Abstract
Enhancement of callus induction and its regeneration efficiency through in vitro techniques has been optimized for 2 abiotic stresses (salt and air desiccation) using 3 rice genotypes viz. BR10, BRRI dhan32 and BRRI dhan47. The highest frequency of callus induction was obtained for BRRI dhan32 (64.44%) in MS medium supplemented with 2, 4-D (2.5 mgL−1) and Kin (1.0 mgL−1). Different concentrations of NaCl (2.9, 5.9, 8.8 and 11.7 gL−1) were used and its effect was recorded on the basis of viability of calli (VC), relative growth rate (RGR), tolerance index (TI) and relative water content (RWC). It was observed that in all cases BRRI dhan47 showed highest performance on tolerance to VC (45.33%), RGR (1.03%), TI (0.20%) and RWC (10.23%) with 11.7 gL−1 NaCl. Plant regeneration capability was recorded after partial air desiccation pretreatment to calli for 15, 30, 45 and 60 h. In this case BRRI dhan32 gave maximum number of regeneration (76.19%) when 4 weeks old calli were desiccated for 45 h. It was observed that air desiccation was 2-3 folds more effective for enhancing green plantlet regeneration compared to controls. Furthermore, desiccated calli also showed the better capability to survive in NaCl induced abiotic stress; and gave 1.9 fold (88.80%) increased regeneration in 11.7 gL−1 salt level for BRRI dhan47. Analysis of variance (ANOVA) showed that the genotypes, air desiccation and NaCl had significant effect on plant regeneration at P < 0.01.
Keywords: abiotic stress, Bangladeshi Indica rice cultivars, callus induction, mature embryos, Oryza sativa, plant regeneration
Abbreviations
- MS
Murashinge and Skoog
- 2,4-D
2,4-Dichlorophenoxy acetic acid
- NAA
α-napthalene acetic acid
- BAP
6-Benzyl amino purine
- Kin
kinetin
- CH
casein hydrolysate
- w
week
- d
day
- h
hour
- VCn
number of viable callus
- ICn
number of inoculated callus
Introduction
Rice (Oryza sativa L.) belongs to the family Gramineae is a cereal crop and staple food over the world and also main food crop in Bangladesh. The population of rice eaters are increasing day by day and the number of rice consumers will probably 2 fold by 2020.1 In spite of many abiotic stresses are played negative role on rice and other crop production.2-5 Drought and salinity are the major abiotic stresses that adversely affect the overall metabolic activities and cause plant demise.6,7 Certain crops reduced their production capabilities in high saline conditions.8 Over 2 million acres of agricultural land has been estimated which lost from production per every year due to occurrence of high Na+ and Cl− ions in soil.9 The coastal area of Bangladesh is 20% of the country and over 30% of net cultivable area and it expends inside up to 150 km from the coast.10 Agricultural genetics is one of the most important parts to solve the recent global issue. In biotechnology, genotype strongly influences the potentiality of tissue culture, and identification of superior performance is a key step to transform gene in rice.11 In vitro production of rice plants provides efficient and convenient system to produce rice lines rapidly.12 The application of biotechnology in combination with conventional breeding methods such as doubled haploid breeding may help to increase food production properly.13-15 For rice improvement, in vitro culture method was successfully initiated by culturing of excised immature embryos in nutrients media.16,17 Successful plant regeneration by embryo-derived calli has been reported by Raina,18 Vasil19, Crougham and Chu.20 For the development of highly reproducible reproduction system through somatic embryogenesis using mature embryos in rice were studied by Verma et al.21 Joyia et al.22 optimized the responsive age of the cells to regeneration which was a prime character for efficient plant regeneration. However, many factors affect plant regeneration frequency in rice e.g. genotype, development stage of callus in the explants and hormonal composition of medium.23-25 A comprehensive study on rice genotypes for both callus induction and plant regeneration has been done for 500 rice varieties by Kamia et al.26 The identification and screening of useful cultivars for embryogenic callus formation and subsequent plant regeneration through in vitro system is a key step in rice genetic improvement.27,28 An efficient plant regeneration in Bangladeshi indica rice is still poses a major problem for genetic manipulation through innovative approaches.14,29 The optimal desiccation periods were 72 h and 48 h for Malaysian rice cultivars of MR232 and MR220 respectively, where plant regeneration enhanced up to 2-5 folds.30 They suggested that partial desiccation can be useful in stimulating regeneration response. Desiccation treatment reduced hyperhydricity of the calli.31 Tsukahara and Hirosawa32 reported that dehydration for 24 h of cell suspension derived calli of japonica rice increased shoot regeneration from 3 to 47%. Three-fold increased in shoot regeneration frequency following partial desiccation for 24 h of suspension cells in indica rice.33 Chand and Sahrawat34 carried out partial desiccation of embryogenic calli prior to transfer to regeneration medium and found increasing regeneration frequency of desiccation treatment to callus cultures of cv. Safari17 and Kasturi. In sugarcane desiccation improves somatic embryogenesis and the calli exhibited a greater regeneration frequency that is very important for genetic transformation work.35 Three and 4 hours desiccated calli reduced fresh weight due to reduction of water content and stimulated callus growth, globularization and embryo formation in 2 date palm cultivars.31 Desiccation also has been reported to promote somatic embryo differentiation and development to other crops like as soybean,36,37 wheat,38 spruce39 and cassava.40 In recent years tissue culture techniques are being used as a useful tool to elucidate the mechanism involved in salt tolerance.41,42 Several metals are essential nutrients for plants, yet they become toxic at high levels and deleteriously affect crop yield and quality.43 Using Bangladeshi indica rice cultivars till there is not enough report on successful regeneration on high salt stress and other stress pretreatment factors for tolerance level. Therefore, the present study has been undertaken to determine the effect of partial air desiccation and by NaCl on somatic embryogenesis derived from mature seeds and their subsequent regeneration using 3 rice cultivars.
Results
Effect of media on callus induction (CI)
Two basal media (MS and N6) consisting of 4 hormonal combinations (H1 - H4) were used for their effectiveness on callus induction. All the steps in callus induction, somatic embryogenesis (direct) and plant regeneration in rice are shown in Fig. 1A–H. The results indicated that all of the responding genotypes showed well embryogenic response to induce callus (Fig. 1A and B). Out of 3 genotypes, BRRI dhan32 (64.44%) and BRRI dhan47 (52.78%) performed highest number of CI in MS + H2. The maximum callus induction (53.26%) was recorded for BR10 in MS + H1 (Fig. 2). On the other hand, BR10, BRRI dhan47 and BRRI dhan32 gave minimum callusing 47.13, 47.71 and 55.34% in N6 + H4, respectively. It was observed that all the responding genotypes showed better performance on callusing in MS medium than N6. Analysis of variance showed the significant differences within the studied genotypes and the media examined at p < 0.01 level (Table 1).
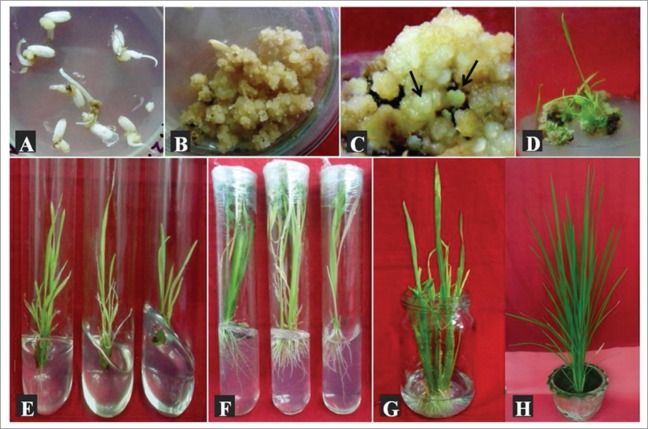
Figure 1.
