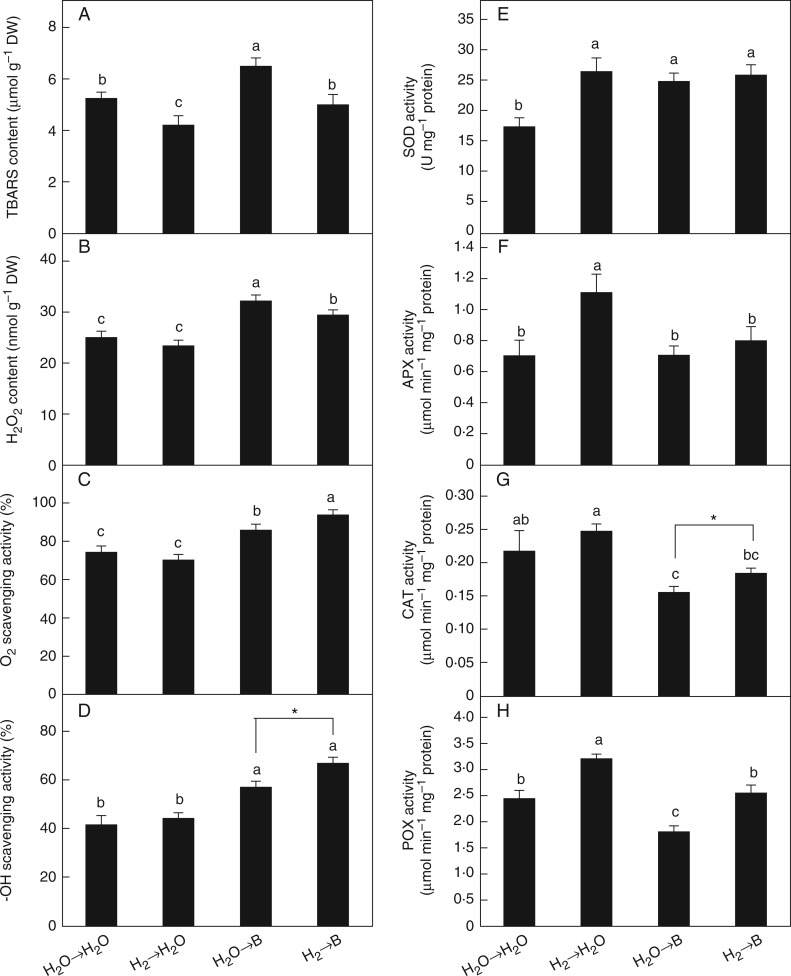
Excess B usually leads to ROS imbalance. To examine whether the beneficial role of H2 in B toxicity was related to the modulation of ROS imbalance, we measured TBARS content and accumulation of ROS. Pretreatment with 0·39 mm H2 significantly suppressed the accumulation of TBARS caused by excess B (Fig. 6A). This result was consistent with the changes in ROS, showing that B-triggered H2O2 production was partially alleviated by H2 (Fig. 6B). The scavenging activities of and •OH in rice seedlings were increased (Fig. 6C, D). These results suggest that H2 has a protective function against B-induced lipid peroxidation and oxidative stress in rice.
Fig. 6.
Changes in TBARS and H2O2 contents, ROS scavenging activities and antioxidant enzyme activities in rice seedlings under B toxicity. Seeds were presoaked in water in the presence or absence of 0·39 mm H2 for 24 h and then transferred to water (→H2O) or 10 mm H3BO3 solution (→B). TBARS content (A) was measured after 72 h of the treatments, and H2O2 level (B), scavenging activities of (C) and •OH (D), and activities of SOD (E), APX (F), CAT (G) and POX (H) were measured after 48 h of treatment. Values are means ± s.e. of three independent experiments with at least three replicates for each. Different letters and * denote significant differences at P < 0·05 according to Duncan’s multiple comparison test.
As antioxidant enzymes are mainly responsible for scavenging ROS, the activities of antioxidant enzymes were measured. The results showed that B-inhibited CAT (Fig. 6G) and in particular POX (Fig. 6H) activities were differentially improved by H2 pretreatment. Slight but non-significant increased activities of SOD (Fig. 6E) and APX (Fig. 6F) were observed.
