Abstract
Folic acid is a member of the B-vitamin family and is essential for amino acid metabolism. Adequate intake of folic acid is vital for metabolism, cellular homeostasis, and DNA synthesis. Since the initial discovery of folic acid in the 1940s, folate deficiency has been implicated in numerous disease states, primarily those associated with neural tube defects in utero and neurological degeneration later in life. However, in the past decade, epidemiological studies have identified an inverse relation between both folic acid intake and blood folate concentration and cardiovascular health. This association inspired a number of clinical studies that suggested that folic acid supplementation could reverse endothelial dysfunction in patients with cardiovascular disease (CVD). Recently, in vitro and in vivo studies have begun to elucidate the mechanism(s) through which folic acid improves vascular endothelial function. These studies, which are the focus of this review, suggest that folic acid and its active metabolite 5-methyl tetrahydrofolate improve nitric oxide (NO) bioavailability by increasing endothelial NO synthase coupling and NO production as well as by directly scavenging superoxide radicals. By improving NO bioavailability, folic acid may protect or improve endothelial function, thereby preventing or reversing the progression of CVD in those with overt disease or elevated CVD risk.
Keywords: endothelial function, folic acid, 5-methyl tetrahydrofolate, nitric oxide
INTRODUCTION
Folic acid has long been known to be essential for the prevention of both macrocytic anemia in pregnant women and neural tube defects.1 Initially identified in the 1940s, folic acid is critical for neural development in utero, and mandated folic acid fortification of foods has led to population-wide increases in blood folate concentrations in developed nations.2–4 Over the past decade, however, novel investigations of folate deficiency or disturbances in folate metabolism have revealed that additional supplementation of folic acid, ie, above the current average daily intake, may be an important strategy for maintaining or improving cardiovascular health.5–7 Specifically, high doses of folic acid are a well-tolerated intervention to improve nitric oxide (NO) bioavailability in individuals with compromised endothelial function.8–11 The purpose of this review is to examine emerging evidence that high-dose folic acid supplementation improves NO synthase (NOS) coupling and subsequent NO bioavailability and thus may be efficacious in the prevention and treatment of cardiovascular disease (CVD).
Folate, a B vitamin, is a generic term used for compounds that have structure and functions similar to those of folic acid. Folic acid, however, is synthetically produced and is used in fortified foods and supplements on the basis that it is converted to biologically active, fully reduced forms by dihydrofolate reductase (DHFR). Mammals lack the necessary enzymes to synthesize folate de novo and therefore depend entirely on the ingestion of preformed folates and/or folic acid supplementation to meet their biological needs. Dietary sources of natural folates include green leafy vegetables, mushrooms, legumes, and liver. The bioavailability of folates in natural foods, however, is approximately 50% of that of the synthetic form of folic acid and varies greatly, depending on the food consumed.12 Consequently, supplementation with synthetic folic acid, such as that found in dietary supplements and used in food fortification, may be required to obtain the benefits of improved endothelial function observed in clinical randomized controlled trials of folic acid.8,13,14
FOLATE METABOLISM
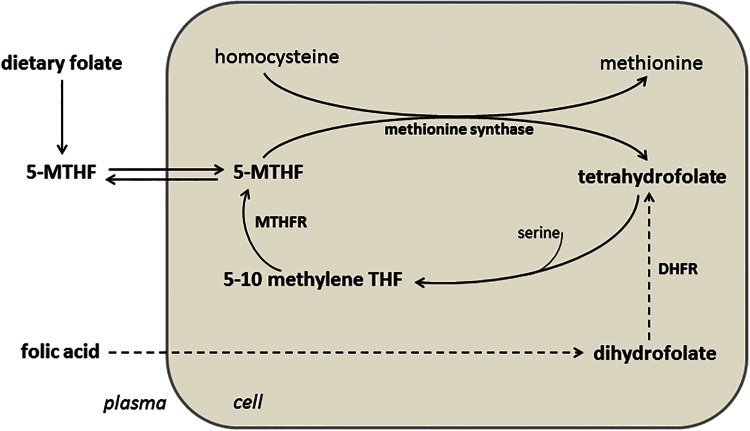
Folate metabolism is closely linked to homocysteine metabolism, and, as a result, increased plasma folate is associated with decreased plasma homocysteine (Figure 1). This homocysteine-lowering effect of folate has been suggested to contribute to the cardiovascular benefit conferred by folic acid supplementation. The role of hyperhomocysteinemia in CVD risk and the potential therapeutic role of folic acid supplementation for hyperhomocysteinemia are outside the scope of this review but are examined in detail elsewhere.15–17 Recent studies, however, suggest that folate’s role in improving vascular health via increased NOS coupling and NO bioavailability is independent of its homocysteine-lowering effect.7,10,18,19
Figure 1.
Metabolism of folate. Abbreviations: 5-MTHF, 5-methyl tetrahydrofolate; MTHFR, 5,10-methylene tetrahydrofolate reductase; DHFR, dihydrofolate reductase; THF, tetrahydrofolate.
Folate is one of the so-called B-complex vitamins, a group of small water-soluble molecules that act as cofactors for specific enzymes, thereby enabling them to carry out their metabolic functions. In this context, the chief function of folate (in its chemically reduced bioactive form tetrahydrofolate) is to enable enzymes to transfer one-carbon groups.20 5-Methyl tetrahydrofolate (5-MTHF) is the primary metabolite of folate that enters the circulation from the intestinal cells when tetrahydrofolate is converted to 5-MTHF. The conversion of folate to 5-MTHF is limited, however, and if enough folate is consumed orally, unmetabolized folate enters the circulation,21 is taken up by cells, and is then reduced by DHFR to tetrahydrofolate20 (Figure 1). 5-Methyl tetrahydrofolate is associated with improvements in NOS coupling and NO synthesis in vivo,10,11,19,22 and, as such, both DHFR23 and 5,10-methylene tetrahydrofolate reductase (MTHFR) are essential for the beneficial effects of high-dose folic acid supplementation on NO bioavailability.19
FOLIC ACID AND NITRIC OXIDE SYNTHASE COUPLING
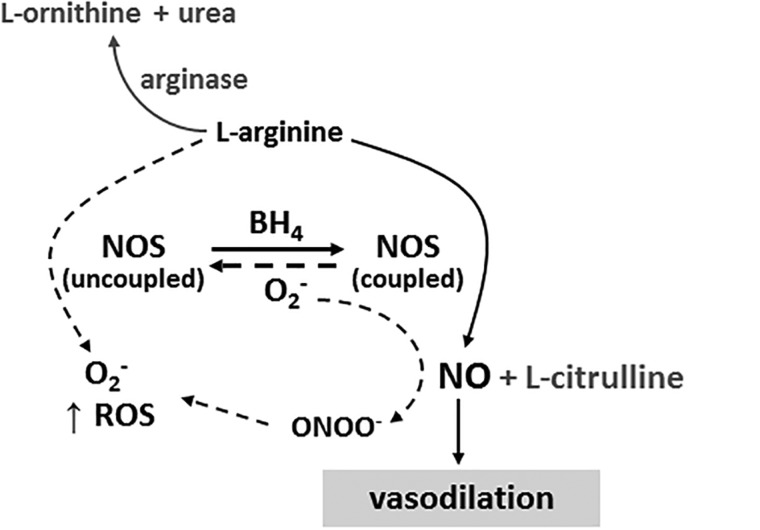
The vascular endothelium is a cellular monolayer that plays a critical role in cardiovascular physiology in both health and disease. In the healthy endothelium, NO is synthesized by the constitutively expressed monoxygenase enzyme endothelial nitric oxide synthase (eNOS). Nitric oxide is a potent vasodilator that improves vascular health and function through its antithrombotic, antiangiogenic, and anti-inflammatory properties. The NOS enzyme is a dimer that relies on the presence of the essential cofactor tetrahydrobiopterin (BH4) and available substrate to couple the oxidation of L-arginine to the reduction of molecular oxygen to produce NO.24,25 When either substrate or cofactor bioavailability is limited, or when oxidant stress is elevated, the NOS dimer can destabilize and uncouple,26 resulting in the production of superoxide radicals rather than NO27,28 (Figure 2).