Callus induction, somatic embryogenesis (direct) and plant regeneration in rice. (A) Calli derived from mature seeds after one week of culture, (B) Proliferation of embryogenic calli (4–5 weeks old), (C) Development of somatic embryos on mature embryo induced calli (indicated by arrows), (D) Formation of shoots from germinated somatic embryos, (E) Elongated shoots, (F) Rooted shoots (regenerated plantlets), (G) Acclimatized plantlets, (H) Hardening of regenerated plantlets in pot culture.
Figure 2.
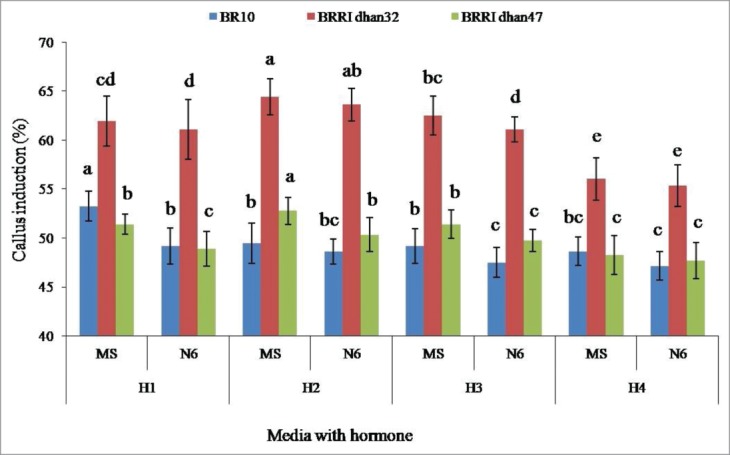
Effect of media and hormonal combinations on callus induction in 3 rice genotypes. H1 = 0.5 mgL−1 2, 4-D + 1.0 mgL−1 Kin, H2 = 2.5 mgL−1 2, 4-D + 1.0 mgL−1 Kin + 300 mgL−1 L-proline + 400 mgL−1 CH, H3 = 0.5 mgL−1 NAA + 2.0 mgL−1 Kin + 400 mgL−1 L-proline + mgL−1 CH, H4 = 2.5 mgL−1 IAA + 0.5 mgL−1 Kin + 300 mgL−1 L-proline + 400 mgL−1 CH. Within the bars of same color, different letter (s) shows significant difference at p < 0.05 according to DMRT.
Table 1.
Analysis of variance (ANOVA) subjected to callus induction, partial air desiccation and NaCl treatments in 3 rice genotypes
| Stress | Subject of ANOVA (Data source) | Source of variation | DF | Mean sum of square |
|---|---|---|---|---|
| — | Callus Induction (CI) (Fig. 1) | Genotype (G) | 2 | 442.476** |
| Media (M) | 7 | 2.858** | ||
| G × M | 14 | — | ||
| Salt induced stress | Viability of callus (VC) (Table 1) | Genotype (G) | 2 | 7422.85** |
| Salt concentration (SC) | 4 | 8543.69** | ||
| Callus culture period (CCP) | 3 | 193.27** | ||
| G × SC | 8 | 581.68** | ||
| G × CCP | 6 | 4.36NS | ||
| SC × CCP | 12 | 7.27*NS | ||
| G × SC × CCP | 24 | 8.48 | ||
| Relative growth rate (RGR) (Fig. 3A) | Genotype (G) | 2 | 1.741** | |
| Salt concentration (SC) | 4 | 9.414** | ||
| G × S | 8 | 0.132 | ||
| Tolerance index (TI) (Fig. 3B) | Genotype (G) | 2 | 0.039** | |
| Salt concentration (SC) | 4 | 0.453** | ||
| G × S | 8 | 0.005 | ||
| Relative water content (RWC) (Fig. 3C) | Genotype (G) | 2 | 3.327** | |
| Salt concentration (SC) | 4 | 38.644** | ||
| G × SC | 8 | 2.119 | ||
| Partial air desiccation stress | Plant regeneration (PR) (Table 2) | Genotype (G) | 2 | 604.34** |
| Age of callus (AC) | 3 | 803.94** | ||
| Partial air desiccation (PAD) | 4 | 686.17** | ||
| G × AC | 6 | 18.45NS | ||
| G × PAD | 8 | 45.93NS | ||
| AC × PAD | 12 | 124.71* | ||
| G × AC × PAD | 24 | 35.09 |
* = Significant at P < 0.01, * = Significant at P < 0.05, NS = Non-significant.
Response to in vitro Abiotic Stresses
Viability of callus to NaCl induced stress
To observe the viability of calli 4 concentrations of NaCl were used and data shown in Table 2. The varieties BRRI dhan47, BR10 and BRRI dhan32 gave 53.33, 14.67 and 2.67% viable calli after one week cultured in the highest concentration of NaCl (11.7 gL−1) respectively. In the same salt level, the viability was decreased remarkably after 4 weeks of culture in BRRI dhan47 (45.33%), BR10 (10.67%) and BRRI dhan32 (0.00%). In stress condition, the survival rate of callus was significantly differed on NaCl concentrations, culture periods and rice genotypes (Table 1).
Table 2.
Effect of salt on viability of seed derived callus exposed in different concentrations of NaCl and grown one to 4 weeks for 3 genotypes
| Viable calli (% ± SE) | |||||
|---|---|---|---|---|---|
|
Variety |
NaCl
(gL−1) |
1 w |
2 w |
3 w |
4 w |
| BR10 | Cont. | 93.33 ± 1.33ab | 88.00 ± 2.31a | 86.67 ± 3.53a | 85.33 ± 2.67a |
| 2.9 | 78.67 ± 3.53d | 77.33 ± 2.67cd | 74.67 ± 1.33bc | 73.33 ± 1.33b | |
| 5.9 | 41.33 ± 1.33h | 36.00 ± 2.31g | 33.33 ± 2.67f | 33.33 ± 2.67e | |
| 8.8 | 32.00 ± 2.31i | 32.00 ± 1.33g | 26.67 ± 1.33f | 24.00 ± 2.31f | |
| 11.7 | 14.67 ± 2.67j | 12.00 ± 2.31i | 12.00 ± 2.31gh | 10.67 ± 2.67gh | |
| BRRI dhan32 | Cont. | 86.67 ± 2.67bc | 84.00 ± 2.31ab | 81.33 ± 2.67ab | 81.33 ± 2.67a |
| 2.9 | 62.67 ± 3.53f | 52.00 ± 2.31f | 45.33 ± 2.67e | 33.33 ± 1.33e | |
| 5.9 | 25.33 ± 1.33i | 22.67 ± 1.33h | 18.67 ± 1.33g | 16.00 ± 2.31g | |
| 8.8 | 13.33 ± 1.33j | 8.00 ± 2.31i | 8.00 ± 2.31h | 5.33 ± 1.33hi | |
| 11.7 | 2.67 ± 1.33k | 0.00 ± 0.00j | 0.00 ± 0.00i | 0.00 ± 0.00i | |
| BRRI dhan47 | Cont. | 94.67 ± 1.33a | 89.33 ± 1.33a | 86.67 ± 3.53a | 85.33 ± 1.33a |
| 2.9 | 81.33 ± 3.53cd | 80.00 ± 2.31bc | 77.33 ± 1.33bc | 74.67 ± 1.33b | |
| 5.9 | 74.67 ± 1.33de | 73.33 ± 1.33d | 72.00 ± 2.31c | 72.00 ± 2.31b | |
| 8.8 | 70.67 ± 3.53e | 64.00 ± 2.31e | 64.00 ± 2.31d | 58.67 ± 1.33c | |
| 11.7 | 53.33 ± 1.33g | 49.33 ± 1.33f | 46.67 ± 2.67e | 45.33 ± 3.53d | |
Culture medium was MS +2.5 mgL−1 2, 4-D + 1.0 mgL−1 kin + 0.3 gL−1 L-proline + 0.4 gL−1 CH + NaCl. For each NaCl concentration, number of used callus was 75 in 3 replications and in a column the mean values followed by same letter (s) are not significantly different at p < 0.05 according to DMRT.