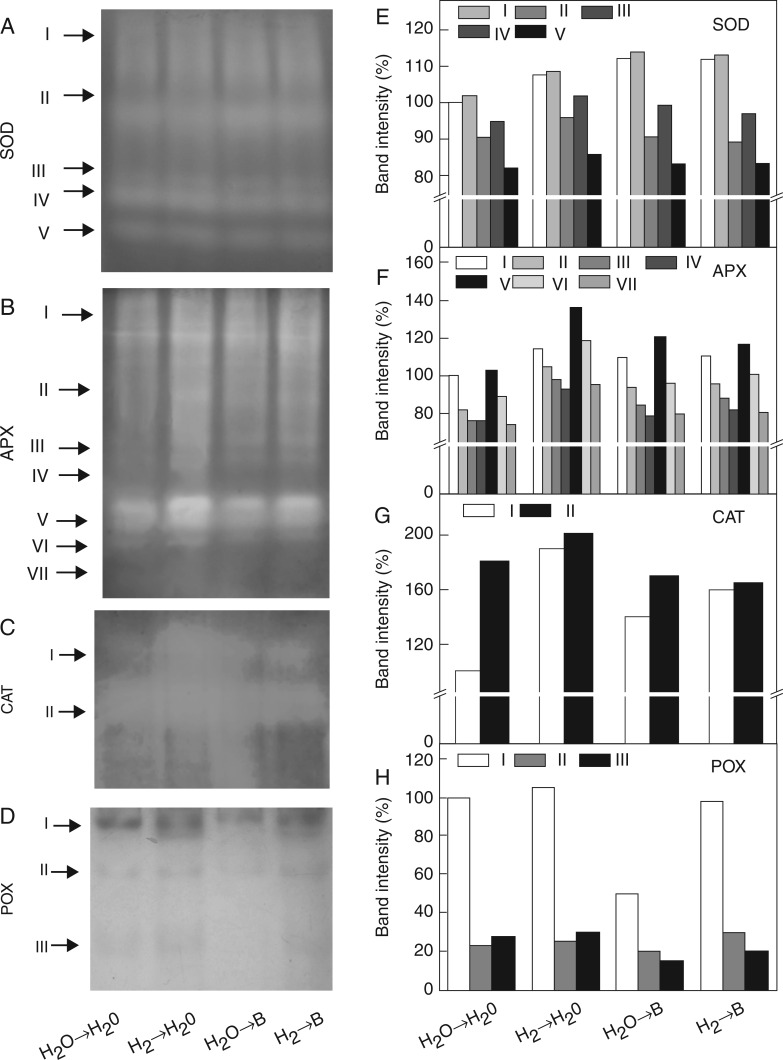
To further confirm the above results, we conducted a non-denaturing polyacrylamide gel electrophoresis (PAGE) analysis (stacking gel 5 %, separating gel 12 %) (Fig. 7). At least five SOD isozymes, seven APX isozymes, two CAT isozymes and three POX isozymes were observed in germinating rice seeds. Similar to the results for total activities shown in Fig. 6, CAT and POX isozyme activities in B-stressed plants were increased by H2 pretreatment, especially CAT-I and in particular POX-I isoforms. Apart from this, no obvious differences were found in the isozyme activities of SOD and APX in the presence or absence of H2 followed by B stress.
Fig. 7.
Changes in isozyme activities of SOD (A, E), APX (B, F), CAT (C, G), and POX (D, H) in rice seedlings. Seeds were presoaked in water in the presence or absence of 0·39 mm H2 for 24 h and then transferred to water (→H2O) or 10 mm H3BO3 solution (→B) for 48 h. To determine in-gel activities of isozymes, extracts of rice seedlings containing 30 μg of protein were loaded onto the native PAGE. After electrophoresis the gels were stained (A–D) and relative activities of different isozymes were determined (E–H). Band intensities of the individual isozymes were expressed as the percentage of corresponding first isozyme of the control samples. Arrows point to bands corresponding to various isozymes.