Figure 2.
Schematic representation of nitric oxide synthesis. Nitric oxide synthase (NOS) requires adequate substrate (L-arginine) and cofactor (BH4) availability to remain in its coupled conformation and produce nitric oxide (NO). In conditions of limited substrate or cofactor bioavailability and/or high oxidative stress, NOS uncouples and produces superoxide (O2−) rather than NO. Abbreviations: BH4, tetrahydrobiopterin; NO, nitric oxide; NOS, nitric oxide synthase; O2−, superoxide; ONOO−, peroxynitrite; ROS, reactive oxygen species.
The critical role of NO bioavailability in vascular biology is well documented, and endothelial dysfunction – the reduced ability of the endothelium to produce NO – is a hallmark of all CVD.28,29 As such, intervention strategies that preserve or restore endothelial production of NO and aid in the prevention and treatment of CVD are highly relevant.30
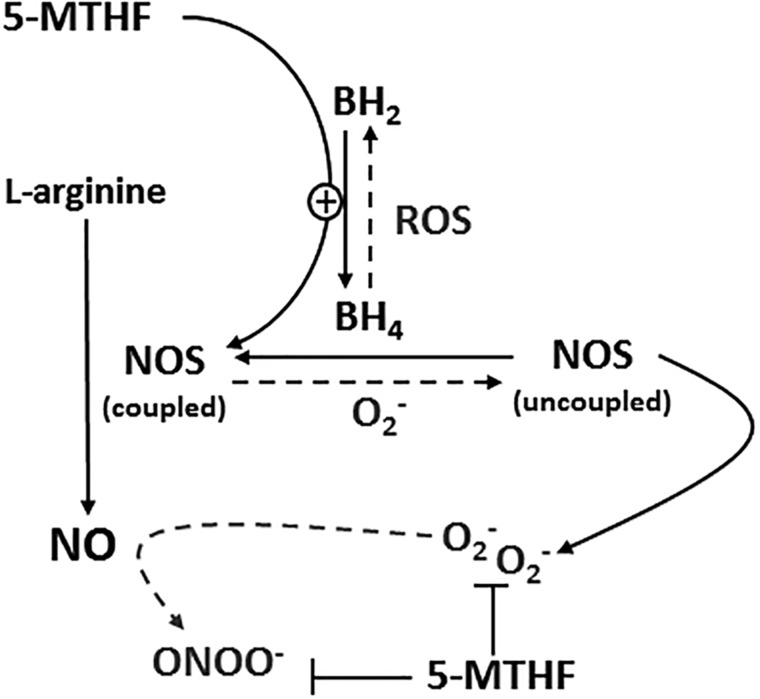
A number of case–control and prospective studies have identified an association between low folate intake, low blood folate concentration, and increased CVD risk.31–39 Conversely, in vivo human studies have shown that high-dose folic acid supplementation can ameliorate endothelial dysfunction as assessed by flow-mediated dilation, an endothelium-dependent stimulus, in populations with overt cardiovascular and metabolic disease22,40–43 and known endothelial dysfunction.9,10 Similarly, high doses of folic acid can prevent NOS dysfunction induced by nitroglycerin and nitrate tolerance in the arterial circulation of healthy subjects, a phenomenon attributed to the protection of NOS coupling and NO synthesis.44 Putative mechanisms by which folic acid supplementation may mitigate vascular endothelial dysfunction and promote NO synthesis are likely mediated by 5-MTHF, the primary circulating metabolite of folic acid.9,11 These include increasing the bioavailability of the essential NOS cofactor BH4 through stabilization of BH4 and/or recycling from dihydrobiopterin (BH2), direct interaction with NOS, and direct scavenging of reactive oxygen species, specifically superoxide radicals (Figure 3).
Figure 3.
Proposed mechanisms by which 5-methyl tetrahydrofolate (5-MTHF) may increase nitric oxide (NO) synthesis and bioavailability. Abbreviations: 5-MTHF, 5-methyl tetrahydrofolate; BH4, tetrahydrobiopterin; BH2, dihydrobiopterin; NO, nitric oxide; NOS, nitric oxide synthase; O2−, superoxide; ONOO−, peroxynitrite; ROS, reactive oxygen species.
FOLIC ACID AND TETRAHYDROBIOPTERIN BIOAVAILABILITY
Tetrahydrobiopterin (BH4) serves as an essential cofactor for pteridine-requiring monoxygenase enzymes and therefore plays a critical role in NOS dimerization and NO production.45 Reduced bioavailability of BH4 contributes to the endothelial dysfunction associated with many cardiovascular pathologies,46–49 and in vivo human studies demonstrate that administration of exogenous BH4 improves endothelium-dependent measures of vessel function in populations that exhibit diminished endothelial function.50–55 Elevated oxidative stress may deplete bioavailable BH4 by direct oxidation of the existing BH4 to BH2 and/or by decreasing de novo BH4 synthesis in vivo.56
Adequate folate bioavailability can contribute to the restoration of BH4 bioavailability by several mechanisms. 5-Methyl tetrahydrofolate can increase the effectiveness of BH4 in eNOS coupling, a phenomenon attributable to improved redox state and/or enhanced binding affinity of BH4 to eNOS.11,57 5-Methyl tetrahydrofolate can also increase the in vivo production of BH4 from its inactive form BH2 by upregulating activity of DHFR in the biopterin recycling pathway.7,58 This upregulated recycling serves to both increase BH4 bioavailability and decrease the presence of BH2, which may act as a competitive inhibitor of BH4 binding to NOS.58,59 The chemical structure of 5-MTHF is very similar to that of BH4. Using a computer modeling system, Hyndman et al.60 demonstrated that 5-MTHF is capable of directly binding to NOS to promote NO production. However, this is in direct contrast to the findings of Stroes et al.,61 who found that 5-MTHF has no effect when eNOS is depleted of BH4 in vitro.
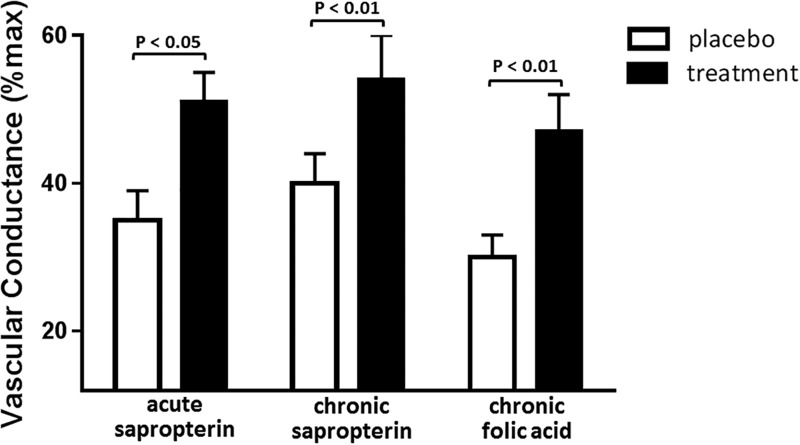
While further studies are necessary to identify the precise mechanisms by which 5-MTHF may increase BH4 bioavailability, in vivo human studies have mechanistically delineated the efficacy of direct 5-MTHF administration and high-dose folic acid supplementation in increasing BH4 bioavailability and improving NO-dependent vasodilation. Intravenous infusion of 5-MTHF prior to vessel harvest in patients undergoing coronary artery bypass graft surgery improves NO-dependent vasodilation, an effect mediated by increased vascular BH4 and an improved ratio of BH4 to total biopterin.11 Similarly, direct administration of 5-MTHF acutely improves NO-mediated vasodilation in the microvasculature of healthy older adults.10 In studies of folic acid supplementation, 7 weeks of oral folic acid treatment in patients with coronary artery disease increased NO-mediated vasomotor responses, decreased vascular superoxide, and improved eNOS coupling, all by means of increased availability of BH4.62 Recently, a series of studies using acute and chronic oral sapropterin (the pharmaceutical analog to BH4) treatment63 and chronic folic acid treatment10 found that 5 mg of folic acid taken once daily for 6 weeks improves endothelium-dependent vasodilation measured in the cutaneous circulation of older adults to the same extent as that observed following pharmaceutical BH4 administration (Figure 4). Further, this increase in vasodilator function was mediated entirely by improvements in NOS synthesis of NO.10,63
Figure 4.