Relative growth rate, tolerance index and relative water content to NaCl stress
Three weeks old calli were used for this study and grown in 4 concentrations of NaCl (2.9, 5.9, 8.8, 11.7 gL−1) up to 4 weeks. Relative growth rate (RGR), tolerance index (TI) and relative water content (RWC) were determined and shown in Figure 3A–C. It was observed that in all cases, significant differences were found among the genotypes. Furthermore, significant differences were also observed in absence of NaCl in the medium (control) on RGR, TI and RWC. However, 1.03, 0.23 and 0.11 RGR values were recorded at 11.7 gL−1 salt stress in BRRI dhan47, BR10 and BRRI dhan32 respectively. On comparison to the controls, RGR values were decreased at 79.88% in BRRI dhan47, 94.26% in BR10 and 97.59% in BRRI dhan32. Since, BRRI dhan47 grew with highest capability in the top most level of NaCl stress (11.7 gL−1). The same genotype carried the highest TI (0.20) which expressed the high capability to grow in abiotic stress condition developed by NaCl. Comparatively lower TI numbers were recorded in other 2 genotypes BR10 (0.02) and BRRI dhan32 (0.06). The WRC values were also lower for BR10 (7.22%) and BRRI dhan32 (7.03%) than BRRI dhan47 (10.23%).
Figure 3.
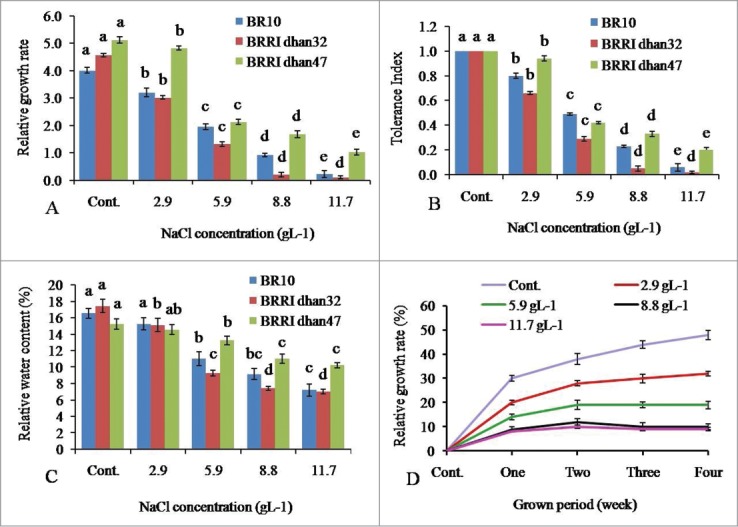
Effect of different concentrations (2.9, 5.9, 8.8, 11.7 gL−1) of NaCl subjected to (A) relative growth rate, (B) tolerance index, (C) relative water content of 3 rice genotypes and (D): Relative growth rate (%) of BRRI dhan47 in different NaCl concentrations in contrast to calli exposure periods. Within the bars of same color, different letter (s) shows significant difference at p < 0.05 according to DMRT.
On spite of showing highest RGR and TI values, BRRI dhan47 was taken to conduct related another extended experiment. To observe the changing pattern of RGR, its values were determined in contrast of stress levels and calli exposure periods (every week up to 4). The results showed that RGR was increased till 2 weeks of calli exposure periods, at all NaCl levels tested. After 2 weeks, RGR was restricted in 5.9, 8.8 and 11.7 gL−1 stress levels, while in 2.9 gL−1 lower rate of increment in RGR value was found (Fig. 3D).
Effect of partial air desiccation to regeneration
Calli of different age groups (3, 4, 5 and 6 w) were partially desiccated (15, 30, 45 and 60 h) and cultured on RM. The significant differences were found within the genotypes, age groups and air desiccation period on regeneration (Table 1 and Fig. 1C and D). The results showed that 4 w old calli of BRRI dhan32 performed highest regeneration (76.19%) among the genotypes when it was desiccated by 45 h (Table 3). The gained regeneration value was 2 fold higher than the control (38.10%). In the same desiccation pretreatment (45 h), other 2 genotypes BR10 and BRRI dhan47 gave 73.02% and 58.73% regeneration respectively; and the results were more than 2 fold higher (Fig. 4). In contrast to the controls, desiccated calli showed around 2–3 folds higher regeneration. Considering our recorded data, the calli of lower age, needed to higher desiccation pretreatment within a range than comparatively aged callus to perform maximum regeneration. However, analysis of variance (ANOVA) showed that the effect of partial air desiccation, age of calli and rice genotype on plant regeneration differed significantly at P < 0.01 (Table 1).
Table 3.
Effect of partial air desiccation period and 4 age groups of calli on plant regeneration for 3 rice genotypes (% ± SE)
| Genotypes | ||||
|---|---|---|---|---|
|
Age of calli
(w) |
Desiccation
(h) |
BR10 |
BRRI
dhan32 |
BRRI
dhan47 |
| 3 | Cont. | 38.10 ± 2.75de | 31.75 ± 3.17jk | 33.33 ± 2.75def |
| 15 | 36.51 ± 1.59e | 39.68 ± 1.59ghij | 36.51 ± 3.17cde | |
| 30 | 47.62 ± 2.75c | 55.56 ± 1.59de | 38.10 ± 2.75cde | |
| 45 | 50.79 ± 3.17c | 68.25 ± 3.17b | 42.86 ± 2.75bc | |
| 60 | 34.92 ± 1.59e | 41.27 ± 1.59fghi | 26.98 ± 1.59fg | |
| 4 | Cont. | 31.75 ± 1.59e | 38.10 ± 2.75hijk | 30.16 ± 1.59efg |
| 15 | 53.97 ± 3.17c | 47.62 ± 2.75efg | 34.92 ± 3.17cdef | |
| 30 | 68.25 ± 3.17ab | 65.08 ± 4.20bc | 42.86 ± 2.75bc | |
| 45 | 73.02 ± 4.20a | 76.19 ± 2.75a | 58.73 ± 3.17a | |
| 60 | 46.03 ± 3.17cd | 58.73 ± 1.59cd | 47.62 ± 2.75bc | |
| 5 | Cont. | 30.16 ± 1.59e | 34.92 ± 3.17ijk | 30.16 ± 1.59efg |
| 15 | 47.62 ± 2.75c | 47.62 ± 2.75efg | 47.62 ± 2.75bc | |
| 30 | 63.49 ± 4.20b | 55.56 ± 1.59de | 39.68 ± 1.59bcd | |
| 45 | 49.21 ± 4.20c | 49.21 ± 3.17ef | 34.92 ± ± 3.17cdef | |
| 60 | 34.92 ± 1.59e | 39.68 ± 1.59ghij | 31.75 ± 1.59defg | |
| 6 | Cont. | 31.75 ± 3.17e | 30.16 ± 1.59k | 26.98 ± 1.59fg |
| 15 | 38.10 ± 2.75de | 44.44 ± 1.59fgh | 34.92 ± 3.17cdef | |
| 30 | 36.51 ± 1.59e | 39.68 ± 3.17ghij | 33.33 ± 2.75def | |
| 45 | 34.92 ± 1.59e | 38.10 ± 2.75hijk | 26.98 ± 1.59fg | |
| 60 | 33.33 ± 2.75e | 31.75 ± 3.17jk | 23.81 ± 2.75g | |
Used regeneration medium, MS + 2.0 mgL−1 BAP + 0.5 mgL−1 NAA + 1.0 mgL−1 Kin was constant for all the age of calli and partial air desiccation pretreatments. For each desiccation pretreatment number of callus was 63 in 3 replications and in a column the mean values followed by same letter (s) are not significantly different at p < 0.05 according to DMRT.