DISCUSSION
Hydrogen alleviated boron toxicity by modulating ROS homeostasis
Excess of B can lead to plant growth inhibition and crop yield reduction (e.g. Reid et al., 2004; Roessner et al., 2006; Miwa et al., 2007). Our results show that rice seed germination, root growth and shoot growth were seriously inhibited by excess B (Table 1, Fig. S1), and a reduction in fresh weight and dry weight was also observed (Fig. 1B–E). The above responses to B toxicity, as well as oxidative damage and membrane peroxidation (Fig. 6A and B), were the most common symptoms occurring in plants (e.g. Chio et al., 2007; Tanaka and Fujiwara, 2008; Wang et al., 2010; Pandey and Archana, 2013). Previous results confirmed that re-establishment of ROS homeostasis is beneficial for plants under B toxicity (e.g. Cervilla et al., 2007, 2009; Ardıc et al., 2009; Aftab et al., 2010). For example, B tolerance of chickpea was closely related to increased capacity of the antioxidant system (Ardıc et al., 2009). Further results showed that exogenously applied H2 (0·39 mm) not only significantly blocked B-inhibited endogenous H2 production (Fig. 1A) but also alleviated the inhibition of rice seed germination and seedling growth (Table 1, Fig. 1 and Fig. S2).
Previous studies revealed that H2 plays an important role in preventive and therapeutic applications by alleviating oxidative damage (e.g. Ohsawa et al., 2007; Buchholz et al., 2008; Huang et al., 2010; Taura et al., 2010; Kawaguchi et al., 2014; Iuchi et al., 2016), and proved that H2 could react directly with cytotoxic ROS due to its ability to rapidly diffuse across membranes (Ohsawa et al., 2007; Taura et al., 2010; Iuchi et al., 2016). Consistently, in our experiments, H2 alleviated B-induced lipid peroxidation and H2O2 overproduction (Fig. 6A, B), which was further confirmed by the enhancement of ROS scavenging ability (Fig. 6C, D) and activities of CAT and POX (Figs 6 and 7). These effects may be beneficial for the improvement of rice seed germination and seedling growth under B toxicity. Similar antioxidant behaviours of exogenous H2 have been reported in studies of plant tolerance of abiotic stresses (e.g. Xie et al., 2012, 2014, 2015; Cui et al., 2013, 2014; Jin et al., 2013; Xu et al., 2013; Su et al., 2014).
Hydrogen alleviated rice growth inhibition and water stress caused by toxic boron
It has been reported that excess B can lead to marked inhibition of root elongation in plants, the critical site for sensing B toxicity being the root apex (e.g. Chio et al., 2007; Tanaka and Fujiwara, 2008; Wang et al., 2010). We also observed a clear decrease in rice root length (Table 1) and water depletion (Fig. 2D–H) in germinating seeds when supplied with excess B. These toxic responses were significantly rescued by H2 pretreatment. In fact, it has been reported that the most severe stress happens when tomatoes are grown under both B toxicity and water stress (Ben-Gal and Shani, 2003), while rain can significantly reduce B toxicity (Reid and Fitzpatrick, 2009). Therefore, we deduced a possible link among root growth inhibition, alteration of water status and the beneficial role of H2 in B-stressed plants.
The expansion of root cells by water absorption is controlled by the osmotic potential in cell sap and the mechanical properties of the cell wall (Pritchard, 1994). Apart from this, lower osmotic potential could play an important role in the maintenance of plant root elongation at low water potential (Rodriguez et al., 1997). In our experiments, a higher level of osmotic potential was observed in rice roots under excessive B (Fig. 2C). This may partly explain the water depletion and growth inhibition in rice roots. By contrast, H2 pretreatment decreased the osmotic potential, thus enhancing water absorption and alleviating root growth inhibition. Our result was also consistent with a study of B-tolerant barley Sahara 3771, showing that restricting osmotic potential to a lower level could maintain root elongation under high B (Chio et al., 2007).