Increased cutaneous vasodilation following oral sapropterin or folic acid administration in healthy older adults. Cutaneous vasodilation response to whole-body heat stress (increase in mean body temperature = 1.0°C) in older adults following placebo and acute sapropterin (10 mg/kg body weight; n = 9), chronic sapropterin (400 mg twice daily for 4 weeks; n = 4), or chronic folic acid (5 mg once daily for 6 weeks; n = 11). Adapted from Stanhewicz et al.64 and Stanhewicz et al.10
Collectively, the biochemical and in vitro data suggesting that increasing bioavailable folate increases NOS coupling and NO synthesis through increased BH4 availability are well supported by the few in vivo human studies that have mechanistically examined this hypothesis. However, few human studies have directly measured plasma 5-MTHF and BH4 bioavailability simultaneously, and further in vivo studies elucidating the mechanistic role of folic acid supplementation in improved BH4 (and subsequent NO) bioavailability in CVD are warranted.
ANTIOXIDANT PROPERTIES OF FOLIC ACID
Elevated oxidative stress due to increased production of reactive oxygen species contributes to NOS uncoupling, quenches available NO, and depletes substrate and cofactor availability, all of which have been implicated in the pathogenesis of endothelial dysfunction in CVD. Antioxidants such as vitamin C can ameliorate endothelial dysfunction observed in patients with overt CVD,65–69 but the efficacy of chronic antioxidant therapy in the form of vitamin C supplementation for the prevention of CVD is controversial. There is evidence that folic acid exerts both direct and indirect antioxidant effects, such as free radical scavenging,70 protection against oxidative modification of human low-density lipoproteins,71 and improvement of cellular antioxidant defense.72,73 5-Methyl tetrahydrofolate reduces superoxide radicals produced by recombinant eNOS and xanthene oxidase in vitro22 and abolishes homocysteine-induced production of superoxide radicals in cultured porcine endothelial cells.74
In vivo human studies examining the antioxidant role of folic acid supplementation in preserved or restored endothelial dysfunction are less conclusive. Endothelial dysfunction observed in healthy humans following an acute oral fat load was abolished by 2 weeks of folic acid pretreatment, an effect associated with a decreased production of malondialdehyde, an end product of lipid peroxidation.75 However, those findings are in contrast to the results of other studies in which folic acid treatment alone had no effect on plasma malondialdehyde concentrations yet still improved vascular endothelial function in patients with coronary artery disease.74,76 In the study by Title et al.,76 plasma malondialdehyde concentrations were lowered in patients with coronary artery disease when folic acid was administered along with the antioxidant vitamins C and E but were unchanged following folic acid treatment alone. There was no added increase in vascular endothelial function in the group receiving folic acid plus vitamins C and E. However, Cagnacci et al.9 recently found that oral supplementation with 5-MTHF at 15 mg/d for 3 weeks decreased oxidative stress in postmenopausal women. This decrease was strongly correlated with a decline in nocturnal blood pressure.
The relevance of folic acid supplementation and increased plasma 5-MTHF as an effective antioxidant strategy for improving endothelial function in vivo remains controversial and unresolved. The scavenging potency of 5-MTHF is approximately 20-fold lower than that of vitamin C.61 Therefore, the high concentrations of 5-MTHF utilized in vitro are not attainable in vivo following oral administration of folic acid. In addition, by increasing the bioavailability of BH4, which is a potent antioxidant, and increasing NOS coupling, thereby reducing superoxide production by the uncoupled dimer, increases in folate bioavailability may reduce oxidative stress secondary to its role in NOS function. This possibility makes it difficult to tease out direct antioxidant effects that folate and/or 5-MTHF may have in vivo.
PHARMACOLOGICAL CONSIDERATIONS OF FOLIC ACID IN THE TREATMENT OR PREVENTION OF ENDOTHELIAL DYSFUNCTION
Supplemental folic acid is readily absorbed in healthy young adults, with ≥90% appearing in the circulation.77–79 Folic acid is absorbed mainly in the small intestine and, at low doses, is converted by the liver and excreted into the systemic circulation mainly as 5-MTHF.79,80 However, at oral doses of 200 µg or greater, the relatively low capacity of the human liver to reduce folic acid is exceeded, and folic acid reaches the systemic circulation unmetabolized.21,80 Human studies of folate supplementation demonstrate that oral folic acid increases plasma and red blood cell folate concentrations in a time- and dose-dependent manner.77,81–83 Furthermore, Hao et al.83 demonstrated that, upon cessation of a chronic dosing regimen, blood folate concentrations declined rapidly but remained elevated above baseline 3 months after cessation of the supplementation in subjects who had taken the highest doses (≥400 µg/d). Studies of age-related changes in the pharmacokinetics of folic acid supplements suggest that age may reduce the absorption of folic acid.84 Interestingly, this same study found that, contrary to young subjects, older adults did not show increased excretion of folic acid following supplementation.84 Overall, human studies of folic acid supplementation report that oral dosing regimens increase plasma and red blood cell folate concentrations in a dose-dependent manner, and that the depletion of these folate stores occurs rapidly after cessation of a treatment regimen. Further study of age- and pathology-specific effects on the absorption, distribution, and elimination of folic acid is warranted.