Figure 4.
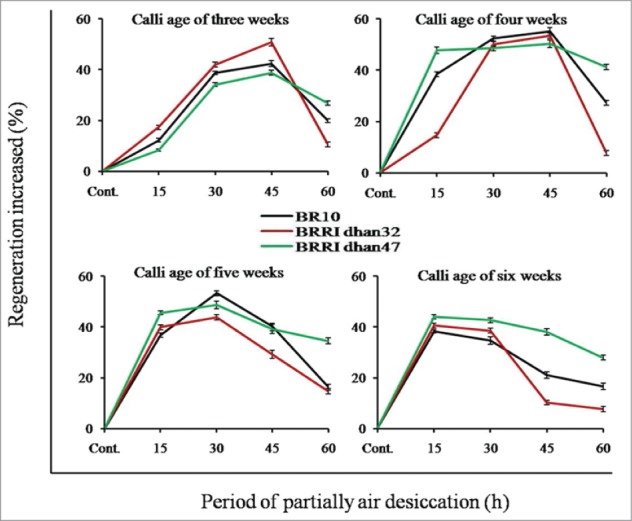
Effect of different period of partial air desiccation in relation to age of calli to enhance plant regeneration. Each curve indicates the percentage value of increased regeneration compare to the control (vertical bar expressed the SE of 3 replicates).
Regeneration response by partially desiccated calli in salt stress
Desiccated callus performed with increased regeneration in NaCl induced stress condition (Table 4). The callus age of 4 weeks were pretreated at 45 h air desiccation and transferred to regeneration medium (RM) supplemented with NaCl levels (2.9, 5.9, 8.8, 11.7 gL−1). The variety BRRI dhan47 gave the highest regeneration (26.98%) at 11.7 gL−1 salt level after desiccation pretreatment. The result was 1.89 fold higher than the control (14.29%). In the same stress level, other 2 varieties BR10 and BRRI dhan32 could not be regenerated from undedicated calli; whereas, after desiccation pretreatment they were been capable for regeneration at 11.11% and 4.76%, respectively.
Table 4.
Effect of desiccation to NaCl stress on regeneration of 3 rice genotypes (% ± SE)
| NaCl (gL−1) | ||||||
|---|---|---|---|---|---|---|
|
Variety |
Desiccation
(h) |
Cont. |
2.9 |
5.9 |
8.8 |
11.7 |
| BR10 | Cont. | 36.51 ± 1.59 | 31.75 ± 1.59 | 9.52 ± 2.75 | 3.17 ± 1.59 | 0.00 ± 0.00 |
| 45 | 71.43 ± 4.76 | 68.25 ± 4.20 | 33.33 ± 2.75 | 14.29 ± 2.75 | 11.11 ± 3.17 | |
| BRRI dhan32 | Cont. | 36.51 ± 1.59 | 25.40 ± 1.59 | 9.52 ± 2.75 | 3.17 ± 1.59 | 0.00 ± 0.00 |
| 45 | 74.60 ± 4.20 | 63.49 ± 3.17 | 20.63 ± 1.59 | 7.94 ± 3.17 | 4.76 ± 2.75 | |
| BRRI dhan47 | Cont. | 30.16 ± 1.59 | 28.57 ± 2.75 | 25.40 ± 3.17 | 23.81 ± 2.75 | 14.29 ± 2.75 |
| 45 | 60.32 ± 4.20 | 58.73 ± 3.17 | 49.21 ± 4.20 | 38.10 ± 2.75 | 26.98 ± 3.17 | |
Regeneration medium was MS + 2.0 mgL−1 BAP + 0.5 mgL−1 NAA + 1.0 mgL−1 Kin + different concentration of NaCl. In each case number of used callus was 63 in 3 replications.
Discussion
In the present study high frequency of callus induction (CI) was found in 3 Bangladeshi indica rice varieties viz. BR10, BRRI dhan32 and BRRI dhan47 (Fig. 1 and 2). Zuraida et al.44 reported that CI frequency depends on genotype, and most indica rice cultivars had poor callusing potentiality. In this case we examined the CI potentiality using 4 hormonal combinations (H1, H2, H3 and H4). Results showed that all the responding genotypes induced callus at high frequency in 2, 4-D than NAA and IAA. Makerly et al.30 recorded highest percentage of CI (41% and 37%) for Malaysian indica rice cultivars MR232 and MR220, respectively, in MS supplemented with NAA; and also reported that the varieties responded lower in 2, 4-D. Present investigation differs with their reports, and mention that in 2, 4-D studied varieties responded high to induce calli. Tiwari et al.45 reported that the optimum hormonal combination as 1.5 mgL−1 2, 4-D + 0.1 mgL−1 NAA + 0.1 mgL-1 mgL−1 BAP with MS for maximum CI. They have recorded 85% and 90% CI for indica rice varieties of Pusa Basmati1 and Kalanamak respectively. In this case, we found that BRRI dhan32 showed the highest CI capability at 64.44% and 63.61% in MS and N6 media respectively (Fig. 2). Out of responding genotypes BRRI dhan37, recorded CI was 52.78% in MS + H2 and 50.33% in N6 + H2. The results of present study defers to the previous reports with lesser frequency of CI. It might be occurred due to the effect of different media with hormonal combinations and the genetic variability of the rice genotypes.
In the experiment on viability test, the calli expressed the survival capability in salt stress condition. Soheilikhah et al.46 recorded 47-64% decreased cell viability in NaCl induced stress for Safflower (Carthamus tinctorius L.) varieties, and mentioned that the accumulation of Na+ ions and osmolytes could play an important role in osmotic adjustment in cells under saline stress. The present investigation significant differences were found among the rice genotypes on cell viability as well as the viability of the calli in NaCl induced stress. The calli of BRRI dhan47 exhibited with highest viability (45.33%) after 4 weeks cultured in 11.7 gL−1 of NaCl (Table 2). BR10 survived with 10.67% viable calli and BRRI dhan32 could not be exhibited when the calli were exposed in 11.7 gL−1 up to 4 weeks. We have observed that the phenomenon was happened due to presence of necrotic cells in the calli. A huge number of necrotic cells turned the calli deep brown or blackish in color, together with survival disability was appeared. It was observed that lower frequency of viable calli was appeared in higher salt concentration than the lower one. On spite of lesser osmotic potentiality and genotypic effect, the varieties might disable to adapt in salt stress condition. The calli of BR10 and BRRI dhan32 were begun to necrosis within a week, and after 4 weeks cultured in 11.7 gL−1 of NaCl level, a few number of calli were existed. On the other hand BRRI dhan47 could be adapted to the NaCl stress and showed highest viability in all cases of salt stresses. Based on survival feature, BRRI dhan32 and BR10 showed sensitivity to NaCl induced in vitro stress; whereas, BRRI dhan47 was appeared as tolerant in nature.