The osmotic potential of the cell is modulated by the content of osmotic solutes and the rate of water flow regulated by AQPs (Javot and Maurel, 2002; Tabuchi et al., 2004). The content of soluble sugar in rice roots was decreased by toxic B and reversed by H2 (Fig. 2B). Soluble sugar in rice roots not only contributes to the osmotic potential required for water uptake and cell elongation (Tabuchi et al., 2004; Chio et al., 2007), but also provides energy for growth. This could explain the improvement of plant growth and water status in H2-pretreated rice plants under B toxicity (Table 1, Figs 1B, C and 2D–H). Apart from this, the enhanced activities of α/β-amylase triggered by H2 facilitated the conversion of starch into sugars (Fig. 2A, B). Similarly, a higher soluble sugar content and lower osmotic potential were found in B-tolerant barely, which contributed to better root growth compared with B-intolerant barely (Chio et al., 2007).
Aquaporins are water channel proteins expressed in the cell membrane of plants, and can facilitate water flow across root tissues (Javot and Maurel, 2002). Five AQP genes were downregulated by excess B; this was reversed by H2 (Fig. 4D–H). These results were consistent with the alleviation of toxic B-induced water stress (Fig. 2D–H). Some AQPs were also identified as boric acid channels, which play roles in B uptake under B limitation, or provide tolerance of B toxicity under excess B (e.g. Pang et al., 2010; Kumar et al., 2014). Interestingly, B concentration in rice roots was reduced by H2 (Fig. 3). The OsPIP2;4 and OsPIP2;7 proteins have been confirmed to be involved in mediating B transport and providing tolerance via efflux of excess B from root and shoot tissues (Kumar et al., 2014). In our tests, the expression levels of PIP2;4 and PIP2;7 genes in B-stressed rice roots were significantly upregulated by H2, which may have contributed to the decreased B accumulation (Figs 3 and 4G, H). The AtTIP5;1 protein is also involved in B toxicity tolerance via vacuolar compartmentation for B (Pang et al., 2010), and the gene expression of TIP5;1 was improved by H2 pretreatment as well (Fig. 4D).
Moreover, OsBOR1 is a B transporter required for efficient B uptake under B limitation (Nakagawa et al., 2007). We found that BOR1 transcript in rice roots was decreased by excess B, an effect that was strengthened by H2 (24 h; Fig. 4A). Apart from this, Bot1 identified in barley might play a role in limiting the net entry of B into the root and in the disposal of B from leaves under high excess boron (Sutton et al., 2007). The gene expression of BOR2, the homologous gene of Bot1 in rice, was increased by H2 pretreatment under excess B (Fig. 4B). BOR4 in Arabidopsis functions in the exclusion of toxic B (Miwa et al., 2007). As expected, the gene expression of BOR4 in rice was increased under toxic B, but no significant difference was observed with H2 pretreatment (Fig. 4C). Above all, the downregulation of BOR1 and upregulation of BOR2 by H2 may contribute to the decreased concentration of B in rice roots (48 h; Fig. 3).
Pectin methylesterase, which catalyses the specific demethylesterification of pectic polysaccharide in plant cell walls, can lead to a stiffening of the cell wall by disrupting pectin gelation status when enhanced enzymatic activity occurs (Richard et al., 1994). Increased activity of PME and upregulated gene expression of PME11, PME14 and PME27 in rice were observed under B toxicity (Fig. 5). Similar changes in the transcription of eight PME genes and PME activity were used to explain the inhibition of rice root elongation caused by aluminium toxicity (Yang et al., 2013). In fact, the rigidified cell may cause an increased pressure potential and suppress the movement of water into the cells (Spollen and Sharp, 1991). This might be another explanation of water depletion in B-stressed rice roots (Fig. 2C–H). By contrast, H2 pretreatment significantly reversed the high PME activity and PME gene expression induced by excess B. We therefore suggest that H2 may alleviate rice root growth inhibition and water stress under toxic B by adjusting the cell wall rigidity and osmotic potential influenced by PME. Similarly, hydrogen sulphide (H2S) improved root elongation inhibition triggered by excess B by targeting cell wall-related PME (Wang et al., 2010). In kiwifruit, H2 was confirmed to suppress the activity of PME and alleviate pectin solubilization (Hu et al., 2014).