In general, folic acid supplementation is considered safe, and there is little evidence that high natural folate intake poses a risk of acute toxicity.85 However, whether there is long-term risk associated with chronic high-dose folic acid intake is debatable.86–90 The current Recommended Dietary Allowance (RDA) for folate is 400 µg/d for adults in the United States, and since the initiation of mandatory fortification in 1998, the prevalence of low serum or red blood cell folate in the US population is ≤1%.91,92 The main safety concern associated with folate supplementation is the potential for folate to mask the diagnosis of pernicious anemia, since high folic acid concentrations treat the anemia but allow the neuropathy to progress undiagnosed.93,94 In fact, in one study of folic acid fortification and vitamin B12 deficiency, high serum folate exacerbated cognitive symptoms and anemia in older adults.95 Thus, vitamin B12 levels should be monitored before and during treatment with supplemental folic acid. Similarly, 5-MTHF, although readily available in the plasma, relies on the vitamin B12-dependent enzyme methionine synthase for conversion to tetrahydrofolate (Figure 1). In cases of B12 deficiency, 5-MTHF may not be incorporated into the cellular folate pool, although whether this step is required for improved endothelial function remains unclear. Despite this, studies from the Framingham Heart Study revealed that the benefits of folate fortification through projected decreases in plasma homocysteine and reduced CVD risk greatly outweigh the risk of masked conditions of anemia.96 Also of note, some evidence suggests that high folic acid supplementation, mainly from fortified grain products, may enhance the development and progression of already existing, yet undiagnosed, premalignant and malignant colorectal cancer lesions.97 Conversely, epidemiological data support the concept that higher folate status offers protection against several cancers98,99 and that folate supplementation may reduce the risk of cancer in populations with low folate status.100 Overall, no confirmed adverse outcomes of folic acid use for homocysteine lowering have been reported for randomized controlled trials in which daily folic acid doses were 800 to 2500 µg.101–103
Although the general population of North America – where fortification of foods is mandated – on average achieves the RDA of 400 µg/d for folate, clinical studies of folate and 5-MTHF in the restoration of endothelial function suggest that higher doses of oral folic acid are required to confer benefits on cardiovascular endpoints. Although no human trials have specifically conducted dose–response studies of supplemental folic acid and endothelial function, it appears that daily doses ≥5 mg are efficacious, while daily doses ≤2.5 mg lower plasma homocysteine concentrations but do not reduce CVD risk (Table 110,22,40,44,57,75,76,102,104–115). The closest example of this is a series of placebo-controlled studies conducted in healthy male volunteers, which found that pretreatment with folic acid at 10 mg/d for 1 week prevented nitroglycerin intolerance and nitroglycerin-induced endothelial dysfunction,44 while folic acid at 1 mg/d for 1 week in the same population using the same protocol did not.104 Similarly, a meta-analysis of randomized controlled clinical trials of folate supplementation for improved blood pressure in hypertensive patients found that daily doses ≥5 mg were required to improve flow-mediated dilation.8 This potential dose–response relation may explain the disparity in the literature between the finding that high doses of folic acid improve cardiovascular endpoints through restoration of endothelial NO bioavailability and the finding that lower doses that simply decrease homocysteine concentrations do not prevent the incidence of cardiovascular events (Table 1).116 Interestingly, these improvements in endothelium-dependent dilation may occur even in the absence of changes in clinical findings (ie, measures of blood pressure) and likely improve overall CVD outcomes through the many atheroprotective properties of NO.8,14 Importantly, there is no additional benefit of higher daily doses of folic acid on plasma homocysteine lowering, further suggesting that the apparent dose dependency of the endothelial response is independent of plasma homocysteine interactions. Interestingly, even very high daily doses of folic acid (up to 40 mg) have not been effective in improving endothelial function, NO bioavailability, or incidence of CVD mortality in patients with chronic kidney disease,103,105,117 although the rationale for the lack of benefit in this population is currently unknown.
Table 1.
Folic acid supplementation trials and cardiovascular outcomes
| Reference | Population | No. of cases | Folic acid dosage | Duration | Primary outcome |
|---|---|---|---|---|---|
| Verhaar et al. (1998)22 | Subjects with familial hypercholesterolemia | 10 patients,10 controls | Intrarterial 5-MTHF | 1 d | Improved FMD |
| Woo et al. (1999)106 | Hyperhomocysteinemic subjects | 17 | 10 mg/d | 8 wk | Improved FMD |
| Title et al. (2000)76 | CAD patients | 75 (25 placebo) | 5 mg/d | 4 mo | Improved FMD |
| Wilmink et al. (2000)75 | Healthy subjects | 20 | 10 mg/d | 2 wk | Prevented postprandial lipid-induced decrease in FMD |
| Doshi et al. (2001)74 | CAD patients | 52 | 5 mg/d | 6 wk | Improved FMD, reduced endothelial superoxide levels |
| Gori et al. (2001)44 | Healthy men | 18 | 10 mg/d | 1 wk | Prevented NTG intolerance and NTG-induced endothelial dysfunction |
| Toole et al. (2004)107 | Ischemic stroke patients | 1827 (high), 1853 (low) | 2.5 mg/d (high), 20 µg/d (low) | 2 y | No effect on vascular outcomes |
| Lekakis et al. (2004)108 | Hypercholesterolemic patients | 34 | 5 mg/d | 4 wk | Improved FMD |
| Bonaa et al. (2006)102 | Patients with recent myocardial infarction | 3749 (937 placebo) | 0.8 mg/d | 3.3 y | No effect on risk of recurrent CVD |
| Title et al. (2006)40 | Patients with type 2 diabetes | 19 | 10 mg/d | 2 wk | Improved FMD |
| Jamison et al. (2007)105 | Chronic kidney disease patients | 2056 | 40 mg/d | 3.2 y | No effect on CVD or all-cause mortality |
| Moens et al. (2007)109 | Post-acute myocardial infarction patients | 40 | 10 mg/d | 6 wk | Improved FMD |
| Mann et al. (2008)110 | Chronic kidney disease patients | 307 treatment, 312 placebo | 2.5 mg/d | 5 y | No reduction in CVD risk |
| Ebbing et al. (2008)111 | Patients undergoing coronary angiography | 3096 (780 placebo) | 0.8 mg/d | 1 y | No effect on total mortality or incidence of cardiovascular events |
| Albert et al. (2008)112 | Women at risk for CVD | 5442 | 2.5 mg/d | 7.3 y | No effect on incidence of cardiovascular events |
| DiFabio et al. (2010)104 | Healthy men | 20 | 1 mg/d | 1 wk | Did not prevent NTG intolerance or NTG-induced endothelial dysfunction |
| VITATOPS Trial Group (2010)113 | Patients with transient ischemic attack or stroke | 616 treatment, 678 placebo | 2 mg/d | 3.4 y | No effect on incidence of major cardiovascular events |
| Mierzecki et al. (2015)114 | Subjects with elevated CVD risk | 97 | 0.4 mg/d | 3 mo | No effect on coagulation or inflammation |
| Stanhewicz et al. (2015)10 | Healthy older adults (>60 y) | 11 | 5 mg/d | 6 wk | Improved NO-dependent dilation |
| Van Dijk et al. (2016)115 | Hyperhomocysteinemic older adults | 271 treatment, 251 placebo | 400 µg/d | 2 y | No effect on endothelial function or inflammatory markers |
Abbreviations: CAD, coronary artery disease; CVD, cardiovascular disease; FMD, flow-mediated dilation; NO, nitric oxide; NTG, nitroglycerin.
Overall, the available clinical data suggest that supplemental folic acid treatment at ≥5 mg/d is effective in improving NO-dependent vasodilation in patients with compromised endothelial function, with the exception of patients with chronic kidney disease. Coupled with the in vitro data, it is assumed that this improvement occurs through increased NOS coupling and subsequent increases in NO bioavailability, independent of decreases in plasma homocysteine. In support of this theory, evidence that lower daily doses of folic acid (≤2.5 mg) effectively lower plasma homocysteine but do not confer cardiovascular benefit suggests that folate, in higher doses, has a primary effect on vascular endothelial function. Further studies of this potential dose–response relation are essential for determining if a threshold exists beyond which folic acid supplementation is effective in reducing or preventing CVD in at-risk populations. Notably, long-term trials (>1 year follow-up) with higher doses of folic acid are lacking, raising the question of whether folic acid is a valid long-term prevention strategy. Prolonged clinical trials of high-dose folic acid are clearly warranted to determine if high-dose treatment regimens are effective in decreasing the incidence of recurrent CVD and CVD-related mortality in patients with attenuated endothelial function.