Remarkable differences were found among the genotypes examined on relative growth rate (RGR), tolerance index (TI) and relative water content (RWC). In the top level of NaCl stress (11.7 gL−1) recorded RGR values were 1.03, 0.23 and 0.11; TI were 0.20, 0.06 and 0.02; and WRC were 10.23, 7.22 and 7.03% for the genotypes BRRI dhan47, BR10 and BRRI dhan32, respectively (Fig. 3A-C). Among 3 genotypes BRRI dhan47 exhibited the highest potentiality to survive in NaCl induced abiotic stress with maximum RGR (1.03), TI (0.20), and WRC (10.23%). The recorded values of the parameters RGR, TI and WRC expressed the higher survival capability against the abiotic stress conducting the physiological activities of BRRI dhan47. On the other hand stress sensitivity was found in BRRI dhan32 and BR10 considering lower value of the parameters. RGR, TI and RWC values were decreased in higher stress level than the lower one. The phenomena might be occurred due to reduction of water availability and lose of turgor pressure (TP) in the cells of the calli. Such physiological causes were reported in previous investigation for Oryza sativa,47 Carthamus tinctorius,46,48 Saccharum sp.,9 Tagetes minuta49 and Triticum durum50,51. Errabi et al.9 mentioned that due to interference of Na+ and Cl- ions on uptake and translocation processes, nutritional imbalance might be created and the growth of callus is declined. However, NaCl treated calli of BRRI dhan47 was least affected by the highest dose of salt stress and exhibited high ability in terms of both cellular viability and growth of callus. BRRI52 mentioned that BRRI dhan47 can tolerate at 12-14 dS m−1 (approximately 7.0-8.9 gL−1) of NaCl stress and it has an ability to survive and grown in saline soil. So that it is considered as salt tolerant rice genotype. Therefore, our investigations are in agreement with the previous report for the genotype BRRI dhan47. Calli of BRRI dhan47 might accumulate less Na+ ion than salt susceptible BR10 and BRRI dhan32. In several species K+ is a major cation and contributor to adjust osmotic potential (OP) under stress condition.50,53 In salt stress Na+ concentration is increased which lead to decrease concentration of K+ among rice genotypes. As a result an imbalance of essential ions be created and cell of salt sensitive varieties could not be survived. Such reports previously mentioned in rice,47,54 sugarcane,9,55 Carthamus tinctorius46 and Cynara cardunculus.56
Before transfer the callus to regeneration medium, partial air desiccation pretreatment enhanced the frequency of plant regeneration. Rance et al.57 reported that 2-4 folds higher regeneration from 3 h desiccated calli than the control in rice genotypes viz. PN1, IR72 and IR64. Compare to undesiccated calli, 2 and 5 folds higher regeneration was recorded from 48 and 72 h desiccation in MR220 and MR232 respectively.30 Approximately similar increment of regeneration was recorded in maize,58,59 sugarcane35 and rice.29,60,61 Under this study we have recorded around 2–3 folds higher regeneration from desiccated calli in BR10, BRRI dhan32 and BRRI dhan47 (Fig. 4, Table 3). Therefore, the obtained results are in agreement with previous findings. In our study, effect of partial air desiccation to regeneration was determined in contrast of callus age. However, an effective relationship was found, and investigated that callus of lower age (within a range) need to comparatively higher period of desiccation to perform maximum regeneration. On the other hand callus of relatively higher age gave maximum regeneration when it was pretreated at lower level of desiccation. The phenomena could be depended on water content (WC) in the cells of the calli. Callus of lower age might contain a big amount of water while they need to higher desiccation in which the calli dehydrated at optimum level. Makerly et al.30 reported that the degrees of water loss differ against same desiccation period in different rice genotype, and an optimal level of water loss (partial air desiccation) could be beneficial to plant regeneration. They also noticed that regeneration varied depending on cultivars and duration of partial desiccation. In date plum cultivar 3 and 4 h partial desiccation reduced fresh weight of calli and stimulated calli growth globularization as well as embryo formation .31 Under this study recorded results showed that partial desiccation strongly influenced the regeneration and played an effective role to enhance the somatic embryogenesis. To regenerate in vitro plant for indica rice genotypes genetic effect along with the age of explants has been reported earlier by Hoque and Mansfield.27 Such effect was noticed in sugarcane,62 coffee,63 rice,64,65 Primula ssp66 However, genetic variability, optimal air desiccation and suitable age of calli might play a vital role to enhance regeneration in rice genotypes. Although at optimum level of desiccation promote the regeneration, yet over desiccation suppressed to embryo formation as well as plant regeneration. Over desiccation created the drought abiotic stress in which regeneration frequency was decreased up to 23.81% at 60 h desiccation to 6 w age of calli for BRRI dhan47 (Table 3). Kranner et al.67 reported that loss of more than 20-50% water content of the cells is been lethal to most of the higher plants. However, the optimum period of partial air desiccation pretreatment was varied on the age of calli as well as the rice genotype significantly.
At optimum period, partially desiccated calli responded of enhanced regeneration in NaCl stress condition than the controls (Table 4). The variety BRRI dhan47 performed 88.80 per cent higher regeneration than the control at the top level of NaCl (11.7 gL−1), after pretreatment of 45 h desiccation. The calli of other 2 genotypes BR10 and BRRI dhan32 were been able to regenerate at 11.7 gL−1 NaCl level; while both were not shown any regeneration without desiccation (control). Makerly et al.30 reported that regeneration capability was varied depending on the duration of desiccation. At optimal period of partial air desiccation characterized the cells of the callus to survive and adapt at adverse physiological stress. Because of reduction of water the cells of desiccated calli might acquired higher osmotic potential (OP). So that ability was developed to uptake water in salt stress condition and could be able to survive. However, in case of BRRI dhan47 partial air desiccation pretreatment was more effective to enhance the capability of regeneration in NaCl induced abiotic stress.
Conclusion
Out of studied 3 Bangladeshi indica rice genotypes, BRRI dhan32 showed better callusing and plant regeneration that might be considered for advance research in biotechnology especially in genetic transformation for varietal improvement. BRRI dhan47 expresses the tolerance features in salt stress, whereas BR10 and BRRI dhan32 showed susceptibility. At optimal level of partial air desiccation pretreatment played a positive role to enhance the plant regeneration along with increased the capability of calli adapt in NaCl stress condition for a suitable rice genotype. Desiccated calli are been enriched in osmotic potential resulting able to exist in salt stress condition.
Materials and Methods
Plant materials
For this study seeds of 3 rice varieties viz. BR10 (Progati), BRRI dhan32 and BRRI dhan47 were collected from Bangladesh Rice Research Institute (BRRI), Regional Station, Rajshahi, Bangladesh.