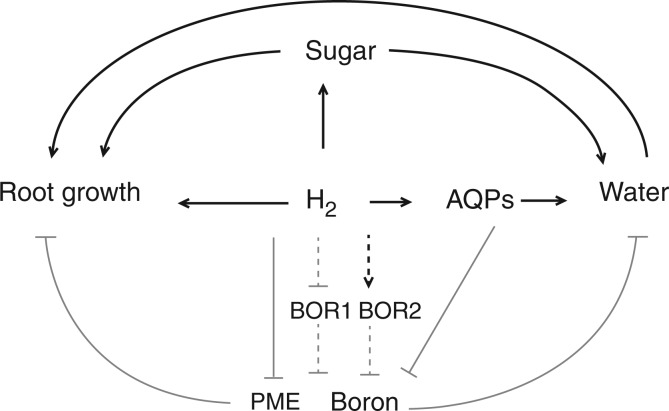
Taking these results together, we suggest the following mechanism of H2-mediated tolerance of B toxicity in rice (Fig. 8). Hydrogen gas keeps osmotic potential low under B toxicity by improving soluble sugar content and AQP-related water flow, and alleviating PME-induced cell wall stiffening. These effects result in enhanced water uptake and facilitate cell growth for rice root elongation. The increased soluble sugar content also provides an energy source for seed germination and seedling growth. Moreover, upregulation of AQP genes and BOR2, along with downregulation of BOR1 transcript, may suppress B accumulation.
Fig. 8.
Simplified scheme of mechanisms involved in H2-mediated tolerance of boron toxicity in rice. Thick lines indicate the promotion response and thin lines indicate the inhibition response. The dashed line denotes a possible signalling cascade.
Conclusions
Our data indicate that H2 alleviates B toxicity in germinating rice seeds. We observed decreased production of endogenous H2 in response to B stress and provide evidence for mechanisms of H2-mediated tolerance of B toxicity in rice: the alleviation of growth inhibition, water stress and ROS imbalance. Further genetic evidence will be required to investigate the functions of the B transporters, including BOR1, BOR2 and AQPs, in the above functions of H2.
SUPPLEMENTARY DATA
Supplementary data are available online at www.aob.oxfordjournals.org and consist of the following. Table S1: sequences of primers used in qRT–PCR. Figure S1: changes in rice seed germination rate under different concentrations of boron. Figure S2: changes in germination rate in rice seeds pretreated with different concentrations of H2 followed by boron stress.
ACKNOWLEDGEMENTS
This work was supported by the National Natural Science Foundation of China (J1210056 and J1310015) and the Priority Academic Program Development of Jiangsu Higher Education Institutions.
LITERATURE CITED
- Aftab T, Khan MMA, Idrees M, Naeem M, Ram M. 2010. Boron induced oxidative stress, antioxidant defence response and changes in artemisinin content in Artemisia annua L. Journal of Agronomy and Crop Science 196: 423–430. [Google Scholar]
- Ardıc M, Sekmen AH, Tokur S, Ozdemir F, Turkan I. 2009. Antioxidant responses of chickpea plants subjected to boron toxicity. Plant Biology 11: 328–338. [DOI] [PubMed] [Google Scholar]
- Association of Official Seed Analysts (AOSA). 1983. Seed vigor testing handbook. Contribution no. 32 to the handbook on seed testing. Springfield, IL: Association of Official Seed Analysts. [Google Scholar]
- Beauchamp C, Fridovich I. 1971. Superoxide dismutase: improved assays and an assay applicable to acrylamide gels. Analytical biochemistry 44: 276–287. [DOI] [PubMed] [Google Scholar]
- Bellincampi D, Dipierro N, Salvi G, Cervone F, De Lorenzo G. 2000. Extracellular H2O2 induced by oligogalacturonides is not involved in the inhibition of the auxin-regulated rolB gene expression in tobacco leaf explants. Plant physiology 122: 1379–1386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ben-Gal A, Shani U. 2003. Water use and yield of tomatoes under limited water and excess boron. Plant and Soil 256: 179–186. [Google Scholar]
- Bradford MM. 1976. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Analytical Biochemistry 72: 248–254. [DOI] [PubMed] [Google Scholar]
- Buchholz B, Kaczorowski DJ, Sugimoto R, et al. 2008. Hydrogen inhalation ameliorates oxidative stress in transplantation induced intestinal graft injury. American Journal of Transplantation 8: 2015–2024. [DOI] [PubMed] [Google Scholar]