CONCLUSION
Bioavailable folates, primarily the circulating metabolite 5-MTHF, contribute to enhanced endothelial function by increasing NO bioavailability within the vascular endothelium. In patient populations in whom endothelial function is compromised, folic acid supplementation at ≥5 mg/d can effectively restore endothelium-dependent vasodilation, even in those who previously met the RDA of 400 µg/d for folate. There are two putative mechanism(s) by which bioavailable folate restores NO bioavailability: (1) increased NOS coupling, via direct interaction with the NOS dimer and/or increased availability of the essential NOS cofactor BH4; and (2) direct scavenging of deleterious reactive oxygen species, which preserves bioavailable NO. It remains unclear if, as previously thought, the homocysteine-lowering effect of folic acid supplementation directly benefits the endothelium; however, careful consideration of the literature suggests that simply lowering plasma homocysteine does not improve CVD outcomes and that higher daily doses (≥5 mg) of folic acid are required to confer endothelial benefits, even if they do not lower plasma homocysteine further. Further in vivo mechanistic research in humans will shed light on the specific role of folate in endothelial NO production and NO bioavailability, while clinical trials of daily folic acid doses ≥5 mg are essential for the assessment of folic acid supplementation as a viable long-term strategy for the prevention and treatment of endothelial dysfunction in CVD. Collectively, folic acid represents a well-tolerated and readily available potential treatment for endothelial dysfunction, which may translate to improved outcomes in patients with overt CVD and/or elevated CVD risk. Further clinical trials should help clarify the long-term efficacy of high-dose folate in the prevention and treatment of CVD.
Acknowledgments
Funding/support. A.E.S. is supported by a grant from the National Institutes of Health (NIH F32 HL129677–01; principal investigator, A.E.S.)
Declaration of interest. The authors have no relevant interests to declare.
References
- 1.Olney RS, Mulinare J. Trends in neural tube defect prevalence, folic acid fortification, and vitamin supplement use. Semin Perinatol. 2002;26:277–285. [DOI] [PubMed] [Google Scholar]
- 2.Hopkins SM, Gibney MJ, Nugent AP, et al. Impact of voluntary fortification and supplement use on dietary intakes and biomarker status of folate and vitamin B-12 in Irish adults. Am J Clin Nutr. 2015;101:1163–1172. [DOI] [PubMed] [Google Scholar]
- 3.McNulty H, Scott JM. Intake and status of folate and related B-vitamins: considerations and challenges in achieving optimal status. Br J Nutr. 2008;99 (suppl 3):S48–S54. [DOI] [PubMed] [Google Scholar]
- 4.Dietrich M, Brown CJ, Block G. The effect of folate fortification of cereal-grain products on blood folate status, dietary folate intake, and dietary folate sources among adult non-supplement users in the United States. J Am College Nutr. 2005;24:266–274. [DOI] [PubMed] [Google Scholar]
- 5.Ashfield-Watt PA, Moat SJ, Doshi SN, et al. Folate, homocysteine, endothelial function and cardiovascular disease. What is the link? Biomed Pharmacother. 2001;55:425–433. [DOI] [PubMed] [Google Scholar]
- 6.Yang HT, Lee M, Hong KS, et al. Efficacy of folic acid supplementation in cardiovascular disease prevention: an updated meta-analysis of randomized controlled trials. Eur J Int Med. 2012;23:745–754. [DOI] [PubMed] [Google Scholar]
- 7.Chalupsky K, Kracun D, Kanchev I, et al. Folic acid promotes recycling of tetrahydrobiopterin and protects against hypoxia-induced pulmonary hypertension by recoupling endothelial nitric oxide synthase. Antioxid Redox Signal. 2015;23:1076–1091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.McRae MP. High-dose folic acid supplementation effects on endothelial function and blood pressure in hypertensive patients: a meta-analysis of randomized controlled clinical trials. J Chiropr Med. 2009;8:15–24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Cagnacci A, Cannoletta M, Xholli A, et al. Folate administration decreases oxidative status and blood pressure in postmenopausal women. Eur J Nutr. 2015;54:429–435. [DOI] [PubMed] [Google Scholar]
- 10.Stanhewicz AE, Alexander LM, Kenney WL. Folic acid supplementation improves microvascular function in older adults through nitric oxide-dependent mechanisms. Clin Sci (London). 2015;129:159–167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Antoniades C, Shirodaria C, Warrick N, et al. 5-methyltetrahydrofolate rapidly improves endothelial function and decreases superoxide production in human vessels: effects on vascular tetrahydrobiopterin availability and endothelial nitric oxide synthase coupling. Circulation. 2006;114:1193–1201. [DOI] [PubMed] [Google Scholar]
- 12.Monch S, Netzel M, Netzel G, et al. Folate bioavailability from foods rich in folates assessed in a short term human study using stable isotope dilution assays. Food Funct. 2015;6:242–248. [DOI] [PubMed] [Google Scholar]
- 13.Tinker SC, Cogswell ME, Hamner HC, et al. Usual folic acid intakes: a modelling exercise assessing changes in the amount of folic acid in foods and supplements, National Health and Nutrition Examination Survey, 2003–2008. Public Health Nutr. 2012;15:1216–1227. [DOI] [PubMed] [Google Scholar]
- 14.Yi X, Zhou Y, Jiang D, et al. Efficacy of folic acid supplementation on endothelial function and plasma homocysteine concentration in coronary artery disease: a meta-analysis of randomized controlled trials. Exp Ther Med. 2014;7:1100–1110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Mao X, Xing X, Xu R, et al. Folic acid and vitamins D and B12 correlate with homocysteine in Chinese patients with type-2 diabetes mellitus, hypertension, or cardiovascular disease. Medicine (Baltimore). 2016;95:e2652 doi:10.1097/MD.0000000000002652. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Debreceni B, Debreceni L. The role of homocysteine-lowering B-vitamins in the primary prevention of cardiovascular disease. Cardiovasc Ther. 2014;32:130–138. [DOI] [PubMed] [Google Scholar]
- 17.Debreceni B, Debreceni L. Why do homocysteine-lowering B vitamin and antioxidant E vitamin supplementations appear to be ineffective in the prevention of cardiovascular diseases? Cardiovasc Ther. 2012;30:227–233. [DOI] [PubMed] [Google Scholar]
- 18.Pullin CH, Ashfield-Watt PA, Burr ML, et al. Optimization of dietary folate or low-dose folic acid supplements lower homocysteine but do not enhance endothelial function in healthy adults, irrespective of the methylenetetrahydrofolate reductase (C677T) genotype. J Am Coll Cardiol. 2001;38:1799–805. [DOI] [PubMed] [Google Scholar]
- 19.Antoniades C, Shirodaria C, Leeson P, et al. MTHFR 677 C>T polymorphism reveals functional importance for 5-methyltetrahydrofolate, not homocysteine, in regulation of vascular redox state and endothelial function in human atherosclerosis. Circulation. 2009;119:2507–2515. [DOI] [PubMed] [Google Scholar]
- 20.Scott JM, Weir DG. Folic acid, homocysteine and one-carbon metabolism: a review of the essential biochemistry. J Cardiovasc Risk. 1998;5:223–227. [PubMed] [Google Scholar]