Methods
Sterilization of seeds and inoculation
Mature seeds were dehusked and surface sterilized with 70% (v/v) ethanol for 1 min and sodium hypochlorite (NaOCl) for 5 minutes. Seeds were then surface sterilized with 0.1% (v/v) mercuric chloride (HgCl2) for 5 min and washed 2-3 times with sterile distilled water. The seeds were then inoculated on callus induction medium (CIM) in Petri dishes. Two basal media, MS and N6, and 4 types of hormonal combinations (H1, H2, H3 and H4) were used for experimental purpose as shown in Fig. 2. Petri dishes were sealed with parafilm and incubated at 25 ± 2°C in dark for callus induction. Ten (10) days old calli were sub-cultured using the same medium and after 3 weeks callus induction (CI) frequencies were recorded. The pH of all media adjusted 5.8.
Application of NaCl to medium and data collection
Four to 5 weeks old embryogenic calli were pretreated and subjected to abiotic stress as NaCl. Four different concentrations of NaCl (2.9, 5.9, 8.8, 11.7 gL−1) were used to medium (MS + H2). Data were recorded by one week interval and up to 4 weeks of culture initiation in salt stress condition. Through visual observation, the viable calli were counted and percentage value of viability was determined as (VCn / ICn) × 100. Calli ages of 4 weeks and approximately uniform size (100 mg) were weighed individually and placed to MS medium that supplemented with different hormones and NaCl. For each treatment calli were weighed individually which was known as initial fresh weight (FWi) and cultured into vessel singly. After 4 weeks, calli were rinsed with sterile distilled water 4-5 times. Excess water of calli was sacked by blotting paper and fresh weight (FWf) was recorded. Relative growth rate (RGR) of callus was determined on fresh weight (FW) using the standard formula RGR = (FWf − FWi) / FWi followed by Smith and McComb.68 To compare variety-related responses to stress conditions, tolerance index (TI), based on RGR was computed according to formula TI = RGR treatment / RGR control as follows by Soheilikhah et al.46
Application of partial air desiccation to calli
To observe the effect of partial desiccation on plant regeneration, 4 (04) age groups of calli derived from mature seeds (3, 4, 5 and 6 w) and 5 (05) desiccation periods (15, 30, 45, 60 and 75 h) were applied to the suitable calli. For air desiccation calli were transferred to empty Petri dishes containing sterile whatman-1 filter papers followed by the standard protocol of Saharan et al.61 The petri dishes were sealed with parafilm and kept at 27 ± 1°C in dark for different desiccation period. After the pretreatment duration the calli were transferred to regeneration medium MS + 2.0 mgL−1 BAP + 0.5 mgL−1 NAA + 1.0 mgL−1 Kin.
Determination of relative water content in calli
Targeted calli were incubated at 60°C for 48 h and after drying, dry mass of calli was weighted. The relative water content (RWC) of callus was calculated using the formula (FW – DW) / DW; where FW = fresh weight, DW = dry weight and percentage value was determined following the method of Al-Khayri and Al-Bahrany.69
Data recording and statistical analysis
The average or mean values were computed from 3 replications with standard error (SE). The experiments were laid out as completely randomized design (CRD). Analysis of variance (ANOVA) and Duncan's Multiple Range Test (DMRT) were done by SPSS16.0 software. To test the homogeneity of means accordance to DMRT, percentage values of replications were used. The seed derived calli without any pretreatment were considered as control.
Funding Statement
The authors are gratefully acknowledges to CRP-ICGEB, Italy for providing research grant for this study.
Disclosure of Potential Conflicts of Interest
No potential conflicts of interest were disclosed.
Acknowledgments
ABS also gratefully acknowledges to the University Grant Commission (UGC) of Bangladesh for providing fellowship to this study. NT thanks Dr Rakhi Chaturvedi, Indian Institute of Technology Guwahati, Assam, India, for her critical suggestions on the manuscript.
References
- 1. Khush GS, Toenniessen GH. Rice Biotechnology. Int Rice Res Inst 1991; 6:VII-VIII. [Google Scholar]
- 2. Somboonwatthanaku I, Dorling S, Leung S, McManus MT. Proline biosynthetic gene expression in tissue cultures of rice (Oryza sativa L.) in response to saline treatment. Plant Cell Tiss Organ Cult 2010; 103:369-76; http://dx.doi.org/ 10.1007/s11240-010-9790-9. [DOI] [Google Scholar]
- 3. Krasensky J, Jonak C. Drought, salt, and temperature stress induced metabolic rearrangements and regulatory networks. J Exp Bot 2012; 63:1593-608; PMID:22291134; http://dx.doi.org/ 10.1093/jxb/err460. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Islam SMS, Tuteja N. Production of abiotic stress tolerant fertile transgenic plants using androgenesis and genetic transformation methods in cereal crops. In: Tuteja N, Gill SS, editors. Crop Improvement Under Adverse Conditions, Springer; New York Heidelberg London: 2013; p. 213-229. [Google Scholar]
- 5. Trivedi DK, Ansari MW, Tuteja N. Multiple abiotic stress responsive rice cyclophilin (OsCYP-25) mediates a wide range of cellular responses. Comm Integr Biol 2013; 6(5):1-7; http://dx.doi.org/ 10.4161/cib.25260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Roychoudury A, Basu S, Sarkar SN, Sengupta DN. Comparative physiological and molecular responses of a common aromatic indica rice cultivar to high salinity with non aromatic indica rice cultivars. Plant Cell Rep 2008; 27:1395-410; PMID:18509653; http://dx.doi.org/ 10.1007/s00299-008-0556-3. [DOI] [PubMed] [Google Scholar]
- 7. Yadav DK, Islam SMS, Tuteja N. Rice heterotrimeric G-protein gamma subunits (RGG1 and RGG2) are differentially regulated under abiotic stress. Plant Signaling & Behavior 2012;7 (7):733-40; PMID:22751322; http://dx.doi.org/ 10.4161/psb.20356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Rani CR, Reema C, Alka S, Singh PK. Salt tolerance of Sorghum bicolor cultivars during germination and seedling growth. Res J Recent Sci 2012; 1(3):1-10. [Google Scholar]
- 9. Errabii T, Gandonou CB, Essalmani H, Abrini J, Idaoma M, Senhaji NS. Effects of NaCl and mannitol induced stress on sugarcane (Saccharum sp.) callus cultures. Acta Physiol Plant 2007; 29:95-102; http://dx.doi.org/ 10.1007/s11738-006-0006-1. [DOI] [Google Scholar]
- 10. Haque SA. Salinity problems and crop production in coastal regions of Bangladesh. Pak J Bot 2006; 38(5):1359-1365 [Google Scholar]