- Cavazza C, Martin L, Mondy S, Gaillard J, Ratet P, Fontecilla-Camps JC. 2008. The possible role of an [FeFe]-hydrogenase-like protein in the plant responses to changing atmospheric oxygen levels. Journal of Inorganic Biochemistry 102: 1359–1365. [DOI] [PubMed] [Google Scholar]
- Cervilla LM, Blasco B, Ríos JJ, Romero L, Ruiz JM. 2007. Oxidative stress and antioxidants in tomato (Solanum lycopersicum) plants subjected to boron toxicity. Annals of Botany 100: 747–756. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cervilla LM, Rosales MA, Rubio-Wilhelmi MM, et al. 2009. Involvement of lignification and membrane permeability in the tomato root response to boron toxicity. Plant Science 176: 545–552. [DOI] [PubMed] [Google Scholar]
- Chio EY, Kolesik P, McNeill A, et al. 2007. The mechanism of boron tolerance for maintenance of root growth in barley (Hordeum vulgare L.). Plant, Cell & Environment 30: 984–993. [DOI] [PubMed] [Google Scholar]
- Collins GG, Jenner CF, Paleg LG. 1972. The metabolism of soluble nucleotides in wheat aleurone layers treated with gibberellic acid. Plant Physiology 49: 404–410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cui W, Gao C, Fang P, Lin G, Shen W. 2013. Alleviation of cadmium toxicity in Medicago sativa by hydrogen-rich water. Journal of Hazardous Materials 260: 715–724. [DOI] [PubMed] [Google Scholar]
- Cui W, Fang P, Zhu K, et al. 2014. Hydrogen-rich water confers plant tolerance to mercury toxicity in alfalfa seedlings. Ecotoxicology and Environmental Safety 105: 103–111. [DOI] [PubMed] [Google Scholar]
- Dubois M, Gilles KA, Hamilton JK, Rebers P, Smith F. 1956. Colorimetric method for determination of sugars and related substances. Analytical Chemistry 28: 350–356. [Google Scholar]
- Durner J, Klessig DF. 1996. Salicylic acid is a modulator of tobacco and mammalian catalases. Journal of Biological Chemistry 271: 28492–28501. [DOI] [PubMed] [Google Scholar]
- Halliwell B, Gutteridge JM, Aruoma OI. 1987. The deoxyribose method: a simple “test-tube” assay for determination of rate constants for reactions of hydroxyl radicals. Analytical Biochemistry 165: 215–219. [DOI] [PubMed] [Google Scholar]
- Hammerschmidt R, Nuckles EM, Kuć J. 1982. Association of enhanced peroxidase activity with induced systemic resistance of cucumber to Colletotrichum lagenarium. Physiological Plant Pathology 20: 73–82. [Google Scholar]
- Hodges DM, DeLong JM, Forney CF, Prange RK. 1999. Improving the thiobarbituric acid-reactive-substances assay for estimating lipid peroxidation in plant tissues containing anthocyanin and other interfering compounds. Planta 207: 604–611. [DOI] [PubMed] [Google Scholar]
- Hu H, Li P, Wang Y, Gu R. 2014. Hydrogen-rich water delays postharvest ripening and senescence of kiwifruit. Food chemistry 156: 100–109. [DOI] [PubMed] [Google Scholar]
- Huang CS, Kawamura T, Toyoda Y, Nakao A. 2010. Recent advances in hydrogen research as a therapeutic medical gas. Free Radical Research 44: 971–982. [DOI] [PubMed] [Google Scholar]
- Iuchi K, Imoto A, Kamimura N, et al. 2016. Molecular hydrogen regulates gene expression by modifying the free radical chain reaction-dependent generation of oxidized phospholipid mediators. Scientific Reports 6: 18971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Janda T, Szalai G, Tari I, Paldi E. 1999. Hydroponic treatment with salicylic acid decreases the effects of chilling injury in maize (Zea mays L.) plants. Planta 208: 175–180. [Google Scholar]