- 21.Kelly P, McPartlin J, Goggins M, et al. Unmetabolized folic acid in serum: acute studies in subjects consuming fortified food and supplements. Am J Clin Nutr. 1997;65:1790–1795. [DOI] [PubMed] [Google Scholar]
- 22.Verhaar MC, Wever RM, Kastelein JJ, et al. 5-methyltetrahydrofolate, the active form of folic acid, restores endothelial function in familial hypercholesterolemia. Circulation. 1998;97:237–241. [DOI] [PubMed] [Google Scholar]
- 23.Gao L, Chalupsky K, Stefani E, et al. Mechanistic insights into folic acid-dependent vascular protection: dihydrofolate reductase (DHFR)-mediated reduction in oxidant stress in endothelial cells and angiotensin II-infused mice: a novel HPLC-based fluorescent assay for DHFR activity. J Mol Cell Cardiol. 2009;47:752–760. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Liu Q, Gross SS. Binding sites of nitric oxide synthases. Methods Enzymol. 1996;268:311–324. [DOI] [PubMed] [Google Scholar]
- 25.Chen DD, Chen LY, Xie JB, et al. Tetrahydrobiopterin regulation of eNOS redox function. Curr Pharm Design. 2014;20:3554–3562. [DOI] [PubMed] [Google Scholar]
- 26.Forstermann U, Li H. Therapeutic effect of enhancing endothelial nitric oxide synthase (eNOS) expression and preventing eNOS uncoupling. Br J Pharmacol. 2011;164:213–223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Forstermann U. Janus-faced role of endothelial NO synthase in vascular disease: uncoupling of oxygen reduction from NO synthesis and its pharmacological reversal. Biol Chem 2006;387:1521–1533. [DOI] [PubMed] [Google Scholar]
- 28.Siragusa M, Fleming I. The eNOS signalosome and its link to endothelial dysfunction. Pflugers Archiv. 2016;468:1125–1137. [DOI] [PubMed] [Google Scholar]
- 29.Kietadisorn R, Juni RP, Moens AL. Tackling endothelial dysfunction by modulating NOS uncoupling: new insights into its pathogenesis and therapeutic possibilities. Am J Physiol Endocrinol Metabol. 2012;302:E481–E495. [DOI] [PubMed] [Google Scholar]
- 30.Li H, Forstermann U. Pharmacological prevention of eNOS uncoupling. Curr Pharm Des. 2014;20:3595–3606. [DOI] [PubMed] [Google Scholar]
- 31.Pancharuniti N, Lewis CA, Sauberlich HE, et al. Plasma homocyst(e)ine, folate, and vitamin B-12 concentrations and risk for early-onset coronary artery disease. Am J Clin Nutr. 1994;59:940–948. [DOI] [PubMed] [Google Scholar]
- 32.Loehrer FM, Angst CP, Haefeli WE, et al. Low whole-blood S-adenosylmethionine and correlation between 5-methyltetrahydrofolate and homocysteine in coronary artery disease. Arterioscler Thromb Vasc Biol. 1996;16:727–733. [DOI] [PubMed] [Google Scholar]
- 33.Verhoef P, Kok FJ, Kruyssen DA, et al. Plasma total homocysteine, B vitamins, and risk of coronary atherosclerosis. Arterioscler Throm Vasc Biol. 1997;17:989–995. [DOI] [PubMed] [Google Scholar]
- 34.Verhoef P, Stampfer MJ, Buring JE, et al. Homocysteine metabolism and risk of myocardial infarction: relation with vitamins B6, B12, and folate. Am J Epidemiol. 1996;143:845–859. [DOI] [PubMed] [Google Scholar]
- 35.Bunout D, Petermann M, Hirsch S, et al. Low serum folate but normal homocysteine levels in patients with atherosclerotic vascular disease and matched healthy controls. Nutrition. 2000;16:434–438. [DOI] [PubMed] [Google Scholar]
- 36.Quere I, Perneger TV, Zittoun J, et al. Red blood cell methylfolate and plasma homocysteine as risk factors for venous thromboembolism: a matched case-control study. Lancet. 2002;359:747–752. [DOI] [PubMed] [Google Scholar]
- 37.Loria CM, Ingram DD, Feldman JJ, et al. Serum folate and cardiovascular disease mortality among US men and women. Arch Intern Med. 2000;160:3258–3262. [DOI] [PubMed] [Google Scholar]
- 38.Voutilainen S, Lakka TA, Porkkala-Sarataho E, et al. Low serum folate concentrations are associated with an excess incidence of acute coronary events: the Kuopio Ischaemic Heart Disease Risk Factor Study. Eur J Clin Nutr. 2000;54:424–428. [DOI] [PubMed] [Google Scholar]
- 39.Giles WH, Kittner SJ, Croft JB, et al. Serum folate and risk for coronary heart disease: results from a cohort of US adults. Ann Epidemiol. 1998;8:490–496. [DOI] [PubMed] [Google Scholar]
- 40.Title LM, Ur E, Giddens K, et al. Folic acid improves endothelial dysfunction in type 2 diabetes – an effect independent of homocysteine-lowering. Vasc Med. 2006;11:101–109. [DOI] [PubMed] [Google Scholar]
- 41.Chambers JC, Ueland PM, Obeid OA, et al. Improved vascular endothelial function after oral B vitamins: sn effect mediated through reduced concentrations of free plasma homocysteine. Circulation. 2000;102:2479–2483. [DOI] [PubMed] [Google Scholar]
- 42.Doshi SN, McDowell IF, Moat SJ, et al. Folic acid improves endothelial function in coronary artery disease via mechanisms largely independent of homocysteine lowering. Circulation. 2002;105:22–26. [DOI] [PubMed] [Google Scholar]
- 43.Schnyder G, Roffi M, Pin R, et al. Decreased rate of coronary restenosis after lowering of plasma homocysteine levels. N Engl J Med. 2001;345:1593–1600. [DOI] [PubMed] [Google Scholar]
- 44.Gori T, Burstein JM, Ahmed S, et al. Folic acid prevents nitroglycerin-induced nitric oxide synthase dysfunction and nitrate tolerance: a human in vivo study. Circulation. 2001;104:1119–1123. [DOI] [PubMed] [Google Scholar]
- 45.Raman CS, Li H, Martasek P, et al. Crystal structure of constitutive endothelial nitric oxide synthase: a paradigm for pterin function involving a novel metal center. Cell. 1998;95:939–950. [DOI] [PubMed] [Google Scholar]
- 46.Schmidt TS, McNeill E, Douglas G, et al. Tetrahydrobiopterin supplementation reduces atherosclerosis and vascular inflammation in apolipoprotein E-knockout mice. Clin Sci (London). 2010;119:131–142. [DOI] [PubMed] [Google Scholar]
- 47.Porkert M, Sher S, Reddy U, et al. Tetrahydrobiopterin: a novel antihypertensive therapy. J Hum Hypertens. 2008;22:401–407. [DOI] [PubMed] [Google Scholar]
- 48.Lang JA, Holowatz LA, Kenney WL. Local tetrahydrobiopterin administration augments cutaneous vasoconstriction in aged humans. J Physiol. 2009;587:3967–3974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Higashi Y, Sasaki S, Nakagawa K, et al. Tetrahydrobiopterin improves aging-related impairment of endothelium-dependent vasodilation through increase in nitric oxide production. Atherosclerosis. 2006;186:390–395. [DOI] [PubMed] [Google Scholar]
- 50.Pierce GL, Jablonski KL, Walker AE, et al. Tetrahydrobiopterin supplementation enhances carotid artery compliance in healthy older men: a pilot study. Am J Hypertens. 2012;25:1050–1054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Ueda S, Matsuoka H, Miyazaki H, et al. Tetrahydrobiopterin restores endothelial function in long-term smokers. J Am Coll Cardiol. 2000;35:71–75. [DOI] [PubMed] [Google Scholar]