- 11. Hoque ME, Ali MS, Karim NH. Embryogenic callus induction and regeneration of elite Bangladeshi Indica rice cultivars. Plant Tiss Cult Biotech 2007; 17(1):65-70. [Google Scholar]
- 12. Khatun R, Islam SMS, Bari MA. Studies on plant regeneration efficiency through in vitro micropropagation and anther culture of twenty five rice cultivars in Bangladesh. J App Sci Res 2010; 6(11):1705-11. [Google Scholar]
- 13. Chauhan H, Khurana P. Use of doubled haploid technology for development of stable drought tolerant bread wheat (Triticum aestivum L.) transgenics. Plant Biotech J 2010; 1-10. [DOI] [PubMed] [Google Scholar]
- 14. Khatun R, Islam SMS, Ara I, Tuteja N, Bari MA. Effect of cold pretreatment and different media in improving anther culture response in rece (Oryza sativa L.) in Bangladesh. Indian J Biotech 2012; 11:458-63. [Google Scholar]
- 15. Islam SMS, Ara I, Tuteja N, Subramaniam S. Efficient microspore isolation methods for high yield embryoids and regeneration in rice (Oryza sativa L.). World Aca Sci, Engi Tech (WASET), Inter J Biol Sci Engi 2013; 7(12):891-96. [Google Scholar]
- 16. Fujiwara A, Ojima K. Physiogical studies of plant roots (part-1), Influence of some environmental conditions on growth of isolated roots of rice and wheat. J Sci Soil Manure Jpn 1955; 28:9-12. [Google Scholar]
- 17. Amemiya A, Akemine H, Toriyama K. The first germinative stage and varietal differences in growth response of cultured embryos in rice plant. Bull Nat Inst Agri Sci 1956; 6:41-60. [Google Scholar]
- 18. Raina SK. Tissue culture in rice improvement: Status and potential. Advan Agro 1989; 42: 339-97; http://dx.doi.org/ 10.1016/S0065-2113(08)60529-5. [DOI] [Google Scholar]
- 19. Vasil IK. Isolation and culture of protoplasts of grasses. Int Rev Cyto Suppl 1983; 16:79-88. [Google Scholar]
- 20. Croughan TP, Chu Q. Rice (Oryza sativa L.): Establishment of callus cultures and the regeneration of plants. In: Bajaj YPS. (Ed). Biotechnology in Agriculture and Forestry, Springer Verlag, Berlin; 1991, 14:19-37. [Google Scholar]
- 21. Verma D, Joshi R, Shukla A, Kumar P. Protocol for in vitro somatic embryogenesis and regeneration of rice (Oryza sativa L.). Indian J Exp Biol 2011; 49:958-63; PMID:22403871. [PubMed] [Google Scholar]
- 22. Joyia FA, Khan MS. Scutellum-derived callus-based efficient and reproducible regeneration system for elite varieties of indica rice in Pakistan. Int J Agr Biol 2013; 15:27-33. [Google Scholar]
- 23. Jain RK. Effects of some factors on plant regeneration from indicarice cells and protoplasts- A review. Indian J Exp Biol 1997; 35:323-31. [Google Scholar]
- 24. Kyozuka J, Otoo E, Shimamoto K. Plant regeneration from protoplast of indicarice: genotype differences in culture response. Theor Appl Genet 1988; 76:887-90; PMID:24232400; http://dx.doi.org/ 10.1007/BF00273677. [DOI] [PubMed] [Google Scholar]
- 25. Islam SMS, Tuteja N. Enhancement of androgenesis by abiotic stress and other pretreatments in major crop species. Plant Sci 2012; 182:134-44; PMID:22118624; http://dx.doi.org/ 10.1016/j.plantsci.2011.10.001. [DOI] [PubMed] [Google Scholar]
- 26. Kamia M, Kamanaka H, Oono K. Intervariental variations in somatic embryogenesis in rice (Oryza sativa L.). Bull Nat Inst Aerobiol Res 1988; 4:127-51. [Google Scholar]
- 27. Hoque EH, Mansfield JW. Effect of genotype and explant age on callus induction and subsequent plant regeneration from root-derived callus of Indica rice genotypes. Plant Cell Tiss Org Cult 2004; 78:217-23; http://dx.doi.org/ 10.1023/B:TICU.0000025640.75168.2d. [DOI] [Google Scholar]
- 28. Islam MM, Ahmed M, Mahalder D. In vitro callus induction and plant regeneration in seed explants of rice (Oryza sativa L.). Res J Agr Biolo Sci 2005; 1(1):72-5. [Google Scholar]
- 29. Alam MJ, Imran M, Hassan L, Rubel MH, Shamsuddoh M. In vitro regeneration of high yielding indica rice (Oryza sativa L.) varieties. J Environ Sci. & Natural Resour 2012; 5(1):173-77. [Google Scholar]
- 30. Makerly H, Rahmata Z, Wagirana A. Potential use of partial desiccation treatment for regeneration system of (O. sativa L.). J Teknologi (Sci & Eng) 2012; 59:97-100. [Google Scholar]
- 31. Ibrahim IA, Hassan MM, Taha RA. Partial desiccation improves plant regeneration of date palm in vitro cultures. Wudpecker J Agr Res 2012; 1(6):208-14. [Google Scholar]
- 32. Tsukahara M, Hirosawa T. Simple dehydration treatment promotes plantlet regeneration of rice (Oryza sativa L.) callus. Plant Cell Rep 1992; 11:550-3; PMID:24213284; http://dx.doi.org/ 10.1007/BF00233090. [DOI] [PubMed] [Google Scholar]
- 33. Jain RK, Jain S, Wu R. Stimulatory effect of water stress on plant regeneration in aromatic indica rice varieties. Plant Cell Rep 1996; 15(6):449-54; PMID:24178428; http://dx.doi.org/ 10.1007/BF00232074. [DOI] [PubMed] [Google Scholar]
- 34. Chand S, Sahrawat AK. Stimulatory effect of partial desiccation on plant regeneration in indica rice (Oryza sativa L.). J Plant Biochem & Biotech 2001; 10:43-7; http://dx.doi.org/ 10.1007/BF03263105. [DOI] [Google Scholar]
- 35. Kaur A, Gosal SS. Desiccation of callus enhances somatic embryogenesis and subsequent shoot regeneration in sugarcane. Indian J Biotech 2009; 8:322-34. [Google Scholar]
- 36. Hammatt N, Davey MR. Somatic embryogenesis and plant regeneration from cultured zygotic embryos of soybean (Glycine max L. Merr.), J Plant Physiol 1987;128(3):219-26; http://dx.doi.org/ 10.1016/S0176-1617(87)80235-3. [DOI] [Google Scholar]
- 37. Buchheim JA, Colburn SM, Ranch JP. Maturation of soybean somatic embryos and the transition to plantlet grown. Plant Physiol 1989; 89:768-77; PMID:16666619; http://dx.doi.org/ 10.1104/pp.89.3.768. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Fazeli-Nasab B, Omidi M, Amiritokaldani M. Callus Induction and Plant Regeneration of Wheat Mature Embryos under Abscisic Acid Treatment. Int J Agr Crop Sci 2012; 4(1):17-23. [Google Scholar]
- 39. Bomal C, Tremblay FM. Effect of Desiccation to low moisture content of germination, synchronization of root emergence and plantlet regeneration of black spruce somatic embryos. Plant Cell Tiss Org Cult 1999; 56:193-200; http://dx.doi.org/ 10.1023/A:1006201414616. [DOI] [Google Scholar]
- 40. Mathews H, Schopke C, Carcamo R, Chavarriaga P. Improvement of somatic embryogenesis I and plant recovery in cassava. Plant Cell Rep 1993; 12:328-33; PMID:24197258; http://dx.doi.org/ 10.1007/BF00237429. [DOI] [PubMed] [Google Scholar]
- 41. Venkataiah P, Christopher T, Subhash K. Selection and characterization of sodium chloride and mannitol tolerant callus lines of red pepper (Capsicum annuum L.). Plant Physiology 2004; 9(2):158-63. [Google Scholar]