- Javot H, Maurel C. 2002. The role of aquaporins in root water uptake. Annals of Botany 90: 301–313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jin Q, Zhu K, Cui W, Xie Y, Han B, Shen W. 2013. Hydrogen gas acts as a novel bioactive molecule in enhancing plant tolerance to paraquat-induced oxidative stress via the modulation of heme oxygenase-1 signalling system. Plant Cell & Environment 36: 956–969. [DOI] [PubMed] [Google Scholar]
- Kawaguchi M, Satoh Y, Otsubo Y, Kazama T. 2014. Molecular hydrogen attenuates neuropathic pain in mice. PLoS One 9: e100352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kumar K, Mosa KA, Chhikara S, Musante C, White JC, Dhankher OP. 2014. Two rice plasma membrane intrinsic proteins, OsPIP2;4 and OsPIP2;7, are involved in transport and providing tolerance to boron toxicity. Planta 239: 187–198. [DOI] [PubMed] [Google Scholar]
- Miwa K, Takano J, Omori H, Seki M, Shinozaki K, Fujiwara T. 2007. Plants tolerant of high boron levels. Science 318: 1417–1417. [DOI] [PubMed] [Google Scholar]
- Nakagawa Y, Hanaoka H, Kobayashi M, Miyoshi K, Miwa K, Fujiwara T. 2007. Cell-type specificity of the expression of Os BOR1, a rice efflux boron transporter gene, is regulated in response to boron availability for efficient boron uptake and xylem loading. The Plant Cell 19: 2624–2635. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakano Y, Asada K. 1981. Hydrogen peroxide is scavenged by ascorbate-specific peroxidase in spinach chloroplasts. Plant and Cell Physiology 22: 867–880. [Google Scholar]
- Nishikimi M, Rao NA, Yagi K. 1972. The occurrence of superoxide anion in the reaction of reduced phenazine methosulfate and molecular oxygen. Biochemical and Biophysical Research Communications 46: 849–854. [DOI] [PubMed] [Google Scholar]
- Ohsawa I, Ishikawa M, Takahashi K, et al. 2007. Hydrogen acts as a therapeutic antioxidant by selectively reducing cytotoxic oxygen radicals. Nature Medicine 13: 688–694. [DOI] [PubMed] [Google Scholar]
- Pandey N, Archana 2013. Antioxidant responses and water status in Brassica seedlings subjected to boron stress. Acta Physiologiae Plantarum 35: 697–706. [Google Scholar]
- Pang Y, Li L, Ren F, et al. 2010. Overexpression of the tonoplast aquaporin AtTIP5;1 conferred tolerance to boron toxicity in Arabidopsis. Journal of Genetics and Genomics 37: 389–397. [DOI] [PubMed] [Google Scholar]
- Pinhero RG, Rao MV, Paliyath G, Murr DP, Fletcher RA. 1997. Changes in activities of antioxidant enzymes and their relationship to genetic and paclobutrazol-induced chilling tolerance of maize seedlings. Plant Physiology 114: 695–704. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pritchard J. 1994. The control of cell expansion in roots. New Phytologist 127: 3–26. [DOI] [PubMed] [Google Scholar]
- Reid R, Fitzpatrick K. 2009. Influence of leaf tolerance mechanisms and rain on boron toxicity in barley and wheat. Plant Physiology 151: 413–420. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reid RJ, Hayes JE, Post A, Stangoulis JCR, Graham RD. 2004. A critical analysis of the causes of boron toxicity in plants. Plant, Cell & Environment 27: 1405–1414. [Google Scholar]
- Ren C, Kermode AR. 2000. An increase in pectin methyl esterase activity accompanies dormancy breakage and germination of yellow cedar seeds. Plant Physiology 124: 231–242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Renwick GM, Giumarro C, Siegel SM. 1964. Hydrogen metabolism in higher plants. Plant Physiology 39: 303–306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Richard L, Qin LX, Gadal P, Goldberg R. 1994. Molecular cloning and characterisation of a putative pectin methylesterase cDNA in Arabidopsis thaliana (L.). FEBS Letters 355: 135–139. [DOI] [PubMed] [Google Scholar]