- 52.Alexander LM, Kutz JL, Kenney WL. Tetrahydrobiopterin increases NO-dependent vasodilation in hypercholesterolemic human skin through eNOS-coupling mechanisms. Am J Physiol Regul Integr Comp Physiol. 2013;304:R164–R169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Moreau KL, Meditz A, Deane KD, et al. Tetrahydrobiopterin improves endothelial function and decreases arterial stiffness in estrogen-deficient postmenopausal women. Am J Physiol Heart Circ Physiol 2012;302:H1211–H1218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Eskurza I, Myerburgh LA, Kahn ZD, et al. Tetrahydrobiopterin augments endothelium-dependent dilatation in sedentary but not in habitually exercising older adults. J Physiol. 2005;568:1057–1065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Stanhewicz AE, Bruning RS, Smith CJ, et al. Local tetrahydrobiopterin administration augments reflex cutaneous vasodilation through nitric oxide-dependent mechanisms in aged human skin. J Appl Physiol. 2012;112:791–797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Vasquez-Vivar J, Kalyanaraman B, Martasek P, et al. Superoxide generation by endothelial nitric oxide synthase: the influence of cofactors. Proc Natl Acad Sci U S A. 1998;95:9220–9225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Kaufman S. Some metabolic relationships between biopterin and folate: implications for the “methyl trap hypothesis”. Neurochem Res. 1991;16:1031–1036. [DOI] [PubMed] [Google Scholar]
- 58.Crabtree MJ, Channon KM. Synthesis and recycling of tetrahydrobiopterin in endothelial function and vascular disease. Nitric Oxide. 2011;25:81–88. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Sugiyama T, Levy BD, Michel T. Tetrahydrobiopterin recycling, a key determinant of endothelial nitric-oxide synthase-dependent signaling pathways in cultured vascular endothelial cells. J Biol Chem. 2009;284:12691–12700. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Hyndman ME, Verma S, Rosenfeld RJ, et al. Interaction of 5-methyltetrahydrofolate and tetrahydrobiopterin on endothelial function. Am J Physiol Heart Circ Physiol. 2002;282:H2167–H2172. [DOI] [PubMed] [Google Scholar]
- 61.Stroes ES, van Faassen EE, Yo M, et al. Folic acid reverts dysfunction of endothelial nitric oxide synthase. Circ Res. 2000;86:1129–1134. [DOI] [PubMed] [Google Scholar]
- 62.Shirodaria C, Antoniades C, Lee J, et al. Global improvement of vascular function and redox state with low-dose folic acid: implications for folate therapy in patients with coronary artery disease. Circulation. 2007;115:2262–2270. [DOI] [PubMed] [Google Scholar]
- 63.Stanhewicz AE, Alexander LM, Kenney WL. Oral sapropterin acutely augments reflex vasodilation in aged human skin through nitric oxide-dependent mechanisms. J Appl Physiol (1985). 2013;115:972–978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Stanhewicz AE, Alexander LM, Kenney WL. Oral sapropterin augments reflex vasoconstriction in aged human skin through noradrenergic mechanisms. J Appl Physiol (1985). 2013;115:1025–1031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Frei B. On the role of vitamin C and other antioxidants in atherogenesis and vascular dysfunction. Proc Soc Exp Biol Med. 1999;222:196–204. [DOI] [PubMed] [Google Scholar]
- 66.Cross JM, Donald AE, Nuttall SL, et al. Vitamin C improves resistance but not conduit artery endothelial function in patients with chronic renal failure. Kidney Int. 2003;63:1433–1442. [DOI] [PubMed] [Google Scholar]
- 67.Ellis GR, Anderson RA, Lang D, et al. Neutrophil superoxide anion-generating capacity, endothelial function and oxidative stress in chronic heart failure: effects of short- and long-term vitamin C therapy. J Am Coll Cardiol. 2000;36:1474–1482. [DOI] [PubMed] [Google Scholar]
- 68.Ting HH, Timimi FK, Boles KS, et al. Vitamin C improves endothelium-dependent vasodilation in patients with non-insulin-dependent diabetes mellitus. J Clin Invest. 1996;97:22–28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Holowatz LA, Kenney WL. Local ascorbate administration augments NO- and non-NO-dependent reflex cutaneous vasodilation in hypertensive humans. Am J Physiol Heart Circ Physiol. 2007;293:H1090–H1096. [DOI] [PubMed] [Google Scholar]
- 70.Joshi R, Adhikari S, Patro BS, et al. Free radical scavenging behavior of folic acid: evidence for possible antioxidant activity. Free Radic Biol Med. 2001;30:1390–1399. [DOI] [PubMed] [Google Scholar]
- 71.Nakano E, Higgins JA, Powers HJ. Folate protects against oxidative modification of human LDL. Br J Nutr. 2001;86:637–639. [DOI] [PubMed] [Google Scholar]
- 72.Henning SM, Swendseid ME, Ivandic BT, et al. Vitamins C, E and A and heme oxygenase in rats fed methyl/folate-deficient diets. Free Radic Biol Med. 1997;23:936–942. [DOI] [PubMed] [Google Scholar]
- 73.Durand P, Prost M, Blache D. Pro-thrombotic effects of a folic acid deficient diet in rat platelets and macrophages related to elevated homocysteine and decreased n-3 polyunsaturated fatty acids. Atherosclerosis. 1996;121:231–243. [DOI] [PubMed] [Google Scholar]
- 74.Doshi SN, McDowell IF, Moat SJ, et al. Folate improves endothelial function in coronary artery disease: an effect mediated by reduction of intracellular superoxide? Arterioscler Thromb Vasc Biol. 2001;21:1196–1202. [DOI] [PubMed] [Google Scholar]
- 75.Wilmink HW, Stroes ES, Erkelens WD, et al. Influence of folic acid on postprandial endothelial dysfunction. Arterioscler Thromb Vasc Biol. 2000;20:185–188. [DOI] [PubMed] [Google Scholar]
- 76.Title LM, Cummings PM, Giddens K, et al. Effect of folic acid and antioxidant vitamins on endothelial dysfunction in patients with coronary artery disease. J Am Coll Cardiol. 2000;36:758–765. [DOI] [PubMed] [Google Scholar]
- 77.Krumdieck CL, Fukushima K, Fukushima T, et al. A long-term study of the excretion of folate and pterins in a human subject after ingestion of 14C folic acid, with observations on the effect of diphenylhydantoin administration. Am J Clin Nutr. 1978;31:88–93. [DOI] [PubMed] [Google Scholar]
- 78.Clifford AJ, Arjomand A, Dueker SR, et al. The dynamics of folic acid metabolism in an adult given a small tracer dose of 14C-folic acid. Adv Exp Med Biol. 1998;445:239–251. [DOI] [PubMed] [Google Scholar]
- 79.Bhandari SD, Gregory JF III. Folic acid, 5-methyl-tetrahydrofolate and 5-formyl-tetrahydrofolate exhibit equivalent intestinal absorption, metabolism and in vivo kinetics in rats. J Nutr. 1992;122:1847–1854. [DOI] [PubMed] [Google Scholar]
- 80.Wright AJ, Dainty JR, Finglas PM. Folic acid metabolism in human subjects revisited: potential implications for proposed mandatory folic acid fortification in the UK. Br J Nutr. 2007;98:667–675. [DOI] [PubMed] [Google Scholar]
- 81.Venn BJ, Green TJ, Moser R, et al. Increases in blood folate indices are similar in women of childbearing age supplemented with [6S]-5-methyltetrahydrofolate and folic acid. J Nutr. 2002;132:3353–3355. [DOI] [PubMed] [Google Scholar]
- 82.Lamers Y, Prinz-Langenohl R, Moser R, et al. Supplementation with [6S]-5-methyltetrahydrofolate or folic acid equally reduces plasma total homocysteine concentrations in healthy women. Am J Clin Nutr. 2004;79:473–478. [DOI] [PubMed] [Google Scholar]
- 83.Hao L, Yang QH, Li Z, et al. Folate status and homocysteine response to folic acid doses and withdrawal among young Chinese women in a large-scale randomized double-blind trial. Am J Clin Nutr. 2008;88:448–457. [DOI] [PubMed] [Google Scholar]
- 84.de Meer K, Smulders YM, Dainty JR, et al. [6S]5-methyltetrahydrofolate or folic acid supplementation and absorption and initial elimination of folate in young and middle-aged adults. Eur J Clin Nutr. 2005;59:1409–1416. [DOI] [PubMed] [Google Scholar]
- 85.Campbell NR. How safe are folic acid supplements? Arch Intern Med. 1996;156:1638–1644. [PubMed] [Google Scholar]