- 42. Gu R, Liu Q, Pie D, Jiang X. Understanding saline and osmotic tolerance of Populus euphratica suspended cells. Plant Cell Tiss Org Cult 2004; 78:261-65; http://dx.doi.org/ 10.1023/B:TICU.0000025666.84313.92. [DOI] [Google Scholar]
- 43. Ogo Y, Kakei Y, Itai RN, Kobayashi T, Nakanishi H, Nishizawa NK. Tissue-specific transcriptional profiling of iron-deficient and cadmium-stressed rice using laser capture microdissection. Plant Signaling & Behavior 2014; 9:e29427; PMID: 24893047; http://dx.doi.org/ 10.4161/psb.29427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Zuraida AR, Suri R, Zaliha WS, Sreeramanan S. Regeneration of Malaysian Indica rice (Oryza sativa L.) variety MR232 via optimized somatic embryogenesis system. J Phyto 2010; 2(3):30-8. [Google Scholar]
- 45. Tiwari AK, Shamim M, Prakash RS, Singh KDN. Plant regeneration efficiency of two scented Indica rice varieties Pusa Basmati 1 and Kalanamak. Plant Tiss Cult Biotech 2012; 22(2):163-69. [Google Scholar]
- 46. Soheilikhah Z, Karimi N, Ghasmpour HR, Zebarjadi AR. Effects of saline and mannitol induced stress on some biochemical and physiological parameters of Carthamus tinctorius L. varieties callus cultures. Aus J Crop Sci 2013; 7(12):1866-74. [Google Scholar]
- 47. Lutts S, Kinet JM, Bouharmont J. Effects of various salts and of mannitolon ion and proline accumulation in relation to osmotic adjustment in rice (Oryza sativa L.) callus cultures. J Plant Physiol 1996; 149:186-95; http://dx.doi.org/ 10.1016/S0176-1617(96)80193-3. [DOI] [Google Scholar]
- 48. Zebarjadi AR, Ghasempour HR, Soheilikhah Z. Effects of drought stress on biochemical and physiological parameters in callus cultures of Carthamus tinctorius varieties. Acta Agr Hung 2010; 58(4):395-406; http://dx.doi.org/ 10.1556/AAgr.58.2010.4.8. [DOI] [Google Scholar]
- 49. Mohamed MAH, Harris PJC, Henderson J. In vitro selection and characterization of a drought tolerant clone of Tagetes minuta. Plant Sci 2000; 159:213-22; PMID:11074274; http://dx.doi.org/ 10.1016/S0168-9452(00)00339-3. [DOI] [PubMed] [Google Scholar]
- 50. Bajji M, Lutts S, Kinet JM. Physiological changes after exposure to and recovery from polyethylene glycol-induced water deficit in callus cultures issued from durum wheat (Triticum durum Desf.) cultivars differing in drought resistance. J Plant Physiol 2000; 156: 75-83; http://dx.doi.org/ 10.1016/S0176-1617(00)80275-8. [DOI] [Google Scholar]
- 51. Lutts S, Almansouri M, Kinet JM. Salinity and water stress have contrasting effects on the relationship between growth and cell viability during and after stress exposure in durum wheat callus. Plant Sci 2004; 167:9-18; http://dx.doi.org/ 10.1016/j.plantsci.2004.02.014. [DOI] [Google Scholar]
- 52. Modern BRRI. Rice Cultivation. 16th Edt. BD, Gazipur: 2011; p. 5-11. [Google Scholar]
- 53. Santos-Diaz MS, Ochoa-Alejo N. PEG-tolerant cell clones of chilli pepper (Capsicum annuum L.): Growth, osmotic potentials and solute accumulation. Plant Cell Tiss Org Cult 1994; 37:1-8; http://dx.doi.org/ 10.1007/BF00048110. [DOI] [Google Scholar]
- 54. Basu S, Gangopadhyay G, Mukherjee BB. Salt tolerance in rice in vitro: implication of accumulation of Na+, K+ and proline. Plant Cell Tiss Org Cult 2002; 69:55-64; http://dx.doi.org/ 10.1023/A:1015028919620. [DOI] [Google Scholar]
- 55. Patade VY, Suprasanna P, Bapat VA. Effects of salt stress in relation to osmotic adjustment on sugarcane (Saccharum officinarum L.) callus cultures. Plant Growth Regul 2008; 55:169-73; http://dx.doi.org/ 10.1007/s10725-008-9270-y. [DOI] [Google Scholar]
- 56. Benlloch-González M, Fournier JM, Ramos J, Benlloch M. Strategies underlying salt tolerance in halophytes are present in Cynara cardunculus. Plant Sci 2005; 168:653-59; http://dx.doi.org/ 10.1016/j.plantsci.2004.09.035. [DOI] [Google Scholar]
- 57. Rance IM, Tian W, Mathews H, Kochko AD, Fauquet C. Partial desiccation of mature embryo-derived calli, a simple treatment that dramatically enhances the regeneration ability of indica rice. Plant Cell Rep 1994; 13:647-51; PMID:24196246; http://dx.doi.org/ 10.1007/BF00232938. [DOI] [PubMed] [Google Scholar]
- 58. Deng S, Dong Z, Zhan K, Yanmin H, Yin D, Cui D. Moderate desiccation dramatically improves shoot regeneration from maize (Zea mays L.) callus. In Vitro Cell Dev Biol 2009; 45:99-103. [Google Scholar]
- 59. Stipešević B, Kladivko EJ. Effects of winter wheat cover crop desiccation times on soil moisture, temperature and early maize growth. Plant Soil Environ 2005; 51(6):255-61. [Google Scholar]
- 60. Biswas A, Mandal AB. Plant regeneration in different genotypes of indica rice. Indian J Biotech 2007; 6:532-40. [Google Scholar]
- 61. Saharan V, Yadav RC, Yadav NR, Chapagain BP. High frequency plant regeneration from desiccated calli of indica rice (Oryza sativa L.), African J Biotech 2004; 3(5):256-59. [Google Scholar]
- 62. Gandonou, Errabii T, Abrini J, Idaomar M, Chibi F, Skali S. Effect of genotype on callus induction andplant regeneration from leaf explants of sugarcane (Saccharum sp.). African J Biotech 2005; 4:1250-55. [Google Scholar]
- 63. Molina MD, Aponte EM, Cortina H, Moreno G. The effect of genotype and explant age on somatic embryogenesis of coffee. Plant Cell Tiss Org Cult 2000; 71:117-23; http://dx.doi.org/ 10.1023/A:1019965621041. [DOI] [Google Scholar]
- 64. Beena C. Genetic effect on the regeneration of the calli of rice. Indian J Crop Sci 2006; 1(1-2):207-8. [Google Scholar]
- 65. Katiyar SR, Chandel G, Singh P, Pratibha R. Genetic variation and effect of 2,4-D in in vitro plant regeneration in indica rice cultivars. Oryza 1999; 36(3):254-6. [Google Scholar]
- 66. Schween G, Schwenkel HG. Effect of genotype on callus induction, shoot regeneration, and phenotypic stability of regenerated plants in greenhouse of Primula ssp. Plant Cell Tiss Org Cult 2003; 72:53-61; http://dx.doi.org/ 10.1023/A:1021227414880. [DOI] [Google Scholar]
- 67. Kranner I, Beckett RP, Wornik S, Zorn M, Pfeifhofer HW. Revival of a resurrection plant correlates with its antioxidant status. Plant J 2002; 31:13-24; PMID:12100479; http://dx.doi.org/ 10.1046/j.1365-313X.2002.01329.x. [DOI] [PubMed] [Google Scholar]
- 68. Smith MK, McComb JA. Use of callus culture to detect NaCl tolerance in cultivars of three species of pasture legumes. Aus. J Plant Physiol 1981; 8:437-42. [Google Scholar]
- 69. Al-Khayri JM, Al-Bahrany AM. Growth, water content and proline accumulation in drought stressed callus of date palm. Biol Plant 2004; 48:105-8; http://dx.doi.org/ 10.1023/B:BIOP.0000024283.74919.4c. [DOI] [Google Scholar]