- Rodriguez HG, Roberts JK, Jordan WR, Drew MC. 1997. Growth, water relations, and accumulation of organic and inorganic solutes in roots of maize seedlings during salt stress. Plant Physiology 113: 881–893. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roessner U, Patterson JH, Forbes MG, Fincher GB, Langridge P, Bacic A. 2006. An investigation of boron toxicity in barley using metabolomics. Plant Physiology 142: 1087–1101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spollen WG, Sharp RE. 1991. Spatial distribution of turgor and root growth at low water potentials, Plant Physiology 96: 438–443. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Su N, Wu Q, Liu Y, et al. 2014. Hydrogen-rich water reestablishes ROS homeostasis but exerts differential effects on anthocyanin synthesis in two varieties of radish sprouts under UV-A irradiation. Journal of Agricultural and Food Chemistry 62: 6454–6462. [DOI] [PubMed] [Google Scholar]
- Sutton T, Baumann U, Hayes J, et al. 2007. Boron-toxicity tolerance in barley arising from efflux transporter amplification. Science 318: 1446–1449. [DOI] [PubMed] [Google Scholar]
- Tabuchi A, Kikui S, Matsumoto H. 2004. Differential effects of aluminium on osmotic potential and sugar accumulation in the root cells of Al-resistant and Al-sensitive wheat. Physiologia Plantarum 120: 106–112. [DOI] [PubMed] [Google Scholar]
- Tanaka M, Fujiwara T. 2008. Physiological roles and transport mechanisms of boron: perspectives from plants. Pflügers Archiv 456: 671–677. [DOI] [PubMed] [Google Scholar]
- Taura A, Kikkawa YS, Nakagawa T, Ito J. 2010. Hydrogen protects vestibular hair cells from free radicals. Acta Oto-laryngologica 130: 95–100. [DOI] [PubMed] [Google Scholar]
- Wang BL, Shi L, Li YX, Zhang WH. 2010. Boron toxicity is alleviated by hydrogen sulfide in cucumber (Cucumis sativus L.) seedlings. Planta 231: 1301–1309. [DOI] [PubMed] [Google Scholar]
- Woodbury W, Spencer AK, Stahmann MA. 1971. An improved procedure using ferricyanide for detecting catalase isozymes. Analytical Biochemistry 44: 301–305. [DOI] [PubMed] [Google Scholar]
- Xie Y, Mao Y, Lai D, Zhang W, Shen W. 2012. H2 enhances Arabidopsis salt tolerance by manipulating ZAT10/12-mediated antioxidant defence and controlling sodium exclusion. PLoS One 7: e49800. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xie Y, Mao Y, Zhang W, Lai D, Wang Q, Shen W. 2014. Reactive oxygen species-dependent nitric oxide production contributes to hydrogen-promoted stomatal closure in Arabidopsis. Plant Physiology 165: 759–773. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xie Y, Zhang W, Duan X, et al. 2015. Hydrogen-rich water-alleviated ultraviolet-B-triggered oxidative damage is partially associated with the manipulation of the metabolism of (iso) flavonoids and antioxidant defence in Medicago sativa. Functional Plant Biology 42: 1141–1157. [DOI] [PubMed] [Google Scholar]
- Xu S, Zhu S, Jiang Y, et al. 2013. Hydrogen-rich water alleviates salt stress in rice during seed germination. Plant and Soil 370: 47–57. [Google Scholar]
- Yang XY, Zeng ZH, Yan JY, et al. 2013. Association of specific pectin methylesterases with Al-induced root elongation inhibition in rice. Physiologia Plantarum 148: 502–511. [DOI] [PubMed] [Google Scholar]
- Zeng J, Zhang M, Sun X. 2013. Molecular hydrogen is involved in phytohormone signaling and stress responses in plants. PLoS One 8: e71038. [DOI] [PMC free article] [PubMed] [Google Scholar]