- 86.Solomons NW. Food fortification with folic acid: has the other shoe dropped? Nutr Rev. 2007;65:512–515. [DOI] [PubMed] [Google Scholar]
- 87.Johnston RB., Jr Will increasing folic acid in fortified grain products further reduce neural tube defects without causing harm?: consideration of the evidence. Pediatr Res. 2008;63:2–8. [DOI] [PubMed] [Google Scholar]
- 88.Wald NJ, Oakley GP. Should folic acid fortification be mandatory? Yes. BMJ. 2007;334:1252 doi:http://dx.doi.org/10.1136/bmj.39232.493252.47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Hubner RA, Houlston RD, Muir KR. Should folic acid fortification be mandatory? No. BMJ 2007;334:1253 doi:http://dx.doi.org/10.1136/bmj.39232.496227.47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Ulrich CM, Potter JD. Folate supplementation: too much of a good thing? Cancer Epidemiol Biomarkers Prev. 2006;15:189–193. [DOI] [PubMed] [Google Scholar]
- 91.Bailey LB, Stover PJ, McNulty H, et al. Biomarkers of nutrition for development – folate review. J Nutr. 2015;145:1636S–1680S. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Yetley EA, Johnson CL. Folate and vitamin B-12 biomarkers in NHANES: history of their measurement and use. Am J Clin Nutr. 2011;94:322S–3231S. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Scott J, Weir D. Folate/vitamin B12 inter-relationships. Essays Biochem. 1994;28:63–72. [PubMed] [Google Scholar]
- 94.Johnson MA. If high folic acid aggravates vitamin B12 deficiency what should be done about it? Nutr Rev. 2007;65:451–458. [DOI] [PubMed] [Google Scholar]
- 95.Morris MS, Jacques PF, Rosenberg IH, et al. Folate and vitamin B-12 status in relation to anemia, macrocytosis, and cognitive impairment in older Americans in the age of folic acid fortification. Am Clin Nutr. 2007;85:193–200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Tucker KL, Mahnken B, Wilson PW, et al. Folic acid fortification of the food supply. Potential benefits and risks for the elderly population. JAMA. 1996;276:1879–1885. [DOI] [PubMed] [Google Scholar]
- 97.Mason JB, Dickstein A, Jacques PF, et al. A temporal association between folic acid fortification and an increase in colorectal cancer rates may be illuminating important biological principles: a hypothesis. Cancer Epidemiol Biomarkers Prev. 2007;16:1325–1329. [DOI] [PubMed] [Google Scholar]
- 98.Cole BF, Baron JA, Sandler RS, et al. Folic acid for the prevention of colorectal adenomas: a randomized clinical trial. JAMA. 2007;297:2351–2359. [DOI] [PubMed] [Google Scholar]
- 99.Blount BC, Mack MM, Wehr CM, et al. Folate deficiency causes uracil misincorporation into human DNA and chromosome breakage: implications for cancer and neuronal damage. Proc Natl Acad Sci U S A. 1997;94:3290–3295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Qin X, Shen L, Zhang R, et al. Effect of folic acid supplementation on cancer risk among adults with hypertension in China: a randomized clinical trial [published online March 17, 2016]. Int J Cancer. doi: 10.1002/ijc.30094. [DOI] [PubMed] [Google Scholar]
- 101.Loscalzo J. Homocysteine trials – clear outcomes for complex reasons. N Engl J Med. 2006;354:1629–1632. [DOI] [PubMed] [Google Scholar]
- 102.Bonaa KH, Njolstad I, Ueland PM, et al. Homocysteine lowering and cardiovascular events after acute myocardial infarction. N Engl J Med. 2006;354:1578–1588. [DOI] [PubMed] [Google Scholar]
- 103.Nataatmadja M, Cho Y, Fahim M, et al. Recent clinical trials of pharmacologic cardiovascular interventions in patients with chronic kidney disease: an update. Rev Recent Clin Trials. 2016;11:12–32. [DOI] [PubMed] [Google Scholar]
- 104.DiFabio JM, Gori T, Thomas G, et al. Daily low-dose folic acid supplementation does not prevent nitroglycerin-induced nitric oxide synthase dysfunction and tolerance: a human in vivo study. Can J Cardiol. 2010;26:461–465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Jamison RL, Hartigan P, Kaufman JS, et al. Effect of homocysteine lowering on mortality and vascular disease in advanced chronic kidney disease and end-stage renal disease: a randomized controlled trial. JAMA. 2007;298:1163–1170. [DOI] [PubMed] [Google Scholar]
- 106.Woo KS, Chook P, Lolin YI, et al. Folic acid improves arterial endothelial function in adults with hyperhomocystinemia. J Am Coll Cardiol. 1999;34:2002–2006. [DOI] [PubMed] [Google Scholar]
- 107.Toole JF, Malinow MR, Chambless LE, et al. Lowering homocysteine in patients with ischemic stroke to prevent recurrent stroke, myocardial infarction, and death: the Vitamin Intervention for Stroke Prevention (VISP) randomized controlled trial. JAMA. 2004;291:565–575. [DOI] [PubMed] [Google Scholar]
- 108.Lekakis JP, Papamichael CM, Papaioannou TG, et al. Oral folic acid enhances endothelial function in patients with hypercholesterolaemia receiving statins. Eur J Cardiovasc Prev Rehabil. 2004;11:416–420. [DOI] [PubMed] [Google Scholar]
- 109.Moens AL, Claeys MJ, Wuyts FL, et al. Effect of folic acid on endothelial function following acute myocardial infarction. Am J Cardiol. 2007;99:476–481. [DOI] [PubMed] [Google Scholar]
- 110.Mann JF, Sheridan P, McQueen MJ, et al. Homocysteine lowering with folic acid and B vitamins in people with chronic kidney disease – results of the renal Hope-2 study. Nephrol Dial Transplant. 2008;23:645–653. [DOI] [PubMed] [Google Scholar]
- 111.Ebbing M, Bleie O, Ueland PM, et al. Mortality and cardiovascular events in patients treated with homocysteine-lowering B vitamins after coronary angiography: a randomized controlled trial. JAMA. 2008;300:795–804. [DOI] [PubMed] [Google Scholar]
- 112.Albert CM, Cook NR, Gaziano JM, et al. Effect of folic acid and B vitamins on risk of cardiovascular events and total mortality among women at high risk for cardiovascular disease: a randomized trial. JAMA. 2008;299:2027–2036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.VITATOPS Trial Study Group. B vitamins in patients with recent transient ischaemic attack or stroke in the VITAmins TO Prevent Stroke (VITATOPS) trial: a randomised, double-blind, parallel, placebo-controlled trial. Lancet Neurol. 2010;9:855–865. [DOI] [PubMed] [Google Scholar]
- 114.Mierzecki A, Makarewicz-Wujec M, Kloda K, et al. Influence of folic acid supplementation on coagulation, inflammatory, lipid, and kidney function parameters in subjects with low and moderate content of folic acid in the diet. Kardiol Pol. 2015;73:280–286. [DOI] [PubMed] [Google Scholar]
- 115.van Dijk SC, Enneman AW, Swart KM, et al. Effect of vitamin B12 and folic acid supplementation on biomarkers of endothelial function and inflammation among elderly individuals with hyperhomocysteinemia. Vasc Med. 2016;21:91–98. [DOI] [PubMed] [Google Scholar]
- 116.Marti-Carvajal AJ, Sola I, Lathyris D. Homocysteine-lowering interventions for preventing cardiovascular events. Cochrane Database Syst Rev. 2015;1:CD006612 doi:10.1002/14651858.CD006612.pub4. [DOI] [PubMed] [Google Scholar]
- 117.Nursalim A, Siregar P, Widyahening IS. Effect of folic acid, vitamin B6 and vitamin B12 supplementation on mortality and cardiovascular complication among patients with chronic kidney disease: an evidence-based case report. Acta Med Indones. 2013;45:150–156. [PubMed] [Google Scholar]